Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics
Abstract
1. Introduction
2. Materials and Experimental Procedure
3. Results and Discussion
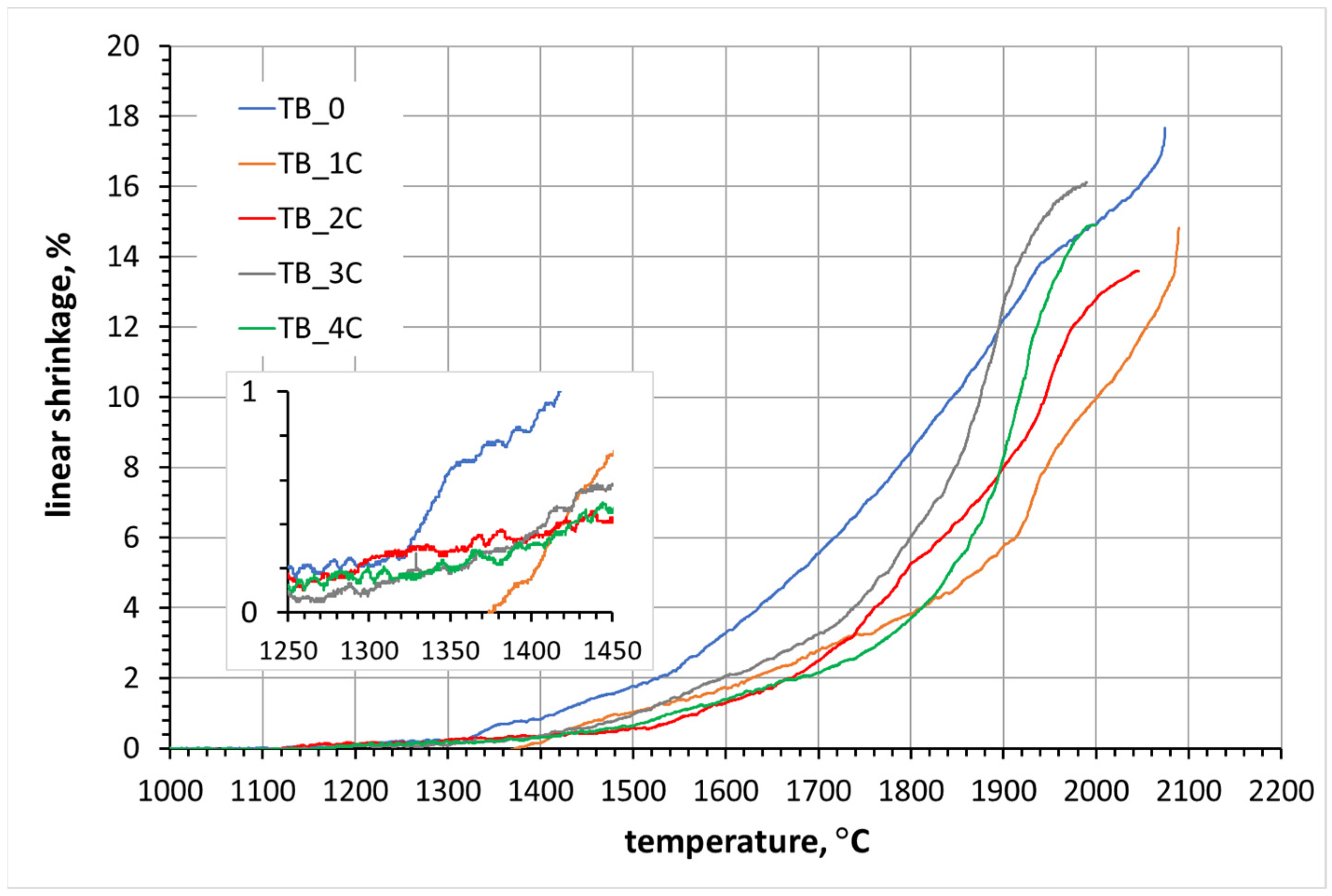
3.1. Dilatometric Analysis
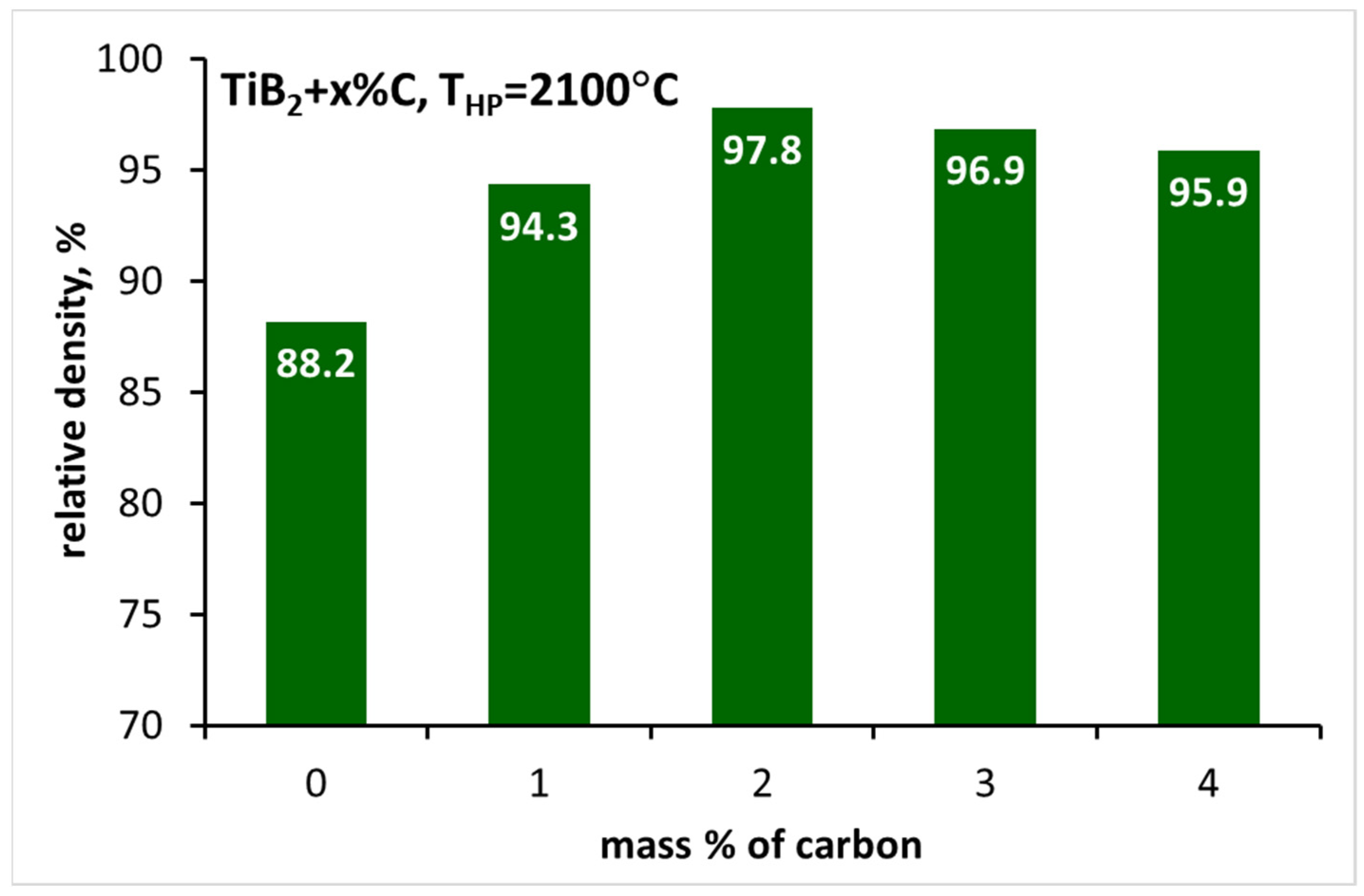
3.2. Sintering of TiB2 with Various Amounts of Carbon
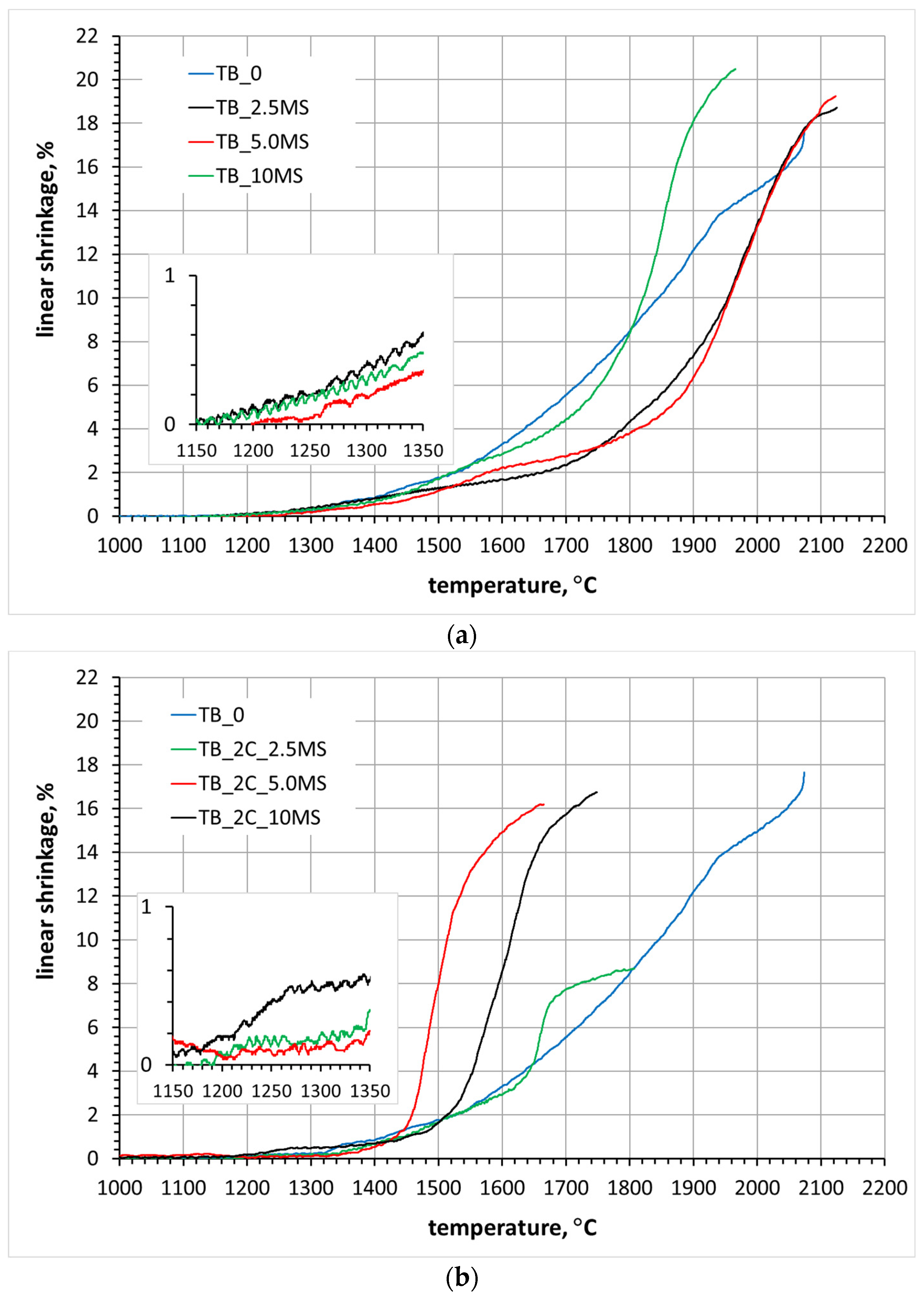
