Effect of Cubic Crystal Morphology on Thermal Characteristics and Mechanical Sensitivity of PYX
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Antisolvent Crystallization Experiment
2.3. Characterization
3. Results and Discussion
3.1. Morphology and Particle Size
3.2. Structure
3.3. Thermal Characteristics
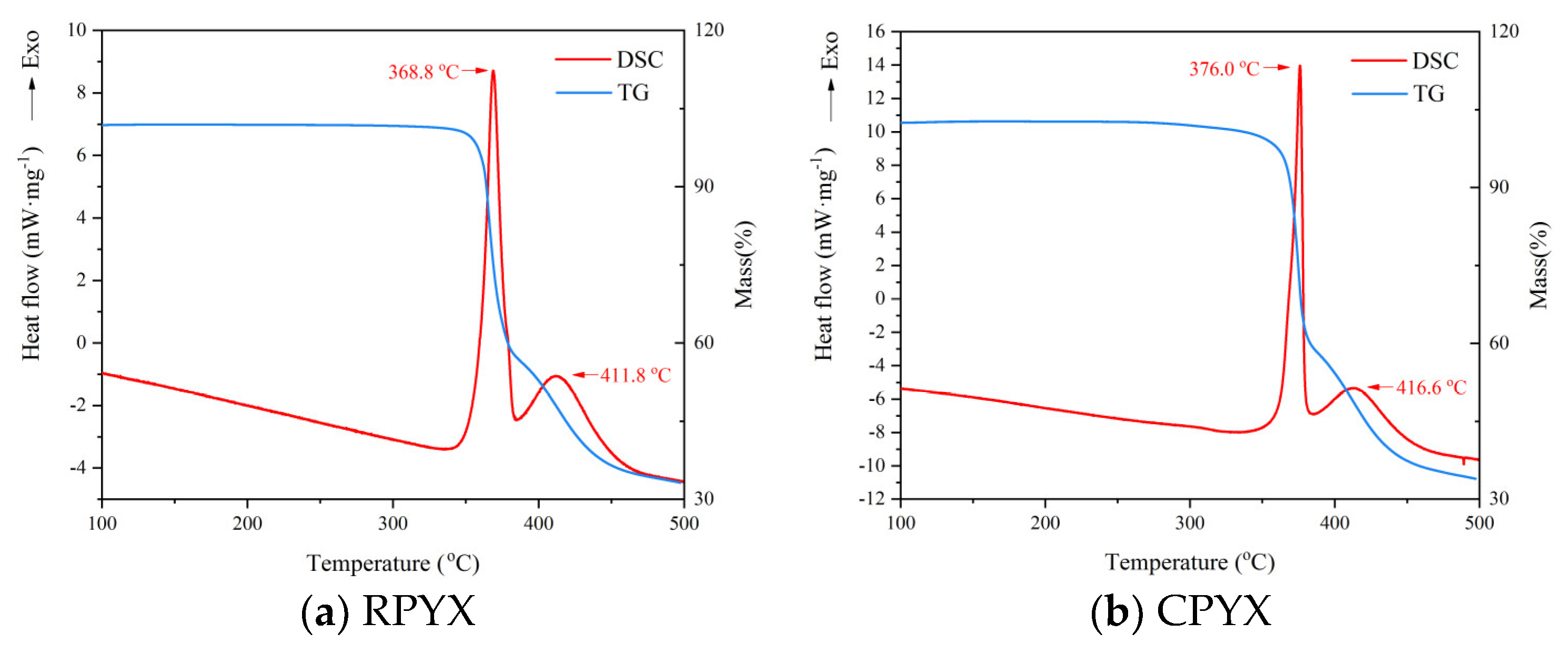
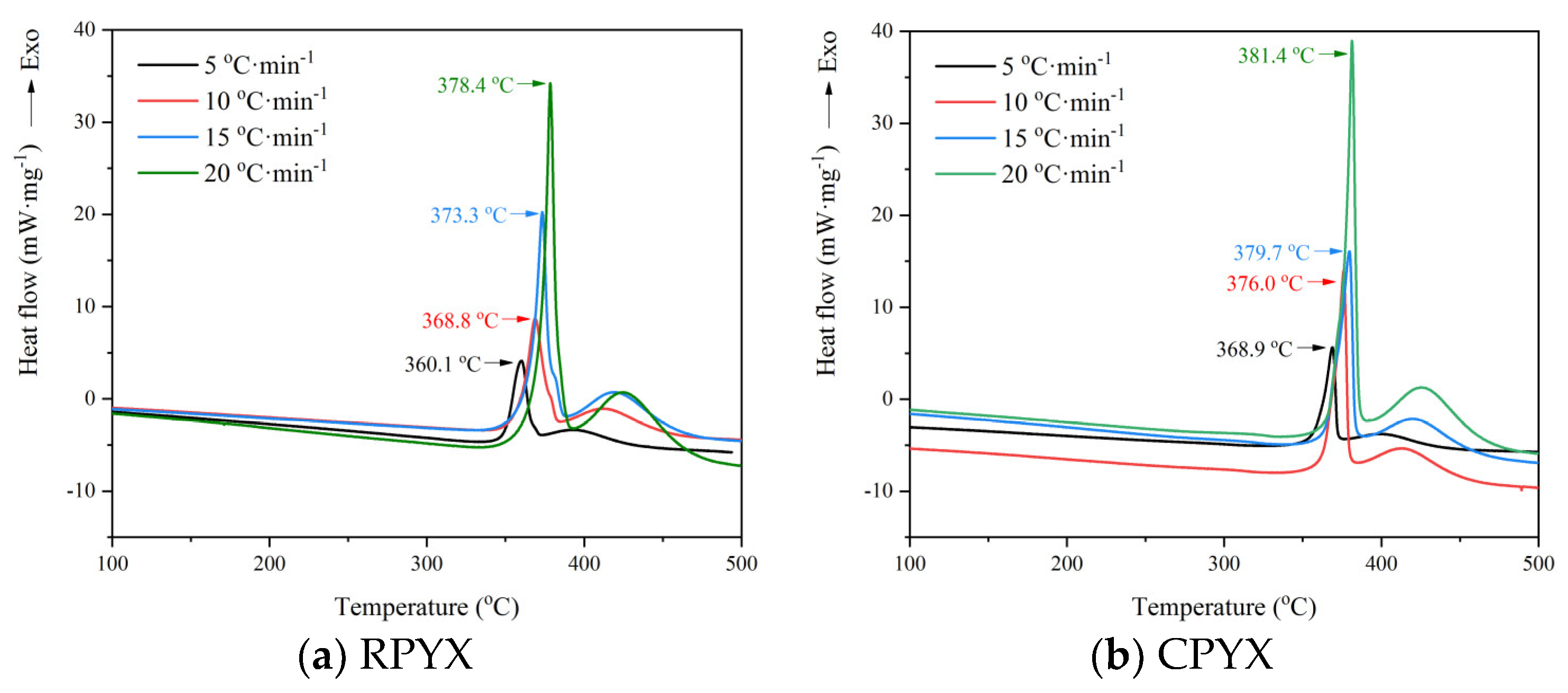
3.3.1. Thermal Decomposition Behavior
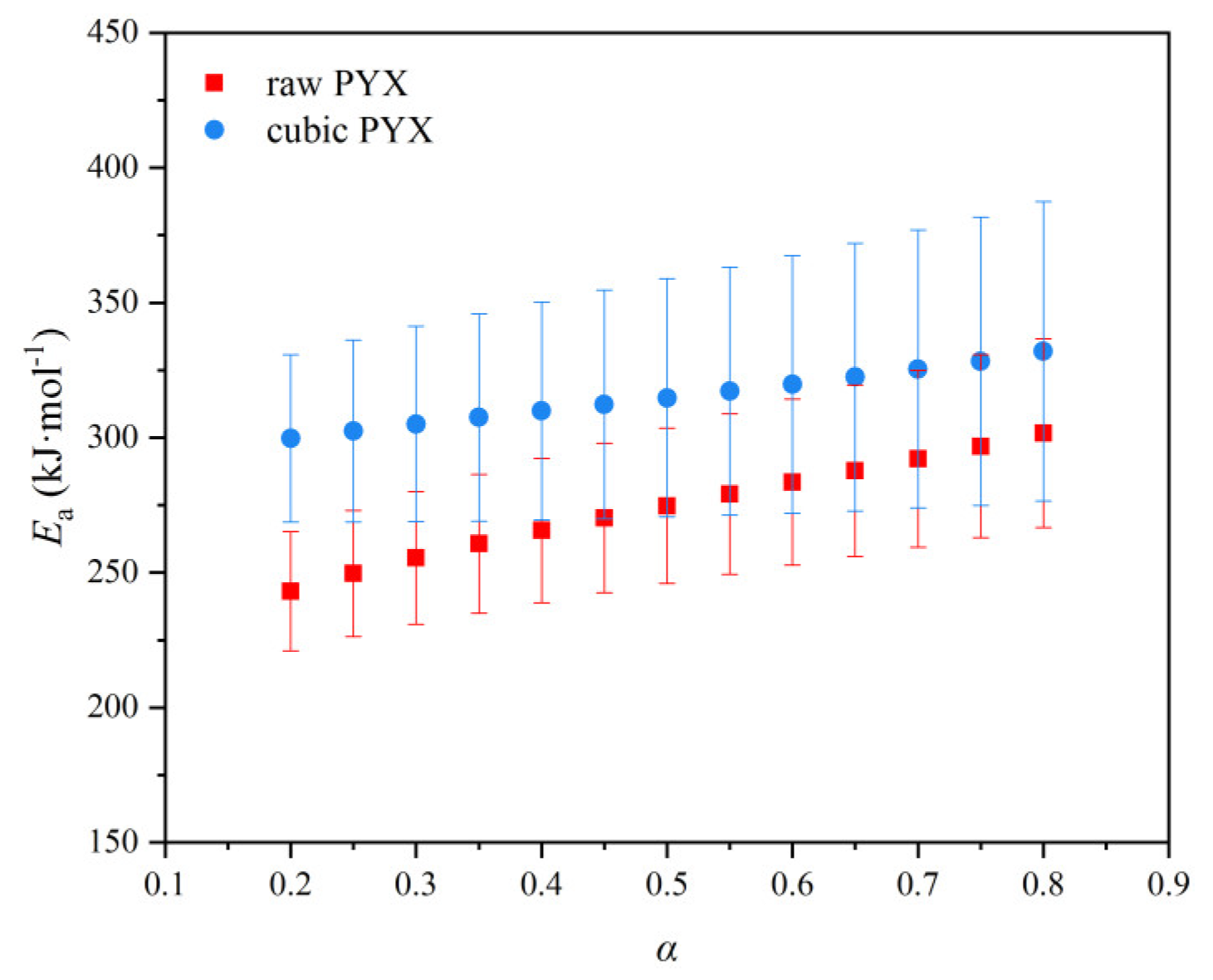
3.3.2. Thermal Decomposition Kinetics
3.3.3. Thermal Safety Parameters
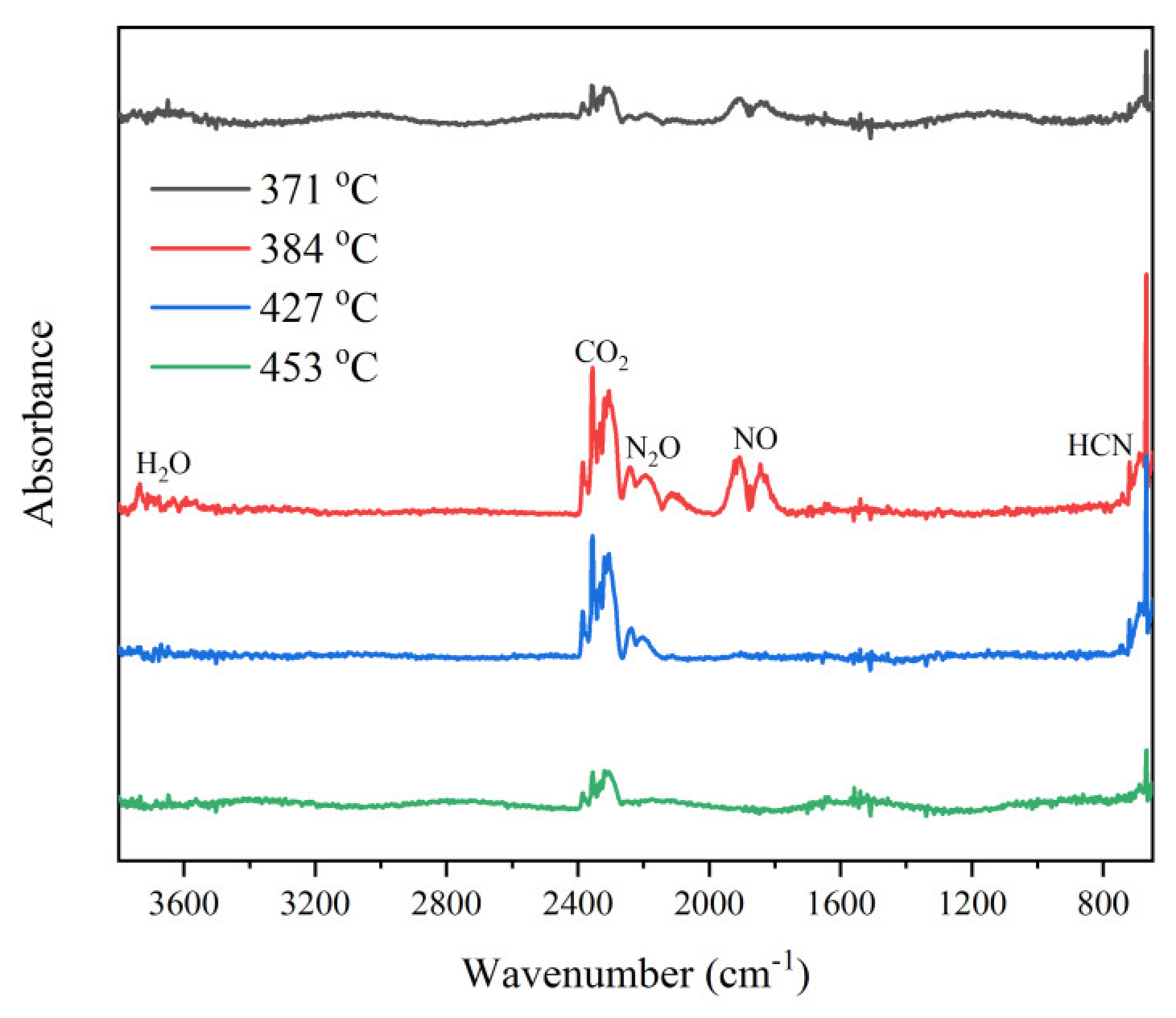
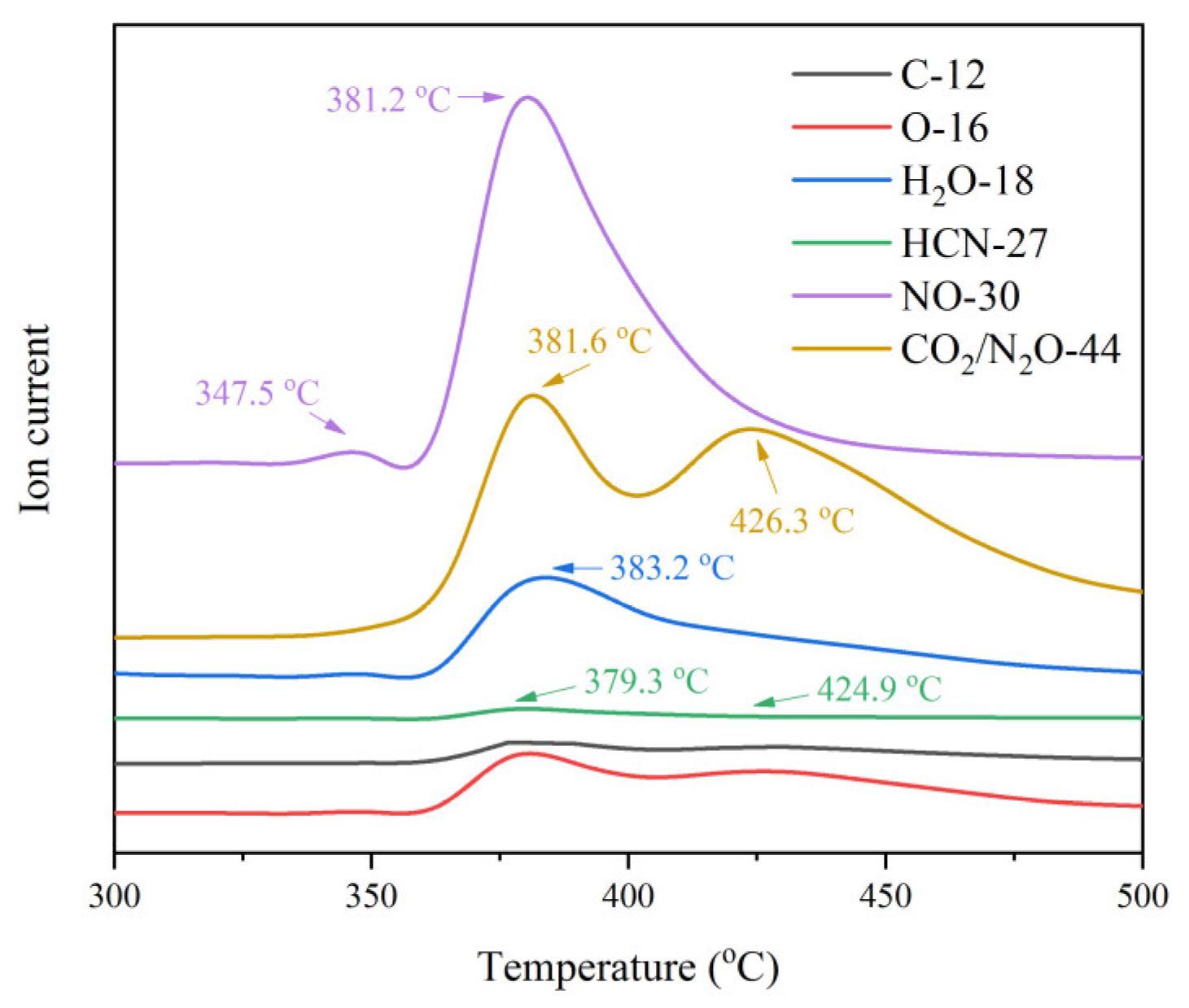
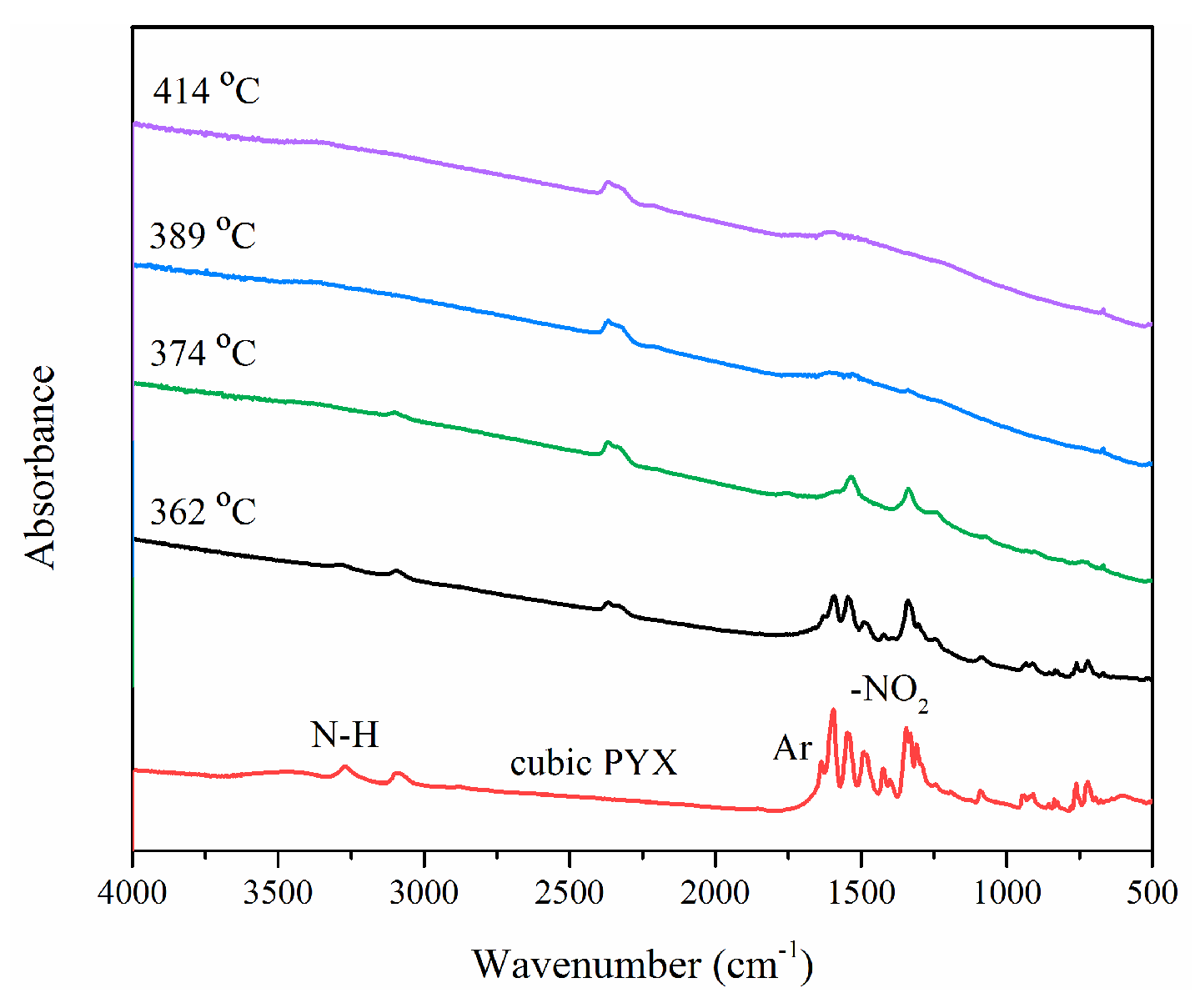
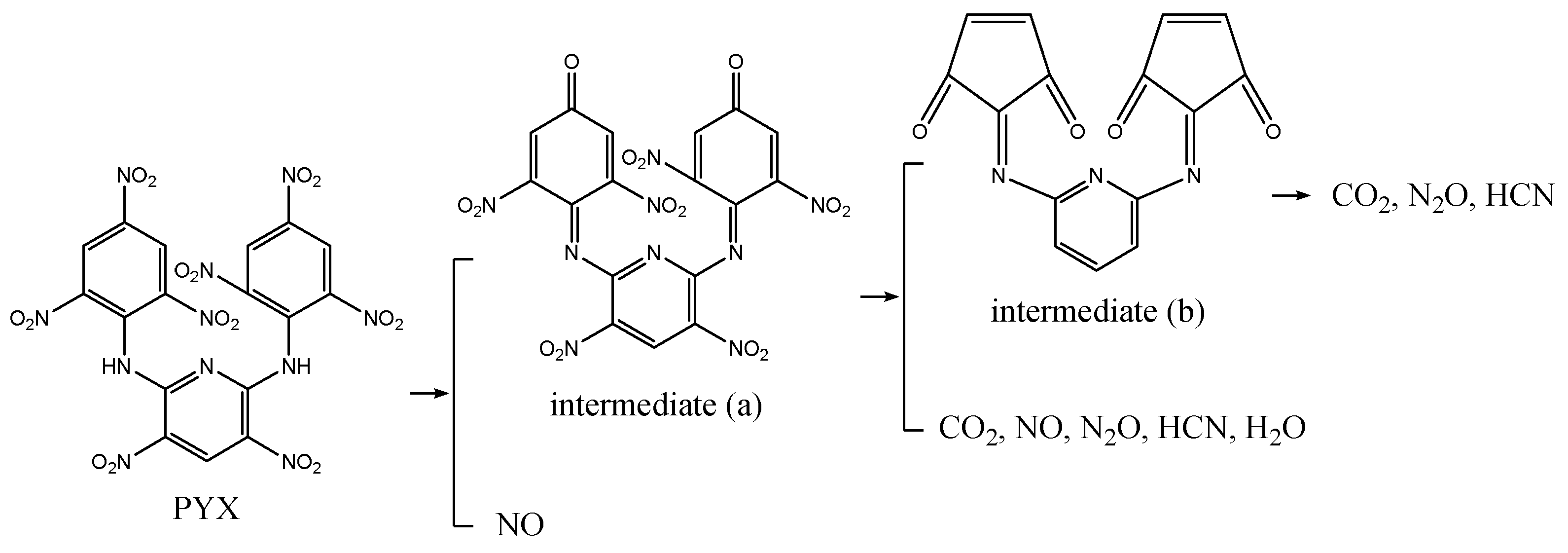
3.3.4. Thermal Decomposition Mechanism of CPYX
3.4. Mechanical Sensitivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutowski, Ł.; Cudziło, S. Synthesis and Properties of Novel Nitro-Based Thermally Stable Energetic Compounds. Def. Technol. 2021, 3, 775–784. [Google Scholar] [CrossRef]
