Numerical Simulations of the Impact of CaO/Al2O3 on the Structure and Crystallization Behavior of Red Mud
Abstract
:1. Introduction
2. Materials and Methods
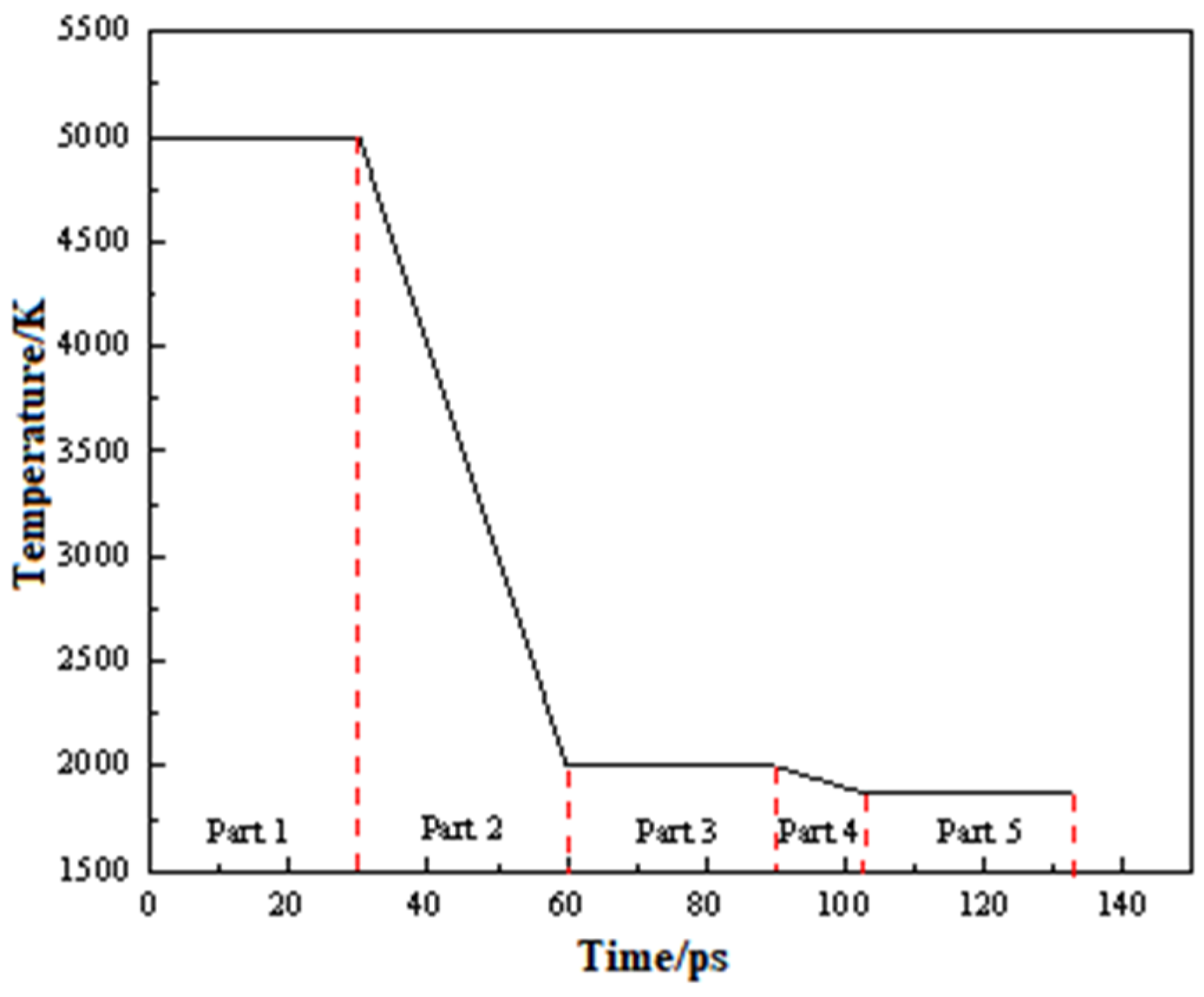
2.1. Simulation Method
2.2. Thermodynamics Calculations