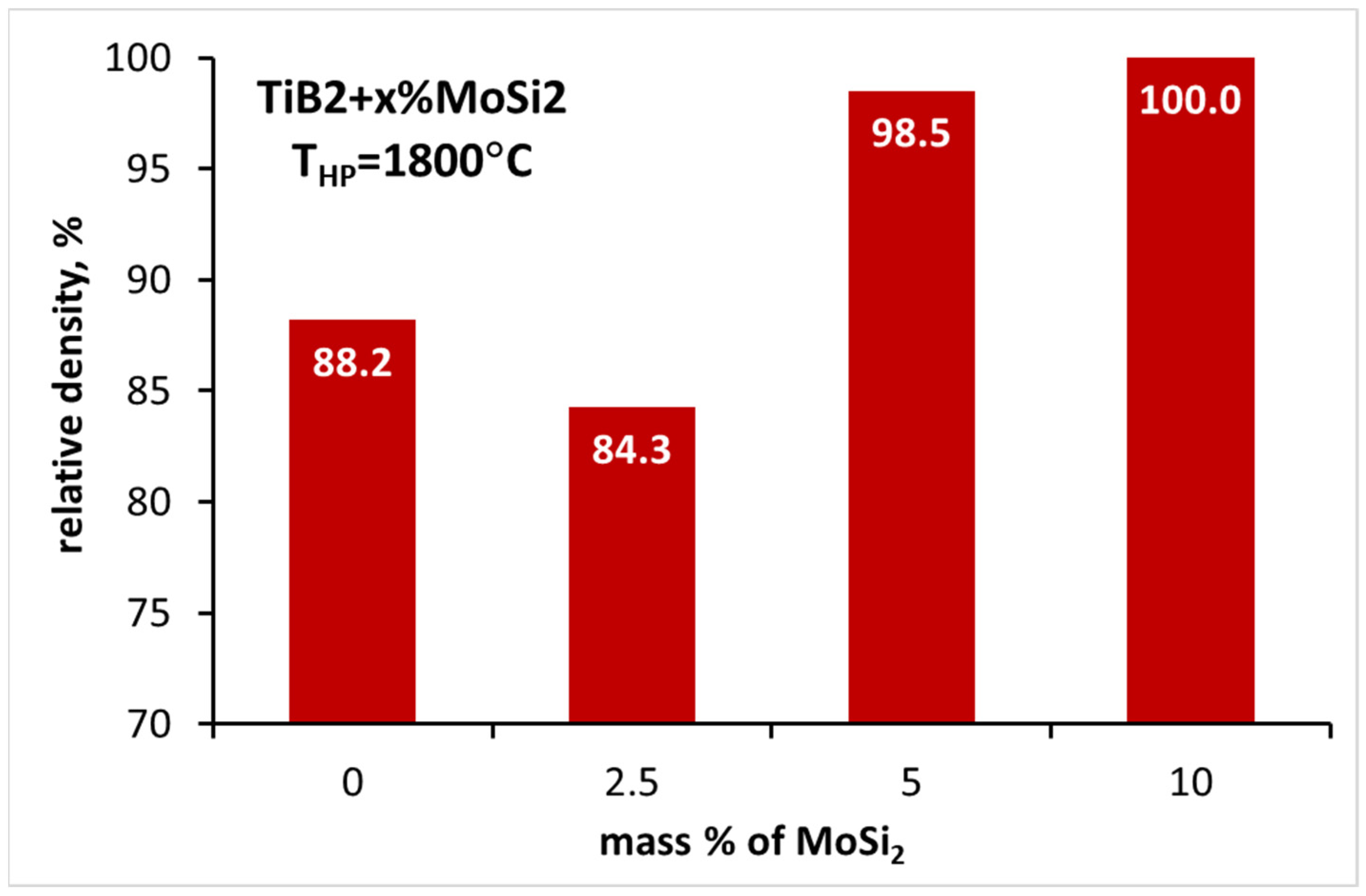
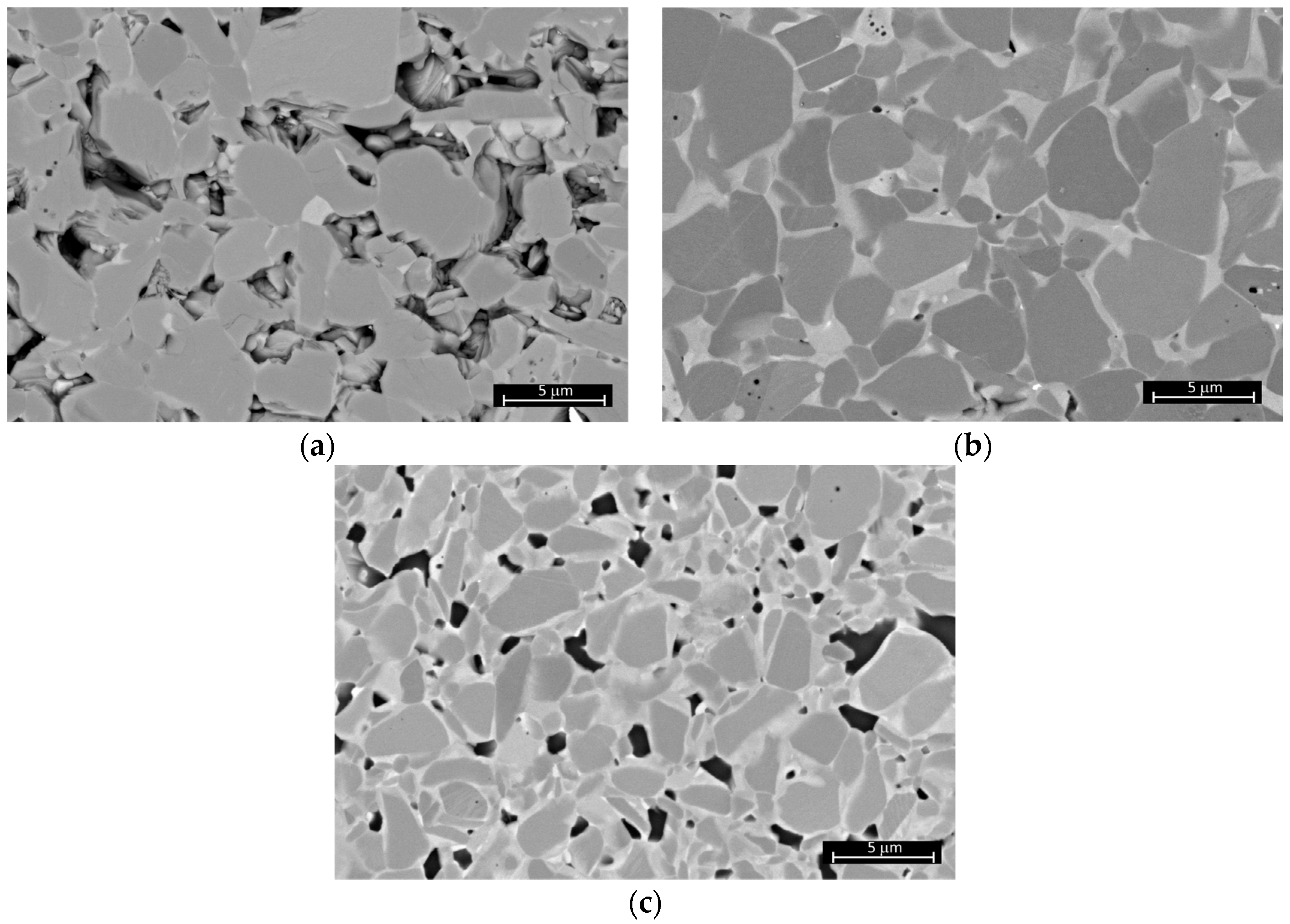
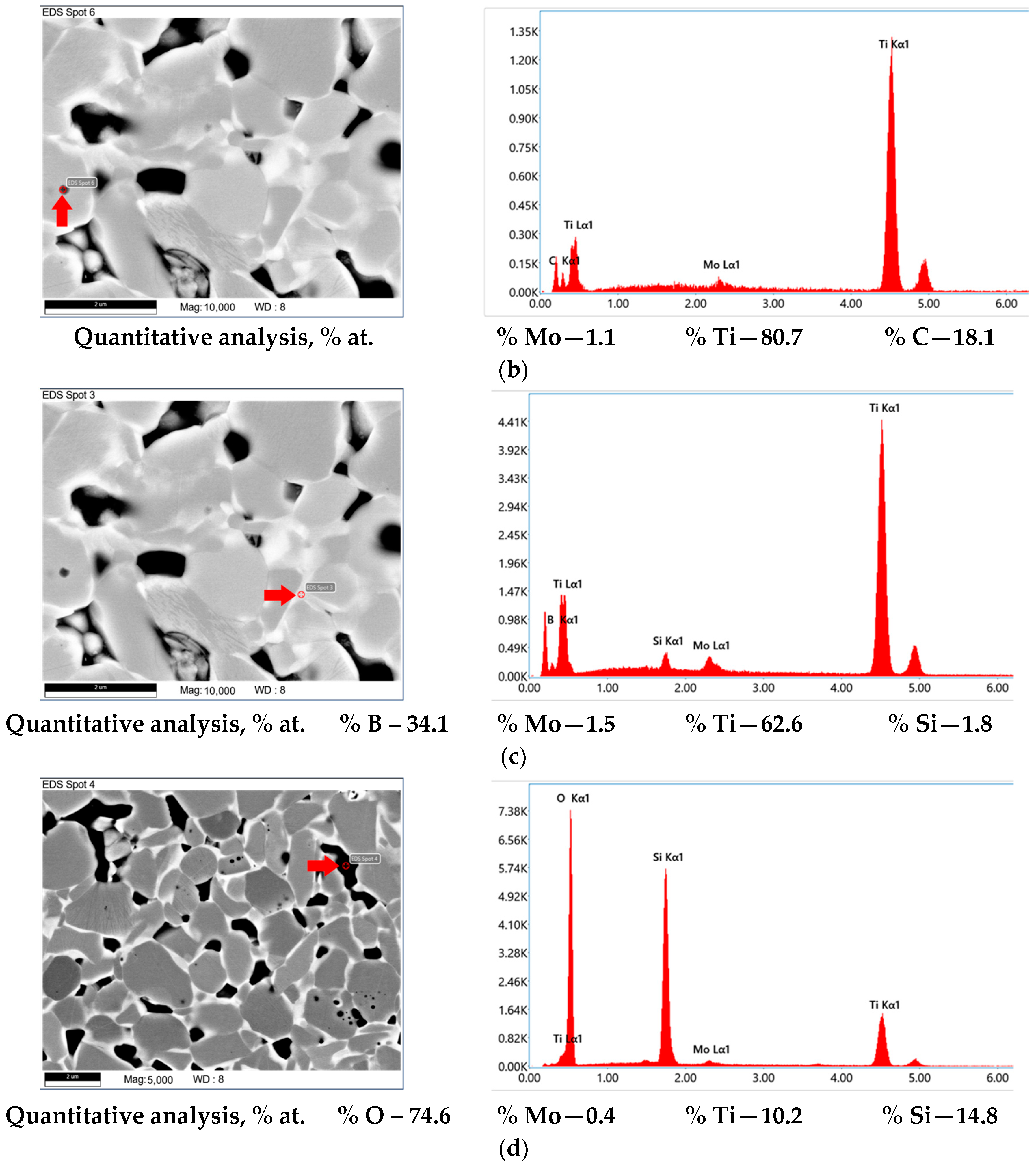
3.3. Sintering of TiB2 with Various Amounts of MoSi2
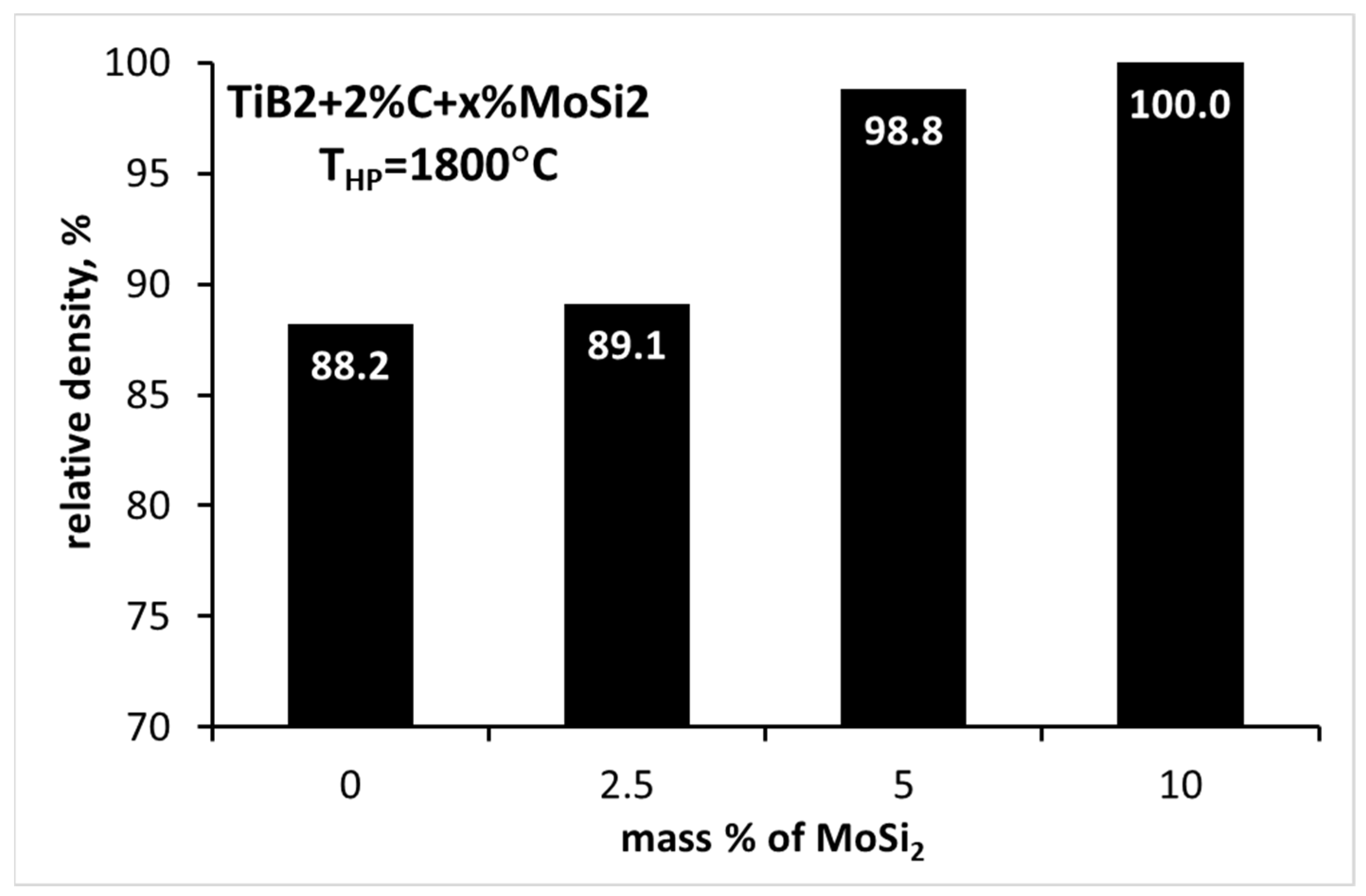
3.4. Sintering of TiB2 with 2 wt.% Carbon Addition and Various Amounts of MoSi2
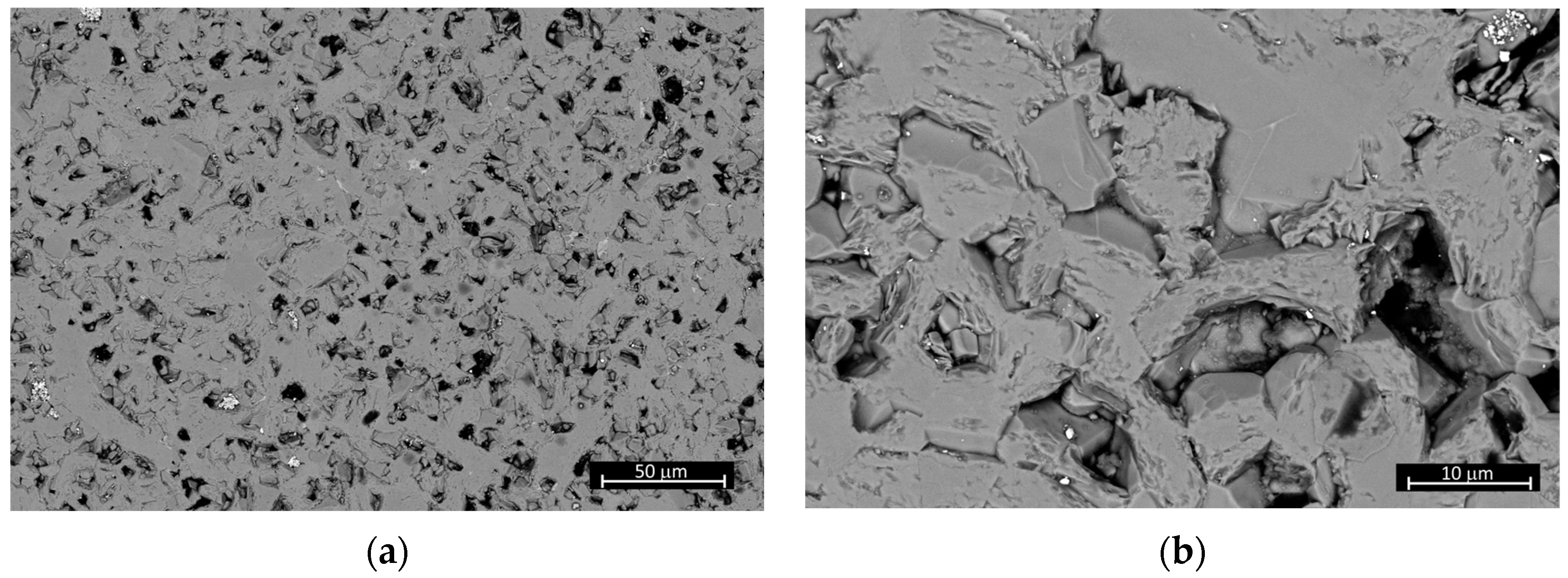
3.5. Mechanical Properties of the TiB2-MoSi2-C Ceramics
4. Results and Discussion
5. Conclusions
- 1.
- The hot pressing of TiB2 with MoSi2 or carbon and MoSi2 with carbon resulted in single-phase polycrystals and composites with density higher than 95%. Both additives used can be considered as TiB2 sintering activators.
- 2.
- It is noteworthy that the use of MoSi2 as a sintering activating additive significantly reduces the sintering temperature of titanium boride down to 1800 °C.
- 3.
- With the addition of carbon, it is possible to obtain single-phase polycrystals, in which only the TiB2 phase is present. On the other hand, with the addition of MoSi2 as well as the combined addition of MoSi2 and carbon, it is possible to obtain solid composites with a core–shell microstructure, characteristic for cermets.
- 4.
- Carbon is an effective reducer of oxide impurities during TiB2 sintering.
- 5.
- Due to the presence of liquid phases from the Si–B–O–Mo system and plastic deformation of MoSi2, it becomes possible to obtain dense composites based on TiB2.
- 6.