- Pagoria, P. A Comparison of the Structure, Synthesis, and Properties of Insensitive Energetic Compounds. Propellants Explos. Pyrotech. 2016, 41, 452–469. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B. Review of Promising Insensitive Energetic Materials. Cent. Eur. J. Energ.Mater. 2017, 14, 821–843. [Google Scholar] [CrossRef] [PubMed]
- Gołofit, T.; Szala, M. Origin of PYX thermal stability investigation with calorimetric and spectroscopic methods. J. Therm. Anal. Calorim. 2017, 3, 2047–2054. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Q.; Duan, B.H.; Lu, X.M.; Bai, X.; Yan, Q.L. Comparative study on thermal behavior of three highly thermostable energetic materials: z-TACOT, PYX, and TNBP. FirePhysChem 2021, 1, 61–69. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, G.X.; Gong, X.D. Theoretical Investigations on Structure, Density, Detonation Properties, and Sensitivity of the Derivatives of PYX. J. Comput. Chem. 2012, 33, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ren, Y.Y.; He, L.; An, C.W.; Wen, S.F.; Shen, F.F. Molecular dynamics simulation of the interface interaction and mechanical properties of PYX and polymer binder. AIP Adv. 2022, 12, 235307. [Google Scholar] [CrossRef]
- Ma, C.M.; Liu, Z.L.; Xu, X.J.; Yao, Q.Z. Research Progress on the Synthesis of Energetic Pyridines. Chin. J. Org. Chem. 2014, 34, 1288–1299. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Stierstorfer, J.; Weyrauther, M.; Witkowski, T.G. Synthesis and Investigation of 2,6-Bis(picrylamino)-3,5-dinitropyredine (PYX) and Its Salts. Chem. Eur. J. 2016, 22, 8619–8626. [Google Scholar] [CrossRef]
- Zhou, J.H.; Yu, Q.; Chen, J.; Liao, L.Y. Study on the thermal stability of heat-resistance explosives TATB, PYX and LLM-105. Chem. Res. Appl. 2014, 16, 1802–1804. [Google Scholar]
- Agrawal, J.P. Past, Present & Future of Thermally Stable Explosives. Cent. Eur. J. Energ. Mater. 2012, 9, 273–290. [Google Scholar]
- Zhou, J.W.; Zhang, C.J.; Wang, Y.B.; Zhang, M.M.; Li, Y. Status and Research Progress of Heat Resistant Explosives. J. Ord. Equip. Eng. 2016, 37, 111–115. [Google Scholar]
- Li, H.Z. Research Progress and Suggestion for the Modification of the Explosive Crystal Characteristics. Chin. J. Energ. Mater. 2020, 28, 847–888. [Google Scholar]
- Huang, M.; Duan, X.H. Crystal Control and Characterization of Explosives, 1st ed.; Northwestern Polytechnical University Press: Xi’an, China, 2020; pp. 55–81. [Google Scholar]