2.3. Molecular Dynamics Simulations
3. Results
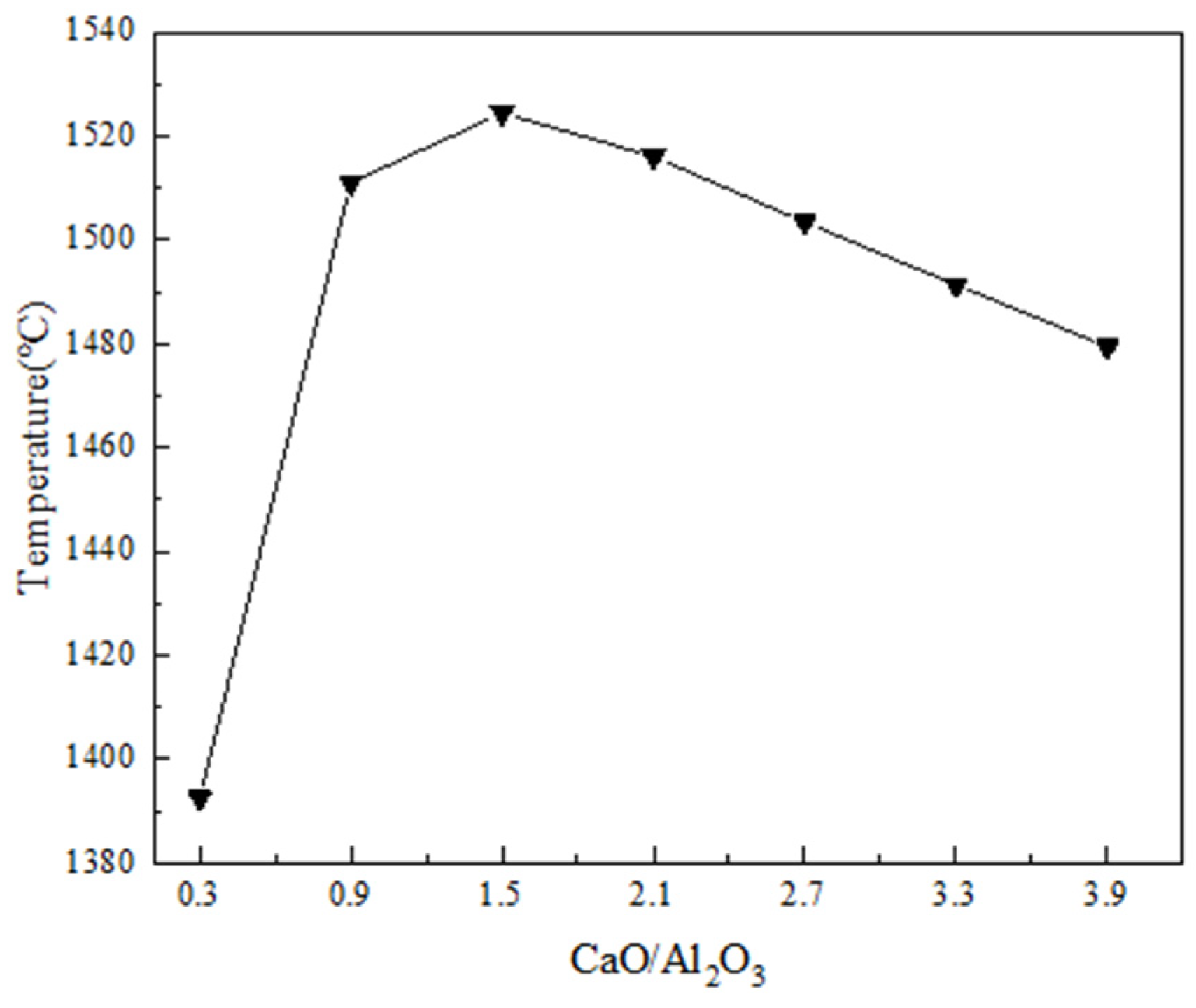
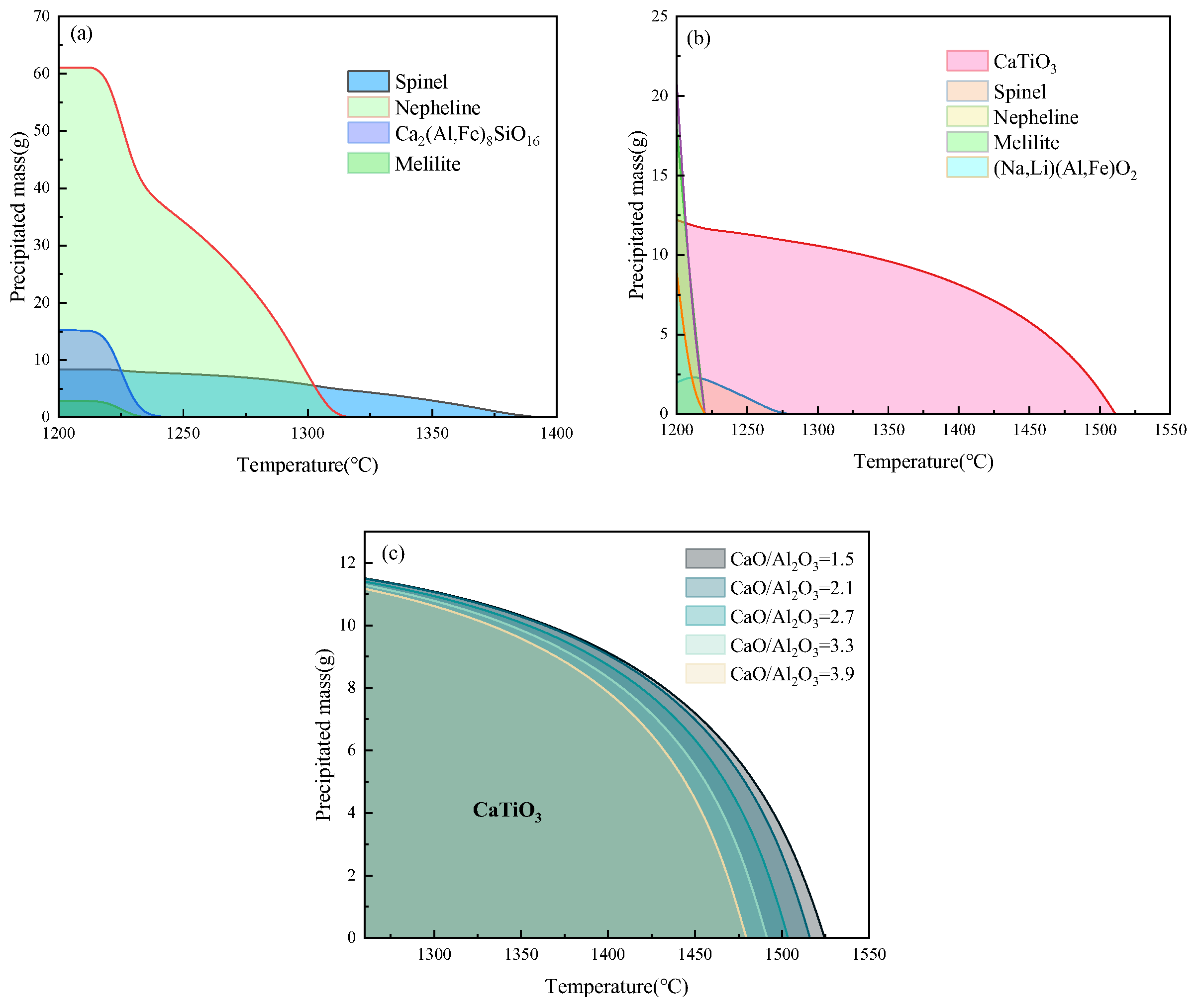
3.1. Calculation of Red Mud Melting Point (Liquidus Temperature)
3.2. Analysis of the Red Mud Melt Structure
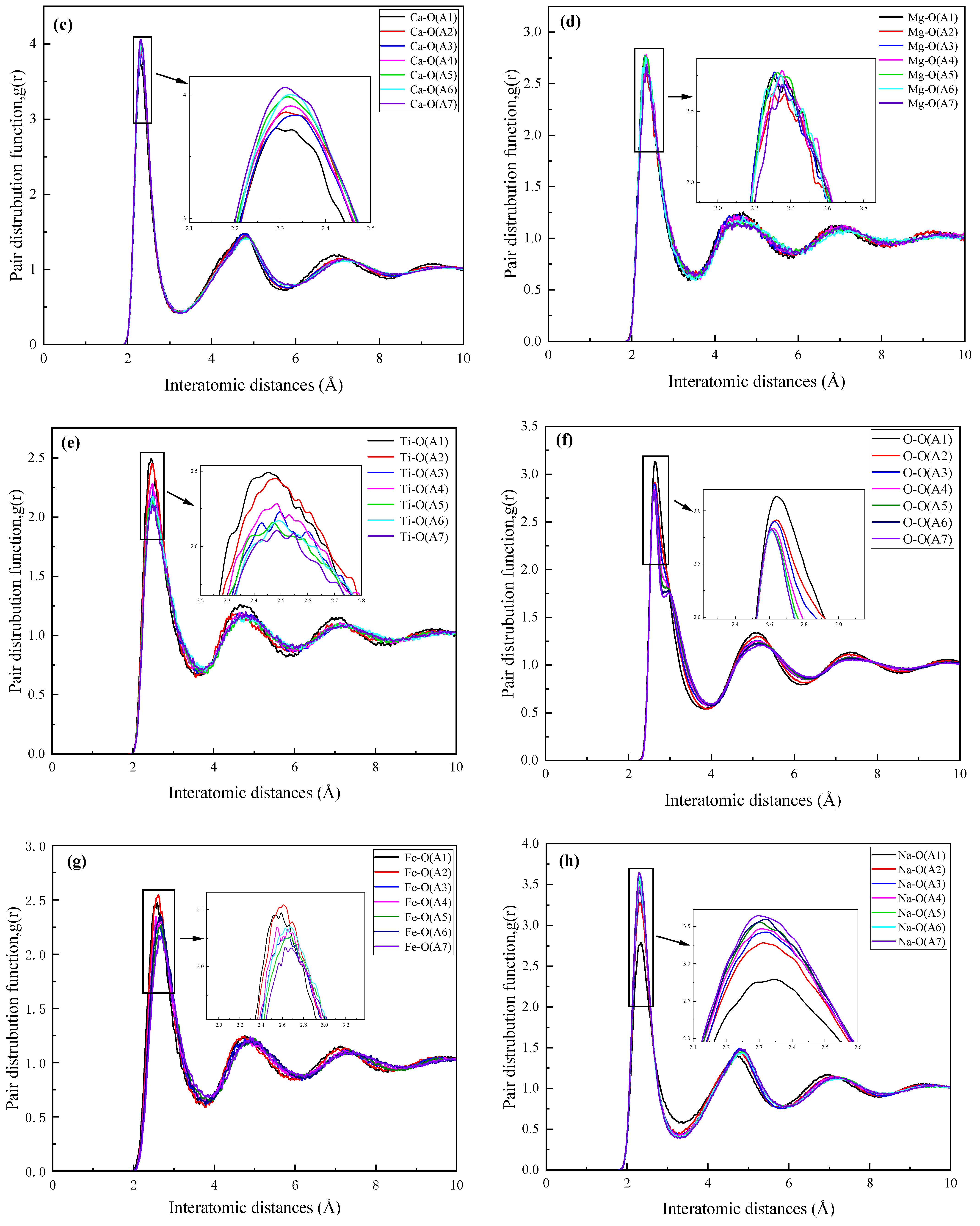
