Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors
Abstract
:1. Introduction
2. Technology for Obtaining Ceramic Nanotube Nets
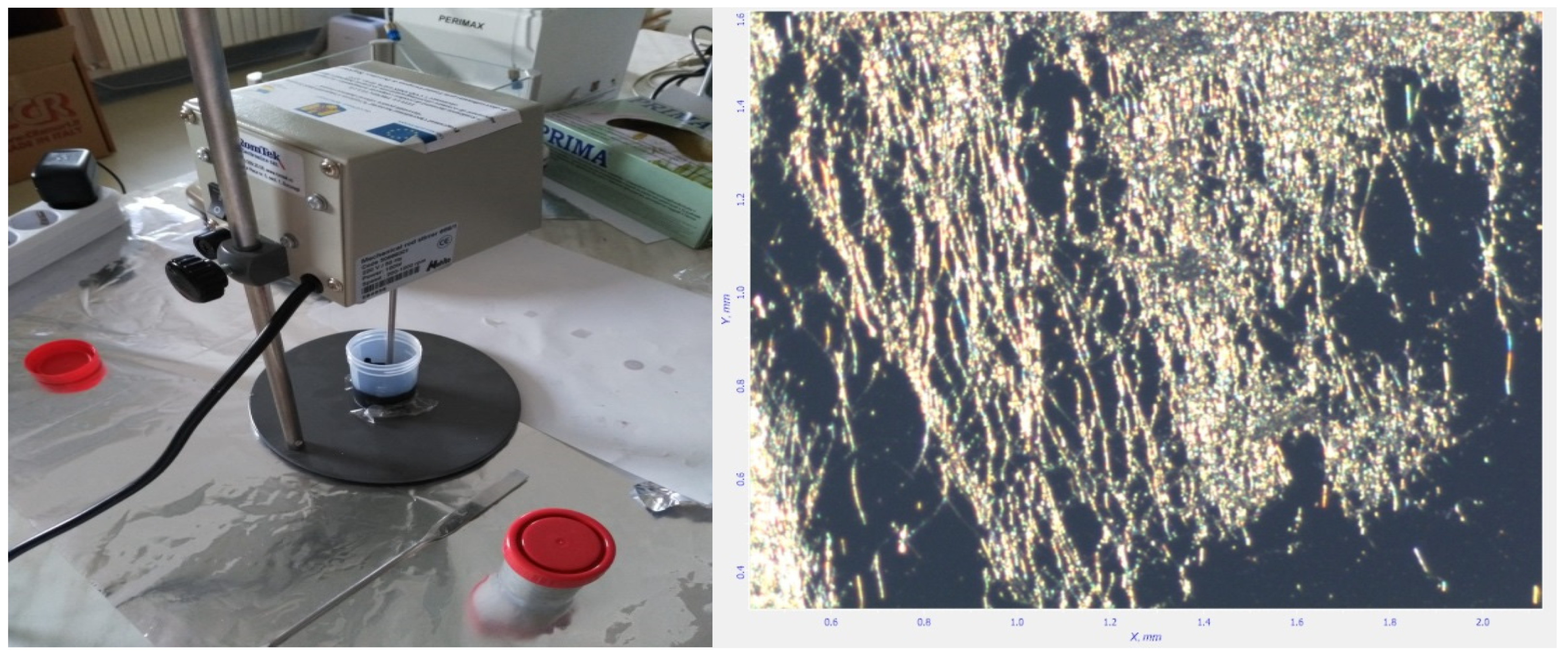
2.1. Technological Equipment
2.2. Materials and Preparation Method
- (i)
- Preliminary manufacturing of polymer fibers nets of poly(methyl methacrylate) (PMMA), with a molecular weight (Mw) of 300,000, from a solution of 10 wt%, with dimethylformamide (DMF) as solvent.
- (ii)
- Magnetron deposition of ceramic films
- (iii)
- Thermal treatment of nanotubes
2.3. Characterization Equipment
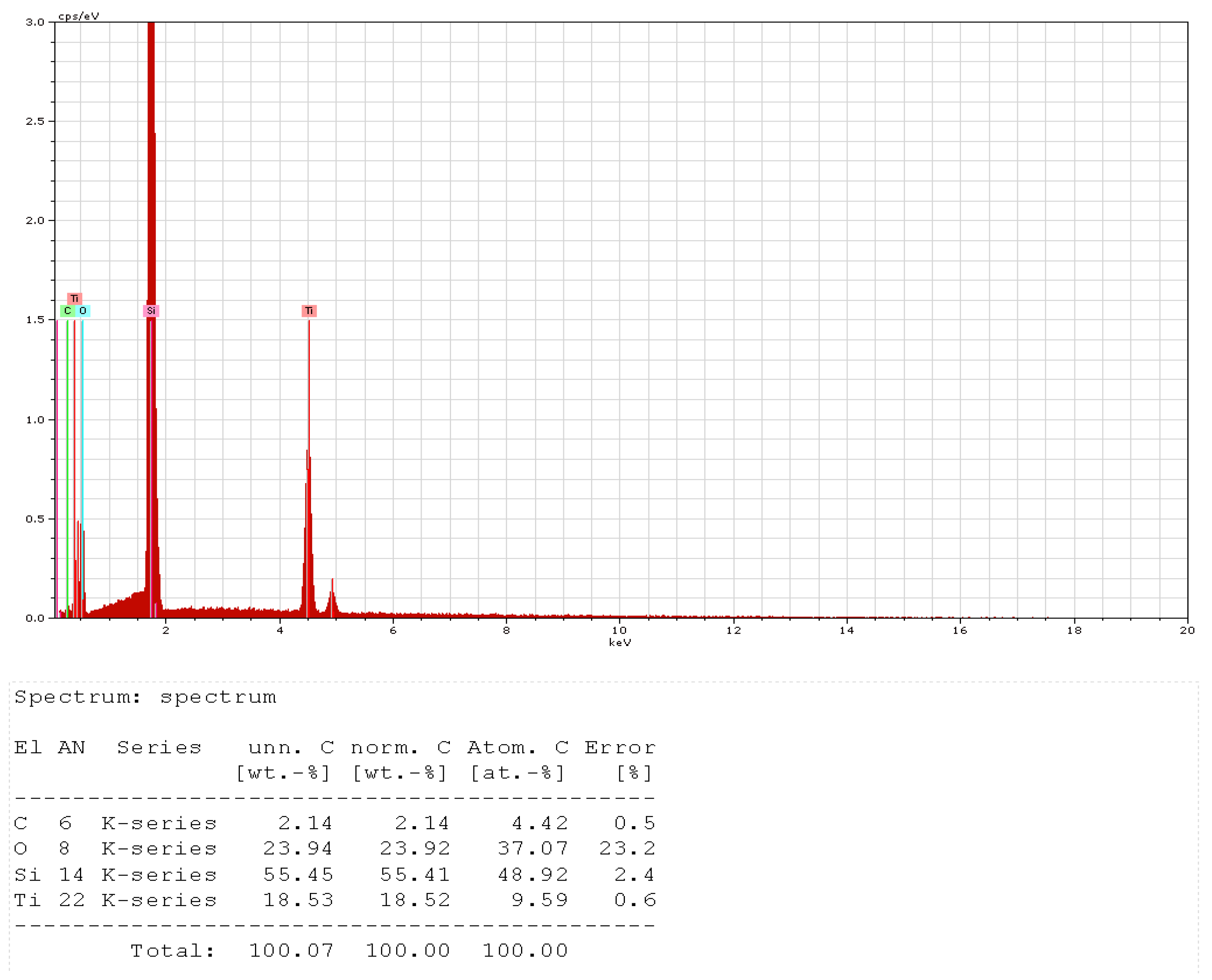
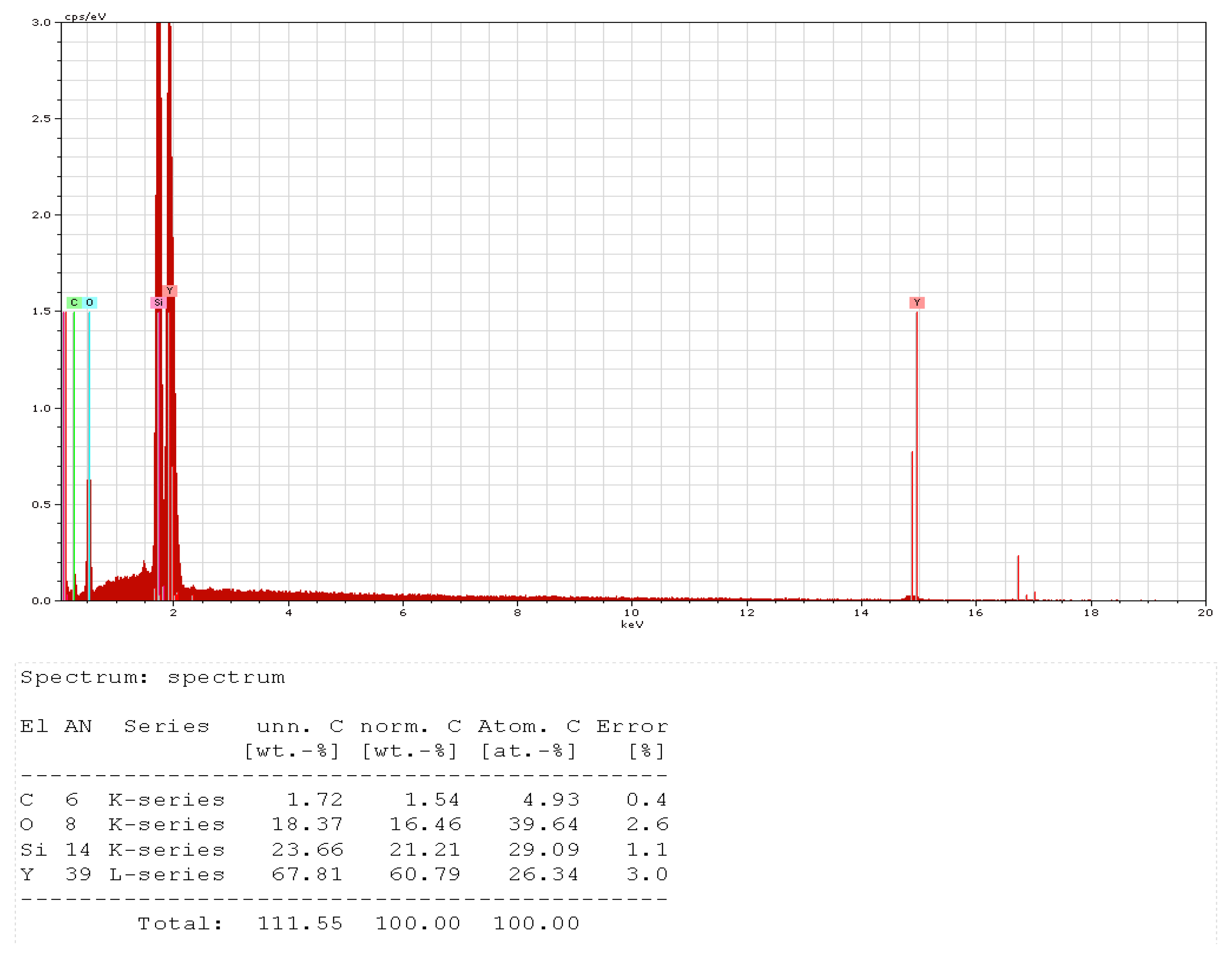
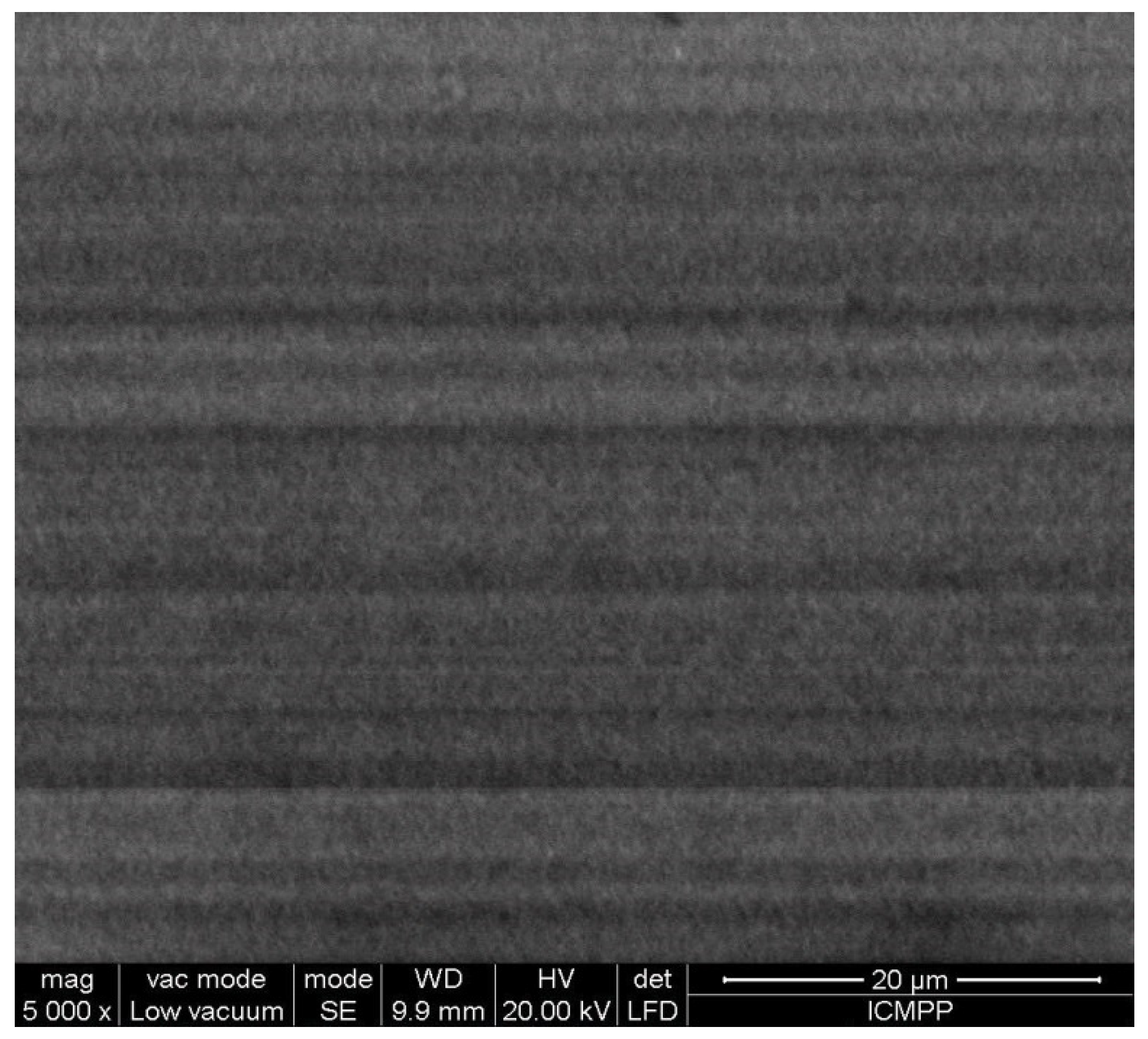
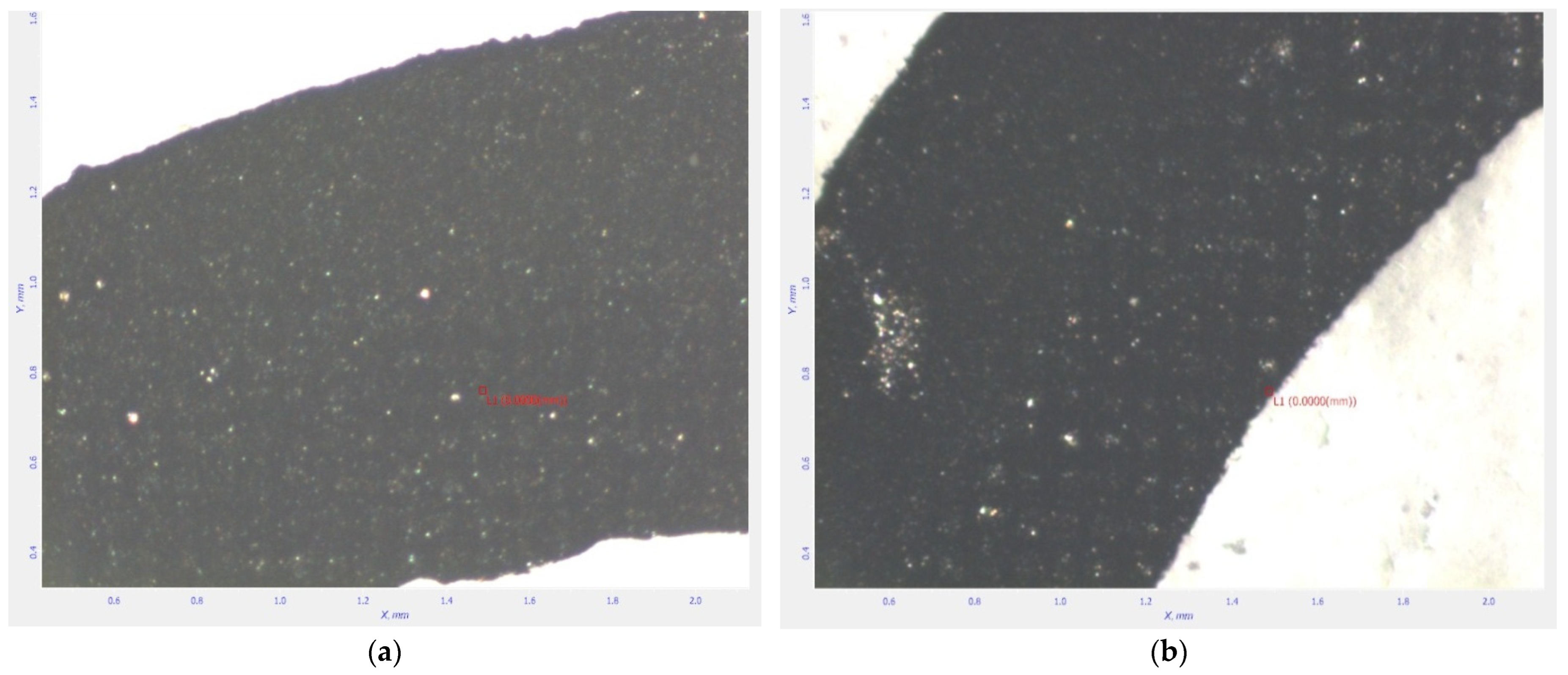
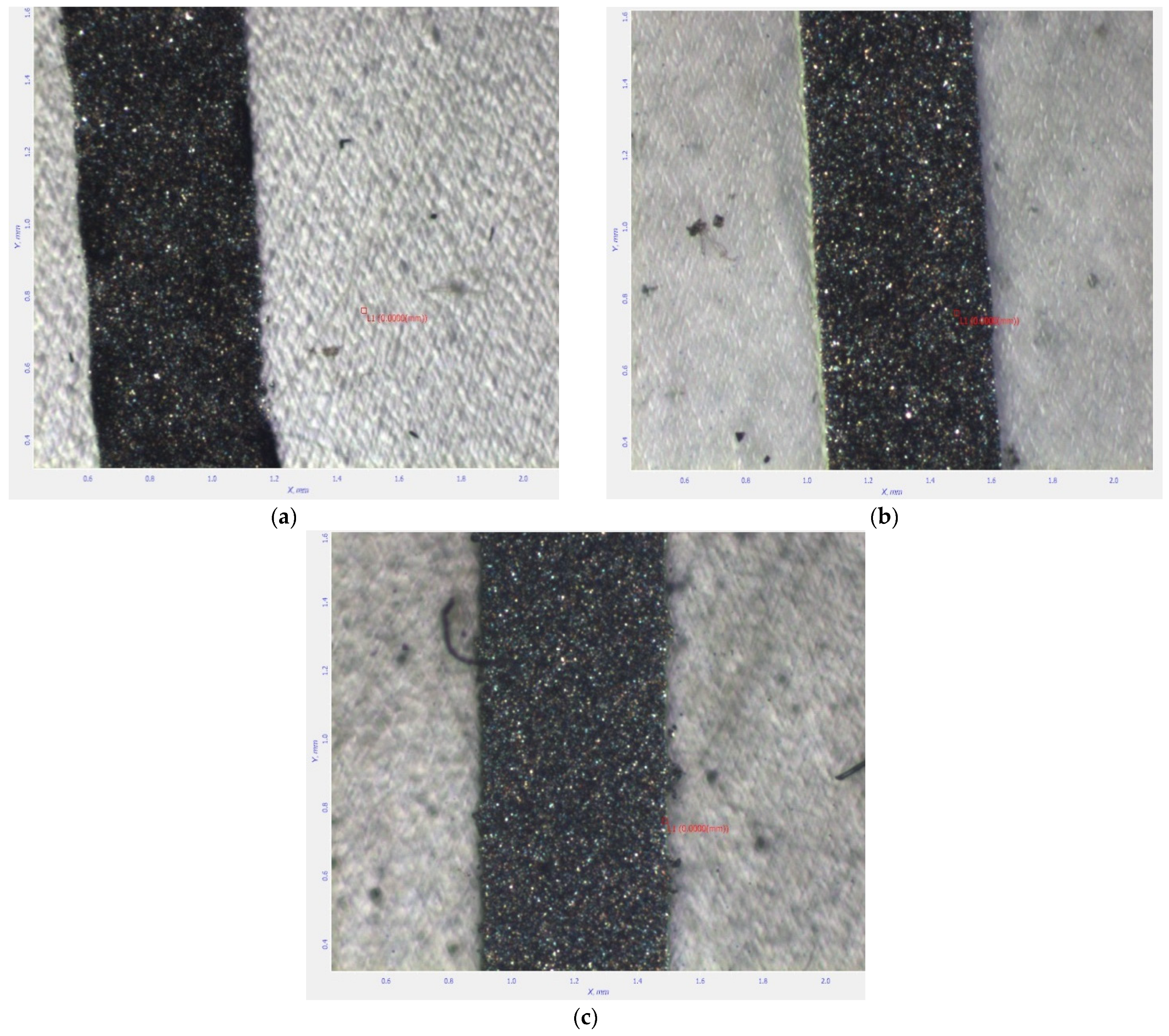
- Optical scanning microscopy SEM and energy-dispersive X-ray spectroscopy (EDX) were performed with a field emission and focused ion beam scanning electron microscope (SEM) model Tescan Lyra III XMU (Libušina tř. 21 623 00, Brno-Kohoutovice, Czech Republic).