- Wang, B.G.; Chen, Y.F.; Zhang, J.L. Influencing Factors of Preparation of Ultra-fine PYX by Solvent and Non-solvent Technique. Acta Armamentarii 2008, 42, 1035–1038. [Google Scholar]
- Liu, L.; Wang, P.; Zeng, G.Y.; Jiao, Z.Q.; Zhang, J. Preparation and Characterization of Ultrafine PYX. Initiat. Pyrotech. 2009, 2, 17–19. [Google Scholar]
- Liu, Q.H.; An, W.Q.; He, J.Y.; Leng, J.P.; Zhao, Q.; Pan, H.X.; Chen, J.; Guo, J.C.; Li, Y.X.; Cao, D.L. Crystallization and Characterization of Spherical Ammonium Perchlorate. Chin. J. Explos. Propellants 2021, 44, 139–146. [Google Scholar]
- Zhou, X.Q.; Zhang, Q.; Xu, R.; Chen, D.; Hao, S.L.; Nie, F.D.; Li, H.Z. A Novel Spherulitic Self-Assembly Strategy for Organic Explosives: Modifying the Hydrogen Bonds by Polymeric Additives in Emulsion Crystallization. Cryst. Growth Des. 2018, 18, 2417–2423. [Google Scholar] [CrossRef]
- Li, J.S.; Wu, S.W.; Lu, K.T. Study on Preparation of Insensitive and Spherical High Bulk Density Nitroguanidine with Controllable Particle Size. Propellants Explos. Pyrotech. 2016, 41, 312–320. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Shan, J.H.; Chen, D.; Li, H.Z. Tuning the Crystal Habits of Organic Explosives by Antisolvent Crystallization: The Case Study of 2,6-dimaino-3,5-dinitropyrazine-1-oxid (LLM-105). Crystals 2019, 9, 392. [Google Scholar] [CrossRef]
- Zhao, X.H.; He, D.; Ma, X.P.; Liu, X.Y.; Xu, Z.S.; Chen, L.Z.; Wang, J.L. Preparation, characterization of spherical 1,1-diamino-2,2-dinitroethene (FOX-7), and study of its thermal decomposition characteristics. RSC Adv. 2021, 11, 33522–33530. [Google Scholar] [CrossRef]
- Zheng, R.X.; Liu, H.N.; Xiu-tian-feng, E.; Zhu, Y.; Meng, Z.H. Thermal behaviors and Decomposition Mechanism of PNIMMO with CL-20. J. Anal. Appl. Pyrol. 2024, 179, 106457. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, B.T. A New Method of Analyzing Thermogravimetric Data. B. Chem. Soc. Jpn 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Pol. Sym. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zeman, S.; Zhang, J.G.; He, P.; Musila, T.; Bartoskova, M. Multi-stage decomposition of 5-aminotetrazole derivatives: Kinetics and reaction channels for the rate-limiting steps. Phys. Chem. Chem. Phys. 2014, 16, 24282–24291. [Google Scholar] [CrossRef]
