Exploring Various Crystal and Molecular Structures of Gabapentin—A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Pharmaceutical Properties of GBP
3.1. Pharmacological Properties
3.1.1. Mechanism of Action
3.1.2. Pharmacokinetics
3.2. Medical Uses
3.2.1. Neuropathic Pain
3.2.2. Seizures
3.2.3. Drug Dependence
3.2.4. Restless Legs Syndrome
3.3. Dosages
3.3.1. Dosages in Epilepsy
3.3.2. Dosages in Neuropathic Pain
3.4. Side Effects
4. Overview of the Crystal Structures of Systems Containing GBP
5. Polymorphism of Anhydrous and Hydrated Forms of GBP
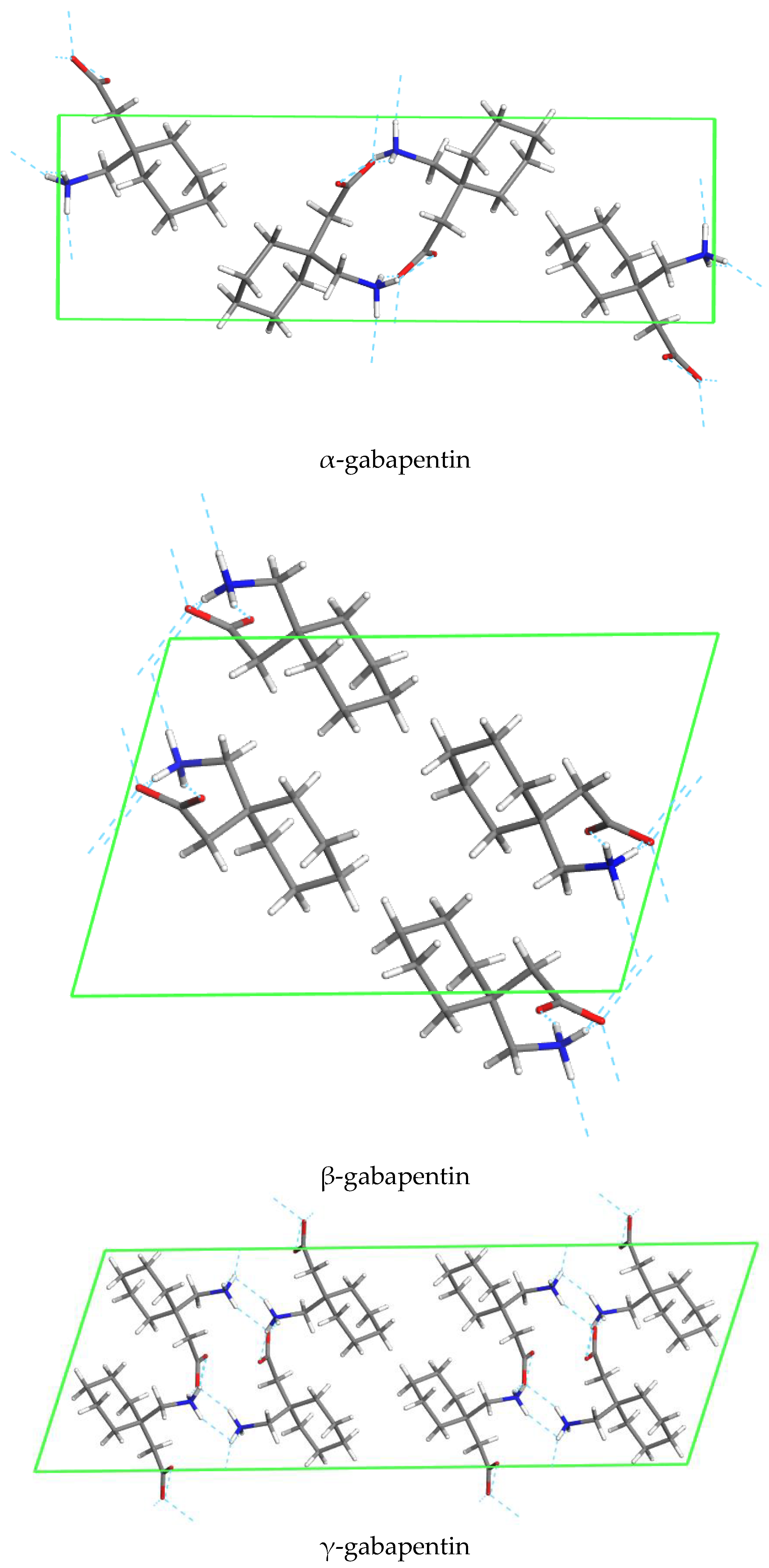
5.1. Polymorhphism of Anhydrous GBP—Structures QIMKIGXX
5.2. Polymorhphism of GBP Monohydrate—Structures QIMKOMXX
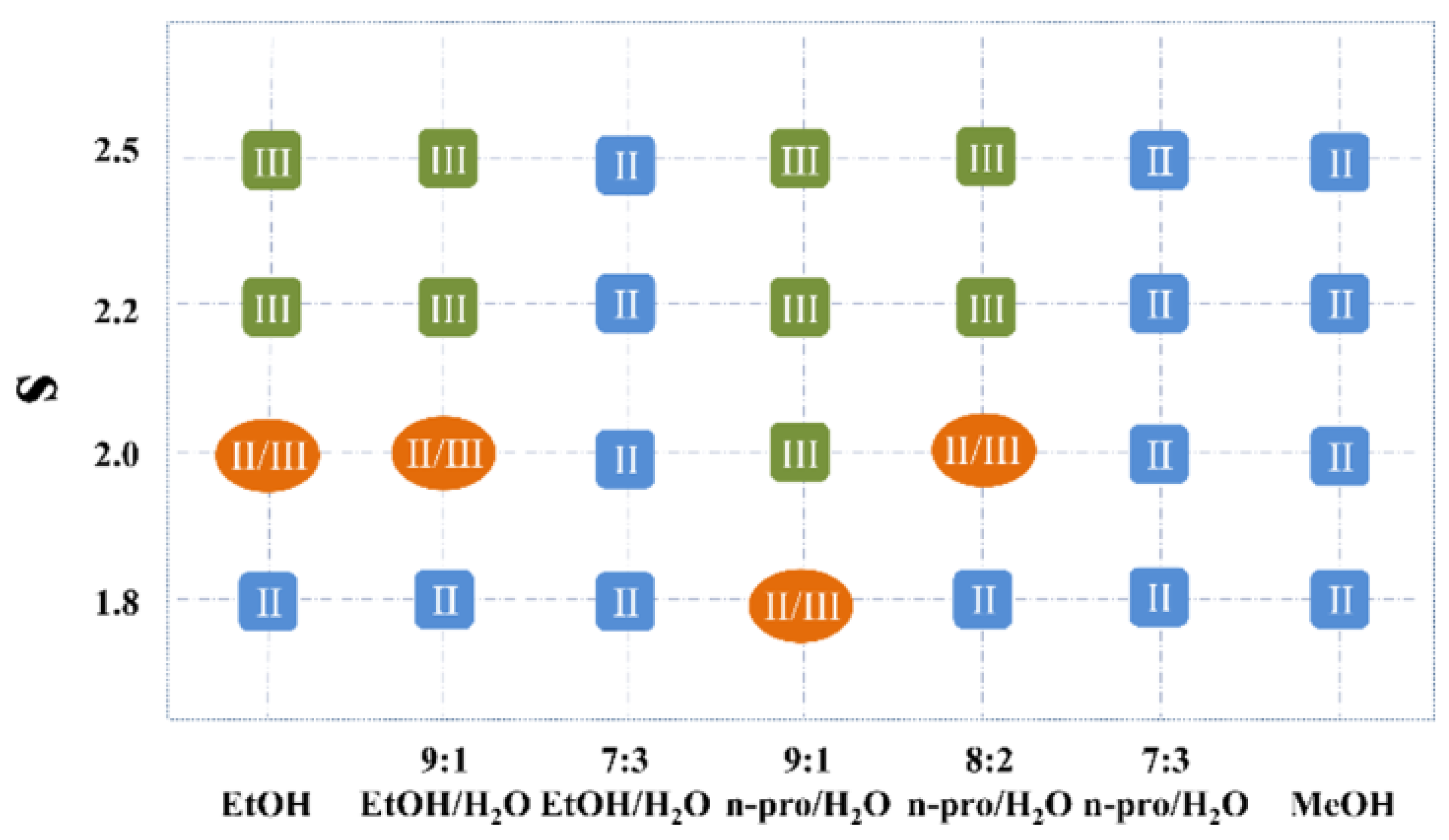
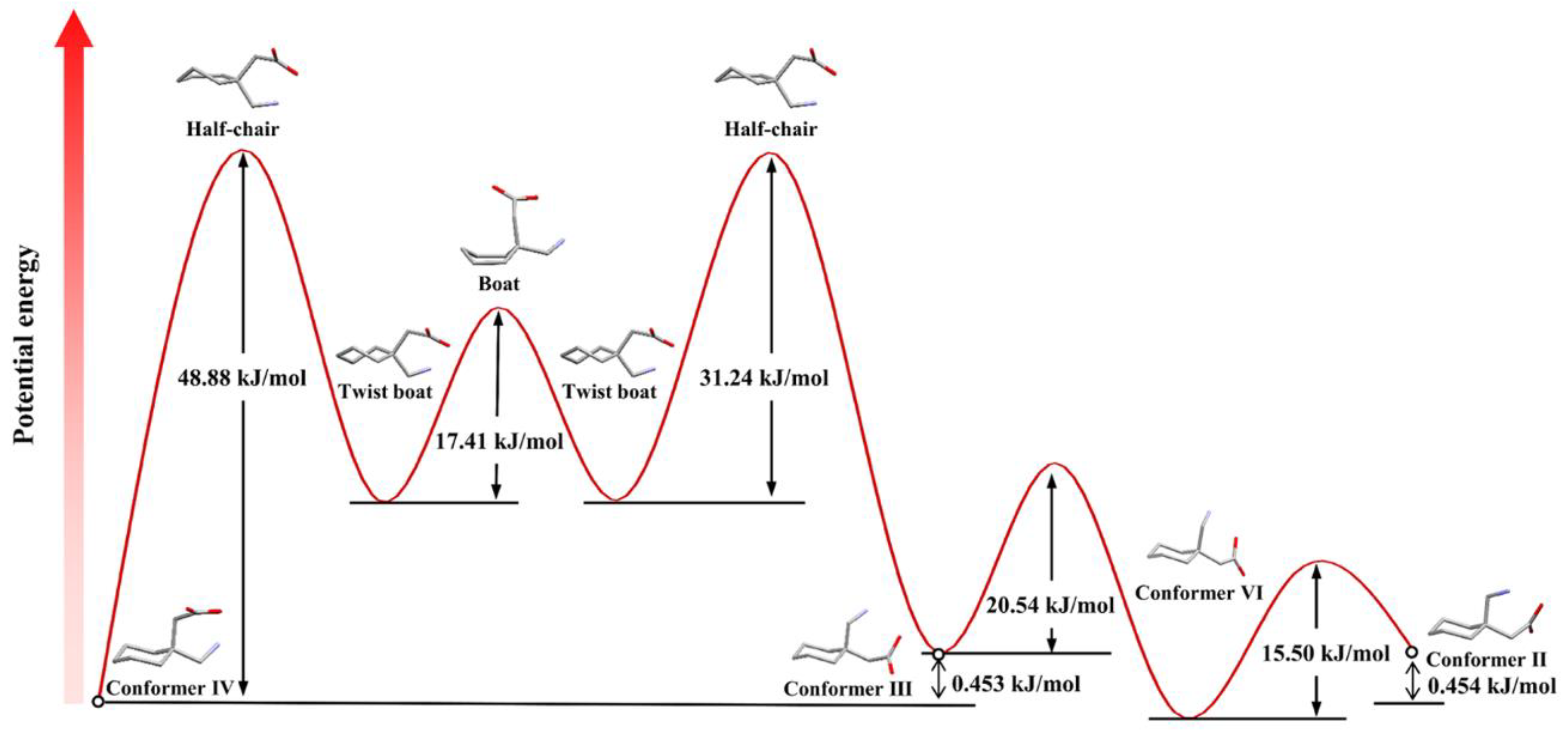
6. Conformational Analysis of GBP in Solution
7. Conformational Analysis of GBP in a Solid State
| ZI |
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herbst, M.; Komisarek, D.; Strothmann, T.; Vasylyeva, V. A Lection in Humbleness: Crystallization of Chiral and Zwitterionic APIs Baclofen and Phenibut. Crystals 2022, 12, 1393. [Google Scholar] [CrossRef]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Al-Salmi, F.A.; Altalhi, T.A.; El-Megharbel, S.M. Potential Therapeutic Effects of New Ruthenium (III) Complex with Quercetin: Characterization, Structure, Gene Regulation, and Antitumor and Anti-Inflammatory Studies (RuIII/Q Novel Complex Is a Potent Immunoprotective Agent). Crystals 2021, 11, 367. [Google Scholar] [CrossRef]
- Udaykumar Rao, B.; Maqdoom, F.; Nikalje, A.P. Determination of gabapentin in bulk drug and in pharmaceutical dosage form by HPLC method. J. Chil. Chem. Soc. 2009, 54, 424–427. [Google Scholar] [CrossRef]
- Rose, M.A.; Kam, P.C. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462. [Google Scholar] [CrossRef]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277. [Google Scholar] [CrossRef]
- Duncan, J.S.; Sander, J.W.; Sisodiya, S.M.; Walker, M.C. Adult epilepsy. Lancet 2006, 367, 1087–1100. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Brigo, F.; Lattanzi, S.; Blumcke, I. Adult epilepsy. Lancet 2023, 402, 412–424. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edginton, P.R.; Kessler, M.; Macrae, C.F.; Mccabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- CCDC Search Structures Application. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 22 January 2024).
- Model the Biosphere|BIOVIA—Dassault Systèmes. Available online: https://www.3ds.com/products-services/biovia/ (accessed on 22 January 2024).
- Cremer-Pople Parameter Calculator. Available online: http://enzyme13.bt.a.u-tokyo.ac.jp/CP/ (accessed on 22 January 2024).
