Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1(Fe) under Various Surface Conditions and Power Modes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodizing Process
2.3. Examination Methods Analytical Techniques
3. Results and Discussion
3.1. Voltage–Current Density Behavior
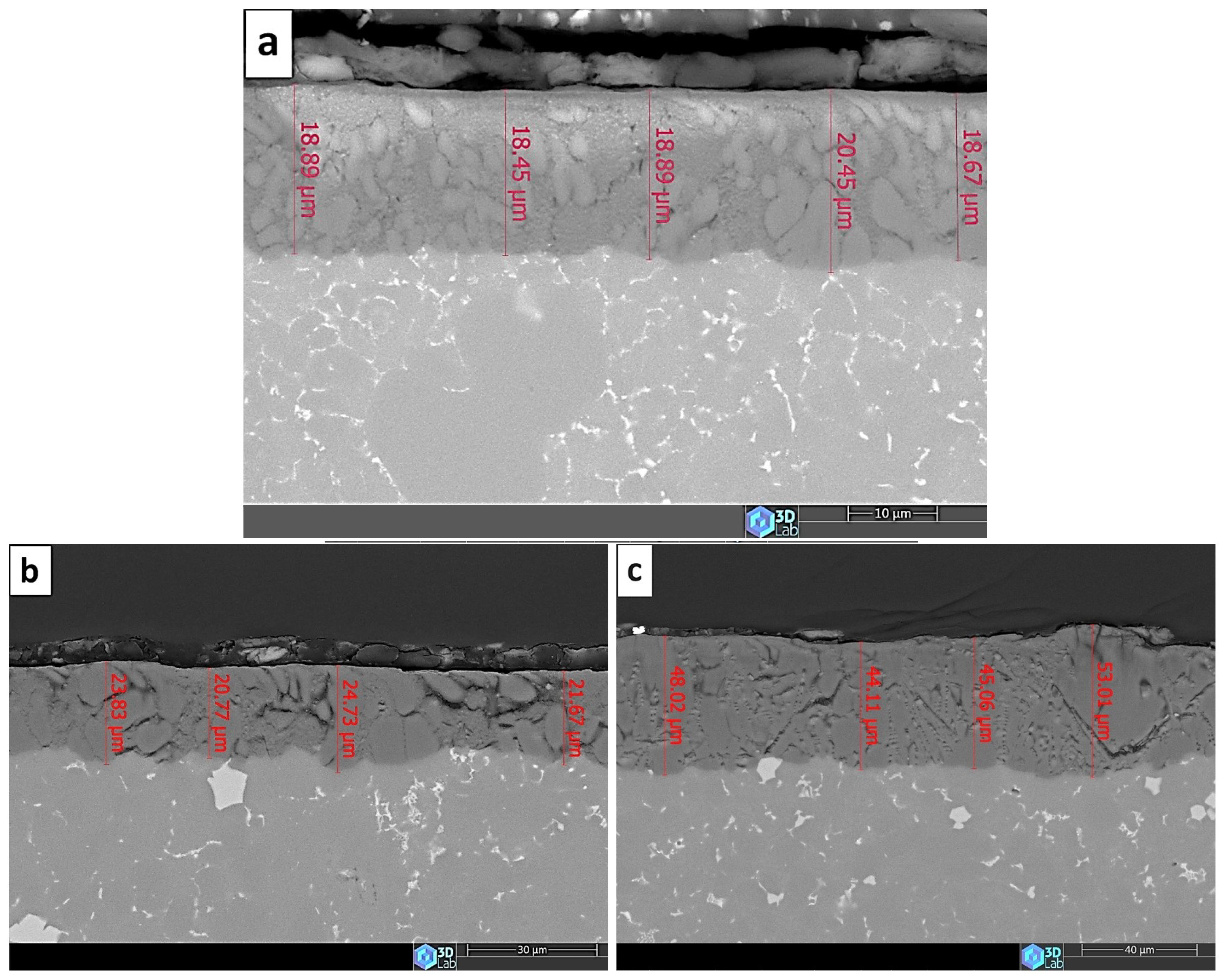
3.2. The Anodic Layer Thicknesses
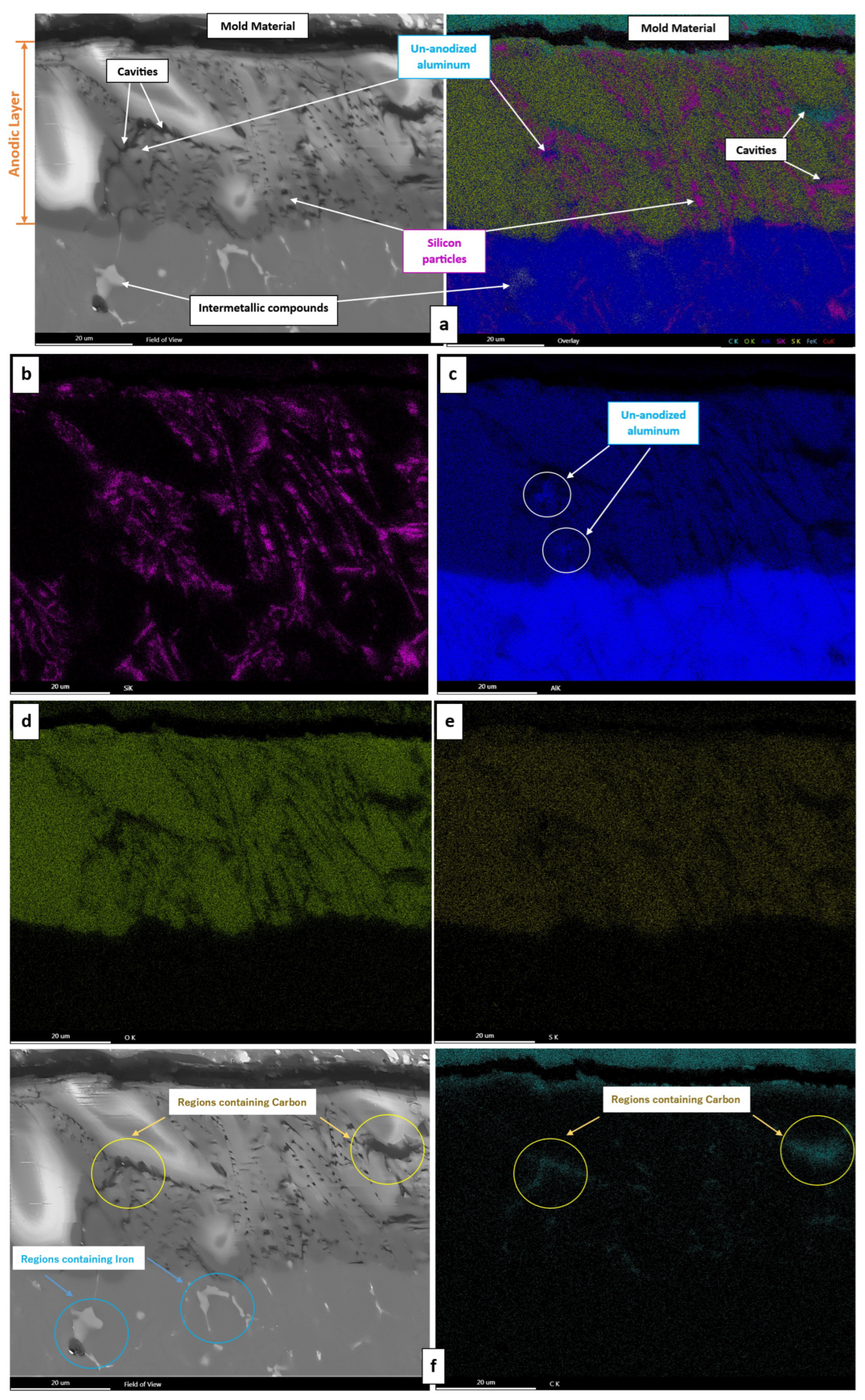
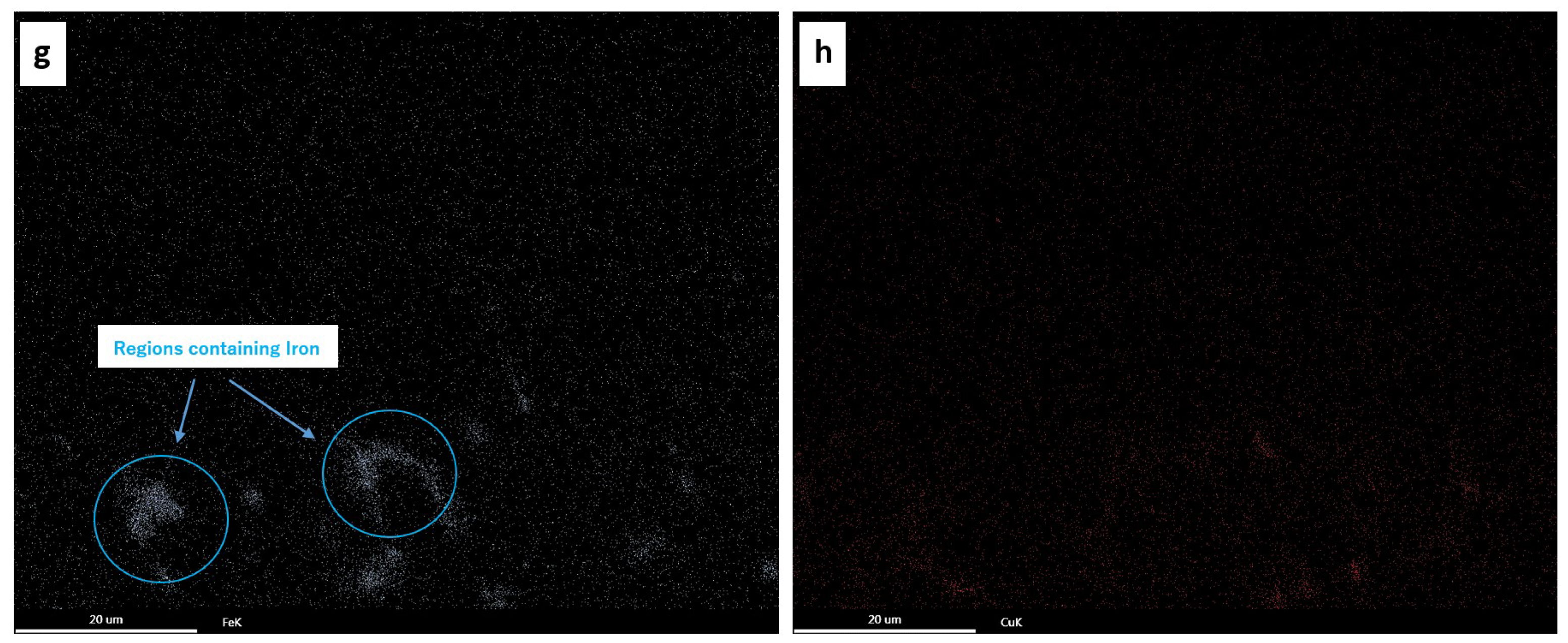