- Hu, R.Z.; Ning, B.K.; Yu, Q.S.; Zhang, T.L.; Liu, R.; Yang, Z.Q.; Gao, S.L.; Zhao, H.A.; Shi, Q.Z. Estimation of the Critical Temperature of Thermal Explosion for Energetic Materials Using Non-isothermal Analysis Method. Chin. J. Energ. Mater. 2003, 11, 18–23. [Google Scholar]
- Ji, W.; Xu, Y.X. Preparation and Characterization of RDX/NC/AP/Al Composite Energetic Microspheres Based on Zero-oxygen Balance. Chin. J. Energ. Mater. 2022, 30, 528–534. [Google Scholar]
- Feng, X.J.; Zhang, K.; Xue, L.X.; Pan, W. Thermal Decomposition Mechanism of Molecular Perovskite Energetic Material (C6NH14)(NH4)(ClO4)3(DAP-4). Propellants Explos. Pyrotech. 2022, 47, e202100362. [Google Scholar] [CrossRef]
- Tsong, W.; Robaugh, D.; Mallard, W.G. Single-Pulse Shock-Tube Studies on C-NO2 Bond Cleavage during the Decomposition of Some Nitro Aromatic Compounds. J. Phys. Chem. 1986, 90, 5968–5973. [Google Scholar] [CrossRef]

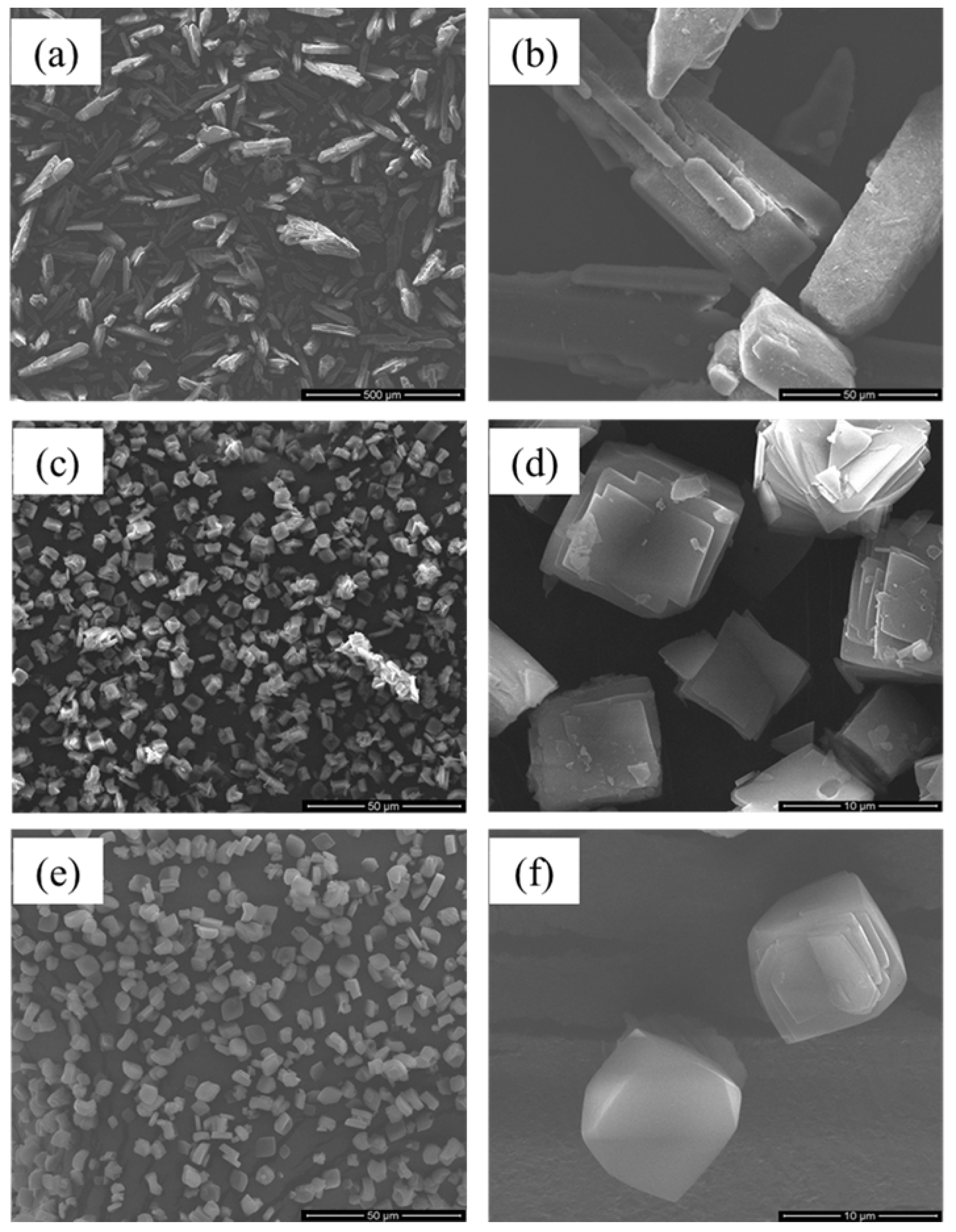

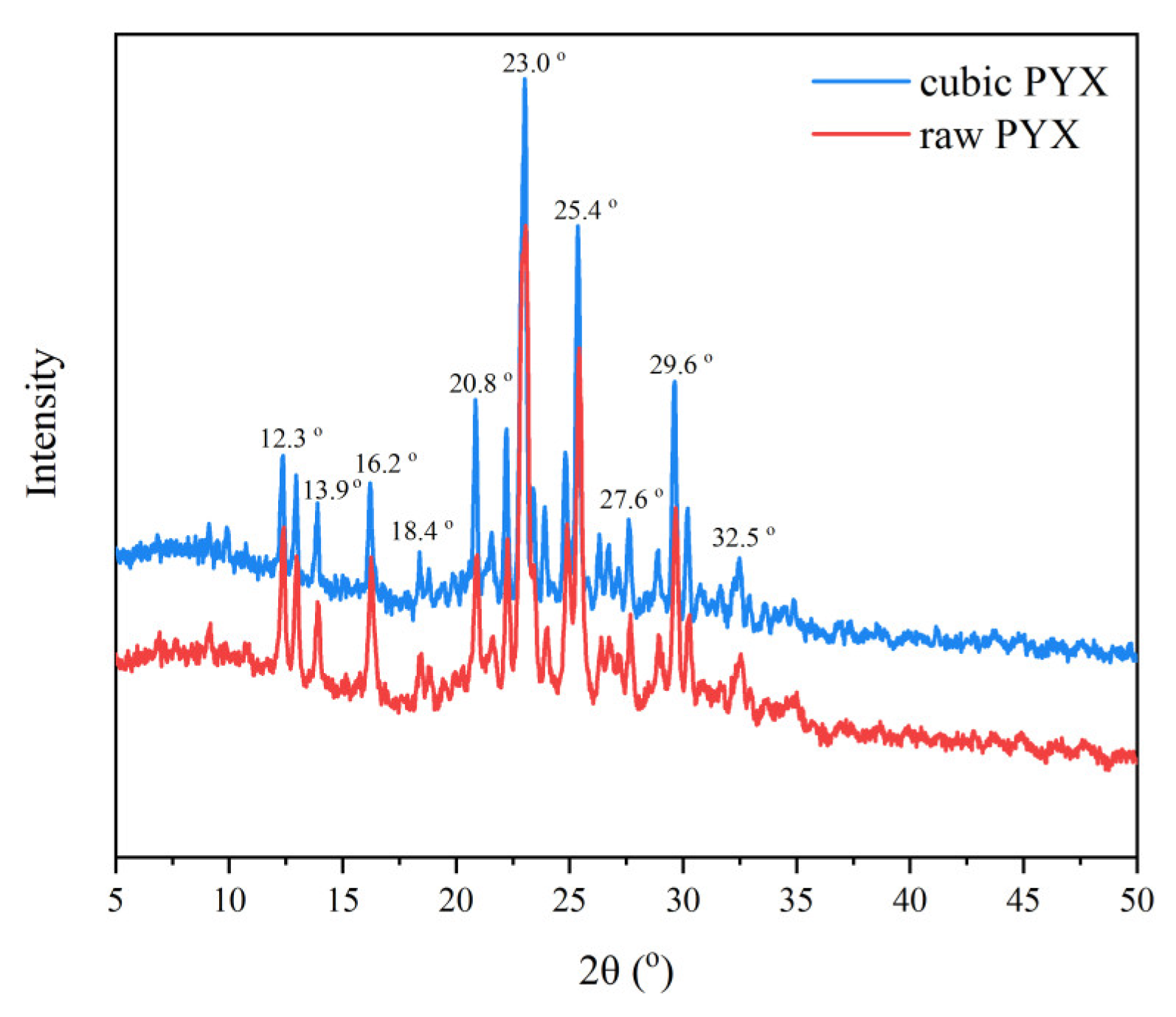
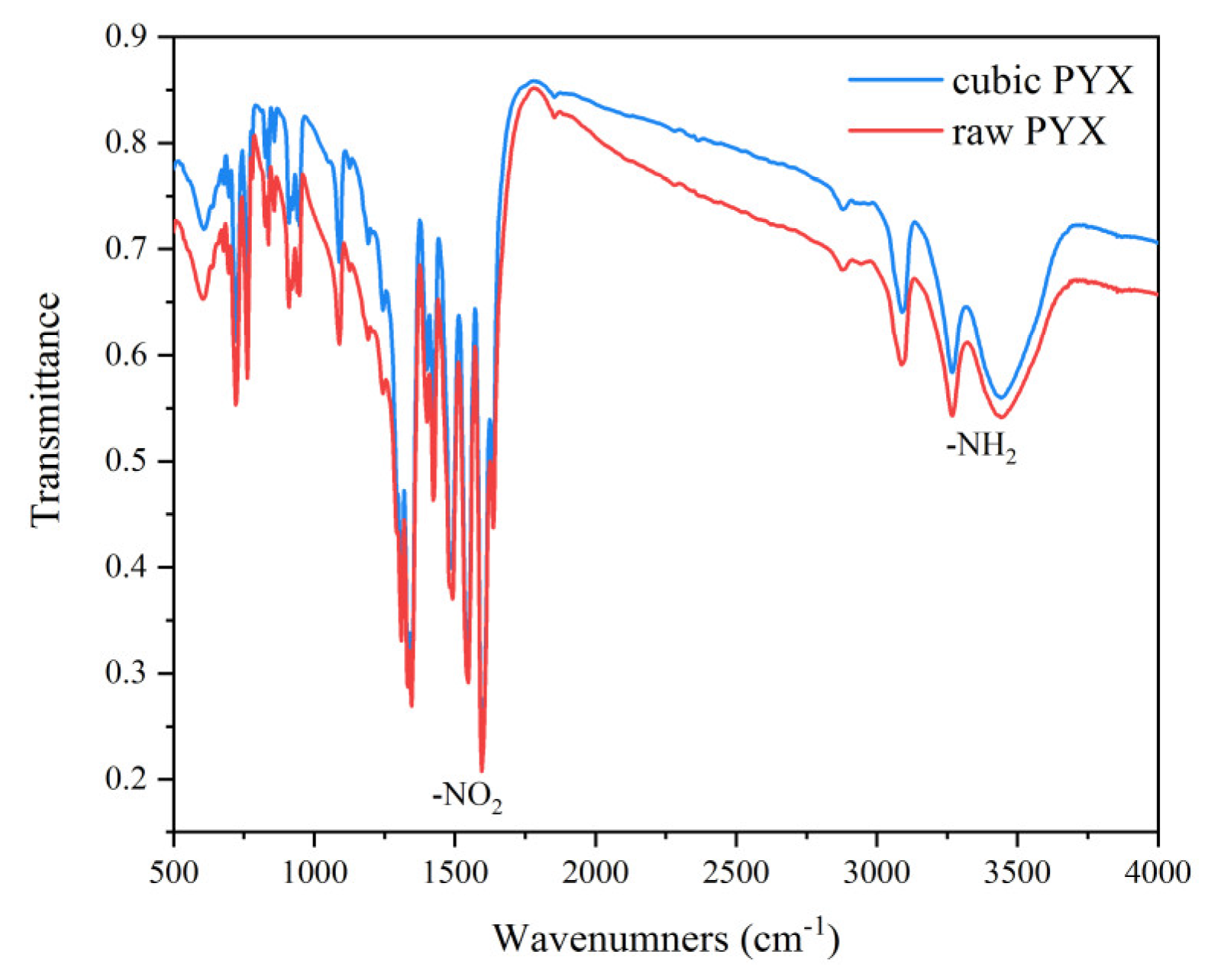

| Sample | d10/µm | d50/µm | d90/µm | N | S/(m2·g−1) |
|---|---|---|---|---|---|
| RPYX | 26.21 | 66.90 | 152.16 | 0.94 | 0.17 |
| CPYX | 3.54 | 10.65 | 21.66 | 0.85 | 1.39 |