- Chincholkar, M. Gabapentinoids: Pharmacokinetics, pharmacodynamic and considerations for clinical practice. Br. J. Pain 2020, 14, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.V.; Havens, J.R.; Walsh, S.L. Gabapentin misuse, abuse and diversion: A systematic review. Addiction 2016, 111, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Maneuf, Y.P.; Luo, Z.D.; Lee, K. α2δ and the mechanism of action of gabapentin in the treatment of pain. Semin. Cell Dev. Biol. 2006, 17, 565–570. [Google Scholar] [CrossRef]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. A Comparison of the Pharmacokinetics and Pharmacodynamics of Pregabalin and Gabapentin. Clin. Pharmacokinet 2010, 49, 661–669. [Google Scholar] [CrossRef]
- Jackson, C.; Clanahan, M.J.; Joglekar, K.; Decha-Umphai, S.T. Hold the gaba: A case of gabapentin-induced hepatotoxicity. Cureus 2018, 10, e2269. [Google Scholar] [CrossRef]
- Hakami, T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef]
- Minozzi, S.; Cinquini, M.; Amato, L.; Farrell, M.F.; Reisser, A.A.R.L.; Pani, P.P.; de Lima, M.S.; Soares, B.G.O.; Vecchi, S. Anticonvulsants for cocaine dependence. Cochrane Database Syst. Rev. 2015, 2015, CD006754. [Google Scholar] [CrossRef] [PubMed]
- Marschall, K.; Gowing, L.; Ali, R.; Le Foll, B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst. Rev. 2014, 12, CD008940. [Google Scholar] [CrossRef] [PubMed]
- Pani, P.P.; Trogu, E.; Pacini, M.; Maremmani, I. Anticonvulsants for alcohol dependence. Cochrane Database Syst. Rev. 2014, 2014, CD008544. [Google Scholar] [CrossRef]
- Hornyak, M.; Scholz, H.; Kohnen, R.; Bengel, J.; Kassubek, J.; Trenkwalder, C. What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med. Rev. 2014, 18, 153–164. [Google Scholar] [CrossRef]
- Aurora, R.N.; Kristo, D.A. The treatment of restless legs syndrome and periodic limb movement disorder in adults—An update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 2012, 35, 1039–1062. [Google Scholar] [CrossRef]
- Tozihi, M.; Bahrami, H.; Zarifian, M.; Vahedpour, M. Protonation of gabapentin: Ion mobility spectrometry and computational study. Eur. J. Mass Spectrom. 2023, 29, 220–230. [Google Scholar] [CrossRef]
- Morozov, Y.N.; Mikhalev, S.P.; Shabatin, V.P.; Kolotilov, P.N.; Sergeev, G.B. Cryomodification of drugs: Micronization and crystalline structures of 1-(aminomethyl)-cyclohexaneacetic acid. Mosc. Univ. Chem. Bull. 2010, 65, 237–243. [Google Scholar] [CrossRef]
- Sinha, L.; Karabacak, M.; Narayan, V.; Cinar, M.; Prasad, O. Molecular structure, electronic properties, NLO, NBO analysis and spectroscopic characterization of Gabapentin with experimental (FT-IR and FT-Raman) techniques and quantum chemical calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 298–307. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Abbas, A.M.; Zaitone, S.A.; Ammar, A.M.; Sallam, S.A. Copper (II) ternary complexes with gabapentin and neurotransmitters as antiepileptic drug. J. Mol. Struct. 2019, 1180, 861–877. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Helal, M.A.; Ammar, A.M. Zinc ternary complexes with gabapentin and neurotransmitters: Synthesis, spectral, thermal and molecular docking studies. J. Mol. Struct. 2020, 1199, 126951. [Google Scholar] [CrossRef]
- Ibers, J.A. Gabapentin and gabapentin monohydrate. Acta Crystallogr. C 2001, 57, 641–643. [Google Scholar] [CrossRef]
- Ananda, K.; Aravinda, S.; Vasudev, P.G.; Raja, K.M.P.; Sivaramakrishnan, H.; Nagarajan, K.; Shamala, N.; Balaram, P. Stereochemistry of gabapentin and several derivatives: Solid state conformations and solution equilibria. Curr. Sci. 2003, 85, 1002–1011. [Google Scholar]
- Wang, Y.; Du, S.; Wu, S.; Zhang, D.; Yu, B.; Bekele, H.K.; Gong, J. Thermodynamic and molecular investigation into the solubility, stability and self-assembly of gabapentin anhydrate and hydrate. J. Chem. Thermodyn. 2017, 113, 132–143. [Google Scholar] [CrossRef]
- Reece, H.A.; Levendis, D.C. Polymorphs of gabapentin. Acta Crystallogr C 2008, 64, 105–108. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Rubini, K.; Polito, M.; Brescello, R.; Cotarca, L.; Duarte, M.T.; Andre, V.; Fatima, M.; et al. Polymorphic gabapentin: Thermal behaviour, reactivity and interconversion of forms in solution and solid-state. New J. Chem. 2008, 32, 1788–1795. [Google Scholar] [CrossRef]
- Vasudev, P.G.; Aravinda, S.; Ananda, K.; Veena, S.D.; Nagarajan, K.; Shamala, N.; Balaram, P. Crystal Structures of a New Polymorphic Form of Gabapentin Monohydrate and the E and Z Isomers of 4-Tertiarybutylgabapentin. Chem. Biol. Drug Des. 2009, 73, 83–96. [Google Scholar] [CrossRef]
- Zhao, C.; Su, X.; Fang, L.; Shang, Z.; Li, Z.; Gong, J.; Wu, S. Multivariate Analysis of a Highly Effective Drug Combination Tablet Containing the Antiepileptic Drug Gabapentin to Enhance Pharmaceutical Properties with a Multicomponent Crystal Strategy. Cryst. Growth Des. 2022, 12, 7234–7247. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Levendis, D.C.; Buth, G.; Kuhs, W.F.; Shankland, N.; Sowa, H. Searching for novel crystal forms by in situ high-pressure crystallisation: The example of gabapentin heptahydrate. CrystEngComm 2010, 12, 2354–2360. [Google Scholar] [CrossRef]
- Jasinski, J.P.; Butcher, R.J.; Yathirajan, H.S.; Mallesha, L.; Mohana, K.N.; Narayana, B. Crystal Structure of a Second Polymorph of Gabapentin Hydrochloride Hemihydrate with a Three-Center Bifurcated Hydrogen Bond. J. Chem. Crystallogr. 2009, 39, 777–780. [Google Scholar] [CrossRef]
- André, V.; Marques, M.M.; da Piedade, M.F.M.; Duarte, M.T. An ester derivative of the drug gabapentin: pH dependent crystal stability. J. Mol. Struct. 2010, 973, 173–179. [Google Scholar] [CrossRef]
- André, V.; Fernandes, A.; Santos, P.P.; Duarte, M.T. On the Track of New Multicomponent Gabapentin Crystal Forms: Synthon Competition and pH Stability. Cryst. Growth Des. 2011, 11, 2325–2334. [Google Scholar] [CrossRef]
- Wenger, M.; Bernstein, J. An Alternate Crystal Form of Gabapentin: A Cocrystal with Oxalic Acid. Cryst. Growth Des. 2008, 8, 1595–1598. [Google Scholar] [CrossRef]
- Hongqi, L.; Yathirajan, H.S.; Mellesha, L.; Mohana, K.N.; Narayana, B. Gabapentinium picrate. Acta Cryst. E 2009, 65, 783. [Google Scholar]
- Shaikjee, A.; Levendis, D.C.; Marques, H.M.; Mampa, R. A gold(III) complex and a tetrachloroaurate salt of the neuroepileptic drug gabapentin. Inorg. Chem. Commun. 2011, 14, 534–538. [Google Scholar] [CrossRef]
- Reddy, L.; Bethune, S.J.; Kampf, J.W.; Rodríguez-Hornedo, N. Cocrystals and Salts of Gabapentin: pH Dependent Cocrystal Stability and Solubility. Cryst. Growth Des. 2009, 9, 378–385. [Google Scholar] [CrossRef]
- Vries, E.J.C.; Gamble, C.; Shaikjee, A. [1-(Carboxymethyl)cyclohexyl]- methanaminium nitrate. Acta Cryst. E 2011, 67, 513. [Google Scholar] [CrossRef]
- Razzaq, S.N.; Khan, I.U.; Şahin, O.; Büyükgüngör, O. [1-(Carboxymethyl)cyclohexyl]methanaminium dihydrogen phosphate. Acta Cryst. E 2010, 66, 1074. [Google Scholar] [CrossRef]
- Fowler, D.A.; Tian, J.; Barnes, C.; Teat, S.J.; Atwood, J.L. Cocrystallization of C-butyl pyrogallol[4]arene and C-propan-3-ol pyrogallol[4]arene with gabapentin. CrystEngComm 2011, 13, 1446–1449. [Google Scholar] [CrossRef]
- Spiel, P.D.; Sikligar, K.; Baker, G.A.; Kelley, S.P.; Atwood, J.L. Cocrystallization of C-Propyl Pyrogallol[4]arene and the Pharmaceutical Gabapentin. J Chem. Crystallogr. 2018, 49, 119–124. [Google Scholar] [CrossRef]
- Kumari, H.; Zhang, J.; Erra, L.; Barbour, L.J.; Deakyne, C.A.; Atwood, J.L. Cocrystals of gabapentin with C-alkylresorcin[4]arenes. CrystEngComm 2013, 15, 4045. [Google Scholar] [CrossRef]
- Queresma, S.; André, V.; Antunes, A.M.M.; Cunha-Silva, L.; Duarte, M.T. Gabapentin Coordination Networks: Mechanochemical Synthesis and Behavior under Shelf Conditions. Cryst. Growth Des. 2013, 13, 5007–5017. [Google Scholar] [CrossRef]
- Vries, E.J.C.; Gamble, C.; Shaikjee, A. Tetraaquatetrakis{μ2-[1-(carboxylatomethyl)cyclohexyl]methanaminium}-bis(μ3-hydroxido)bis(nitrato-κ2O,O’)-tetrazinc(II). Acta Cryst. E 2011, 67, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Grepioni, F.; Maini, L.; Brescello, R.; Cotarca, L. Simple and quantitative mechanochemical preparation of the first zinc and copper complexes of the neuroleptic drug gabapentin. CrystEngComm 2008, 10, 469–471. [Google Scholar] [CrossRef]
- Mungalimane, A.; Shetty, V. CCDC 982169: Experimental Crystal Structure Determination, 2017. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 22 January 2024).
