Tribological Behavior and Wear Mechanism of Cu-SiO2 Sintered Composite under Different Sliding Speeds
Abstract
:1. Introduction
2. Materials and Methods
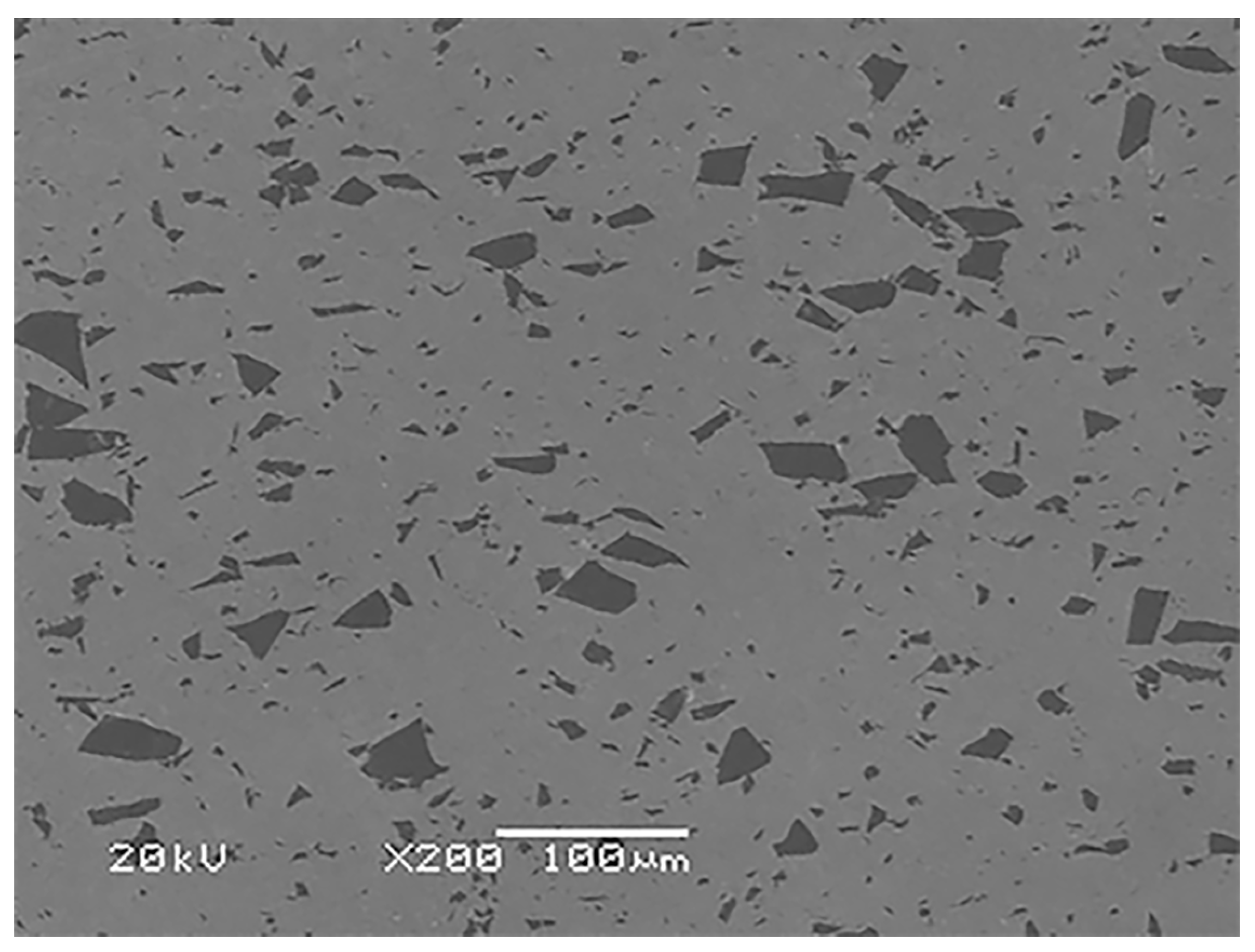
2.1. Materials
2.2. Sliding Wear Tests
2.3. Morphology and Composition Characterization
3. Results
3.1. Friction Coefficient, Wear Rate and Bulk Temperature
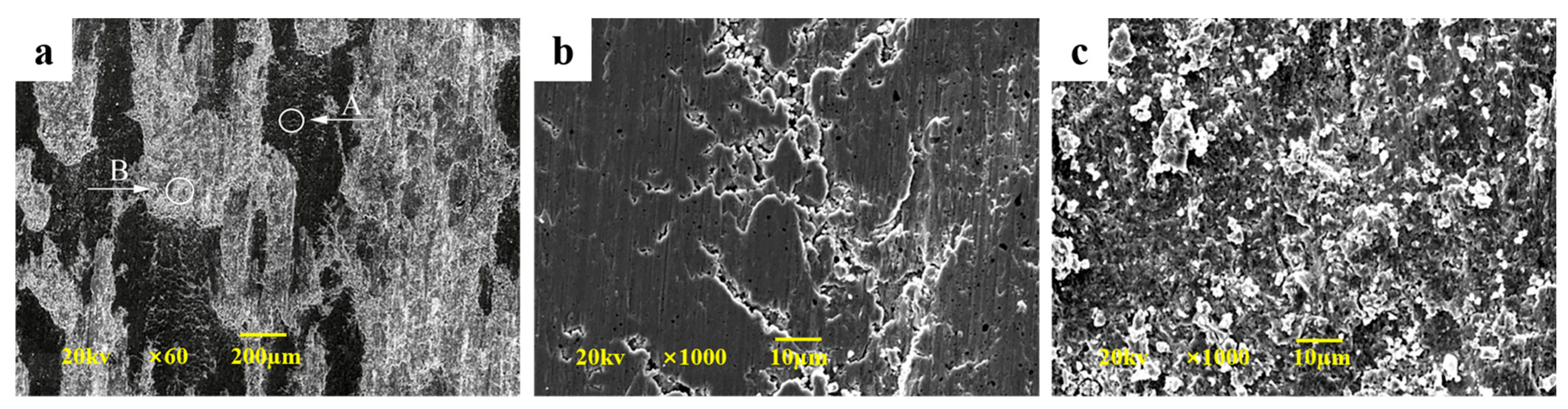
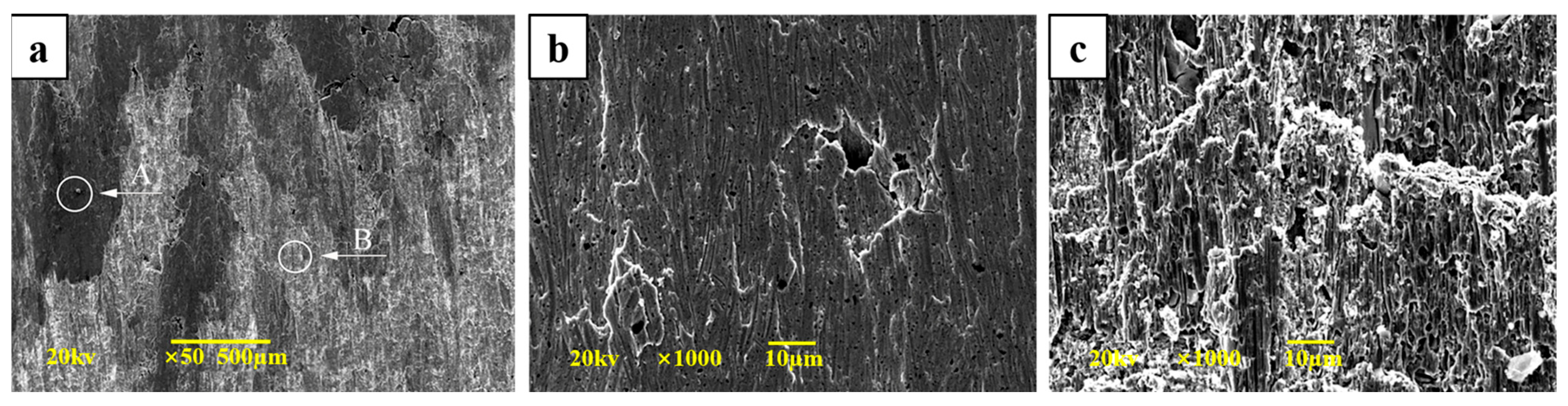
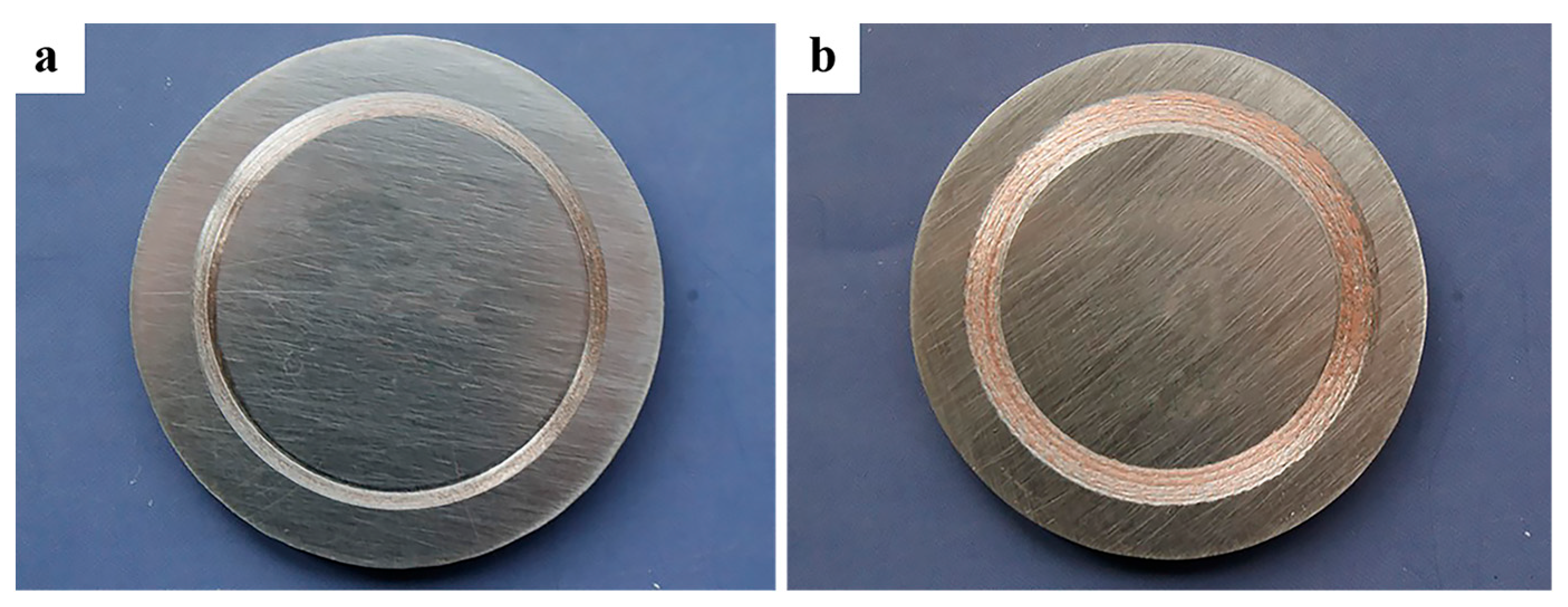
3.2. Morphology of Cu-SiO2 Worn Surface
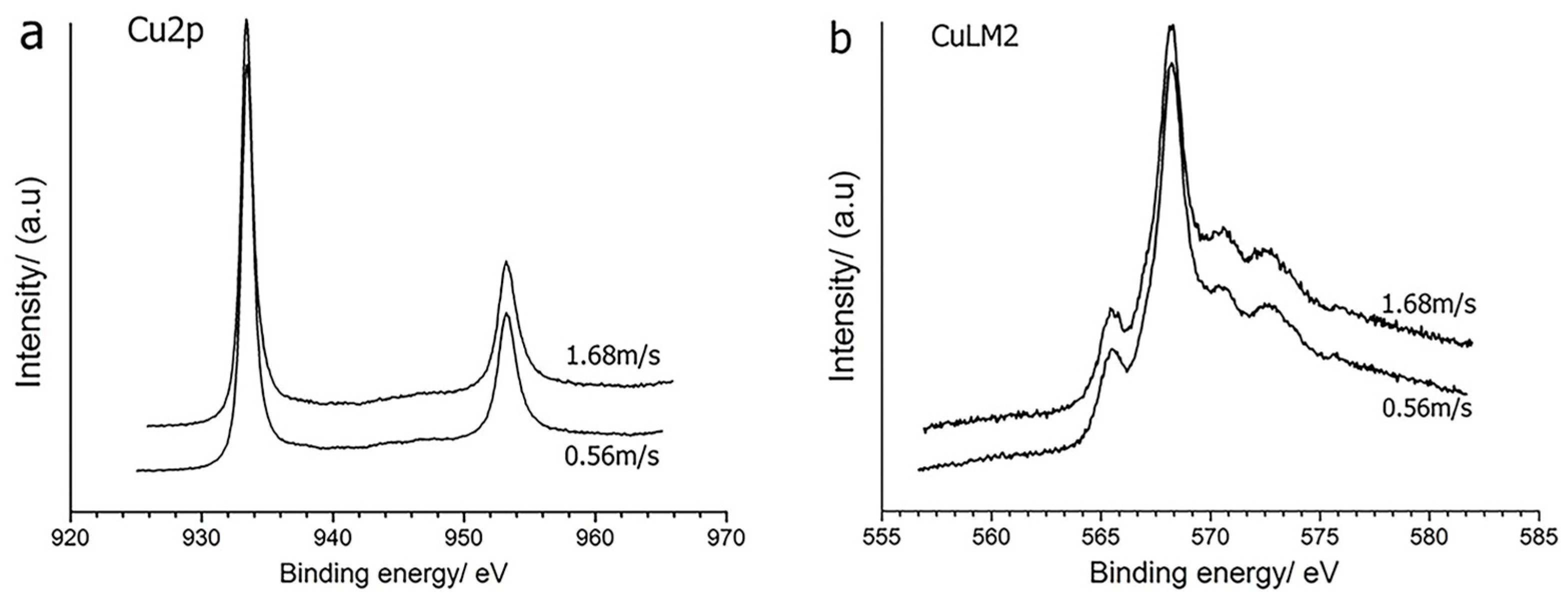
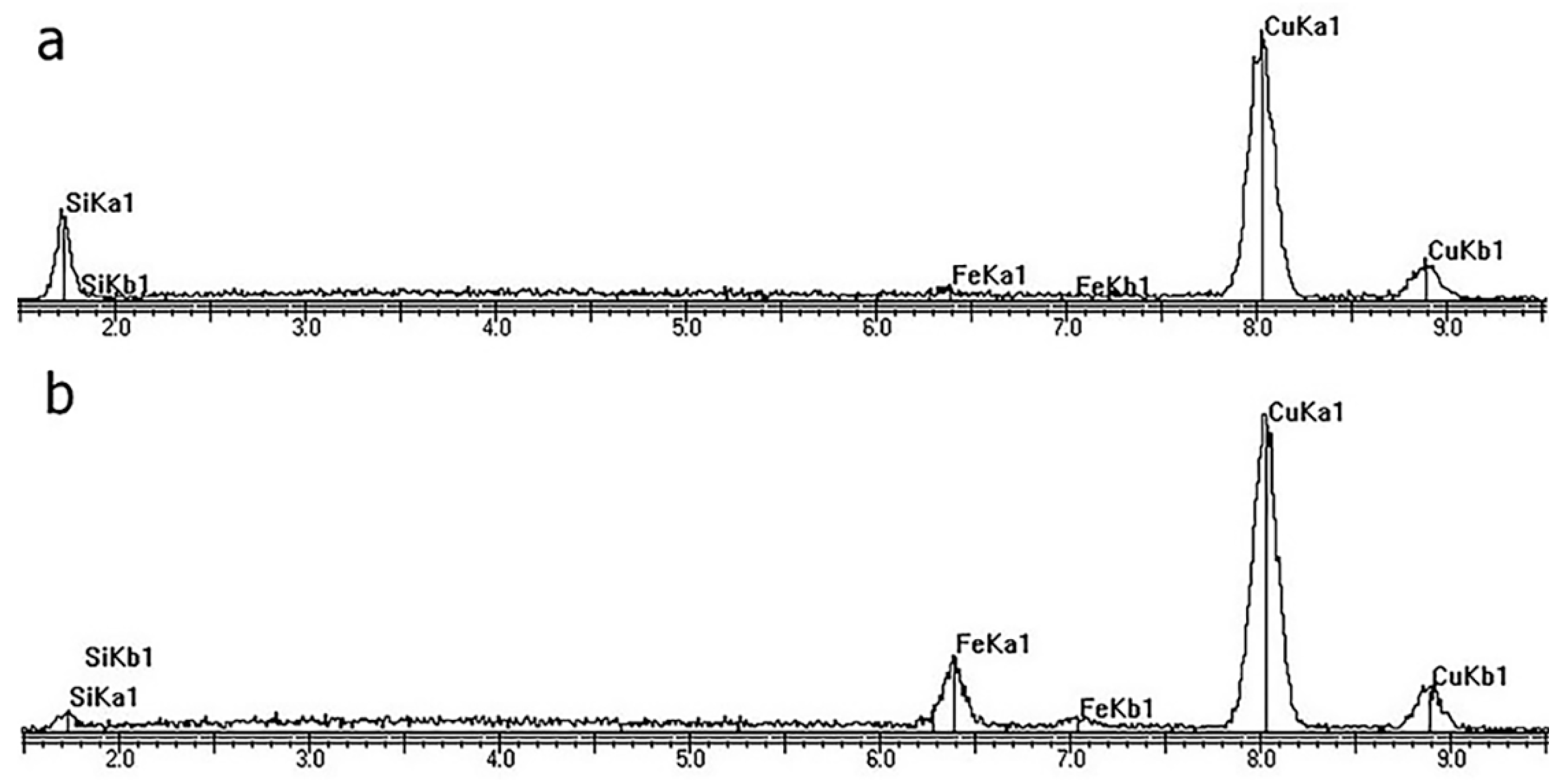
3.3. Composition of Cu-SiO2 Worn Spot
3.3.1. XPS of Cu-SiO2 Worn Spot
3.3.2. LRS of Cu-SiO2 Worn Spot
3.4. Morphology of Worn Surface/Subsurface