- Structural characterization was carried out by X-ray diffraction (XRD) using CuKα radiation with Ni filter Bruker AXS D8 Advance (Bruker AXS, Billerica, MA, USA) with CuKα radiation (λ = 0.154 nm). Diffraction patterns were recorded at room temperature in Bragg-Brentano geometry at an angle 2θ from 20° to 65° at a rate of 0.6°/min (2θ)/min.
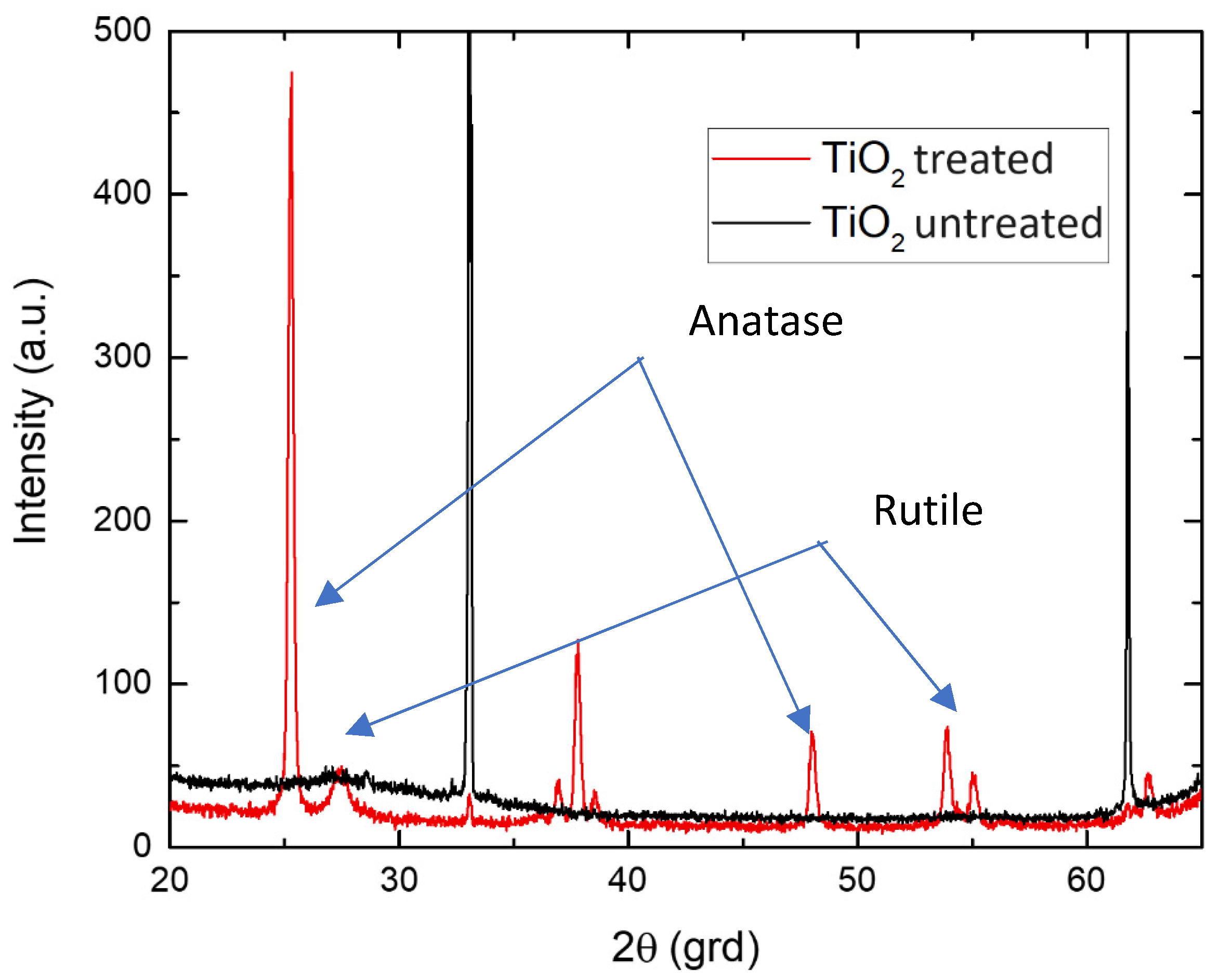
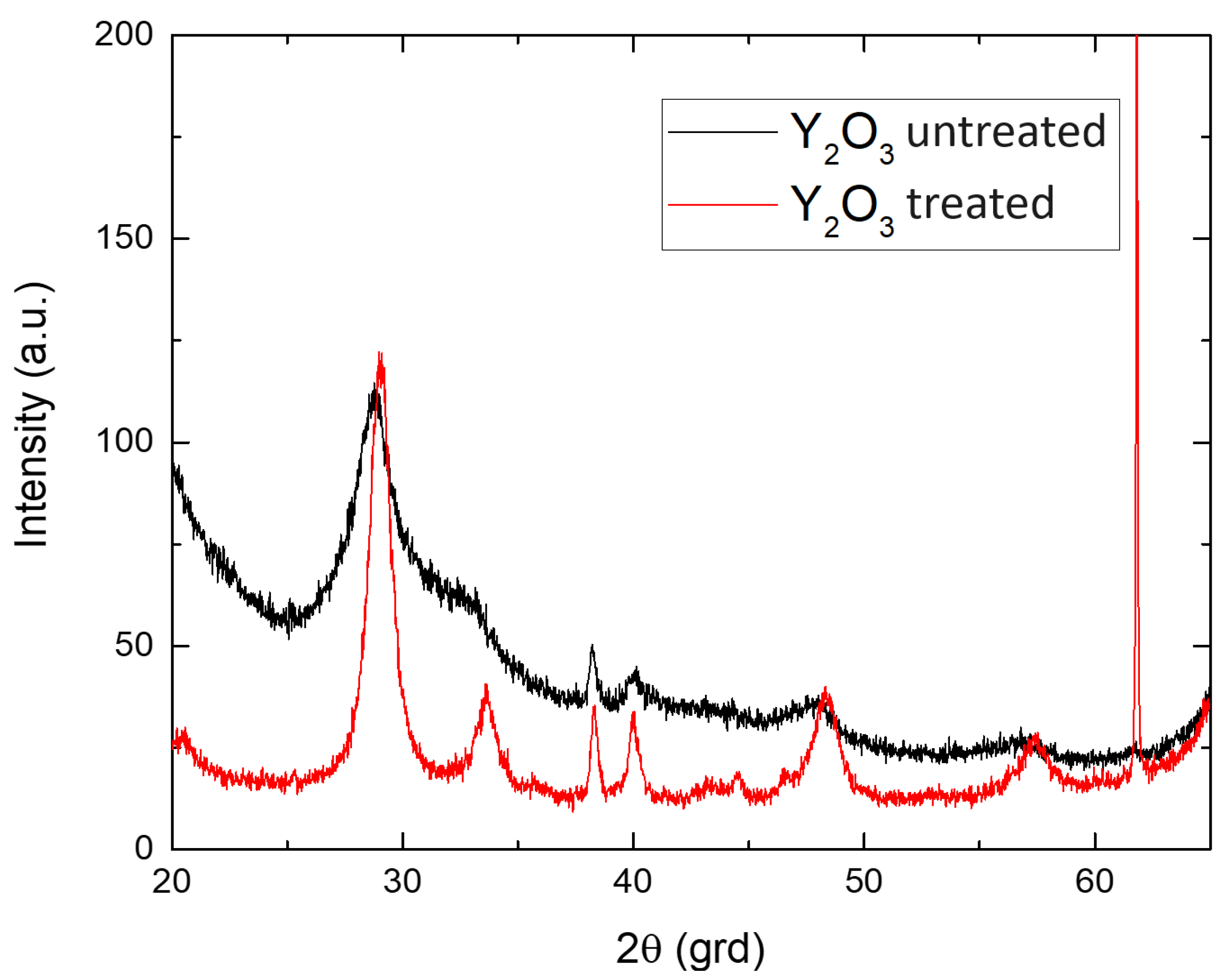
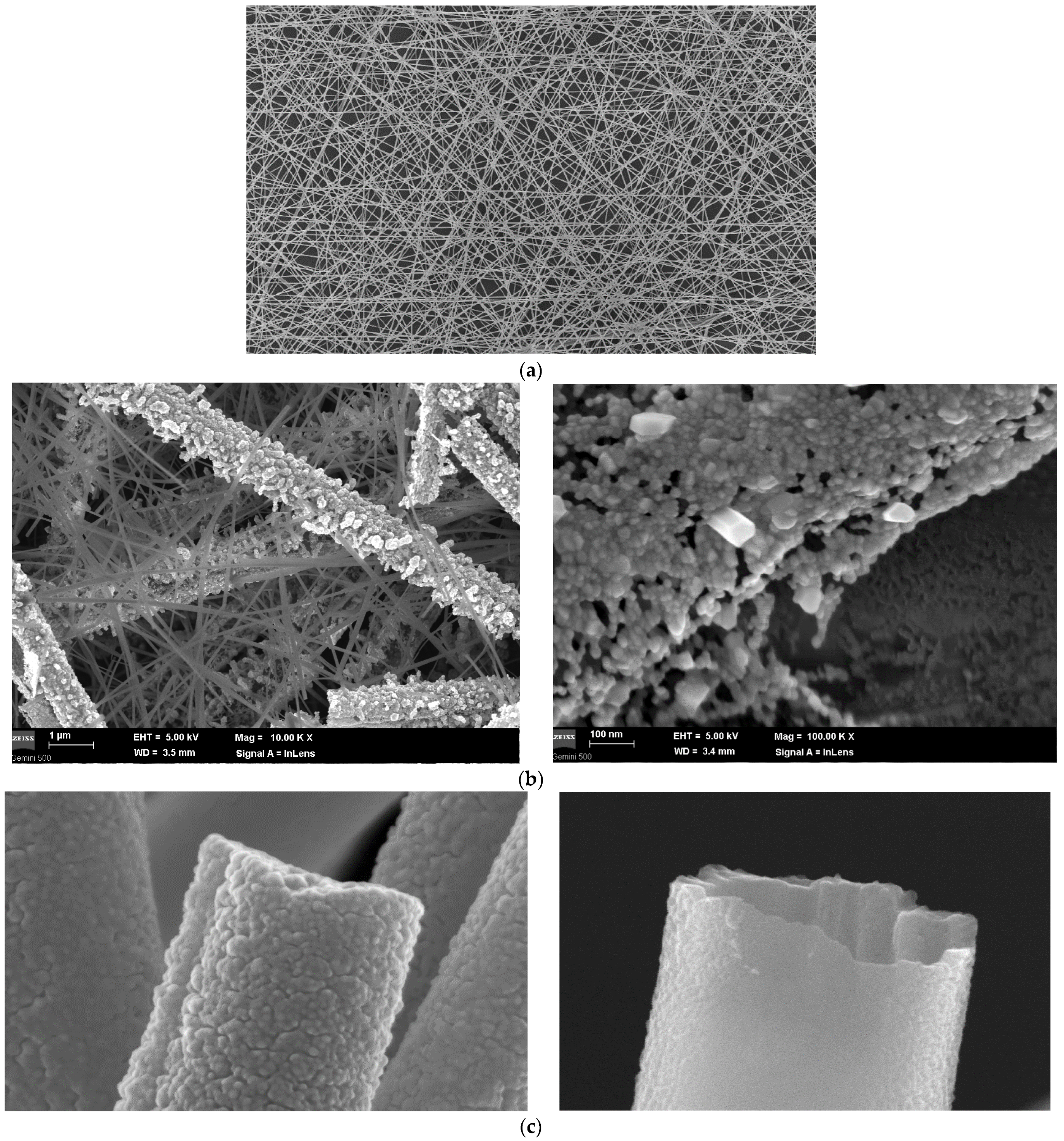
2.4. Results and Discussion
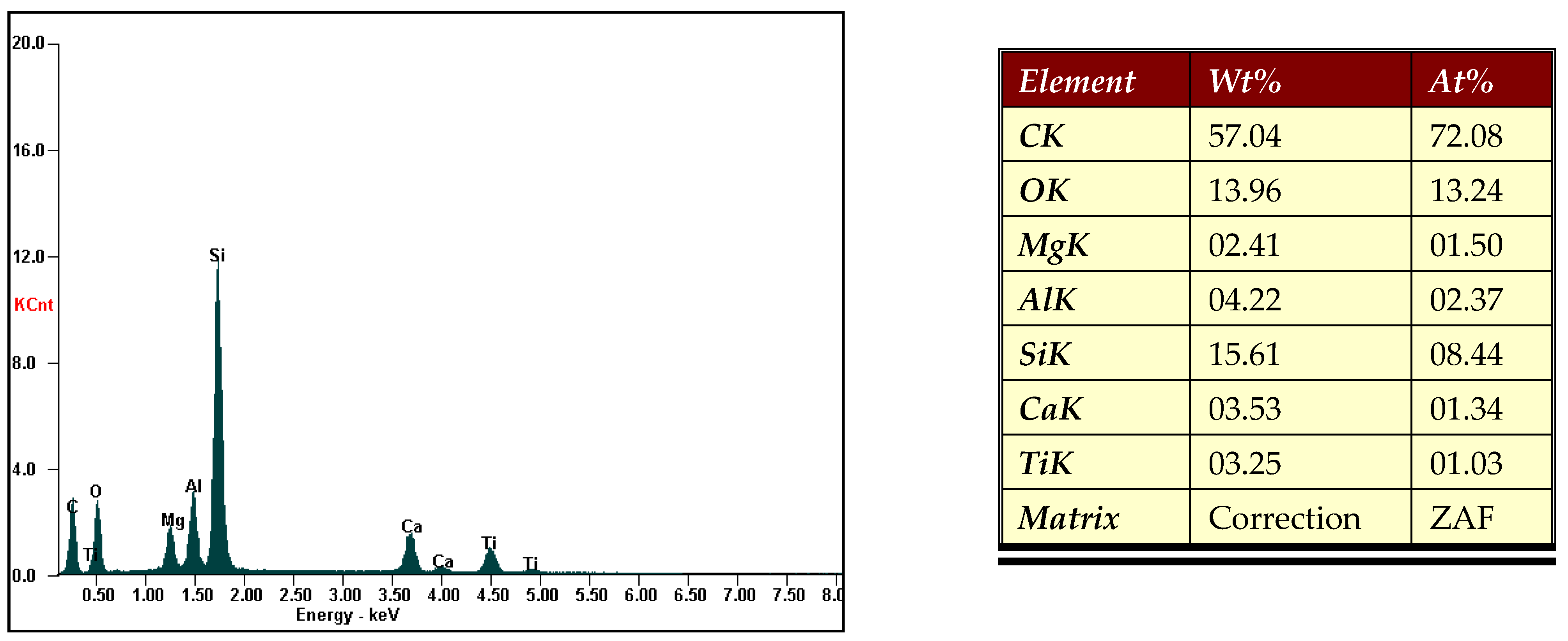
SEM Analysis
3. Preparation of Specialized Inks for Screen-Printed Sensors
3.1. Preparation of Carbon Nanotubes Containing Ink with Ceramic Nanotubes
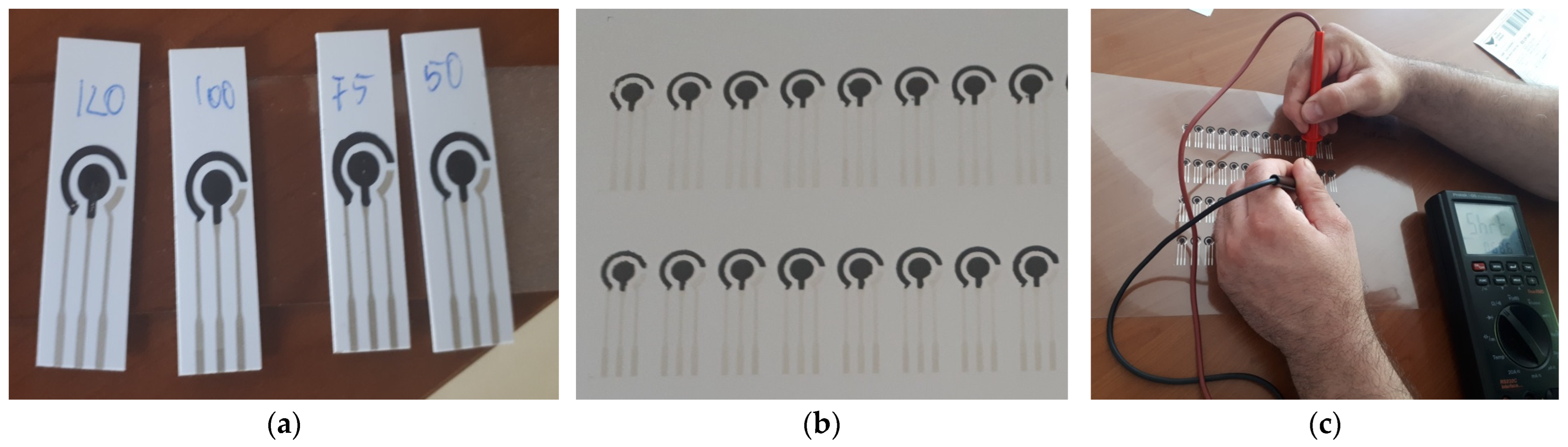
3.2. Experimental Printing and Testing Electrodes for a Classical Electrochemical Sensor
3.3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Collares, F.M.; Sampaio de Melo, M.A. Metal Oxide Nanoparticles and Nanotubes: Ultrasmall Nanostructures to Engineer Antibacterial and Improved Dental Adhesives and Composites. Bioengineering 2021, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, T.; Bae, C.; Shin, H.; Kim, J. Fabrication and applications of metal-oxide nano-tubes. JOM J. Miner. Met. Mater. Soc. 2010, 62, 44–49. [Google Scholar] [CrossRef]