- Pesachovich, M.; Singer, C.; Pilarski, G. Preparation of Gabapentin. U.S. Patent No. 6,255,526,B1, 2 July 2001. [Google Scholar]
- Satyanarayana, C.; Ramanjaneyulu, G.S.; Kumar, I.V.S. World Intellectual Property Organization, Publication No. WO 2004/110342; WIPO: Karlsruhe, Germany, 2004. [Google Scholar]
- Liu, Y.; Wang, Y.; Huang, X.; Li, X.; Zong, S.; Wang, N.; Hao, H. Conformational Selectivity and Evolution Affected by the Desolvation Process. Cryst. Growth Des. 2022, 22, 1283–1291. [Google Scholar] [CrossRef]
- Bryans, J.S.; Horwell, D.C.; Ratcliffe, G.S.; Recevaur, J.M.; Rubin, J.R. An In Vitro Investigation into Conformational Aspects of Gabapentin. Bioorg. Med. Chem. 1999, 8, 715–721. [Google Scholar] [CrossRef]
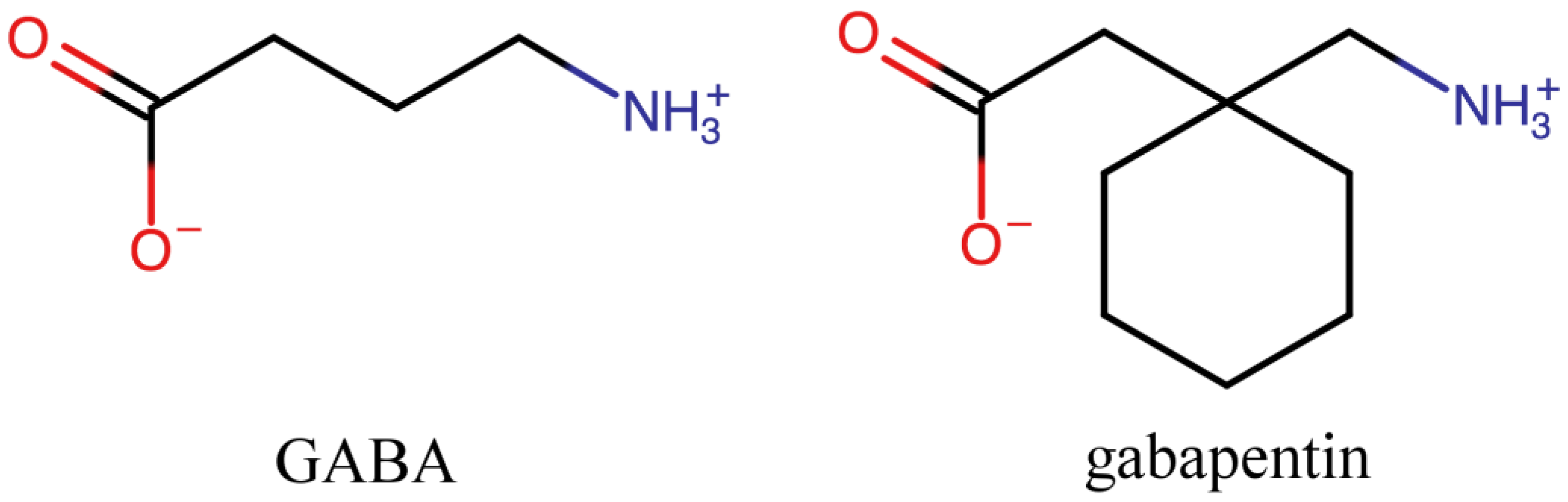
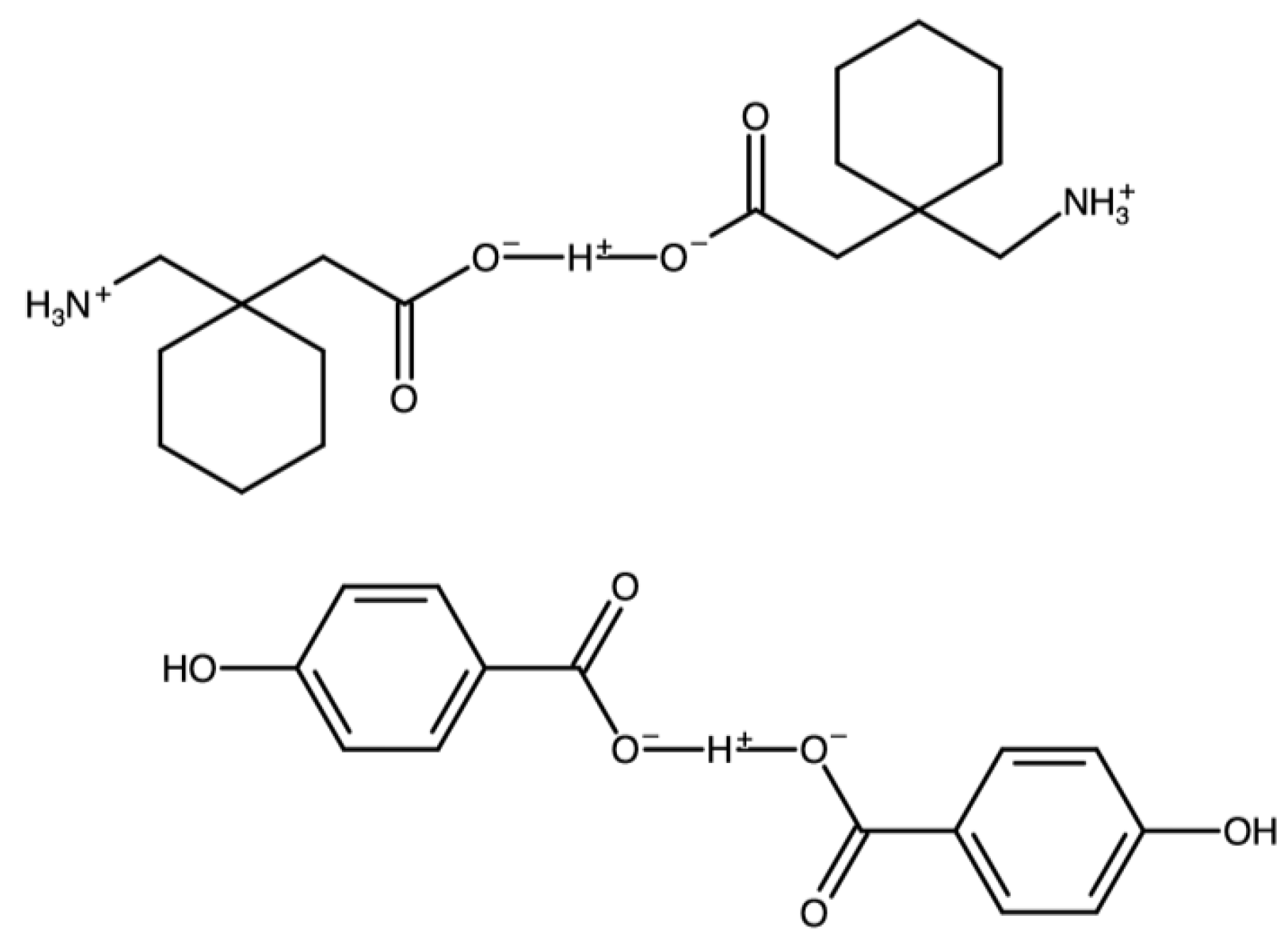



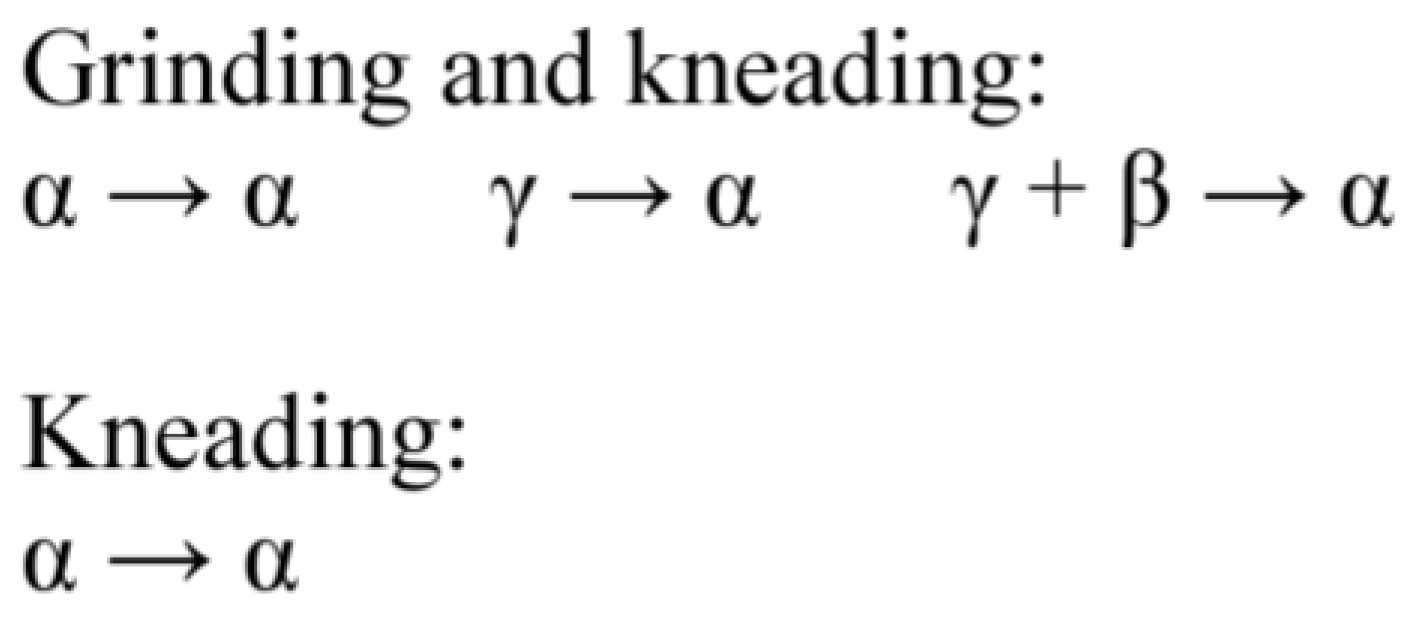

| Chemical Name | CCDC Refcode | Molecular Structure | Reference and Year of Depositon | Methods of Physicochemical Analysis Other than SCXRD |
|---|---|---|---|---|
| Gabapentin polymorphs | ||||
| Gabapentin α (II) polymorph | QIMKIG | 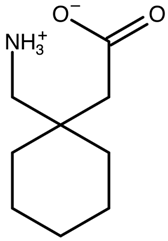 | [28] 2001 | |
| QIMKIG01 | [29] 2004 | 1H IS NMR | ||
| QIMKIG06 | [30] 2017 | PXRD TGA Optical microscopy GC Solubility measurements MD | ||
| Gabapentin β (IV) polymorph | QIMKIG02 | [31] 2008 | ||
| QIMKIG04 | [32] 2009 |
PXRD DSC Melting point HSM Stability at 50 and 100% RH | ||
| Gabapentin γ (III) polymorph I | QIMKIG03 | [31] 2008 | ||
| QIMKIG05 | [32] 2009 |
PXRD DSC Melting point HSM Stability at 50 and 100% RH | ||
| Gabapentin hydrates | ||||
| Gabapentin monohydrate | QIMKOM (I) polymorph | 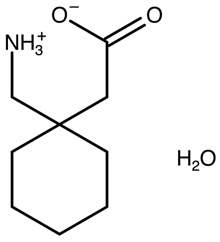 | [28] 2001 | |
| QIMKOM01 (I) polymorph | [33] 2010 | |||
| QIMKOM03 (I) polymorph | [30] 2017 | PXRD TGA Optical microscopy GC Solubility measurement MD | ||
| QIMKOM02 (II) polymorph | [33] 2010 | |||
| QIMKOM04 (II) polymorph | [34] 2022 | PXRD DSC TGA FTIR RM DVS SEM | ||
| Gabapentin heptahydrate | YUZTET |  | [35] 2010 | |
| Gabapentin hydrochloride hemihydrate | AWUWIY C 2/c polymorph | 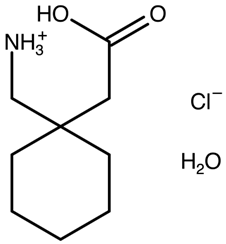 | [29] 2004 | 1H IS NMR |
| AWUWIY01 I 2/a polymorph | [36] 2010 | |||
| AWUWIY02 C 2/c polymorph | [37] 2016 | PXRD DSC | ||
| Gabapentin salts | ||||
| Gabapentin with terephthalic acid | AVILOH | 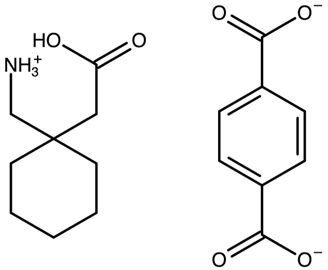 | [38] 2011 | PXRD HSM DSC TGA FTIR |
| Gabapentin hemikis(oxalate) | SOCYUF |  | [39] 2008 | PXRD HSM DSC |
| Gabapentinium picrate | LORQIT |  | [40] 2009 | |
| Gabapentin–[AuCl4]−salt | AVAVAV |  | [41] 2011 | 1H IS NMR |
|
Gabapentin 1-hydroxy-2-naphthoate | FOXPEO |  | [42] 2010 | PXRD DSC RM IR |
|
Gabapentin RS-mandelate | FOXPOY | 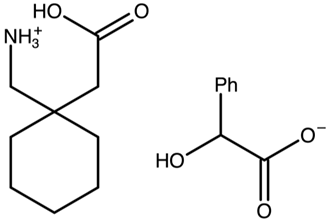 | [42] 2010 | PXRD DSC RM IR |
| Gabapentin salicylate | FOXPAK | 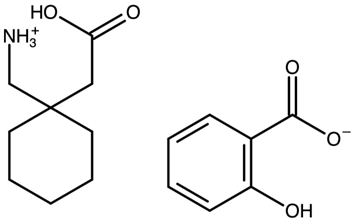 | [42] 2010 | PXRD DSC RM IR |
| Gabapentin nitrate | IKATIY |  | [43] 2011 | |
| Gabapentinium dihydrogen phosphate | NUPXAY |  | [44] 2010 | |
| Cocrystals and host-guest complexes | ||||
| Gabapentin with trimesic acid | AVILUN | 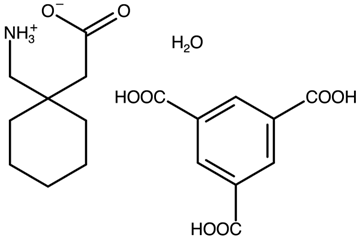 | [38] 2011 | PXRD HSM DSC TGA FTIR |
| Gabapentin 4-aminobenzoic acid | DESNOI |  | [34] 2022 | PXRD DSC TGA FTIR RM DVS SEM |
|
Gabapentin 3-hydroxybenzoic acid | FOXNOW | 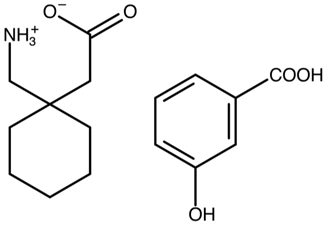 | [42] 2010 | PXRD DSC HPLC RM IR |
| Pyrogallol[4]arene with gabapentin | ELUNOP | 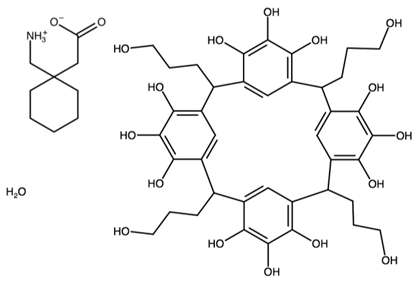 | [45] 2011 | |
| C-butylpyrogallol[4]arene bis(gabapentin) | ANISAS | 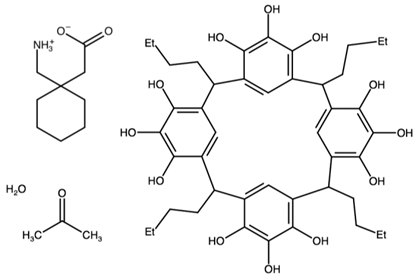 | [45] 2011 | |