3.4.1. Morphologies of Cu-SiO2 Worn Spot
3.4.2. Morphology of 1045 Steel Worn Surface
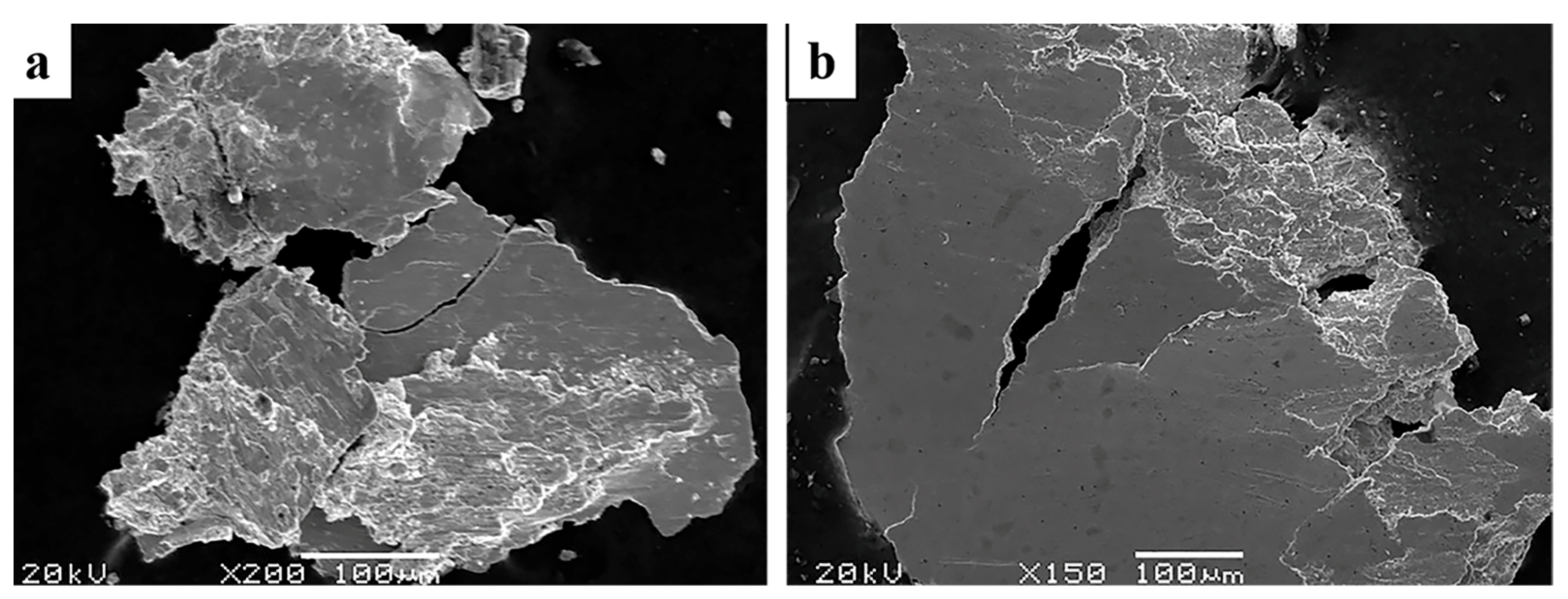
3.5. Morphology of Wear Debris
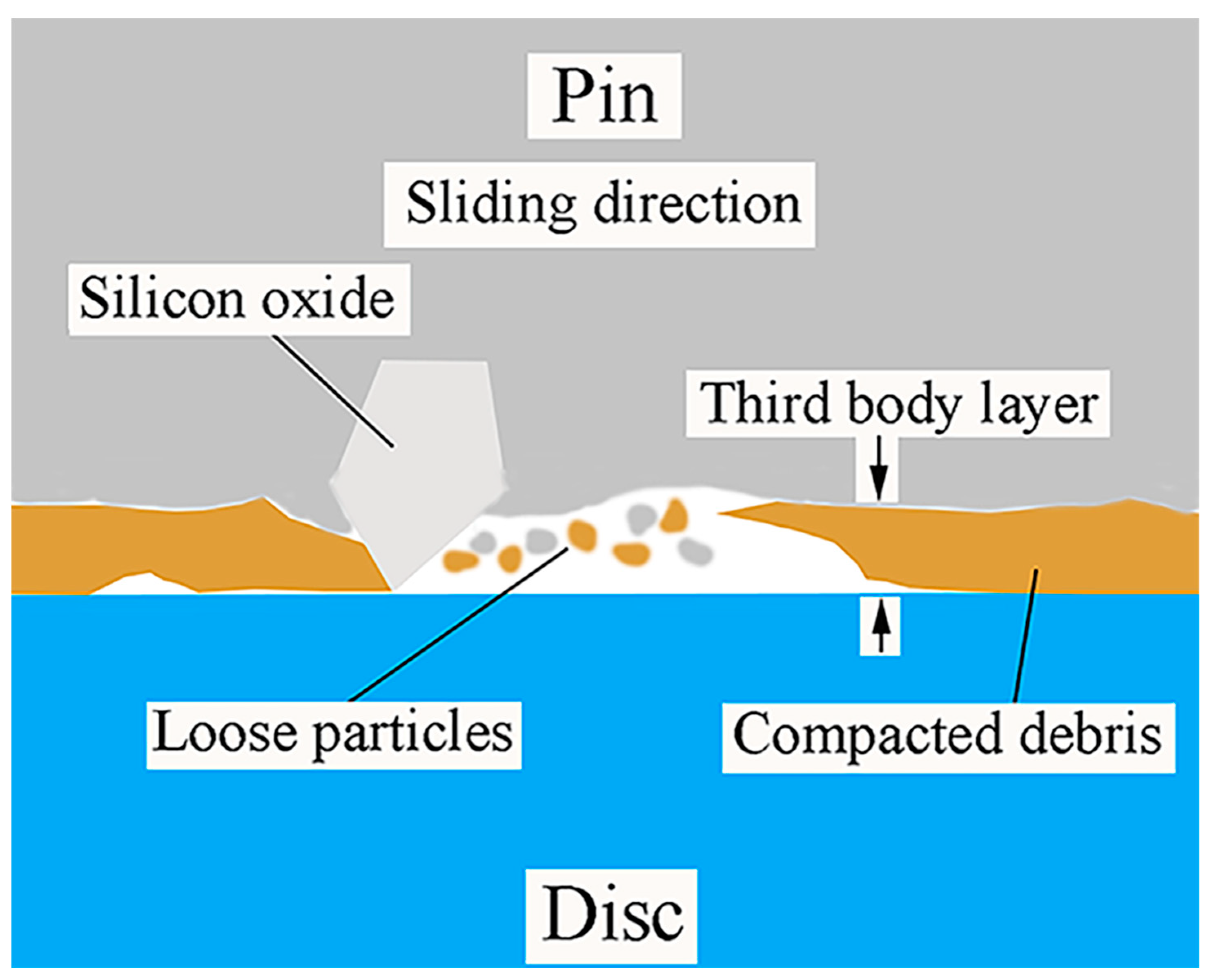
4. Formation Mechanism of Tribolayer
5. Discussion
6. Conclusions
- (1)
- Abrasive wear and adhesive wear are the dominant mechanisms at the 0.56 m/s condition.
- (2)
- Delamination wear and oxidation wear are the dominant wear mechanisms at the 1.68 m/s condition.
- (3)
- Plastic and thermal deformation cause the evolution in morphology and structure of the tribolayer of Cu-SiO2.
- (4)
- There is a certain correlation between the friction coefficient and the variation in friction temperature during