| Sample | β/(K·min−1) | Te/K | Tp/K | 103·Tp−1/K−1 | Kissinger Method | Ozawa Method |
|---|---|---|---|---|---|---|
| ln(β/Tp2)/(min−1·K−1) | lgβ/(K·min−1) | |||||
| RPYX | 5 | 623.0 | 633.1 | 1.5795 | −11.2918 | 0.6990 |
| 10 | 630.1 | 641.8 | 1.5581 | −10.6260 | 1.0000 | |
| 15 | 635.7 | 646.3 | 1.5473 | −10.2345 | 1.1761 | |
| 20 | 640.0 | 651.4 | 1.5352 | −9.9625 | 1.3010 | |
| CPYX | 5 | 630.7 | 641.9 | 1.5579 | −11.3194 | 0.6990 |
| 10 | 635.5 | 649.0 | 1.5408 | −10.6483 | 1.0000 | |
| 15 | 638.8 | 652.7 | 1.5321 | −10.2542 | 1.1761 | |
| 20 | 644.7 | 654.4 | 1.5281 | −9.9717 | 1.3010 |
| Sample | Kissinger Method | Ozawa Method | Friedman Method | |||
|---|---|---|---|---|---|---|
| EaK/(kJ·mol−1) | lgA/s−1 | rk2 | EaO/(kJ·mol−1) | ro2 | EaF/(kJ·mol−1) | |
| RPYX | 257.16 | 19.15 | 0.9908 | 251.66 | 0.9913 | 273.91 |
| CPYX | 365.91 | 27.93 | 0.9944 | 358.20 | 0.9953 | 315.14 |
| Sample | Te0/K | Tp0/K | ΔS≠/(J·mol−1·K−1) | ΔH≠/(kJ·mol−1) | ΔG≠/(kJ·mol−1) |
|---|---|---|---|---|---|
| RPYX | 614.2 | 615.4 | 107.35 | 252.04 | 185.98 |
| CPYX | 620.3 | 630.0 | 261.46 | 360.67 | 195.95 |
| Sample | Tp/°C | TSADT/°C | Tb/°C |