| bis(C-butylpyrogallol[4]arene) tetrakis(gabapentin) | ANISEW |  | [45] 2011 | |
| [1-(azaniumylmethyl)cyclohexyl]acetate 2,8,14,20-tetrapropylpentacyclo[19.3.1.13,7.19,13.115,19]octacosa-1(25),3(28),4,6,9(27),10,12,15(26),16,18,21,23-dodecaene-4,5,6,10,11,12,16,17,18,22,23,24-dodecol 1,2-dichlorobenzene | EFIVIB | 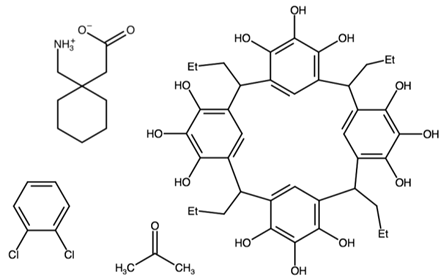 | [46] 2019 | |
| 2,8,14,20-tetrahexyl-4,6,10,12,16,18,22,24-octahydroxycalix(4)arene (1-(aminomethyl)cyclohexyl)acetic acid methanol solvate monohydrate | SESKEI |  | [47] 2013 | PXRD |
| Complexes with metals | ||||
| tetrakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-di-erbium tetrachloride octahydrate | VIXBUB | [Er2GBP6(H2O)2Cl2]Cl4 · 8H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| octakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-tetraaqua-tri-lanthanum nonachloride dodecahydrate | VIXCAI | [La3GBP10(H2O)4]Cl9 · 12H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-manganese(ii) | VIXCEM | [MnGBP2(H2O)2Cl2] | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-manganese(ii) | VIXCEM01 | [MnGBP2(H2O)2Cl2] | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| bis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-tetrakis(μ2-chloro)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-tri-manganese | VIXCIQ | [Mn3GBP4(H2O)2Cl6] | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| tetrakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-di-neodymium tetrachloride octahydrate | VIXCUC | [Nd2GBP6(H2O)2Cl2]Cl4 · 8H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| tetrakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-diaqua-dichloro-di-yttrium tetrachloride octahydrate | VIXDAJ | [Y2GBP6(H2O)2Cl2]Cl2 · 8H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| catena-[pentakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato-O,O′)-tris(μ2-[1-(ammoniomethyl)cyclohexyl]acetato-O,O,O′)-penta-aqua-tri-yttrium nonachloride decahydrate] | VIXDEN | [Y3GBP8(H2O)5]Cl9 · 10H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| tetradecakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-decaaqua-tetrachloro-hexa-cerium tetradecachloride icosahydrate | VIXQAW | [Ce6GBP16(H2O)10Cl4]Cl14 · 20H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| octakis(μ2-[1-(ammoniomethyl)cyclohexyl]acetato)-bis([1-(ammoniomethyl)cyclohexyl]acetato)-tetraaqua-tri-cerium nonachloride dodecahydrate | VIXQEA | [Ce3GBP10(H2O)4]Cl9·12H2O | [48] 2014 | PXRD DSC TGA HPLC 1H IS NMR |
| bis(μ3-hydroxo)-tetrakis(μ2-[1-(carboxylatomethyl)cyclohexyl]methanaminium)-tetraaqua-bis(nitrato)-tetra-zinc(ii) tetranitrate | UQUMOJ | [Zn4(OH)2(NO3)2(C9H17-NO2)4(H2O)4](NO3)4 | [49] 2011 | |
| Dichloro-bis(1-(ammoniomethyl)cyclohexane acetate)-zinc(ii) | DOBBIG | [ZnCl2(GBP)2] | [50] 2008 | PXDR |
| Dichloro-bis(1-(ammoniomethyl)cyclohexane acetate)-copper(ii) | DOBBOM | [CuCl2(GBP)2] | [50] 2008 | PXDR |
| (gabapentin)-penta-aqua-nickel(ii) sulfate monohydrate | JALXEC | [Ni(H2O)5(GBP)]SO4 · H2O | [51] 2014 | |
| bis{[1-(aminomethyl)cyclohexyl]acetato}-bis{[1-(aminomethyl)cyclohexyl]acetic acid}-diaqua-cobalt(ii) | JALXAY | [Co(H2O)2(GBP)4] | [51] 2014 | |
| Other | ||||
| Gabapentin hydrogenbis(4-hydroxybenzoate) | FOXNUC | 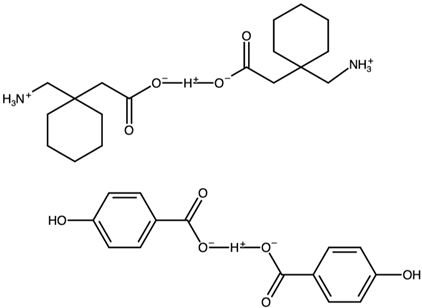 | [42] 2010 | PXDR DSC HPLC RM IR |
| Refcode | Polymorph | Space Group | a [Å] | b [Å] | c [Å] | α [°] | β [°] | γ [°] | V [Å3] | Z | Z′ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| QIMKIG | α | 14 P21/c | 5.88 | 6.92 | 22.26 | 90.00 | 90.08 | 90.00 | 905.17 | 4 | 1 |
| QIMKIG01 | α | 14 P21/c | 5.90 | 6.92 | 22.48 | 90.00 | 90.06 | 90.00 | 918.09 | 4 | 1 |