sliding wear of Cu-SiO2 and 1045 steels. The addition of SiO2, inducing the accumulation of frictional heat at the friction interface, leads to an increase in the average temperature of the contact surface, and induces sticking welding and transfer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rigney, D.A. Transfer, mixing and associated chemical and mechanical processes during the sliding of ductile materials. Wear 2000, 245, 1–9. [Google Scholar] [CrossRef]
- Rigney, D.A.; Karthikeyan, S. The evolution of tribomaterial during sliding: A brief introduction. Tribol. Lett. 2009, 39, 3–7. [Google Scholar]
- Godet, M. The third-body approach: A mechanical view of wear. Wear 1984, 100, 437–452. [Google Scholar] [CrossRef]
- Riahi, A.R.; Alpas, A.T. The role of tribo-layers on the sliding wear behaviour of graphitic aluminium matrix composites. Wear 2001, 251, 1396–1407. [Google Scholar] [CrossRef]
- Descartes, S.; Berthier, Y. Rheology and flows of solid third bodies: Background and application to an MoS1.6 coating. Wear 2002, 252, 546–556. [Google Scholar] [CrossRef]
- Österle, W.; Urban, I. Friction layers and friction films on PMC brake pads. Wear 2004, 257, 215–226. [Google Scholar] [CrossRef]
- Emge, A.; Karthikeyan, S.; Rigney, D. The effects of sliding velocity and sliding time on nanocrystalline tribolayer development and properties in copper. Wear 2009, 267, 562–567. [Google Scholar] [CrossRef]