- The produced materials have potential in high-temperature applications due to the high melting point of TiB2 and very good properties, such as high hardness, high fracture toughness and low deformability. Nevertheless, further testing of their thermal and chemical properties is required.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, B.; Raju, G.B.; Suri, A.K. Processing and Properties of Monolithic TiB2 Based Materials. Int. Mater. Rev. 2006, 51, 352–374. [Google Scholar] [CrossRef]
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on Ultra-High Temperature Boride Ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-High Temperature Ceramics: Materials for Extreme Environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Basu, B.; Balasubramaniam, R.; Suri, A.K.; Subramanian, C.; Fotedar, R.K. Processing and Properties of TiB2 with MoSi2 Sinter-Additive: A First Report. J. Am. Ceram. Soc. 2006, 89, 131–138. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A. Efficacy of HfN as Sintering Aid in the Manufacture of Ultrahigh-Temperature Metal Diborides-Matrix Ceramics. J. Mater. Res. 2004, 19, 3576–3585. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A. Effect of the Addition of Silicon Nitride on Sintering Behaviour and Microstructure of Zirconium Diboride. Scr. Mater. 2002, 46, 223–228. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A. Beneficial Effects of AlN as Sintering Aid on Microstructure and Mechanical Properties of Hot-pressed ZrB2. Adv. Eng. Mater. 2003, 5, 508–512. [Google Scholar] [CrossRef]
- Li, L.-H.; Kim, H.-E.; Son Kang, E. Sintering and Mechanical Properties of Titanium Diboride with Aluminum Nitride as a Sintering Aid. J. Eur. Ceram. Soc. 2002, 22, 973–977. [Google Scholar] [CrossRef]
- Park, J.; Koh, Y.; Kim, H.; Hwang, C.S.; Kang, E.S. Densification and Mechanical Properties of Titanium Diboride with Silicon Nitride as a Sintering Aid. J. Am. Ceram. Soc. 1999, 82, 3037–3042. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Divakar, C.; Singh, A.K.; Upadhyaya, G.S. Synthesis and Sintering of TiB2 and TiB2–TiC Composite under High Pressure. Mater. Sci. Eng. A 2000, 279, 275–281. [Google Scholar] [CrossRef]
- Kang, E.S.; Kim, C.H. Improvements in Mechanical Properties of TiB2 by the Dispersion of B4C Particles. J. Mater. Sci. 1990, 25, 580–584. [Google Scholar] [CrossRef]
- Kang, E.S.; Jang, C.W.; Lee, C.H.; Kim, C.H.; Kim, D.K. Effect of Iron and Boron Carbide on the Densification and Mechanical Properties of Titanium Diboride Ceramics. J. Am. Ceram. Soc. 1989, 72, 1868–1872. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, F.; Zamora, V.; Guiberteau, F.; Ortiz, A.L. Solid-State Spark Plasma Sintering of Super Wear Resistant B4C–SiC–TiB2 Triplex-Particulate Composites. Ceram. Int. 2023, 49, 5532–5537. [Google Scholar] [CrossRef]
- Torizuka, S.; Sato, K.; Harada, J.; Yamamoto, H.; Nishio, H. Microstructure and Sintering Mechanism of TiB2-ZrO2-SiC Composite. J. Ceram. Soc. Jpn. 1992, 100, 392–397. [Google Scholar] [CrossRef]
- Torizuka, S.; Sato, K.; Nishio, H.; Kishi, T. Effect of SiC on Interfacial Reaction and Sintering Mechanism of TiB2. J. Am. Ceram. Soc. 1995, 78, 1606–1610. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Raju, G.B.; Basu, B. Understanding Influence of MoSi2 Addition (5 Weight Percent) on Tribological Properties of TiB2. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2998–3013. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Balasubramaniam, R.; Basu, B.; Suri, A.K.; Mungole, M.N. Oxidation of Monolithic TiB2 and TiB2–20wt.% MoSi2 Composite at 850 °C. J. Eur. Ceram. Soc. 2006, 26, 187–192. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Subramanian, C.; Fotedar, R.K.; Kumar, S.; Gonal, M.R.; Suri, A.K. A New TiB2+CrSi2 Composite—Densification, Characterization and Oxidation Studies. Int. J. Refract. Met. Hard. Mater. 2010, 28, 529–540. [Google Scholar] [CrossRef]
- Raju, G.B.; Basu, B. Densification, Sintering Reactions, and Properties of Titanium Diboride With Titanium Disilicide as a Sintering Aid. J. Am. Ceram. Soc. 2007, 90, 3415–3423. [Google Scholar] [CrossRef]
- Raju, G.B.; Mukhopadhyay, A.; Biswas, K.; Basu, B. Densification and High-Temperature Mechanical Properties of Hot Pressed TiB2-(0-10 Wt.%) MoSi2 Composites. Scr. Mater. 2009, 61, 674–677. [Google Scholar] [CrossRef]
- Silvestroni, L.; Kleebe, H.-J.; Lauterbach, S.; Müller, M.; Sciti, D. Transmission Electron Microscopy on Zr- and Hf-Borides with MoSi2 Addition: Densification Mechanisms. J. Mater. Res. 2010, 25, 828–834. [Google Scholar] [CrossRef]
- Sciti, D.; Silvestroni, L.; Celotti, G.; Melandri, C.; Guicciardi, S. Sintering and Mechanical Properties of ZrB2–TaSi2 and HfB2–TaSi2 Ceramic Composites. J. Am. Ceram. Soc. 2008, 91, 3285–3291. [Google Scholar] [CrossRef]
- Silvestroni, L.; Sciti, D. Densification of ZrB2-TaSi2 and HfB2-TaSi2 Ultra-High-Temperature Ceramic Composites. J. Am. Ceram. Soc. 2011, 94, 1920–1930. [Google Scholar] [CrossRef]
- Watanahe, T.; Shoubu, K. Mechanical Properties of Hot-Pressed TiB2-ZrO2 Composites. J. Am. Ceram. Soc. 1985, 68, C-34. [Google Scholar] [CrossRef]
- Telle, R.; Meyer, S.; Petzow, G.; Franz, E.D. Sintering Behaviour and Phase Reactions of TiB2 with ZrO2 Additives. Mater. Sci. Eng. A 1988, 105–106, 125–129. [Google Scholar] [CrossRef]
- Schneider, J.; Zum Gahr, K.-H.; Müller, R.; Franz, E.-D. Einfluß Des ZrO22 -Zusatzes Auf Mechanische Eigenschaften Und Den Ungeschmierten Gleitverschleiß von TiB2-ZrO2-Mischkeramiken. Materwiss Werksttech 1996, 27, 359–366. [Google Scholar] [CrossRef]
- Muraoka, Y.; Yoshinaka, M.; Hirota, K.; Yamaguchi, O. Hot Isostatic Pressing of TiB2-ZrO2(2 Mol% Y2O3) Composite Powders. Mater. Res. Bull. 1996, 31, 787–792. [Google Scholar] [CrossRef]
- Graziani, T.; Bellosi, A. Sintering and Characterization of TiB2-B4C-Zr02 Composites. Mater. Manuf. Process. 1994, 9, 767–780. [Google Scholar] [CrossRef]
- Stobierski, L.; Gubernat, A. Sintering of Silicon CarbideI. Effect of Carbon. Ceram. Int. 2003, 29, 287–292. [Google Scholar] [CrossRef]
- Gubernat, A. Pressureless Sintering of Single-Phase Tantalum Carbide and Niobium Carbide. J. Eur. Ceram. Soc. 2013, 33, 2391–2398. [Google Scholar] [CrossRef]
- Kornaus, K.; Rączka, M.; Gubernat, A.; Zientara, D. Pressureless Sintering of Binderless Tungsten Carbide. J. Eur. Ceram. Soc. 2017, 37, 4567–4576. [Google Scholar] [CrossRef]
- Zhang, S.C.; Hilmas, G.E.; Fahrenholtz, W.G. Pressureless Sintering of ZrB2–SiC Ceramics. J. Am. Ceram. Soc. 2008, 91, 26–32. [Google Scholar] [CrossRef]
- Zhu, S.; Fahrenholtz, W.G.; Hilmas, G.E.; Zhang, S.C. Pressureless Sintering of Zirconium Diboride Using Boron Carbide and Carbon Additions. J. Am. Ceram. Soc. 2007, 90, 3660–3663. [Google Scholar] [CrossRef]
- Khoeini, M.; Nemati, A.; Zakeri, M.; Tamizifar, M.; Samadi, H. Comprehensive Study on the Effect of SiC and Carbon Additives on the Pressureless Sintering and Microstructural and Mechanical Characteristics of New Ultra-High Temperature ZrB2 Ceramics. Ceram. Int. 2015, 41, 11456–11463. [Google Scholar] [CrossRef]
- Brown-Shaklee, H.J.; Fahrenholtz, W.G.; Hilmas, G.E. Densification Behavior and Microstructure Evolution of Hot-Pressed HfB2. J. Am. Ceram. Soc. 2011, 94, 49–58. [Google Scholar] [CrossRef]
- ICSD (Inorganic Crystal Structure Database) Card 98-003-0330 of Titanium Boride, (n.d.). Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 1 February 2024).