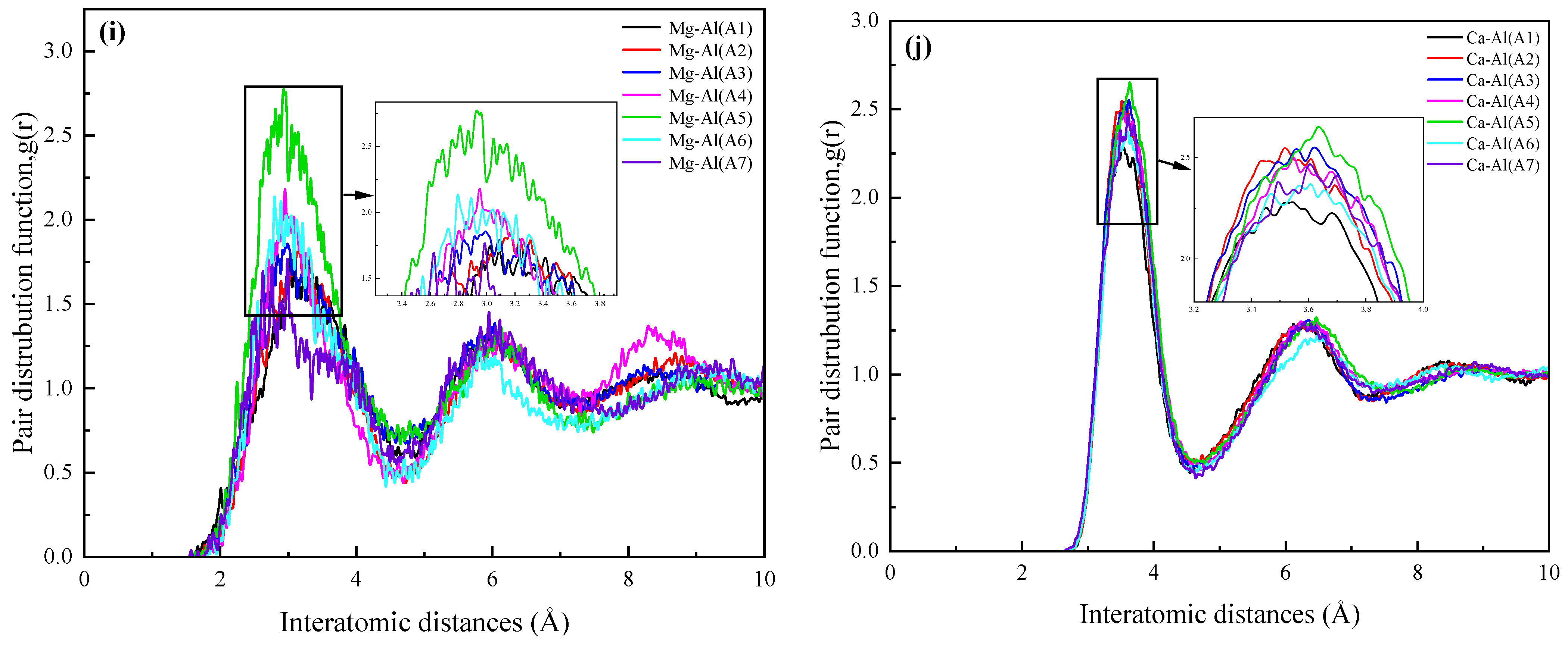
3.2.1. Radial Distribution Function
3.2.2. Oxygen Components
4. Conclusions
- (1)
- Overall, the melting point of red mud shows a significant increase firstly and then a slow decrease with the increase in CaO/Al2O3 when the CaO/Al2O3 is in the range of 0.3 to 3.9. When CaO/Al2O3 in red mud increases to 0.9, the initial crystalline phase changes from MgAl2O4 to CaTiO3 during melt cooling and a significant increase in melting point temperature occurs.