| QIMKIG06 | α | 14 P21/c | 5.90 | 6.92 | 22.46 | 90.00 | 90.00 * | 90.00 | 917.68 | 4 | 1 |
| QIMKIG02 | β | 14 P21/c | 14.54 | 6.63 | 9.83 | 90.00 | 105.92 | 90.00 | 911.91 | 4 | 1 |
| QIMKIG04 | β | 14 P21/c | 14.74 | 6.67 | 9.89 | 90.00 | 106.09 | 90.00 | 933.39 | 4 | 1 |
| QIMKIG03 | γ | 15 C2/c | 30.55 | 5.93 | 10.88 | 90.00 | 108.32 | 90.00 | 1870.58 | 8 | 1 |
| QIMKIG05 | γ | 15 C2/c | 30.58 | 5.93 | 10.92 | 90.00 | 108.31 | 90.00 | 1879.87 | 8 | 1 |
| Refcode | Polymorph | Space Group | a [Å] | b [Å] | c [Å] | α [°] | β [°] | γ [°] | V [Å3] | Z | Z′ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monohydrates | QIMKOM | I | 14 P21/c | 14.57 | 9.22 | 7.65 | 90.00 | 93.38 | 90.00 | 1025.19 | 4 | 1 |
| QIMKOM01 | I | 14 P21/c | 14.63 | 9.31 | 7.67 | 90.00 | 93.16 | 90.00 | 1043.11 | 4 | 1 | |
| QIMKOM03 | I | 14 P21/c | 14.63 | 9.30 | 7.65 | 90.00 | 93.11 | 90.00 | 1039.08 | 4 | 1 | |
| QIMKOM04 | II | 61 P b c a | 9.22 | 7.64 | 29.00 | 90.00 | 90.00 | 90.00 | 2040.90 | 8 | 1 | |
| QIMKOM02 | II | 61 P b c a | 29.14 | 9.30 | 7.66 | 90.00 | 90.00 | 90.00 | 2074.35 | 8 | 1 | |
| Heptahydrate | YUZTET | 2 P-1 | 6.80 | 7.32 | 15.84 | 86.30 | 78.92 | 72.71 | 737.49 | 2 | 1 |
| Refcode | Ionization | Bond Lengths [Å] | Puckering Parameters | CH2NH2 Position | |||
|---|---|---|---|---|---|---|---|
| C-N | C-O* | C-O** | Θ [°] | Q [Å] | |||
| ANISAS | ZI | 1.4868 | 1.2576 | 1.2577 | 176.540 | 0.558 | AX |
| ANISAS * | ZI | 1.5035 | 1.2643 | 1.2625 | 4.859 | 0.552 | AX |
| ANISEW | ZI | 1.4500 | 1.2560 | 1.2445 | 140.528 | 0.670 | AX |
| ANISEW * | ZI | 1.4927 | 1.2288 | 1.2326 | 177.246 | 0.554 | EQ |
| ANISEW ** | ZI | 1.5001 | 1.2445 | 1.2746 | 177.950 | 0.551 | EQ |
| ANISEW *** | ZI | 1.2746 | 1.2635 | 1.2308 | 12.361 | 0.580 | AX |
| AVAVAV | cation | 1.4948 | 1.3141 | 1.2200 | 178.679 | 0.558 | EQ |
| AVILOH | cation | 1.4959 | 1.3167 | 1.2226 | 178.345 | 0.552 | EQ |
| AVILUN | ZI | 1.4867 | 1.2383 | 1.2867 | 177.094 | 0.554 | EQ |
| AWUWIY | cation | 1.4822 | 1.3164 | 1.2012 | 178.293 | 0.548 | EQ |
| AWUWIY01 | cation | 1.4875 | 1.3274 | 1.2105 | 178.258 | 0.554 | EQ |
| AWUWIY02 | cation | 1.4986 | 1.3182 | 1.1957 | 179.201 | 0.533 | EQ |
| DESNOI | ZI | 1.4946 | 1.2627 | 1.2552 | 176.808 | 0.559 | EQ |
| DOBBIG | ZI | 1.4884 | 1.2799 | 1.2427 | 174.637 | 0.566 | EQ |
| DOBBIG2 | ZI | 1.4796 | 1.2823 | 1.2229 | 2.219 | 0.550 | AX |
| DOBBOM | ZI | 1.4706 | 1.2889 | 1.2349 | 177.149 | 0.543 | EQ |
| DOBBOM2 | ZI | 1.4706 | 1.2889 | 1.2349 | 177.100 | 0.543 | EQ |
| EFIVIB | ZI | 1.4846 | 1.2818 | 1.2456 | 173.788 | 0.550 | EQ |
| ELUNOP | ZI | 1.4979 | 1.2519 | 1.2380 | 176.896 | 0.569 | EQ |
| FOXNOW | ZI | 1.4958 | 1.2659 | 1.2575 | 174.871 | 0.558 | EQ |
| FOXNUC | cation | 1.4916 | 1.2803 | 1.2451 | 175.027 | 0.560 | EQ |
| FOXNUC * | cation | 1.4916 | 1.2803 | 1.2451 | 174.99 | 0.561 | EQ |
| FOXPAK | cation | 1.4953 | 1.3290 | 1.2103 | 175.327 | 0.560 | EQ |
| FOXPAK * | cation | 1.4942 | 1.3279 | 1.2099 | 5.628 | 0.559 | AX |
| FOXPEO | cation | 1.4886 | 1.3013 | 1.2189 | 2.754 | 0.557 | AX |
| FOXPOY | cation | 1.4863 | 1.3289 | 1.2116 | 178.246 | 0.557 | EQ |
| IKATIY | cation | 1.4837 | 1.3131 | 1.2131 | 2.010 | 0.559 | AX |
| JALXAY | ZI | 1.4809 | 1.2603 | 1.2481 | 178.968 | 0.550 | EQ |
| JALXAY * | ZI | 1.4737 | 1.2500 | 1.2623 | 178.26 | 0.537 | EQ |
| JALXAY ** | ZI | 1.4924 | 1.2705 | 1.2372 | 1.58 | 0.535 | AX |
| JALXAY *** | ZI | 1.4954 | 1.2691 | 1.2325 | 2.302 | 0.544 | AX |
| JALXEC | ZI | 1.4966 | 1.2804 | 1.2522 | 176.202 | 0.550 | EQ |
| JALXEC * | ZI | 1.4952 | 1.2851 | 1.2460 | 176.013 | 0.545 | EQ |