- Singh, M.; Wiedemeier, H. Chemical Interactions in Diboride-Reinforced Oxide-Matrix Composites. J. Am. Ceram. Soc. 1991, 74, 724–727. [Google Scholar] [CrossRef]
- Harrington, G.J.K.; Hilmas, G.E.; Fahrenholtz, W.G. Effect of Carbon and Oxygen on the Densification and Microstructure of Hot Pressed Zirconium Diboride. J. Am. Ceram. Soc. 2013, 96, 3622–3630. [Google Scholar] [CrossRef]
- BAIK, S.; BECHER, P.F. Effect of Oxygen Contamination on Densification of TiB2. J. Am. Ceram. Soc. 1987, 70, 527–530. [Google Scholar] [CrossRef]
- García, J.; Collado Ciprés, V.; Blomqvist, A.; Kaplan, B. Cemented Carbide Microstructures: A Review. Int. J. Refract. Met. Hard. Mater. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, Z.; Dong, S.; Jiang, D. Pressureless Sintering of High-Density ZrB2 –SiC Ceramic Composites. J. Am. Ceram. Soc. 2006, 89, 3589–3592. [Google Scholar] [CrossRef]
- 8.2: Atomic and Ionic Radius, (n.d.). Available online: https://Chem.Libretexts.Org/@go/Page/98634?Pdf (accessed on 14 October 2023).
- Paul, T.R.; Mondal, M.K.; Mallik, M. Densification Behavior of ZrB2–MoSi2–SiCw Composite Processed by Multi Stage Spark Plasma Sintering. Ceram. Int. 2021, 47, 31948–31972. [Google Scholar] [CrossRef]
- Monteverde, F.; Grohsmeyer, R.J.; Stanfield, A.D.; Hilmas, G.E.; Fahrenholtz, W.G. Densification Behavior of ZrB2-MoSi2 Ceramics: The Formation and Evolution of Core-Shell Solid Solution Structures. J. Alloys Compd. 2019, 779, 950–961. [Google Scholar] [CrossRef]
- Sciti, D.; Monteverde, F.; Guicciardi, S.; Pezzotti, G.; Bellosi, A. Microstructure and Mechanical Properties of ZrB2–MoSi2 Ceramic Composites Produced by Different Sintering Techniques. Mater. Sci. Eng. A 2006, 434, 303–309. [Google Scholar] [CrossRef]
- Hu, D.-L.; Gu, H.; Zou, J.; Zheng, Q.; Zhang, G.-J. Core–rim Structure, Bi-Solubility and a Hierarchical Phase Relationship in Hot-Pressed ZrB2–SiC–MC Ceramics (M = Nb, Hf, Ta, W). J. Mater. 2021, 7, 69–79. [Google Scholar] [CrossRef]
- Rabiezadeh, A.; Hadian, A.M.; Ataie, A. Synthesis and Sintering of TiB2 Nanoparticles. Ceram. Int. 2014, 40, 15775–15782. [Google Scholar] [CrossRef]
- Mashhadi, M.; Shambuli, M.; Safi, S. Effect of MoSi2 Addition and Particle Size of SiC on Pressureless Sintering Behavior and Mechanical Properties of ZrB2–SiC–MoSi2 Composites. J. Mater. Res. Technol. 2016, 5, 200–205. [Google Scholar] [CrossRef]
- Silvestroni, L.; Failla, S.; Neshpor, I.; Grigoriev, O. Method to Improve the Oxidation Resistance of ZrB2 -Based Ceramics for Reusable Space Systems. J. Eur. Ceram. Soc. 2018, 38, 2467–2476. [Google Scholar] [CrossRef]
- Suri, A.K.; Krishnamurthy, N.; Subramanian, C. Issues in the Synthesis and Fabrication of Refractory Carbides, Borides, Silicides and Their Mixtures. Ceram. Eng. Sci. Proc. 2010, 30, 69–80. [Google Scholar]
- Dasgupta, T.; Etourneau, J.; Chevalier, B.; Matar, S.F.; Umarji, A.M. Structural, Thermal, and Electrical Properties of CrSi2. J. Appl. Phys. 2008, 103, 113516. [Google Scholar] [CrossRef]
- Rosenkranz, R.; Frommeyer, G. Microstructures and Properties of the Refractory Compounds TiSi2 and ZrSi2. Int. J. Mater. Res. 1992, 83, 685–689. [Google Scholar] [CrossRef]
- Jeng, Y.-L.; Lavernia, E.J. Processing of Molybdenum Disilicide. J. Mater. Sci. 1994, 29, 2557–2571. [Google Scholar] [CrossRef]
- Nakano, T.; Hagihara, K.; Nakai, Y.; Umakoshi, Y. Plastic Deformation Behavior of NbSi2/MoSi2 Crystals with Oriented Lamellae. Intermetallics 2006, 14, 1345–1350. [Google Scholar] [CrossRef]
- Inui, H.; Moriwaki, M.; Ando, S.; Yamaguchi, M. Plastic Deformation of Single Crystals of CrSi2 with the C40 Structure. Mater. Sci. Eng. A 1997, 239–240, 63–68. [Google Scholar] [CrossRef]
- Fu, Z.; Koc, R. Microstructure and Mechanical Properties of Hot Pressed Submicron TiB2 Powders. Ceram. Int. 2018, 44, 9995–9999. [Google Scholar] [CrossRef]









| The Initial Composition | Name |
|---|---|
| TiB2 | TB_0 |
| TiB2 + 1% C | TB_1C |
| TiB2 + 2% C | TB_2C |
| TiB2 + 3% C | TB_3C |
| TiB2 + 4% C | TB_4C |