- (2)
- The increase in CaO/Al2O3 in red mud mainly affects the bonding of aluminum atoms with oxygen atoms, and higher CaO/Al2O3 leads to the change in bridging oxygen bonds (e.g., Si-O-Al or Al-O-Al) to non-bridging oxygen bonds (e.g., Si-O-Ca or Al-O-Ca), while resulting in a decrease in the degree of polymerization of the red mud melt system.
- (3)
- In summary, when the amount of bridging oxygen in the red mud melt is lower than 50 percent, the melt is prone to preferentially generate high-melting-point substances for precipitation during cooling, which will not be conducive to the preparation of inorganic fibers from the red mud melt. In order to keep the melting point of red mud low during the preparation of fibers, CaO/Al2O3 should be controlled in the range of 0.3 to 0.9.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R.X.; Poon, C.S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Liu, D.G.; Tang, C.J. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Xie, L.Q.; Zhang, T.A.; Lv, G.Z.; Zhu, X.F. Direct calcification-carbonation method for processing of bayer process red mud. Russ. J. Non-Ferr. Met. 2018, 59, 142–147. [Google Scholar] [CrossRef]
- Liu, S.H.; Guan, X.M.; Zhang, S.S.; Dou, Z.Z.; Feng, C.H.; Zhang, H.B.; Luo, S.Q. Sintered bayer red mud based ceramic bricks: Microstructure evolution and alkalis immobilization mechanism. Ceram. Int. 2017, 43, 13004–13008. [Google Scholar] [CrossRef]
- Ning, G.S.; Zhang, B.; Liu, C.J.; Li, S.; Ye, Y.; Jiang, M.F. Large-Scale consumption and zero-waste recycling method of red mud in steel making process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.K.; Huang, B.S.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- He, W.C.; Luo, M.S.; Deng, Y.; Qin, Y.L.; Zhang, S.; Lv, X.W.; Zhao, Y.; Jiang, C.Z.; Pang, Z.D. Preparation of high acidity coefficient slag wool fiber with blast furnace slag and modifying agents. J. Iron Steel Res. Int. 2023, 30, 1440–1450. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Chen, J.Q.; Qi, J.L.; Wang, Y.B.; Ai, Z.R. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018, 44, 6044–6053. [Google Scholar] [CrossRef]
- Yliniemi, J.; Ramaswamy, R.; Luukkonen, T.; Laitinen, O.; De Sousa, A.N.; Huuhtanen, M.; Illikainen, M. Characterization of mineral wool waste chemical composition, organic resin content and fiber dimensions: Aspects for valorization. Waste Manag. 2021, 131, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.Q.; Zhang, Y.Z.; Long, Y.; Zou, Z.S.; Pei, J.J. Crystallisation behaviour of blast furnace slag modified by adding fly ash. Ceram. Int. 2018, 44, 11628–11634. [Google Scholar] [CrossRef]