- Researchers Learn to Control the Dimensions of Metal Oxide Nanotubes. Available online: https://phys.org/news/2007-08-dimensions-metal-oxide-nanotubes.html (accessed on 10 March 2024).
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electro-Spun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, J.; Kim, J. A general approach for synthesis of functional metal oxide nanotubes and their application in dye-sensitized solar cells. RSC Adv. 2014, 4, 2858–2864. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.T.; Xia, Y.; Marquez, M. Electrospinning: A Simple and Versatile Technique for Producing Ceramic Nanofibers and Nanotubes. J. Am. Ceram. Soc. 2006, 89, 1861–1869. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.Y.; Feng, Y.; Yuan, Z.Y.; Su, B.L. One-Dimensional Metal Oxide Nanotubes, Nanowires, Nanoribbons, and Nanorods: Synthesis, Characterizations, Properties and Applications. Crit. Rev. Solid State Mater. Sci. 2012, 37, 1–74. [Google Scholar] [CrossRef]
- Azevedo, J.; Fernández-García, M.P.; Magén, C.; Mendes, A.; Araújo, J.P.; Sousa, C.T. Double-walled iron oxide nanotubes via selective chemical etching and Kirkendall process. Sci. Rep. 2019, 9, 11994. [Google Scholar] [CrossRef] [PubMed]
- Parthangal, P. Direct synthesis of tin oxide nanotubes on microhotplates using carbon nanotubes as templates. J. Mater. Res. 2011, 26, 430–436. [Google Scholar] [CrossRef]
- Falk, M.; Luwan, S.; Tintula, K.; Markus, A.; Sandra, S.; Ulrike, K.; Molina-Luna, L.; Duerrschnabel, M.; Kleebe, H.J.; Ayata, S.; et al. Free-Standing Networks of Core–Shell Metal and Metal Oxide Nanotubes for Glucose Sensing. ACS Appl. Mater. Interfaces 2017, 9, 771–781. [Google Scholar]
- Kang, D.Y. Single-Walled Metal Oxide Nanotubes and Nanotube Membranes for Molecular Separations (Thesis). 2012. Available online: https://core.ac.uk/reader/10189860 (accessed on 10 March 2024).
- Muto, H.; Sato, Y.; Tan, W.K.; Yokoi, A.; Kawamura, G.; Matsuda, A. Controlled formation of carbon nanotubes incorporated ceramic composite granules by electrostatic integrated nano-assembly. Nanoscale 2022, 14, 9669–9674. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Rahat, S.S.M.; Hasan, K.M.Z.; Mondol, M.M.H.; Mallik, A.K. A comprehensive review of carbon nanotube-based metal oxide nanocomposites for supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Gupta, V.; Saleh, T. Syntheses of Carbon Nanotube-Metal Oxides Composites; Adsorption and Photo-Degradation, Volume: Carbon Nanotubes-From Research to Applications. 2011. Available online: https://www.intechopen.com/chapters/16834 (accessed on 10 March 2024). [CrossRef]
- Wang, D.; Sun, W.; Su, C. Carbon Nanotube–Metal Oxide Nanocomposites. In Metal Oxide Nanocomposites: Synthesis and Applications; Scrivener Publishing LLC: Austin, TX, USA, 2020; Chapter 4. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ataei, A. Decoration of Metal Nanoparticles and Metal Oxide Nanoparticles on Carbon Nanotubes. Adv. J. Chem. 2024, 7, 122–145. [Google Scholar] [CrossRef]
- Kumar, N.; Navani, N.K.; Manhas, S.K. Effect of Metal Oxide Nanoparticles on Carbon Nanotube Device Characteristics. J. Electron. Mater. 2021, 50, 528–536. [Google Scholar] [CrossRef]
- Available online: https://eureka.patsnap.com/patent-CN103058173B (accessed on 10 March 2024).