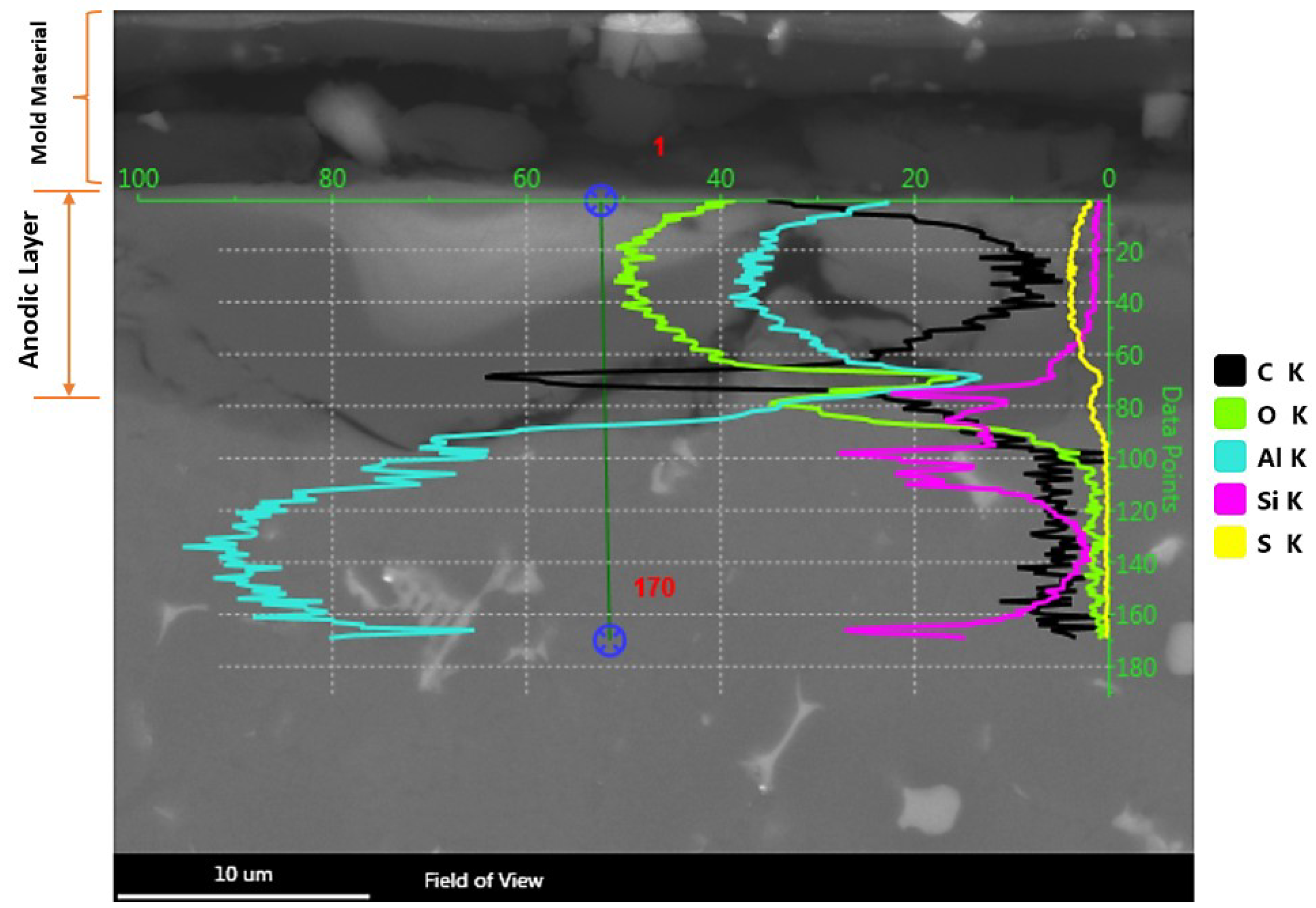
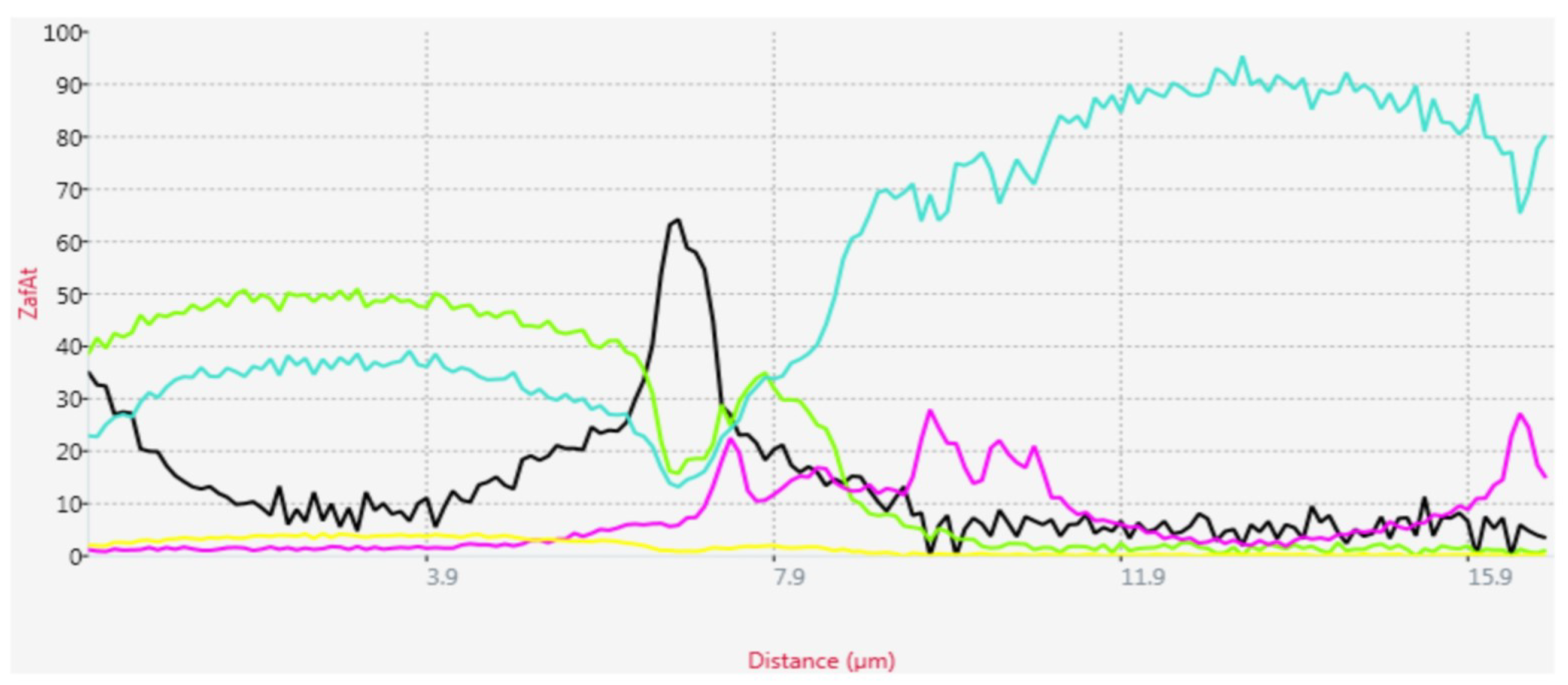
3.3. The Anodic Layer Composition
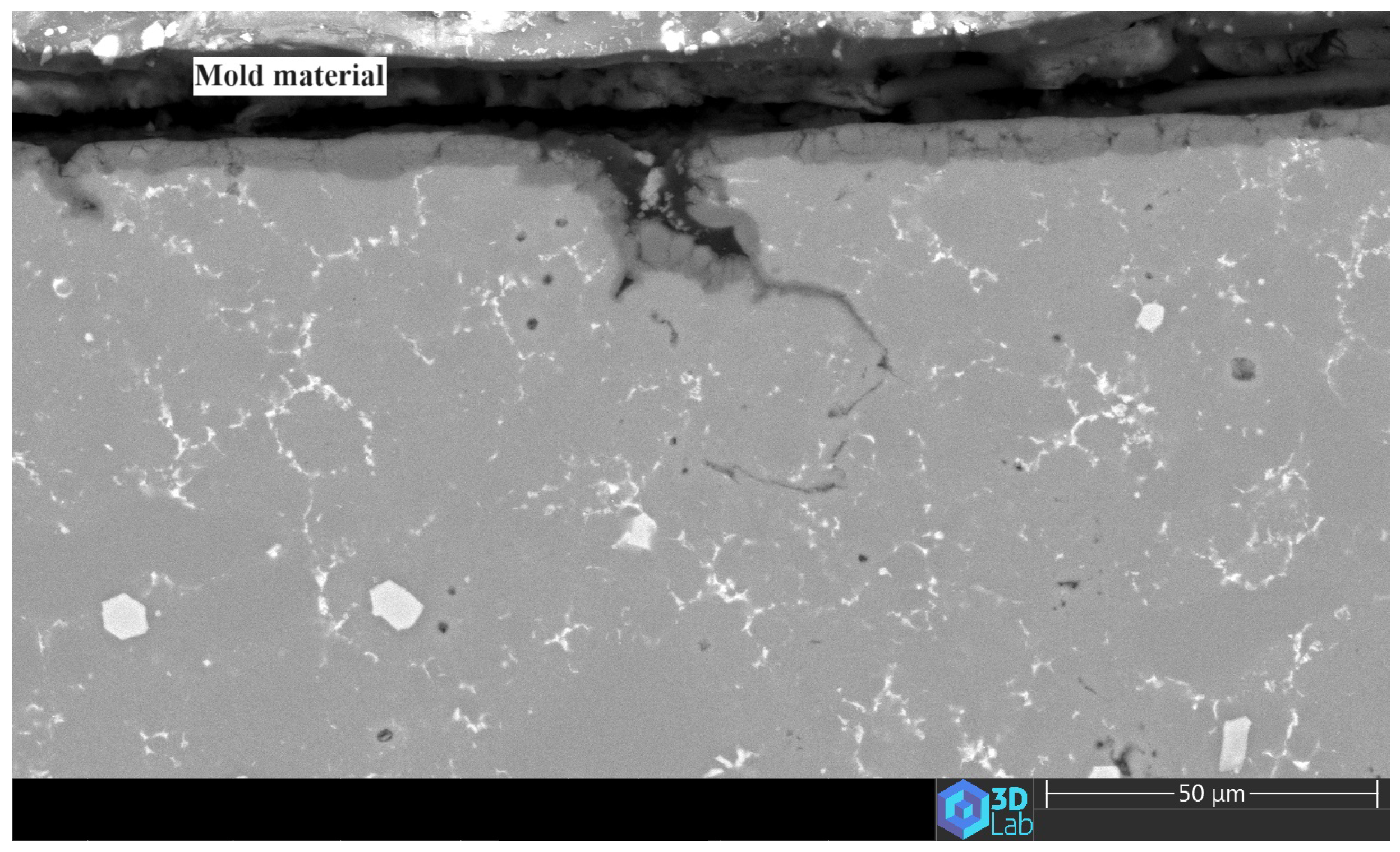
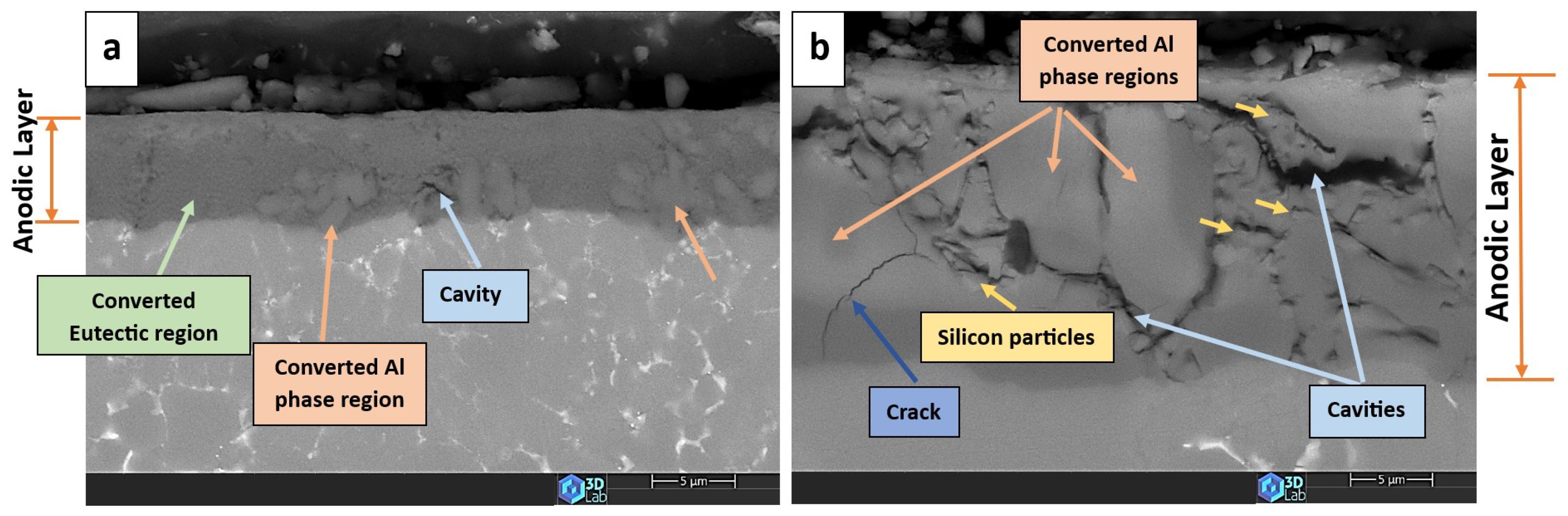