| LORQIT | cation | 1.4968 | 1.3147 | 1.2171 | 176.816 | 0.550 | EQ |
| NUPXAY | cation | 1.4761 | 1.3099 | 1.2135 | 2.498 | 0.554 | AX |
| QIMKIG | ZI | 1.4999 | 1.2716 | 1.2522 | 5.054 | 0.551 | AX |
| QIMKIG01 | ZI | 1.4995 | 1.2677 | 1.2405 | 4.736 | 0.551 | AX |
| QIMKIG02 | ZI | 1.4860 | 1.2657 | 1.2409 | 176.491 | 0.560 | EQ |
| QIMKIG03 | ZI | 1.4836 | 1.2633 | 1.2440 | 2.444 | 0.552 | AX |
| QIMKIG04 | ZI | 1.4859 | 1.2619 | 1.2450 | 177.559 | 0.554 | EQ |
| QIMKIG05 | ZI | 1.4887 | 1.2629 | 1.2504 | 2.451 | 0.556 | AX |
| QIMKIG06 | ZI | 1.4993 | 1.2653 | 1.2458 | 4.719 | 0.550 | AX |
| QIMKOM | ZI | 1.4923 | 1.2680 | 1.2514 | 3.070 | 0.556 | AX |
| QIMKOM01 | ZI | 1.4907 | 1.2630 | 1.2486 | 3.168 | 0.555 | AX |
| QIMKOM02 | ZI | 1.4865 | 1.2647 | 1.2507 | 3.082 | 0.551 | AX |
| QIMKOM03 | ZI | 1.4904 | 1.2599 | 1.2450 | 3.304 | 0.549 | AX |
| QIMKOM04 | ZI | 1.4862 | 1.2582 | 1.2539 | 3.240 | 0.562 | AX |
| SESKEI | ZI | 1.5036 | 1.2752 | 1.2671 | 174.05 | 0.573 | EQ |
| SESKEI * | ZI | 1.4887 | 1.2759 | 1.2723 | 177.625 | 0.54 | EQ |
| SOCYUF | cation | 1.4927 | 1.3154 | 1.2161 | 1.382 | 0.558 | AX |
| UQUMOJ | ZI | 1.4899 | 1.2608 | 1.2603 | 179.039 | 0.559 | EQ |
| UQUMOJ * | ZI | 1.4899 | 1.2608 | 1.2603 | 179.001 | 0.559 | EQ |
| UQUMOJ ** | ZI | 1.4849 | 1.2576 | 1.2526 | 2.610 | 0.553 | AX |
| UQUMOJ *** | ZI | 1.4849 | 1.2576 | 1.2526 | 2.626 | 0.553 | AX |
| VIXBUB | ZI | 1.5026 | 1.2668 | 1.2595 | 178.013 | 0.546 | EQ |
| VIXBUB * | ZI | 1.5099 | 1.2790 | 1.2526 | 2.728 | 0.560 | AX |
| VIXBUB ** | ZI | 1.4960 | 1.2690 | 1.2529 | 4.688 | 0.552 | AX |
| VIXBUB *** | ZI | 1.5026 | 1.2668 | 1.2595 | 178.013 | 0.546 | EQ |
| VIXBUB **** | ZI | 1.5099 | 1.2790 | 1.2526 | 2.728 | 0.560 | AX |
| VIXBUB ***** | ZI | 1.4960 | 1.2690 | 1.2529 | 4.688 | 0.552 | AX |
| VIXCAI | ZI | 1.4912 | 1.2597 | 1.2534 | 1.588 | 0.553 | AX |
| VIXCAI * | ZI | 1.4912 | 1.2597 | 1.2534 | 1.650 | 0.552 | AX |
| VIXCAI ** | ZI | 1.5237 | 1.2662 | 1.2459 | 4.233 | 0.561 | AX |
| VIXCAI *** | ZI | 1.5237 | 1.2662 | 1.2459 | 4.235 | 0.561 | AX |
| VIXCAI **** | ZI | 1.5017 | 1.2792 | 1.2381 | 2000 | 0.543 | AX |
| VIXCAI ***** | ZI | 1.4733 | 1.2658 | 1.2450 | 3.103 | 0.559 | AX |
| VIXCAI ****** | ZI | 1.5246 | 1.2680 | 1.2490 | 2.558 | 0.550 | AX |
| VIXCAI ******* | ZI | 1.5246 | 1.2680 | 1.2490 | 2.625 | 0.550 | AX |
| VIXCAI ******** | ZI | 1.4733 | 1.2658 | 1.2450 | 3.045 | 0.559 | AX |
| VIXCAI ********* | ZI | 1.5017 | 1.2792 | 1.2381 | 1.962 | 0.544 | AX |
| VIXCEM | ZI | 1.4942 | 1.2662 | 1.2569 | 177.542 | 0.553 | EQ |
| VIXCEM * | ZI | 1.4935 | 1.2696 | 1.2318 | 177.542 | 0.553 | AX |
| VIXCEM01 | ZI | 1.4939 | 1.2777 | 1.2418 | 3.161 | 0.547 | EQ |
| VIXCEM01 * | ZI | 1.4942 | 1.2662 | 1.2569 | 2.276 | 0.538 | AX |
| VIXCIQ | ZI | 1.4903 | 1.2851 | 1.2255 | 3.090 | 0.556 | AX |
| VIXCIQ * | ZI | 1.4903 | 1.2851 | 1.2255 | 3.004 | 0.556 | AX |
| VIXCIQ *** | ZI | 1.5012 | 1.2739 | 1.2510 | 175.306 | 0.537 | EQ |
| VIXCIQ **** | ZI | 1.5012 | 1.2739 | 1.2510 | 175.329 | 0.537 | EQ |
| VIXCUC | ZI | 1.5151 | 1.2854 | 1.2538 | 3.097 | 0.551 | AX |
| VIXCUC * | ZI | 1.5151 | 1.2854 | 1.2538 | 3.097 | 0.551 | AX |
| VIXCUC ** | ZI | 1.5136 | 1.2721 | 1.2512 | 3.708 | 0.556 | AX |
| VIXCUC *** | ZI | 1.5035 | 1.2723 | 1.2577 | 178.596 | 0.557 | EQ |
| VIXCUC **** | ZI | 1.5035 | 1.2723 | 1.2577 | 178.596 | 0.557 | EQ |
| VIXCUC ***** | ZI | 1.5136 | 1.2721 | 1.2512 | 3.708 | 0.556 | AX |
| VIXDAJ | ZI | 1.4955 | 1.2703 | 1.2603 | 2.886 | 0.576 | AX |
| VIXDAJ * | ZI | 1.4831 | 1.2623 | 1.2558 | 177.366 | 0.560 | EQ |