- Cho, H.H.; Smith, B.A.; Wnuk, J.D.; Fairbrother, D.H.; Ball, W.P. Influence of Surface Oxides on the Adsorption of Naphthalene onto Multiwalled Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; Hassan, H.S.; Amer, W.A.; Salama, E.; Algarni, H.; Shaaban, E.R. Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling. Materials 2017, 10, 1355. [Google Scholar] [CrossRef]
- Ali, M.A.; Solanki, P.R.; Srivastava, S.; Singh, S.; Agrawal, V.V.; John, R.; Malhotra, B.D. Protein Functionalized Carbon Nanotubes-based Smart Lab-on-a-Chip. ACS Appl. Mater. Interfaces 2015, 7, 5837–5846. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Boghossian, A. Covalent conjugation of proteins onto fluorescent single-walled carbon nanotubes for biological and medical applications. Mater. Adv. 2023, 4, 823–834. [Google Scholar] [CrossRef]
- Hohman, M.; Shin, M.; Rutledge, G.; Brenner, M. Electrospinning and electrically forced jets. II. Applications. Phys. Fluids 2021, 13, 2221–2236. [Google Scholar] [CrossRef]
- Zuo, W.; Zhu, M.; Yang, W.; Yu, H.; Chen, Y.; Zhang, Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the Fiber Diameter during Electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Shivkumar, S. Molecular weight dependent structural regimes during the electrospinning of PVA. Mater. Lett. 2007, 61, 2325–2328. [Google Scholar] [CrossRef]
- Oleiwi, A.; Jabur, A.; Alsalhy, Q. Preparation of Polystyrene/Polyacrylonitrile Blends by Electrospinning Technique. J. Phys. Conf. Ser. 2021, 1879, 022065. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Jin, H.J.; Chin, I.J. Morphological Characterization of Electrospun Nano-Fibrous Membranes of Biodegradable Poly(L-lactide) and Poly(lactide-co-glycolide). Macromol. Symp. 2005, 224, 145–154. [Google Scholar] [CrossRef]
- Nezarati, R.; Eifert, M.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, M.; Murugananthan, M.; Zhang, Y.; Zhang, L. Electrochemically self-doped WO3/TiO2 nanotubes for photocatalytic degradation of volatile organic compounds. Appl. Catal. B Environ. 2020, 260, 118205. [Google Scholar] [CrossRef]
- Kéri, O.; Kocsis, E.; Karajz, D.A.; Nagy, Z.K.; Parditka, B.; Erdélyi, Z.; Szabó, A.; Hernádi, K.; Szilágyi, I.M. Photocatalytic Crystalline and Amorphous TiO2 Nanotubes Prepared by Electrospinning and Atomic Layer Deposition. Molecules 2021, 26, 5917. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, O.; Manolova, N.; Rashkov, I. Electrospun Poly(methyl methacrylate)/TiO2 Composites for Photocatalytic Water Treatment. Polymers 2021, 13, 3923. [Google Scholar] [CrossRef] [PubMed]
- Chawraba, K.; Damiri, F.; Toufaily, J.; Lalevee, J.; Hamieh, T. TiO2 Supported in Polymethyl methacrylate (PMMA) Properties, Preparation, and Photocatalytic Activity for the Degradation of Synthetic Dyes. Lett. Appl. NanoBioSci. 2024, 13, 13. [Google Scholar] [CrossRef]
- Ounas, O.; El Foulani, A.; Lekhlif, B.; Jamal-Eddine, J. Immobilization of TiO2 into a poly methyl methacrylate (PMMA) as hybrid film for photocatalytic degradation of methylene blue. Mater. Today Proc. 2020, 22, 35–40. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.W. Engineering Ceramic Fiber Nanostructures Through Polymer-Mediated Electrospinning, Volume: Polymer-Engineered Nanostructures for Advanced Energy Applications. 2017, pp. 3–30. Available online: https://link.springer.com/chapter/10.1007/978-3-319-57003-7_1 (accessed on 10 March 2024).
- Mafakheri, E.; Salimi, A.; Hallaj, R.; Ramazani, A.; Kashi, M.A. Synthesis of Iridium Oxide Nanotubes by Electrodeposition into Polycarbonate Template: Fabrication of Chromium(III) and Arsenic(III) Electrochemical Sensor. Electroanalysis 2011, 23, 2429–2437. [Google Scholar] [CrossRef]
- Lim, J.H.; Min, S.G.; Malkinskib, L.; Wiley, J. Iron oxide nanotubes synthesized via template-based electrodeposition. Nanoscale 2014, 6, 5289–5295. [Google Scholar] [CrossRef] [PubMed]
- Alkanad, K.; Hezam, A.; Al-Zaqri, N.; Bajiri, M.A.; Alnaggar, G.; Drmosh, Q.A.; Almukhlifi, H.A.; Neratur Krishnappagowda, L. One-Step Hydrothermal Synthesis of Anatase TiO2 Nanotubes for Efficient Photocatalytic CO2 Reduction. ACS Omega 2022, 7, 38686–38699. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, A.; Kubo, T.; Numako, C. Formation Mechanism of TiO2-Derived Titanate Nanotubes Prepared by the Hydrothermal Process. Inorg. Chem. 2010, 49, 5845–5852. [Google Scholar] [CrossRef]
- Gao, H.; Tang, X.; Zhang, H.; He, Y.; Zhou, T.; Shen, J.; Zhu, L.; Si, T. Mechanical strength enhancement of CaZr4P6O24 ceramics with multi-walled carbon nanotubes additions. J. Alloys Compd. 2024, 976, 173310. [Google Scholar] [CrossRef]
- Porras, R.; Bavykin, D.V.; Zekonyte, J.; Walsh, F.C.; Wood, R.J. Titanate nanotubes for reinforcement of a poly(ethylene oxide)/chitosan polymer matrix. Nanotechnology 2016, 27, 195706. [Google Scholar] [CrossRef]
- Hedayati, M.; Taheri-Nassaj, E.; Yourdkhani, A.; Borlaf, M.; Zhang, J.; Calame, M.; Sebastian, T.; Payandeh, S.; Clemens, F.J. BaTiO3 nanotubes by co-axial electrospinning: Rheological and microstructural investigations. J. Eur. Ceram. Soc. 2020, 40, 1269–1279. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Zhang, A. (Eds.) Engineering Ceramic Fiber Nanostructures Through Polymer-Mediated Electrospinning. In Polymer-Engineered Nanostructures for Advanced Energy Applications; Springer: Berlin/Heidelberg, Germany, 2017; Volume 3. [Google Scholar] [CrossRef]
- Moreno, M.; Arredondo, M.; Ramasse, Q.M.; McLaren, M.; Stötzner, P.; Förster, S.; Benavente, E.; Salgado, C.; Devis, S.; Solar, P.; et al. ZnO nucleation into trititanate nanotubes by ALD equipment techniques, a new way to functionalize layered metal oxides. Sci. Rep. 2021, 11, 7698. [Google Scholar] [CrossRef]
- Karacaoglu, E.; Öztürk, E.; Uyaner, M.; Losego, M.D. Atomic layer deposition (ALD) of nanoscale coatings on SrAl2O4-based phosphor powders to prevent aqueous degradation. J. Am. Ceram. Soc. 2020, 103, 3706–3715. [Google Scholar] [CrossRef]
- Liu, H.; Takagi, D.; Ohno, H.; Chiashi, S.; Chokan, T.; Homma, Y. Growth of Single-Walled Carbon Nanotubes from Ceramic Particles by Alcohol Chemical Vapor Deposition. Appl. Phys. Express 2008, 1, 014001. [Google Scholar] [CrossRef]
- Lamnini, S.; Pugliese, D.; Baino, F. Zirconia-Based Ceramics Reinforced by Carbon Nanotubes: A Review with Emphasis on Mechanical Properties. Ceramics 2023, 6, 1705–1734. [Google Scholar] [CrossRef]
- Marinho, T.; Costa, P.; Lizundia, E.; Costa, C.M.; Corona-Galván, S.; Lanceros-Méndez, S. Ceramic Nanoparticles and Carbon Nanotubes Reinforced Thermoplastic Materials for Piezocapacitive Sensing Applications’. Available online: https://repositorium.uminho.pt/bitstream/1822/64639/1/37.pdf (accessed on 10 March 2024).
- Single-Walled Carbon Nanotube-Ceramic Composites and Methods of Use (Patent). Available online: https://patents.google.com/patent/AU2004234395A1/en (accessed on 10 March 2024).