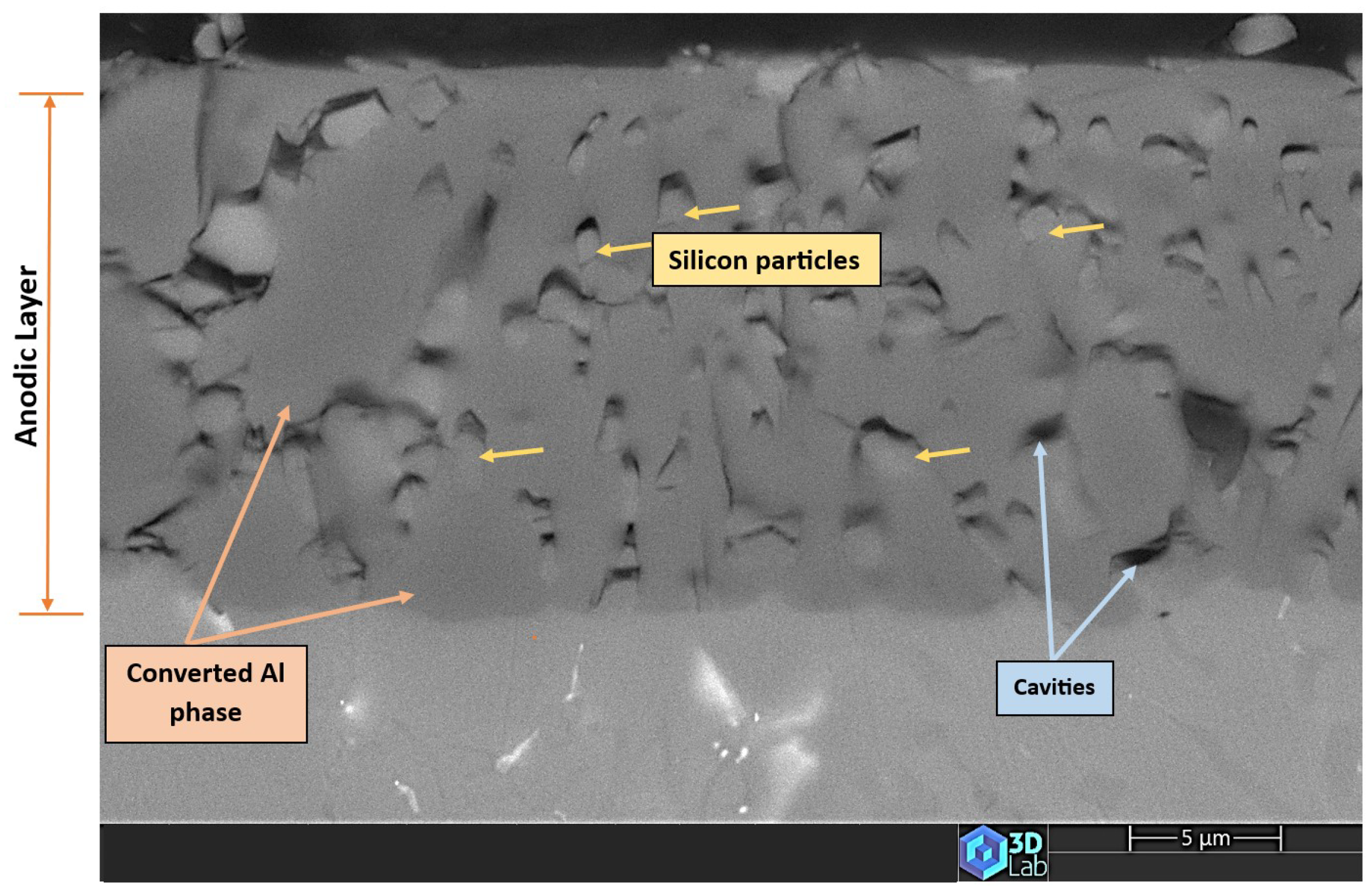
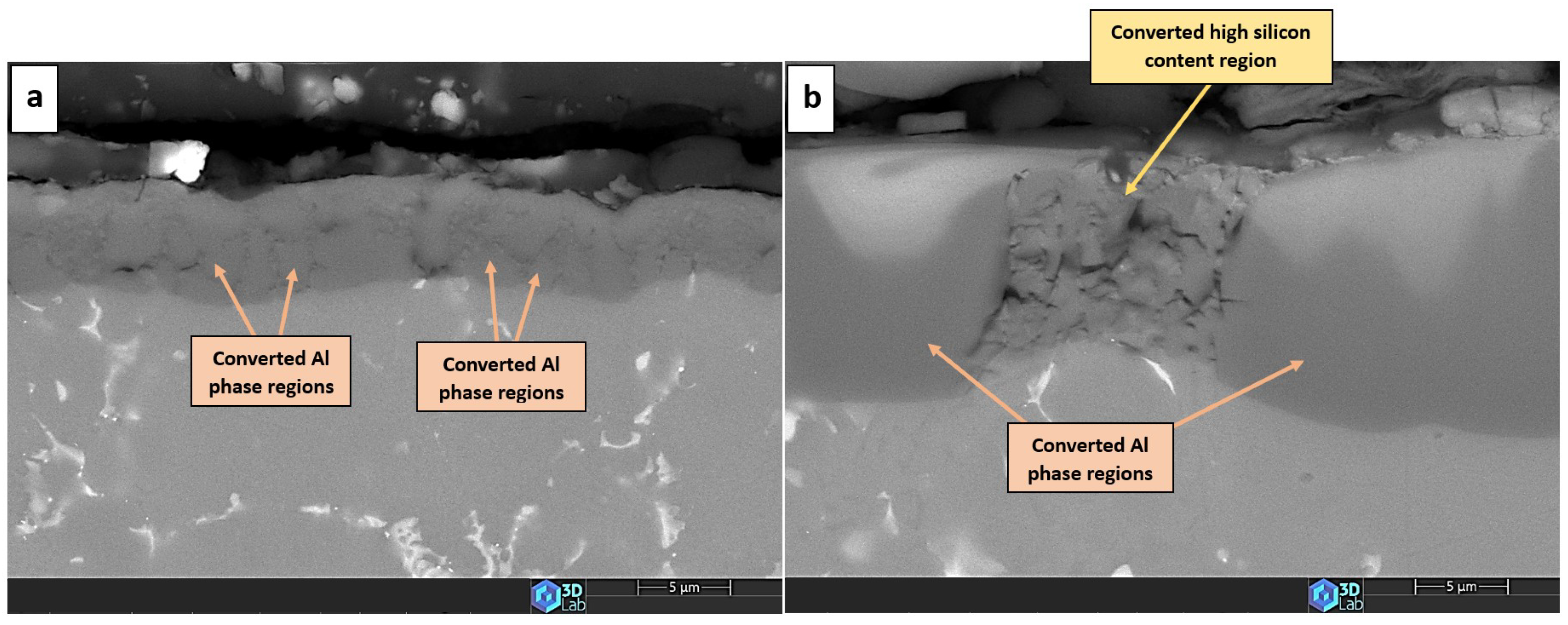
3.4. The Anodic Layer Structures
- Formation of oxygen gas-filled voids: When the oxide front interacts with the silicon phase, it leads to the generation of both SiO2 and gaseous oxygen due to the semiconductor properties of the Si–O bond. Consequently, oxygen gas-filled voids emerge in the aluminium substrate in proximity to the Si particles [31,34].