- Cai, S.; Tian, T.L.; Guo, Z.H.; Zhang, Y.Z. Effect of acidity coefficient on crystallization behaviour of modified blast furnace slag. Ironmak. Steelmak. 2022, 49, 555–561. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, C.X.; Zhou, J.C.; Shangguan, F.Q. Continuous cooling crystallization kinetics of a molten blast furnace slag. J. Non-Cryst. Solids 2012, 358, 20–24. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.L.; Xiao, H.X.; Liu, X.; Yu, G.S.; Chen, X.L. Exploring influence of MgO/CaO on crystallization characteristics to understand fluidity of synthetic coal slags. Chin. J. Chem. Eng. 2023, 53, 1–13. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, Y.J.; Zhang, Y.Z.; Du, P.P.; Ren, Q.Q. Effect of content of Al2O3 and MgO on crystallization of blast furnace slag during fiber formation. J. Cent. South. Univ. 2018, 25, 2373–2379. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, C.; Zhang, Y.Z.; Xing, H.W. Influence of crystallization behavior of gas quenching blast furnace slag on the preparation of amorphous slag beads. Crystals 2020, 10, 30. [Google Scholar] [CrossRef]
- Song, L.; Liu, W.H.; Xin, F.H.; Li, Y.M. “Materials Studio” Simulation Study of the Adsorption and Polymerization Mechanism of Sodium Silicate on Active Silica Surface at Different Temperatures. Int. J. Met. 2021, 15, 1091–1098. [Google Scholar] [CrossRef]
- Liu, H.L.; Song, L. Materials Studio simulation for the adsorption properties of CO2 molecules at the surface of sodium silicate and potassium silicate solution under different pressure conditions. Int. J. Met. 2022, 16, 242–251. [Google Scholar] [CrossRef]
- Bi, Z.S.; Li, K.J.; Jiang, C.H.; Zhang, J.L.; Ma, S.F. Effects of amphoteric oxide (Al2O3 and B2O3) on the structure and properties of SiO2-CaO melts by molecular dynamics simulation. J. Non-Cryst. Solids 2021, 559, 120687. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Q.D.; Liu, N.; Chen, J.Y.; Wang, Z.H.; Tan, C.L.; Sheng, Z.Y. Crystallization kinetics of Ni-Mn alloy thin film using molecular dynamics simulation. Ferroelectrics 2019, 549, 234–240. [Google Scholar] [CrossRef]
- Wang, C.; Lin, P.P.; Liu, X.; Li, G.; Lin, T.S.; He, P.; Long, W.M.; Liu, H.Z. Microstructure evolution and cooperative reinforcement mechanisms of Al2O3/Al2O3 joints brazed by low-melting borosilicate glass. Ceram. Int. 2020, 46, 186–195. [Google Scholar] [CrossRef]
- Pan, X.Y.; Shen, F.M.; Gao, Q.J.; Jiang, X.; Zheng, H.Y. Effect of erosion behavior of FeO-CaO-SiO2-MgO-Al2O3 blast furnace primary slag on Al2O3 substrate. Crystals 2021, 11, 957. [Google Scholar] [CrossRef]