|---|---|---|---|
| RPYX | 368.8 | 341.2 | 350.2 |
| CPYX | 376.0 | 347.3 | 360.6 |
| Sample | Impact Sensitivity | Friction Sensitivity |
|---|---|---|
| RPYX | 76% | 64% |
| CPYX | 40% | 44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wang, Q.; Liu, H.; Li, W.; Zheng, R.; Pang, W. Effect of Cubic Crystal Morphology on Thermal Characteristics and Mechanical Sensitivity of PYX. Crystals 2024, 14, 513. https://doi.org/10.3390/cryst14060513
Luo X, Wang Q, Liu H, Li W, Zheng R, Pang W. Effect of Cubic Crystal Morphology on Thermal Characteristics and Mechanical Sensitivity of PYX. Crystals. 2024; 14(6):513. https://doi.org/10.3390/cryst14060513
Chicago/Turabian StyleLuo, Xi, Qiong Wang, Hongni Liu, Wenjie Li, Ruixue Zheng, and Weiqiang Pang. 2024. "Effect of Cubic Crystal Morphology on Thermal Characteristics and Mechanical Sensitivity of PYX" Crystals 14, no. 6: 513. https://doi.org/10.3390/cryst14060513
APA StyleLuo, X., Wang, Q., Liu, H., Li, W., Zheng, R., & Pang, W. (2024). Effect of Cubic Crystal Morphology on Thermal Characteristics and Mechanical Sensitivity of PYX. Crystals, 14(6), 513. https://doi.org/10.3390/cryst14060513