- Creation of unanodized zones: Unanodized zones form when the oxide front fails to entirely encircle the Si phase, possibly due to its shape or reduced spacing between particles. Consequently, the eutectic silicon phase acts as a barrier shielding the adjacent Al matrix, preventing it from being reached by the oxide front and thus remaining unanodized. The residual metallic Al phase is predominantly detected beneath or amid coarse and interconnected Si eutectic particles [31].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casting Source Magazine. Available online: https://www.castingsource.com/articles/2015/11/01/improving-surface-finishing-die-castings (accessed on 1 February 2024).
- Runge, J.M. The Metallurgy of Anodizing Aluminum; Springer International Publishing: Chicago, IL, USA, 2018; pp. 344–361. [Google Scholar]
- Török, T.I. Anodizing surface treatments of aluminium alloys-Challenges and solutions. BKL Bányászati Kohászati Lapok 2023, 156, 46–50. [Google Scholar]
- Chen, J.; Wen, F.; Liu, C.; Li, W.; Zhou, Q.; Zhu, W.; Zhang, Y.; Guan, R. The microstructure and property of Al-Si alloy improved by the Sc-microalloying and Y2O3 nano-particles. Sci. Technol. Adv. Mater. 2021, 22, 205–217. [Google Scholar]
- Huang, J.M.; Zhao, H.D.; Chen, Z.M. Microstructure and properties of A356 alloy wheels fabricated by low-pressure die casting with local squeeze. J. Mater. Eng. Perform. 2019, 28, 2137–2146. [Google Scholar]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Lorusso, M.; Calignano, F.; Pavese, M.; Andreatta, F. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposure. Corros. Sci. 2019, 152, 101–108. [Google Scholar] [CrossRef]
- Rogov, A.B.; Lyu, H.; Matthews, A.; Yerokhin, A. AC plasma electrolytic oxidation of additively manufactured and cast AlSi12 alloys. Surf. Coat. Technol. 2020, 399, 126116. [Google Scholar] [CrossRef]
- Pezzato, L.; Dabalà, M.; Gross, S.; Brunelli, K. Effect of microstructure and porosity of AlSi10Mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2020, 404, 126477. [Google Scholar]
- Zhu, B.; Zanella, C. Hardness and corrosion behaviour of anodised Al-Si produced by rheocasting. Mater. Des. 2019, 173, 107764. [Google Scholar] [CrossRef]
- Lien, H.H.; Mazumder, J.; Wang, J.; Misra, A. Microstructure evolution and high density of nanotwinned ultrafine Si in hypereutectic Al-Si alloy by laser surface remelting. Mater. Charact. 2020, 161, 110147. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; Del Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard anodizing and plasma electrolytic oxidation of an additively manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Q.; Yuwen, Q.; Li, J. The differences in the formation mechanism of PEO and CPED composited ceramic coatings on Al-12Si alloy. J. Alloys Compd. 2019, 788, 61–66. [Google Scholar] [CrossRef]
- Konieczny, J.; Dobrzański, L.A.; Labisz, K.; Duszczyk, J. The influence of cast method and anodizing parameters on structure and layer thickness of aluminium alloys. J. Mater. Process. Technol. 2004, 157, 718–723. [Google Scholar] [CrossRef]