- Shi, G.Y.; Zhang, T.A.; Dou, Z.H.; Niu, L.P. Dissolution behavior of Al2O3 inclusions in CaO-Al2O3 based slag representing aluminothermic reduction slag. Crystals 2020, 10, 1061. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Y.R.; Wang, Z.Z.; Du, P.P.; Long, Y. Molecular Dynamics Study on the Effect of SiO2/Al2O3 Mass Ratio on the Structural Properties and Viscosity of Molten Fused Red Mud. Minerals 2022, 12, 925. [Google Scholar] [CrossRef]
- Chen, G.; Xian, W.K.; Wang, Q.M.; Li, Y. Molecular simulation-guided and physics-informed mechanistic modeling of multifunctional polymers. Acta. Mech. Sin. 2021, 37, 725–745. [Google Scholar] [CrossRef]
- Sun, W.; Du, J.C. Local ordering and interfacial structure between spinel crystal and aluminosilicate glasses from molecular dynamics simulations. Int. J. Appl. Glass Sci. 2019, 10, 41–56. [Google Scholar] [CrossRef]
- Souza, J.A.; Rino, J.P. A molecular dynamics study of structural and dynamical correlations of CaTiO3. Acta. Mater. 2011, 59, 1409–1423. [Google Scholar] [CrossRef]
- Bi, Z.S.; Li, K.J.; Jiang, C.H.; Zhang, J.L.; Ma, S.F.; Sun, M.M.; Wang, Z.M.; Li, H.T. Performance and transition mechanism from acidity to basicity of amphoteric oxides (Al2O3 and B2O3) in SiO2-CaO-Al2O3-B2O3 system: A molecular dynamics study. Ceram. Int. 2021, 47, 12252–12260. [Google Scholar] [CrossRef]
- Ross, S.; Welsch, A.M.; Behrens, H. Lithium conductivity in glasses of the Li2O-Al2O3-SiO2 system. Phys. Chem. Chem. Phys. 2015, 17, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bai, J.; Huang, Q.; Liu, Z.; Bai, X.J.; Lin, C.T.; Li, W.; Guo, W.P.; Wen, X.D.; Du, S.Y. Coal ash fusion properties from molecular dynamics simulation: The role of calcium oxide. Fuel 2018, 216, 760–767. [Google Scholar] [CrossRef]
- Möncke, D.; Tricot, G.; Winterstein-Beckmann, A.; Wondraczek, L.; Kamitsos, E.I. On the connectivity of borate tetrahedra in borate and borosilicate glasses. Phys. Chem. Glas.-B 2015, 56, 203–211. [Google Scholar]

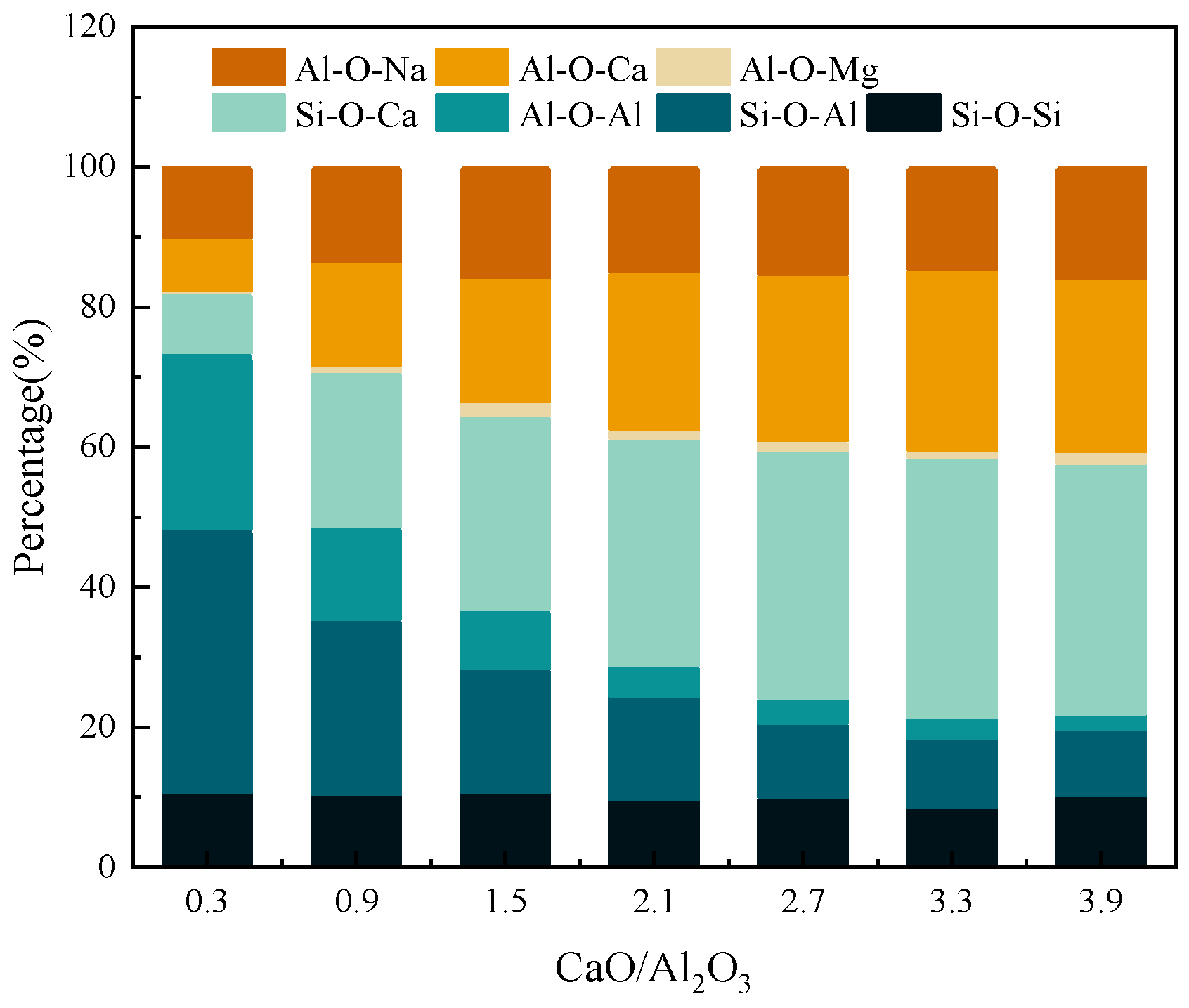


| Red Mud Composition | SiO2 | CaO | Al2O3 | MgO | Na2O | Fe2O3 | TiO2 | K2O | MnO | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass/% | 27.26 | 10.62 | 32.31 | 2.14 | 11.16 | 7.56 | 7.23 | 0.30 | 0.13 | 1.29 |
| Scheme | CaO/Al2O3 | Red Mud Composition/Mass Percentage, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | Al2O3 | SiO2 | Na2O | Fe2O3 | TiO2 | MgO | Total | ||
| A1 | 0.3 | 10.80 | 32.89 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A2 | 0.9 | 20.70 | 22.99 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A3 | 1.5 | 26.20 | 17.49 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A4 | 2.1 | 29.6 | 14.09 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A5 | 2.7 | 31.9 | 11.79 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A6 | 3.3 | 33.5 | 10.19 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| A7 | 3.9 | 34.8 | 8.89 | 27.75 | 11.36 | 7.69 | 7.36 | 2.17 | 100 |