| VIXDAJ ** | ZI | 1.5026 | 1.2591 | 1.2530 | 3.655 | 0.561 | AX |
| VIXDAJ *** | ZI | 1.4955 | 1.2703 | 1.2603 | 2.886 | 0.576 | AX |
| VIXDAJ **** | ZI | 1.4831 | 1.2623 | 1.2558 | 177.366 | 0.560 | EQ |
| VIXDAJ ***** | ZI | 1.5026 | 1.2591 | 1.2530 | 3.655 | 0.561 | AX |
| VIXDEN | ZI | 1.4969 | 1.2808 | 1.2669 | 1.425 | 0.523 | AX |
| VIXDEN * | ZI | 1.5099 | 1.2545 | 1.2459 | 3.392 | 0.541 | AX |
| VIXDEN ** | ZI | 1.4696 | 1.2780 | 1.2735 | 1.068 | 0.555 | AX |
| VIXDEN *** | ZI | 1.5085 | 1.2777 | 1.2535 | 175.266 | 0.562 | EQ |
| VIXDEN **** | ZI | 1.4907 | 1.2592 | 1.2504 | 2.758 | 0.538 | AX |
| VIXDEN ***** | ZI | 1.4868 | 1.2551 | 1.2482 | 178.287 | 0.548 | EQ |
| VIXDEN ****** | ZI | 1.5360 | 1.2633 | 1.2406 | 1.092 | 0.560 | AX |
| VIXDEN ******* | ZI | 1.5009 | 1.2685 | 1.2618 | 175.248 | 0.555 | EQ |
| VIXQAW | ZI | 1.4846 | 1.2798 | 1.2531 | 165.087 | 0.502 | EQ |
| VIXQAW1* | ZI | 1.5082 | 1.2594 | 1.2455 | 1.119 | 0.546 | EQ |
| VIXQAW ** | ZI | 1.4846 | 1.2798 | 1.2531 | 165.042 | 0.502 | AX |
| VIXQAW *** | ZI | 1.4767 | 1.2714 | 1.2353 | 2.893 | 0.545 | EQ |
| VIXQAW **** | ZI | 1.5188 | 1.2885 | 1.2484 | 175.464 | 0.560 | AX |
| VIXQAW ***** | ZI | 1.5079 | 1.2646 | 1.2602 | 1.962 | 0.554 | AX |
| VIXQAW ****** | ZI | 1.4767 | 1.2714 | 1.2353 | 2.908 | 0.545 | AX |
| VIXQAW ******* | ZI | 1.5188 | 1.2885 | 1.2484 | 175.504 | 0.561 | AX |
| VIXQAW ******** | ZI | 1.3978 | 1.2639 | 1.2477 | 177.357 | 0.575 | AX |
| VIXQAW ********* | ZI | 1.5002 | 1.2664 | 1.2502 | 2.227 | 0.555 | AX |
| VIXQAW ********** | ZI | 1.3978 | 1.2639 | 1.2477 | 177.363 | 0.575 | EQ |
| VIXQAW ********** | ZI | 1.5002 | 1.2664 | 1.2502 | 2.309 | 0.554 | AX |
| VIXQAW *********** | ZI | 1.5573 | 1.2680 | 1.2494 | 2.179 | 0.537 | EQ |
| VIXQAW ************ | ZI | 1.5573 | 1.2680 | 1.2494 | 2.176 | 0.536 | AX |
| VIXQAW ************* | ZI | 1.5082 | 1.2594 | 1.2455 | 1.147 | 0.546 | AX |
| VIXQAW ************** | ZI | 1.5079 | 1.2646 | 1.2602 | 1.993 | 0.554 | EQ |
| VIXQEA | ZI | 1.5125 | 1.2583 | 1.2525 | 3.323 | 0.552 | AX |
| VIXQEA * | ZI | 1.4637 | 1.2710 | 1.2577 | 3.144 | 0.543 | AX |
| VIXQEA ** | ZI | 1.5206 | 1.2656 | 1.2646 | 3.821 | 0.553 | AX |
| VIXQEA *** | ZI | 1.5206 | 1.2656 | 1.2646 | 3.826 | 0.553 | AX |
| VIXQEA **** | ZI | 1.5125 | 1.2583 | 1.2525 | 3.323 | 0.552 | AX |
| VIXQEA ***** | ZI | 1.5050 | 1.2728 | 1.2506 | 6.086 | 0.515 | AX |
| VIXQEA ****** | ZI | 1.4637 | 1.2710 | 1.2577 | 3.156 | 0.543 | AX |
| VIXQEA ******* | ZI | 1.5088 | 1.2544 | 1.2483 | 2.799 | 0.553 | AX |
| VIXQEA ******** | ZI | 1.5050 | 1.2728 | 1.2506 | 6.084 | 0.516 | AX |
| VIXQEA ********* | ZI | 1.5088 | 1.2544 | 1.2483 | 2.797 | 0.553 | AX |
| YUZTET | ZI | 1.4922 | 1.2584 | 1.2529 | 176.962 | 0.547 | EQ |
| Average | 1.4932 | 1.2731 | 1.2464 | 0.553 | |||
| Standard deviation | 0.0282 | 0.0184 | 0.0153 | 0.016 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska, J.; Szeleszczuk, Ł. Exploring Various Crystal and Molecular Structures of Gabapentin—A Review. Crystals 2024, 14, 257. https://doi.org/10.3390/cryst14030257
Baranowska J, Szeleszczuk Ł. Exploring Various Crystal and Molecular Structures of Gabapentin—A Review. Crystals. 2024; 14(3):257. https://doi.org/10.3390/cryst14030257
Chicago/Turabian StyleBaranowska, Justyna, and Łukasz Szeleszczuk. 2024. "Exploring Various Crystal and Molecular Structures of Gabapentin—A Review" Crystals 14, no. 3: 257. https://doi.org/10.3390/cryst14030257
APA StyleBaranowska, J., & Szeleszczuk, Ł. (2024). Exploring Various Crystal and Molecular Structures of Gabapentin—A Review. Crystals, 14(3), 257. https://doi.org/10.3390/cryst14030257