- Bouchama, L.; Azzouz, N.; Boukmouche, N.; Chopart, J.P.; Daltin, A.L.; Bouznit, Y. Enhancing aluminium corrosion resistance by two-step anodizing process. Surf. Coat. Technol. 2013, 235, 676–684. [Google Scholar] [CrossRef]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.; Wang, Y.M.; Kuo, H.H.H.; Kia, S. Effect of alloy types on the anodizing process of aluminium. Surf. Coat. Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Caliari, D.; Timelli, G.; Zabala, B.; Igartua, A. Microstructural and tribological investigations of diecast and hard anodized AlSiCu alloys. Surf. Coat. Technol. 2018, 352, 462–473. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, L.; Liu, Z.; Niu, D.; Wang, Y.; Liu, C. The Effects of Different Sealing Techniques for Anodic Film of Al-12.7 Si 0.7 Mg Alloys. Int. J. Electrochem. Sci. 2016, 11, 5234–5344. [Google Scholar]
- Arrabal, R.; Mingo, B.; Pardo, A.; Mohedano, M.; Matykina, E.; Rodríguez, I. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2013, 73, 342–355. [Google Scholar]
- Mingo, B.; Arrabal, R.; Pardo, A.; Matykina, E.; Skeldon, P. 3D study of intermetallics and their effect on the corrosion morphology of rheocast aluminium alloy. Mater. Charact. 2016, 112, 122–128. [Google Scholar] [CrossRef]
- Caliari, D.; Timelli, G.; Salata, T.; Cavagnini, G.; Maestri, S. Surface defects of anodized HPDC Al alloy components. Metall. Ital. 2016, 108, 69–72. [Google Scholar]
- Fratila-Apachitei, L.E.; Duszczyk, J.; Katgerman, L. Vickers microhardness of AlSi (Cu) anodic oxide layers formed in H2SO4 at low temperature. Surf. Coat. Technol. 2003, 165, 309–315. [Google Scholar] [CrossRef]
- Grosselle, F.; Timelli, G.; Bonollo, F.; Molina, R. Correlation between microstructure and mechanical properties of Al-Si diecast engine blocks. Metall. Sci. Technol. 2009, 27, 5–6. [Google Scholar]
- Zhu, B.W.; Seifeddine, S.; Jarfors, A.E.; Leisner, P.; Zanella, C. A study of anodising behaviour of Al-Si components produced by rheocasting. Solid State Phenom. 2019, 285, 39–44. [Google Scholar] [CrossRef]
- Zhang, F.; Örnek, C.; Nilsson, J.O.; Pan, J. Anodisation of aluminium alloy AA7075–Influence of intermetallic particles on anodic oxide growth. Corros. Sci. 2020, 164, 108319. [Google Scholar] [CrossRef]
- Scampone, G.; Timelli, G. Anodizing Al–Si foundry alloys: A critical review. Adv. Eng. Mater. 2022, 24, 2101480. [Google Scholar] [CrossRef]
- Dong, H. Surface Engineering of Light Alloys: Aluminium. Magnesium and Titanium Alloys; Woodhead Publishing: New York, NY, USA, 2010; pp. 84–86. [Google Scholar]
- Labisz, K.; Dobrzański, L.A.; Konieczny, J. Anodization of cast aluminium alloys produced by different casting methods. Arch. Foundry Eng. 2008, 8, 45–50. [Google Scholar]
- Riddar, F.; Hogmark, S.; Rudolphi, Å.K. Comparison of anodised aluminium surfaces from four fabrication methods. J. Mater. Process. Technol. 2012, 212, 2272–2281. [Google Scholar] [CrossRef]