| i | j | Aij/eV | Bij/(Å−1) | Cij/(eV·Å6) |
|---|---|---|---|---|
| O | O | 1.18 × 105 | 7.31 | 0 |
| O | Si | 1.98 × 105 | 5.77 | 0 |
| O | Ca | 1.52 × 105 | 4.93 | 0 |
| O | Fe | 2.74 × 105 | 6.08 | 0 |
| O | Mg | 1.06 × 105 | 4.95 | 0 |
| Al | O | 1.95 × 103 | 3.55 | 0 |
| Ti | O | 2.40 × 105 | 6.06 | 0 |
| Na | O | 1.99 × 103 | 6.25 | 0 |
| Ca | Al | 3.68 × 104 | 6.25 | 0 |
| Al | Mg | 1.72 × 102 | 6.25 | 0 |
| Sample | Density | Number of Atoms | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Si | Al | Ti | Mg | Fe | Na | O | Total | ||
| A1 | 3.31 | 96 | 231 | 322 | 46 | 27 | 48 | 184 | 1324 | 2278 |
| A2 | 3.26 | 185 | 231 | 226 | 46 | 27 | 48 | 184 | 1269 | 2216 |
| A3 | 3.23 | 234 | 231 | 172 | 46 | 27 | 48 | 184 | 1237 | 2179 |
| A4 | 3.21 | 264 | 231 | 138 | 46 | 27 | 48 | 184 | 1216 | 2154 |
| A5 | 3.19 | 284 | 231 | 116 | 46 | 27 | 48 | 184 | 1203 | 2139 |
| A6 | 3.18 | 299 | 231 | 100 | 46 | 27 | 48 | 184 | 1194 | 2129 |
| A7 | 3.17 | 310 | 231 | 88 | 46 | 27 | 48 | 184 | 1187 | 2121 |
| Si–O | Al–O | Ca–O | Mg–O | Ti–O | O–O | Fe–O | Na–O | Mg–Al | Ca–Al | |
|---|---|---|---|---|---|---|---|---|---|---|
| r1 (Å) | ||||||||||
| A1 | 1.59 | 1.77 | 2.29 | 2.37 | 2.45 | 2.63 | 2.59 | 2.35 | 3.25 | 3.55 |
| A2 | 1.59 | 1.77 | 2.31 | 2.37 | 2.49 | 2.65 | 2.61 | 2.31 | 3.17 | 3.51 |
| A3 | 1.59 | 1.77 | 2.35 | 2.31 | 2.49 | 2.63 | 2.69 | 2.33 | 2.91 | 3.61 |
| A4 | 1.59 | 1.79 | 2.33 | 2.35 | 2.49 | 2.61 | 2.55 | 2.31 | 2.95 | 3.55 |
| A5 | 1.59 | 1.77 | 2.31 | 2.33 | 2.47 | 2.61 | 2.69 | 2.31 | 2.97 | 3.63 |
| A6 | 1.59 | 1.75 | 2.31 | 2.35 | 2.49 | 2.61 | 2.63 | 2.33 | 2.79 | 3.61 |
| A7 | 1.59 | 1.75 | 2.31 | 2.35 | 2.49 | 2.61 | 2.63 | 2.29 | 2.99 | 3.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Li, Z.-H.; Du, P.-P.; Long, Y. Numerical Simulations of the Impact of CaO/Al2O3 on the Structure and Crystallization Behavior of Red Mud. Crystals 2024, 14, 526. https://doi.org/10.3390/cryst14060526
Xing L, Li Z-H, Du P-P, Long Y. Numerical Simulations of the Impact of CaO/Al2O3 on the Structure and Crystallization Behavior of Red Mud. Crystals. 2024; 14(6):526. https://doi.org/10.3390/cryst14060526
Chicago/Turabian StyleXing, Lei, Zhi-Hui Li, Pei-Pei Du, and Yue Long. 2024. "Numerical Simulations of the Impact of CaO/Al2O3 on the Structure and Crystallization Behavior of Red Mud" Crystals 14, no. 6: 526. https://doi.org/10.3390/cryst14060526