- Chu, Z.; Peng, J.; Jin, W. Advanced nanomaterial inks for screen-printed chemical sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
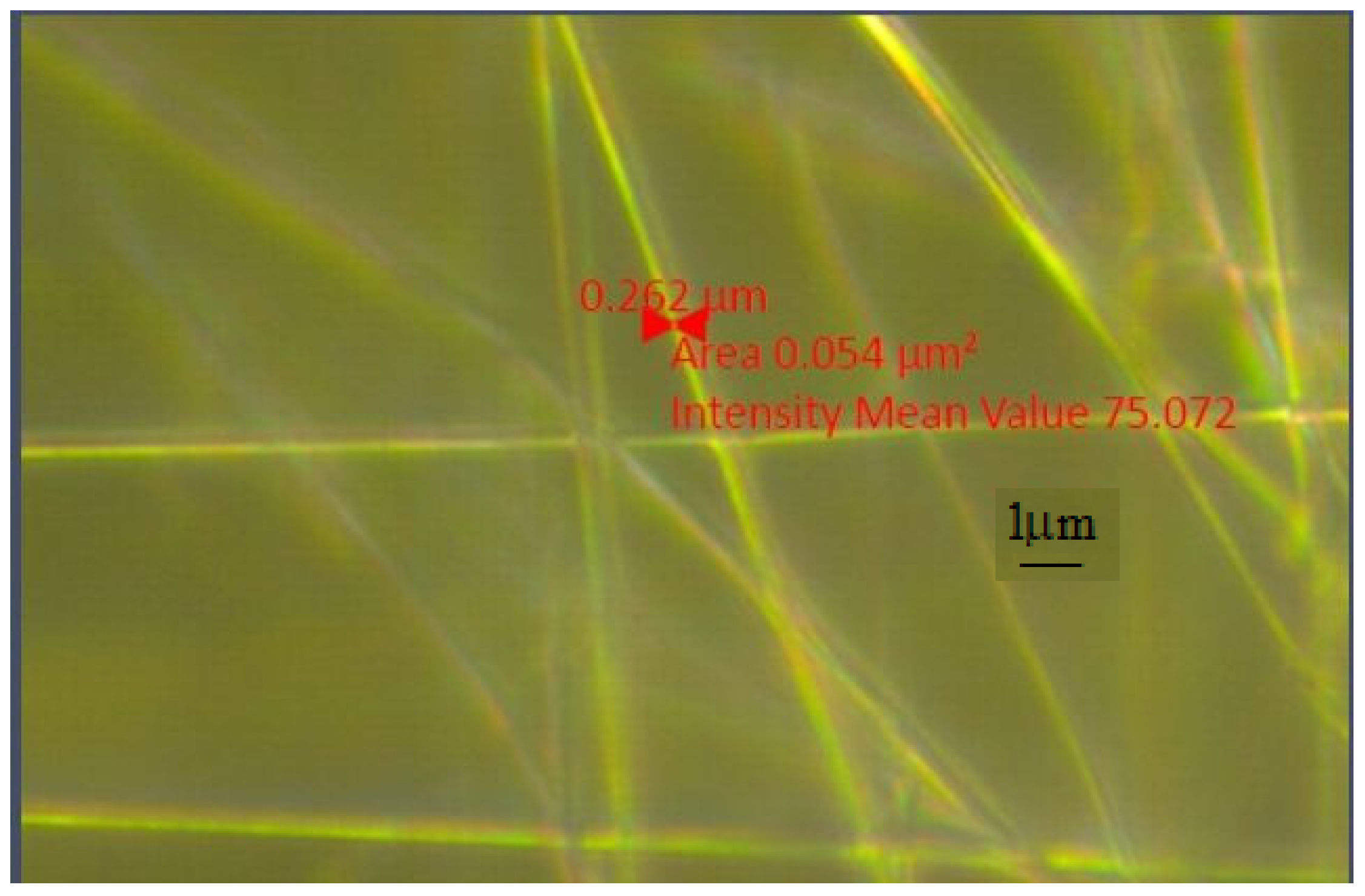
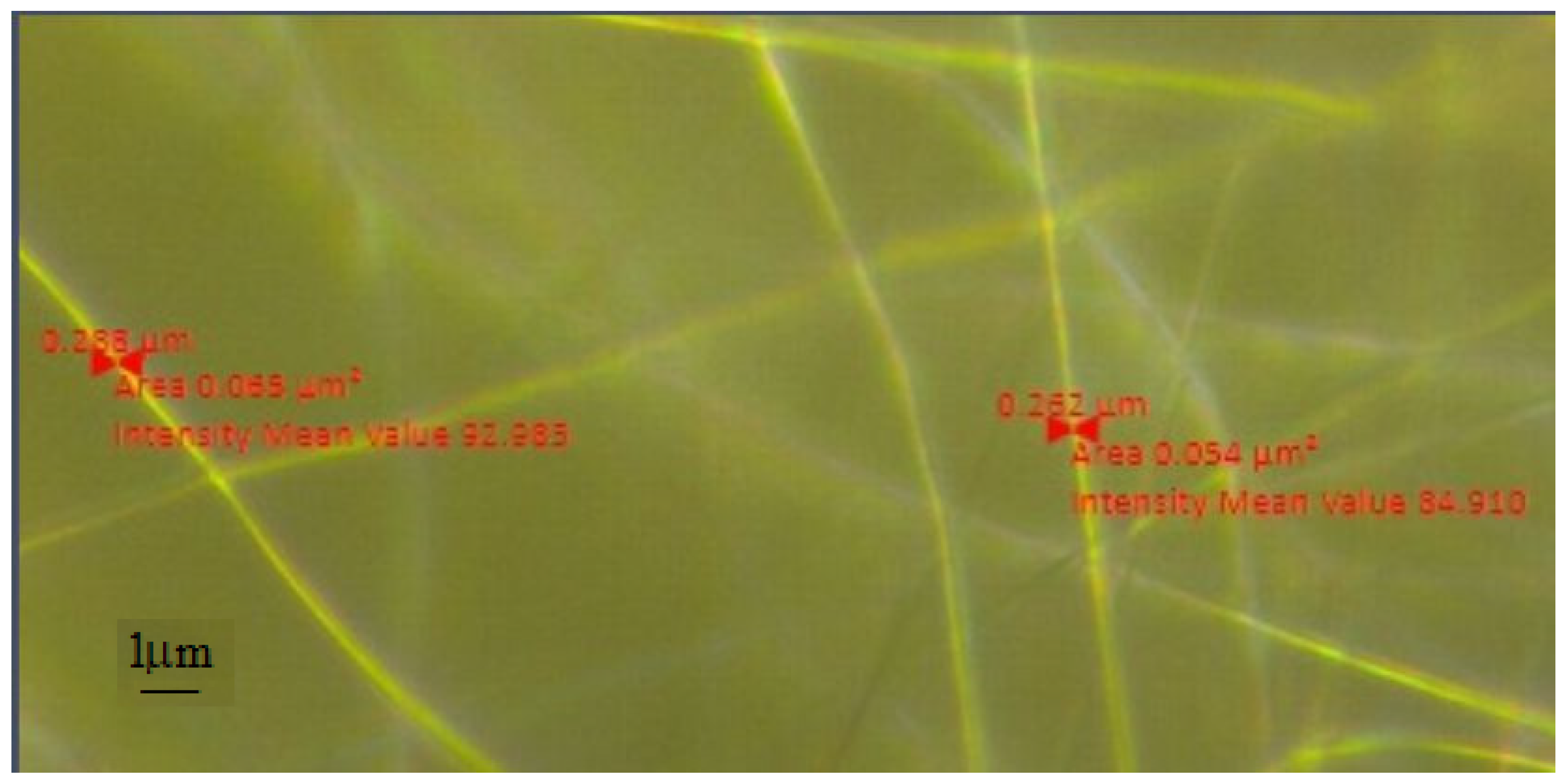
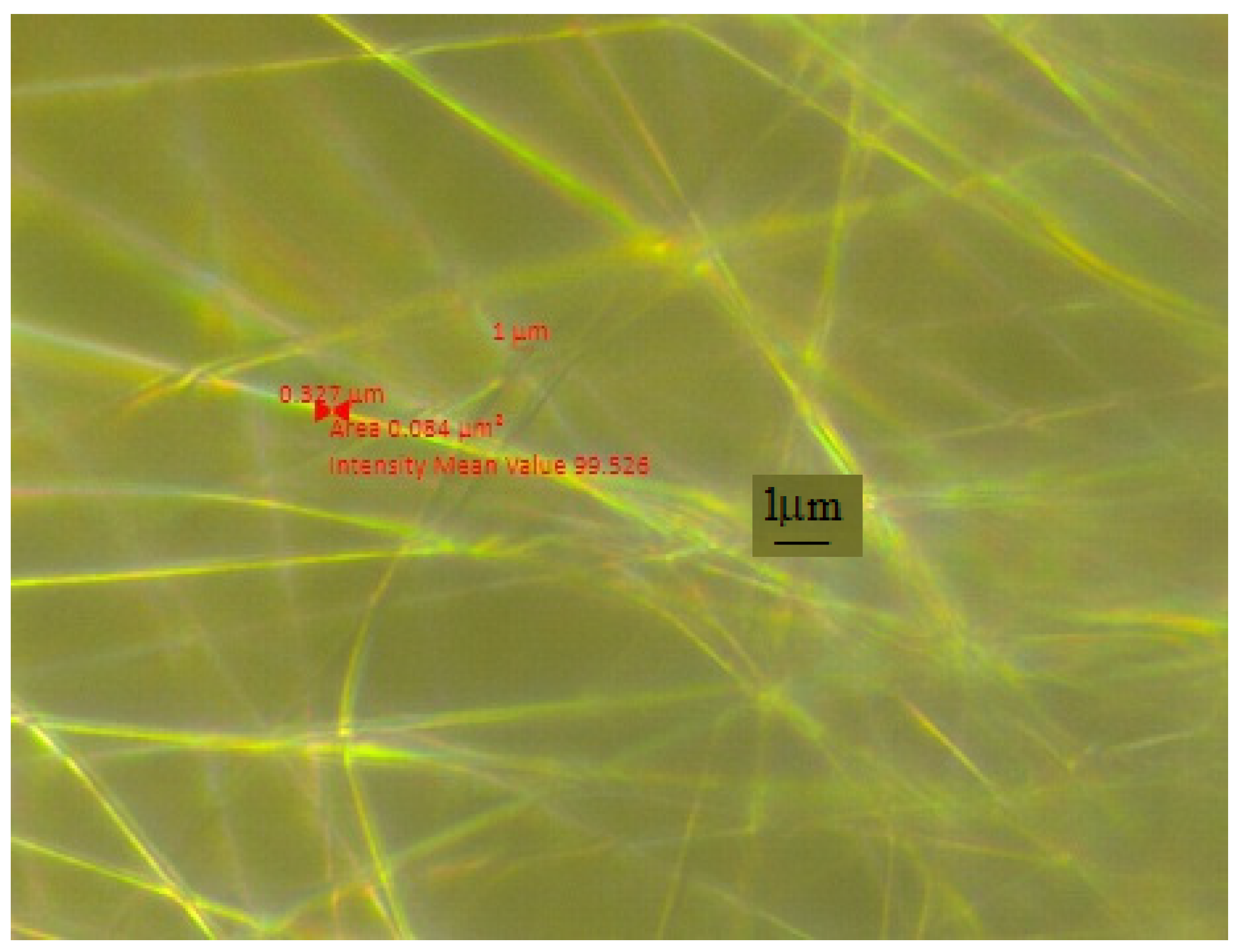



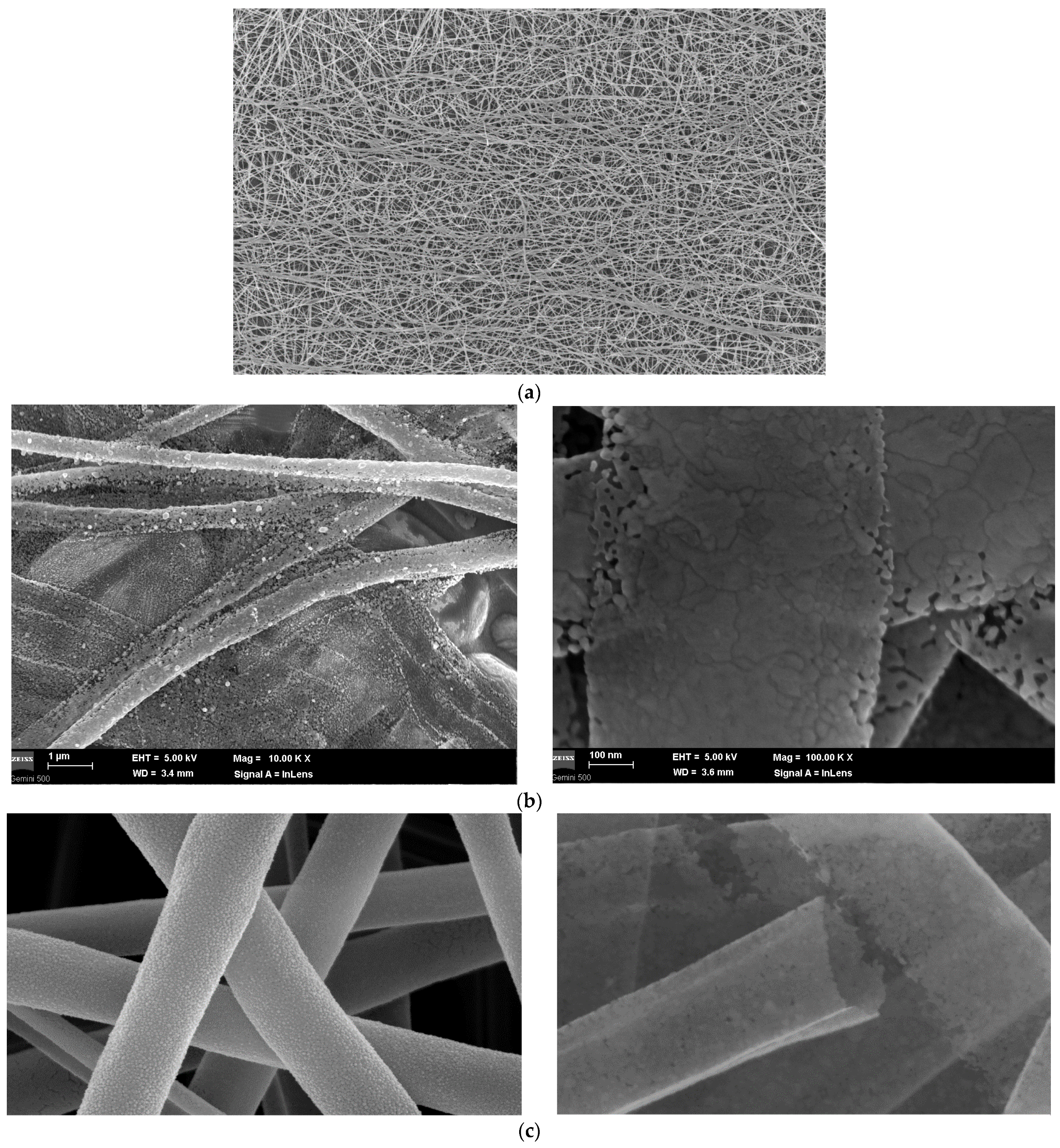
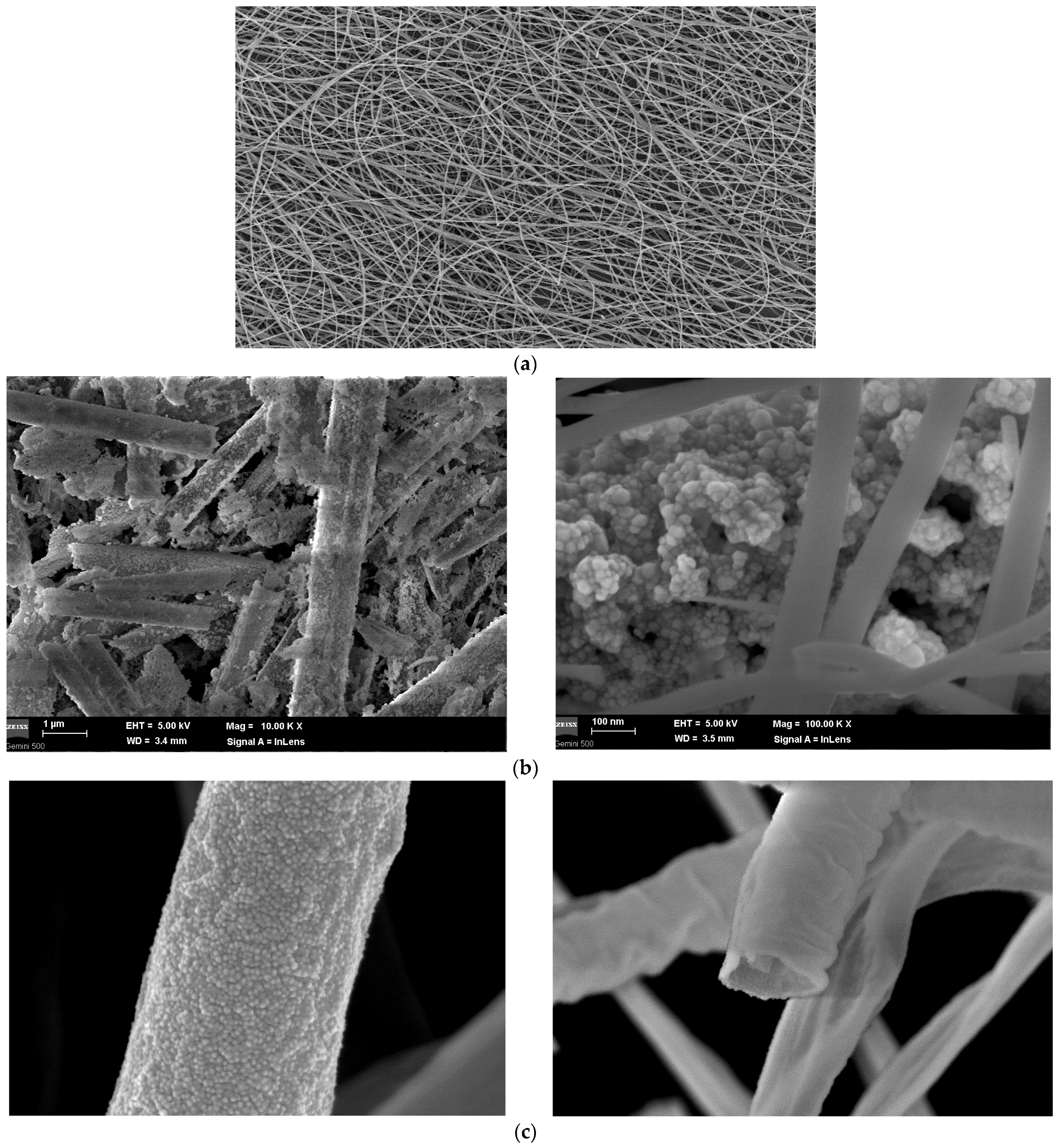


| Sample | PMMA (%) | U (kV) | Distance (mm) | Rotation Speed (rpm) |
|---|---|---|---|---|
| 1 | 10 | 12 | 100 | - |
| 2 | 10 | 15 | 100 | - |
| 3 | 10 | 20 | 100 | - |
| 4 | 10 | 20 | 80 | - |
| 5 | 10 | 15 | 80 | 5 |
| 6 | 10 | 12 | 80 | 5 |
| 7 | 5 | 12 | 80 | 5 |
| 8 | 5 | 15 | 80 | 5 |
| 9 | 5 | 20 | 80 | 5 |
| 10 | 5 | 20 | 100 | 5 |
| 11 | 5 | 15 | 100 | 5 |
| 12 | 5 | 12 | 100 | 5 |
| Characteristic | Value [+/− 5%] |
|---|---|
| Solids content [wt. %] | 48 |
| Density [g/mL] | 2 |
| Viscosity at 10 s−1 | 6500 |
| pH | 6 |
| Resistance [Ω] | Ink Containing TiO2 | Ink Containing Al2O3 | Ink Containing Y2O3 |
|---|---|---|---|
| 75 °C | 5.4 | 7.2 | 6.8 |
| 120 °C | 3.2 | 3.4 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandabat, A.F.; Ciobanu, R.C.; Schreiner, O.D.; Aradoaei, M.; Aradoaei, S.T. Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors. Crystals 2024, 14, 454. https://doi.org/10.3390/cryst14050454
Trandabat AF, Ciobanu RC, Schreiner OD, Aradoaei M, Aradoaei ST. Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors. Crystals. 2024; 14(5):454. https://doi.org/10.3390/cryst14050454
Chicago/Turabian StyleTrandabat, Alexandru Florentin, Romeo Cristian Ciobanu, Oliver Daniel Schreiner, Mihaela Aradoaei, and Sebastian Teodor Aradoaei. 2024. "Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors" Crystals 14, no. 5: 454. https://doi.org/10.3390/cryst14050454