- Abrahami, S.T. Cr (VI)-Free Pre-Treatments for Adhesive Bonding of Aerospace Aluminium Alloys. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2016. [Google Scholar]
- Zhu, B.; Seifeddine, S.; Persson, P.O.; Jarfors, A.E.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, B.H.; Lee, S.M. Effects of physical melt treatments on microstructural evolution and anodizing characteristics of Al-Si casting alloys. Mater. Sci. Forum 2011, 695, 243–246. [Google Scholar] [CrossRef]
- Chauke, L.; Möller, H.; Govender, G. Anodizing of aluminium alloy plates from different series produced by rheo-high pressure die casting. Solid State Phenom. 2015, 217, 247–252. [Google Scholar]
- Fratila-Apachitei, L.E.; Tichelaar, F.D.; Thompson, G.E.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi (Cu) alloys. Electrochim. Acta 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.E.; Duszczyk, J.; Katgerman, L. Voltage transients and morphology of AlSi (Cu) anodic oxide layers formed in H2SO4 at low temperature. Surf. Coat. Technol. 2022, 157, 80–94. [Google Scholar]
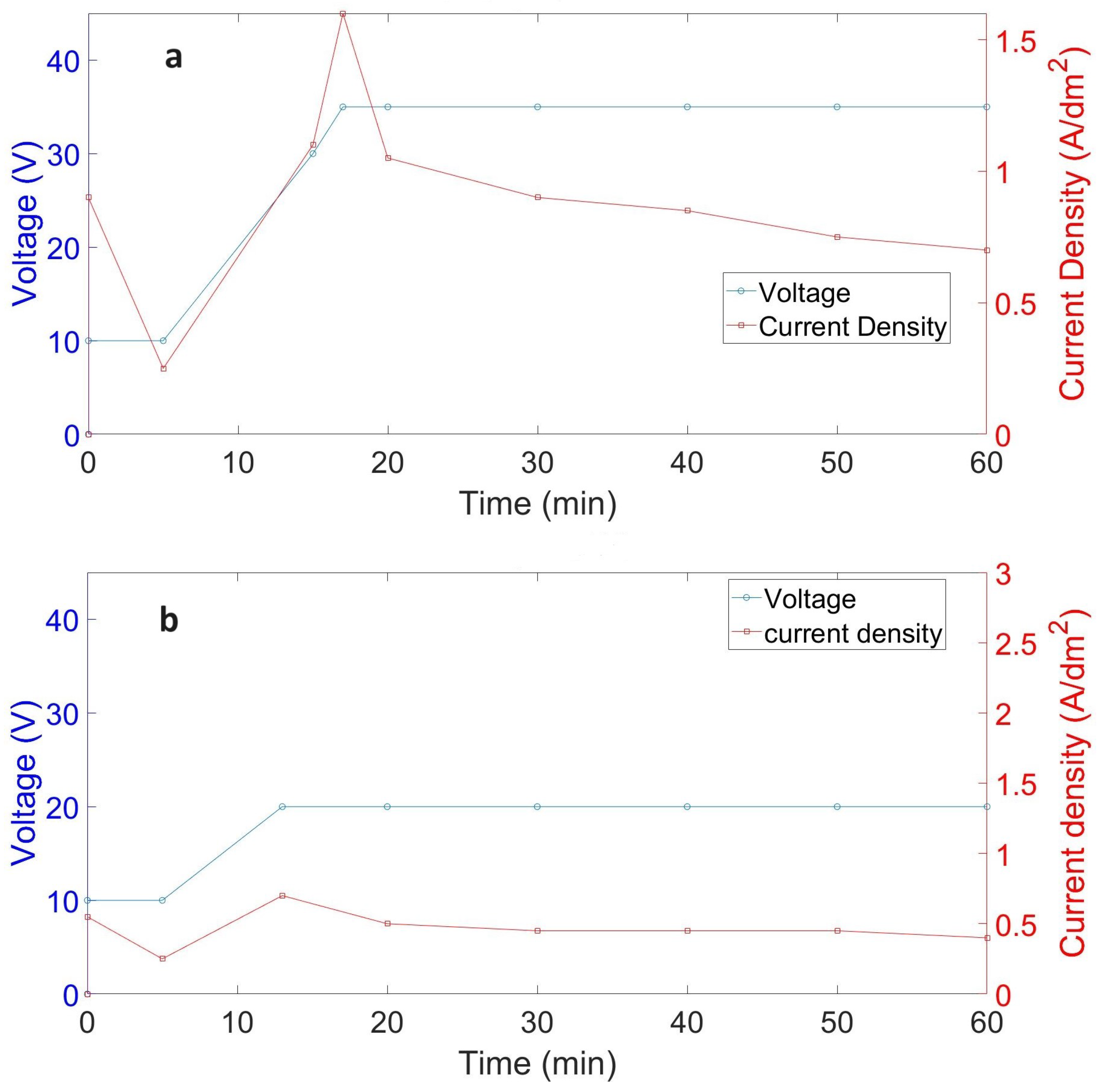

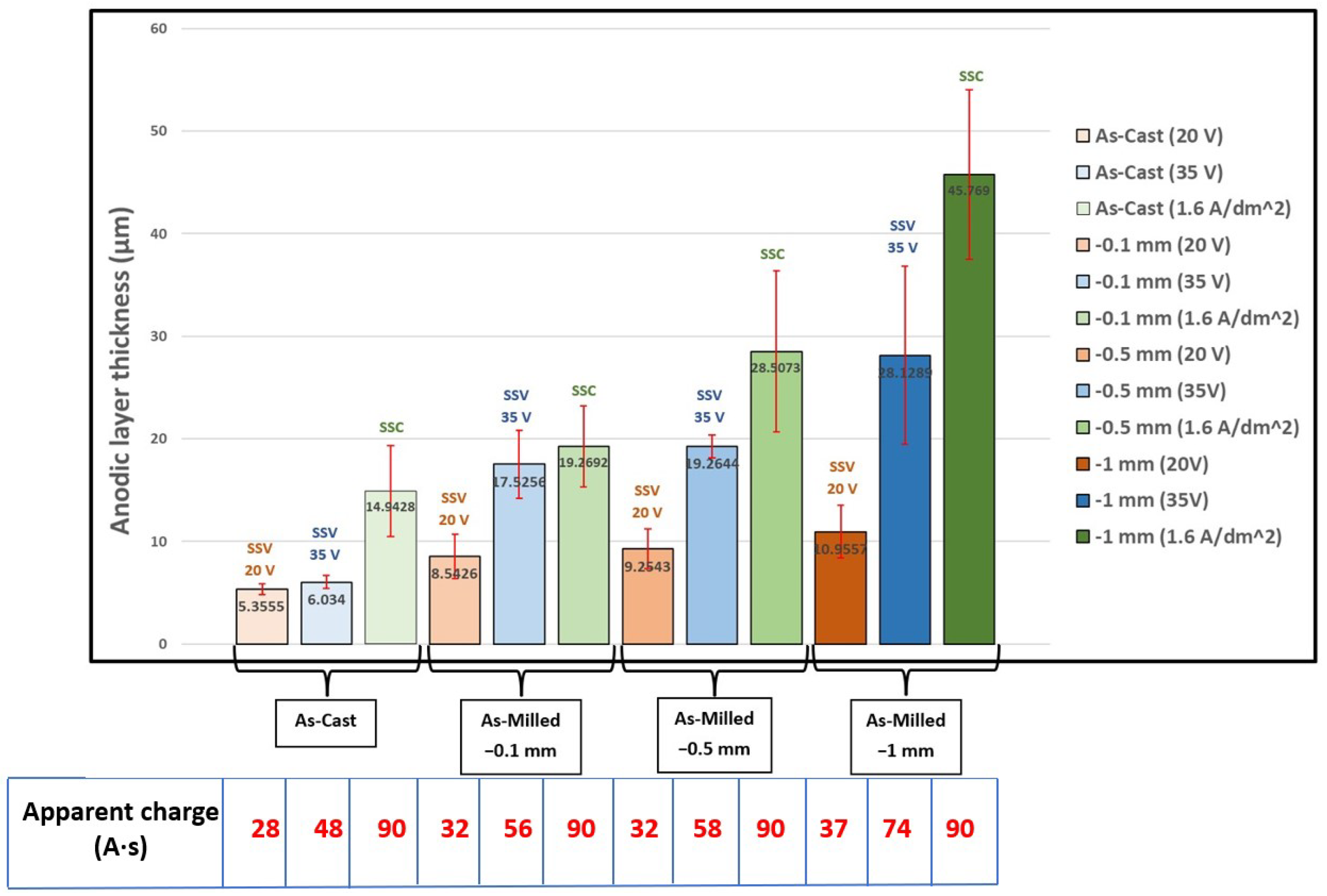


| Sample | Surface Condition | Anodizing State | Anodizing Duration (min) | Theoretical Current Density(max) (A·dm−2) | Apparent Charge (A·s) |
|---|---|---|---|---|---|
| VC1 | As-Cast | SSV (35 V) | 60 | 1.6 | 48 |
| VM11 | ground (−0.1 mm) | SSV (35 V) | 60 | 2.3 | 56 |
| VM12 | ground (−0.5 mm) | SSV (35 V) | 60 | 2.4 | 58 |
| VM13 | ground (−1 mm) | SSV (35 V) | 60 | 2.8 | 74 |
| VC2 | As-Cast | SSV (20 V) | 60 | 0.7 | 28 |
| VM21 | ground (−0.1 mm) | SSV (20 V) | 60 | 0.7 | 32 |
| VM22 | ground (−0.5 mm) | SSV (20 V) | 60 | 0.7 | 32 |
| VM23 | ground (−1 mm) | SSV (20 V) | 60 | 0.9 | 37 |
| CC1 | As-Cast | SSC | 60 | 1.6 | 90 |
| CM1 | ground (−0.1 mm) | SSC | 60 | 1.6 | 90 |
| CM2 | ground (−0.5 mm) | SSC | 60 | 1.6 | 90 |
| CM3 | ground (−1 mm) | SSC | 60 | 1.6 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzouk, E.; Koncz-Horváth, D.; Török, T.I. Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1(Fe) under Various Surface Conditions and Power Modes. Crystals 2024, 14, 352. https://doi.org/10.3390/cryst14040352
Razzouk E, Koncz-Horváth D, Török TI. Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1(Fe) under Various Surface Conditions and Power Modes. Crystals. 2024; 14(4):352. https://doi.org/10.3390/cryst14040352
Chicago/Turabian StyleRazzouk, Emel, Dániel Koncz-Horváth, and Tamás I. Török. 2024. "Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1(Fe) under Various Surface Conditions and Power Modes" Crystals 14, no. 4: 352. https://doi.org/10.3390/cryst14040352
APA StyleRazzouk, E., Koncz-Horváth, D., & Török, T. I. (2024). Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1(Fe) under Various Surface Conditions and Power Modes. Crystals, 14(4), 352. https://doi.org/10.3390/cryst14040352