- Singh, J.B.; Wen, J.G.; Bellon, P. Nanoscale characterization of the transfer layer formed during dry sliding of Cu-15wt.% Ni-8wt.% Sn bronze alloy. Acta Mater. J. 2008, 56, 3053–3064. [Google Scholar] [CrossRef]
- Burton, R.A. Thermal deformation in frictionally heated contact. Wear 1980, 59, 1–20. [Google Scholar] [CrossRef]
- Abdel-Aal, H.A. Temperature fields in dry sliding contact by a hybrid laser-speckle technique. Wear 1995, 181–183, 723–729. [Google Scholar]
- Yu, J.W.; Gong, Z.L. Analysis to the Thermal mechanism of wear in dry friction condition. Lubr. Eng. J. 2005, 171, 173–176. (In Chinese) [Google Scholar]
- Kennedy, F.E. Thermal and thermomechanical effects in dry sliding. Wear 1984, 100, 453–476. [Google Scholar] [CrossRef]
- Abdel-Aal, H.A. On the connection of thermal dilatation to protective layer formation in the fretting of metallic tribo-specimens. Wear 2001, 247, 76–87. [Google Scholar] [CrossRef]
- Abdel-Aal, H.A. Correlating thermal dilatation to protective layer formation in fretting wear. Int. Commun. Heat Mass Transf. 2001, 28, 97–106. [Google Scholar] [CrossRef]
- Kennedy, F.E.; Ballbahadur, A.C.; Lashmore, D.S. The friction and wear of Cu-based silicon carbide particulate metal matrix composites for brake applications. Wear 1997, 203–204, 715–721. [Google Scholar] [CrossRef]
- Dhokey, N.; Paretkar, R. Study of wear mechanisms in copper-based SiCp (20% by volume) reinforced composite. Wear 2008, 265, 117–133. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, J.; Yao, P.; Li, S.; Huang, B. Friction and wear behaviors and mechanisms of Fe and SiO2 in Cu-based P/M friction materials. Wear 2007, 262, 1182–1186. [Google Scholar] [CrossRef]
- Shang, J.; Ma, W.L.; Lu, J.J. Formation of laminar structure under unlubricated friction of Cu-SiO2 composite. Tribol. Lett. J. 2012, 48, 249–254. [Google Scholar] [CrossRef]
- Su, L.; Gao, F.; Han, X.; Chen, J. Effect of copper powder third body on tribological property of copper-based friction materials. Tribol. Int. 2015, 90, 420–425. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Shang, J.; Xue, E. Tribological Behavior and Wear Mechanism of Cu-SiO2 Sintered Composite under Different Sliding Speeds. Crystals 2024, 14, 232. https://doi.org/10.3390/cryst14030232
Chen Q, Shang J, Xue E. Tribological Behavior and Wear Mechanism of Cu-SiO2 Sintered Composite under Different Sliding Speeds. Crystals. 2024; 14(3):232. https://doi.org/10.3390/cryst14030232
Chicago/Turabian StyleChen, Qiangqiang, Jian Shang, and E Xue. 2024. "Tribological Behavior and Wear Mechanism of Cu-SiO2 Sintered Composite under Different Sliding Speeds" Crystals 14, no. 3: 232. https://doi.org/10.3390/cryst14030232
APA StyleChen, Q., Shang, J., & Xue, E. (2024). Tribological Behavior and Wear Mechanism of Cu-SiO2 Sintered Composite under Different Sliding Speeds. Crystals, 14(3), 232. https://doi.org/10.3390/cryst14030232