| TiB2 + 2.5% MoSi2 | TB_2.5MS |
| TiB2 + 5% MoSi2 | TB_5MS |
| TiB2 + 10% MoSi2 | TB_10MS |
| TiB2 + 2% C + 2.5% MoSi2 | TB_2C_2.5MS |
| TiB2 + 2% C + 5% MoSi2 | TB_2C_5MS |
| TiB2 + 2% C + 10% MoSi2 | TB_2C_10MS |
| ICSInitial Phase Composition, wt.% | Phase Composition of the HP Sinters, wt.% |
|---|---|
| 97.5% TiB2, 2.5% MoSi2 | 68.8% TiB2 1, 29.7% TiB2 2, 1.5% MoC |
| 95% TiB2, 5.0% MoSi2 | 69.8% TiB2 1, 28.7% TiB2 2, 1.5% MoC |
| 90% TiB2, 10% MoSi2 | 83.0% TiB2 1, 9.4% TiB2 2, 1.0% MoC, 6.6% MoSi2 |
| Lattice Parameter, Å | Theoretical Unit Cell Parameters of TiB2, [36] | TiB2 + 2.5% MoSi2 | TiB2 + 5.0% MoSi2 | TiB2 + 10% MoSi2 | |||
|---|---|---|---|---|---|---|---|
| TiB2 1 | TiB2 2 | TiB2 1 | TiB2 2 | TiB2 1 | TiB2 2 | ||
| a | 3.028 | 3.030 | 3.028 | 3.030 | 3.030 | 3.029 | 3.029 |
| b | 3.028 | 3.030 | 3.028 | 3.030 | 3.030 | 3.029 | 3.029 |
| c | 3.228 | 3.230 | 3.230 | 3.230 | 3.231 | 3.231 | 3.230 |
| Initial Phase Composition, wt.% | Phase Composition of the HP Sinters, wt.% |
|---|---|
| 95.5% TiB2, 2.0% C, 2.5% MoSi2 | 96.8% TiB2 1, 0.1% TiB2 2, 1.7% TiC, 1.4% SiC |
| 93% TiB2, 2.0% C, 5.0% MoSi2 | 66.3% TiB2 1, 28.9% TiB2 2, 1.8% SiC, 3.0% (Ti,Mo)C2 |
| 88% TiB2, 2.0% C, 10% MoSi2 | 76.1% TiB2 1, 18.6% TiB2 2, 3.1% SiC, 2.2% (Ti,Mo)C2 |
| Lattice Parameter, Å | Theoretical Unit Cell Parameters of TiB2, [36] | TiB2 + 2%C +2.5% MoSi2 | TiB2 + 2%C +5.0% MoSi2 | TiB2 + 2%C +10% MoSi2 | |||
|---|---|---|---|---|---|---|---|
| TiB2 1 | TiB2 2 | TiB2 1 | TiB2 2 | TiB2 1 | TiB2 2 | ||
| a | 3.028 | 3.027 | 3.034 | 3.029 | 3.027 | 3.029 | 3.027 |
| b | 3.028 | 3.027 | 3.034 | 3.029 | 3.027 | 3.029 | 3.027 |
| c | 3.228 | 3.233 | 3.220 | 3.230 | 3.231 | 3.230 | 3.232 |
| Sample | Sintering Temperature (HP), °C | Relative Density, % (*) | Vickers Hardness, GPa | Fracture Toughness, MPa·m0.5 | Young’s Modulus, GPa |
|---|---|---|---|---|---|
| TB_0 | 2150 | 88.2 ± 0.3 | 19.09 ± 6.30 | - | - |
| TB_1C | 2150 | 94.3 ± 0.1 | 26.31 ± 5.86 | - | 526 ± 12 |
| TB_2C | 2150 | 97.8 ± 0.1 | 25.31 ± 0.77 | 5.16 ± 0.28 | 536 ± 9 |
| TB_3C | 2150 | 96.9 ± 0.4 | 23.34 ± 2.17 | 5.26 ± 0.47 | 496 ± 16 |
| TB_4C | 2150 | 95.9 ± 0.3 | 25.68 ± 5.29 | 5.52 ± 0.20 | 542 ± 10 |
| TB_2.5MS | 1800 | 84.3 ± 0.6 | 16.97 ± 2.86 | - | - |
| TB_5MS | 1800 | 98.5 ± 0.2 | 26.21 ± 2.25 | 6.25 ± 0.51 | 536 ± 11 |
| TB_10MS | 1800 | 100.0 ± 0.4 | 26.78 ± 3.37 | 4.86 ± 0.19 | 504 ± 24 |
| TB_2C_2.5MS | 1800 | 89.1 ± 0.8 | 17.19 ± 1.87 | - | 440 ± 14 |
| TB_2C_5MS | 1800 | 98.8 ± 0.4 | 24.88 ± 2.03 | 4.79 ± 0.52 | 543 ± 6 |
| TB_2C_10MS | 1800 | 100.0 ± 0.2 | 24.41 ± 1.90 | 4.17 ± 0.31 | 533 ± 12 |
| Sample | Water Asorbability, % |
|---|---|
| TB_0 | 0.03 ± 0.01 |
| TB_1C | 0.03 ± 0.01 |
| TB_2C | 0.01 ± 0.02 |
| TB_3C | 0.15 ± 0.04 |
| TB_4C | 0.08 ± 0.03 |
| TB_2.5MS | 3.69 ± 0.50 |
| TB_5MS | 0.05 ± 0.02 |
| TB_10MS | 0.04 ± 0.01 |
| TB_2C_2.5MS | 1.29 ± 0.20 |
| TB_2C_5MS | 0.08 ± 0.02 |
| TB_2C_10MS | 0.06 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajdak, M.; Kornaus, K.; Zientara, D.; Moskała, N.; Komarek, S.; Momot, K.; Golis, E.; Zych, Ł.; Gubernat, A. Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics. Crystals 2024, 14, 212. https://doi.org/10.3390/cryst14030212
Sajdak M, Kornaus K, Zientara D, Moskała N, Komarek S, Momot K, Golis E, Zych Ł, Gubernat A. Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics. Crystals. 2024; 14(3):212. https://doi.org/10.3390/cryst14030212
Chicago/Turabian StyleSajdak, Maria, Kamil Kornaus, Dariusz Zientara, Norbert Moskała, Sebastian Komarek, Kinga Momot, Edmund Golis, Łukasz Zych, and Agnieszka Gubernat. 2024. "Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics" Crystals 14, no. 3: 212. https://doi.org/10.3390/cryst14030212
APA StyleSajdak, M., Kornaus, K., Zientara, D., Moskała, N., Komarek, S., Momot, K., Golis, E., Zych, Ł., & Gubernat, A. (2024). Processing, Microstructure and Mechanical Properties of TiB2-MoSi2-C Ceramics. Crystals, 14(3), 212. https://doi.org/10.3390/cryst14030212






