Abstract
Recently, a smart strategy for two-dimensional (2D) materials synthesis has emerged, namely space-confined chemical vapor deposition (CVD). Its extreme case is the microreactor method, in which the growth substrate is face-to-face stacked on the source substrate. In order to grow 2D transition metal dichalcogenides by this method, transition metal oxides, dispersed in very small amounts on the source substrate, are used as source materials in most of the published reports. In this paper, a colloidal dispersion of MoS2 in saline solution is used and MoS2 nanosheets with various shapes, sizes (between 5 and 60 μm) and thicknesses (2–4 layers) have been synthesized. Small MoS2 flakes (regular or defective) are present on the surface of the nanosheets. Catalytic sites, undercoordinated atoms located at the edges of MoS2 flakes and nanosheets, are produced in a high number by a layer-plus-island (Stranski–Krastanov) growth mechanism. Several double-resonance Raman bands (at 147, 177, 187, 225, 247, 375 cm−1) are assignable to single phonon processes in which the excited electron is elastically scattered on a defect. The narrow 247 cm−1 peak is identified as a topological defect-activated peak. These findings highlight the potential of defect engineering in material property optimization, particularly for solar water splitting applications.
1. Introduction
Quasi-two-dimensional (2D) crystalline semiconductor materials are anticipated to form the basis of next-generation high-performance nanoelectronics, optoelectronics, and photoelectrocatalysis. Transition metal dichalcogenides (TMDs) [1] are a prominent class of such 2D materials. The chemical formula of TMDs is MX2, where M is a transition metal element from groups IVB (Ti, Zr or Hf), VB (V, Nb or Ta), VIB (Mo, W), VIIB (Re), or VIIIB (Pd, Pt), while X is a chalcogen element (S, Se, or Te) [2]. These 2D TMDs are crystalline molecular layers (monolayers), thicker than an atom and without dangling bonds, being the building blocks of the corresponding 3D crystals [3]. Each single crystalline MX2 monolayer is a stack of three hexagonal monoelemental planes (X, M, X) bonded together by strong covalent bonds [–X–M–X–] [1,3], while the interactions between monolayers are of the van der Waals type [3].
Since 2004, researchers have been working to develop cost-effective, large-area fabrication techniques for producing 2D semiconductors with high crystallinity and excellent semiconducting properties [4]. Among these methods, vapor deposition, a bottom-up approach, is the most promising. Recently, a new vapor deposition strategy has emerged. It uses a microreactor and represents the extreme case of the method named space-confined vapor deposition [5,6]. In this technique, a confined environment is created by aligning two substrates in a horizontal, face-to-face configuration. The “source substrate”, typically positioned at the bottom, contains the source material, while the “growth substrate”, placed on top, is left blank to allow for material deposition. The microreactor is heated in an inert atmosphere, inside of a quartz tube, using a tube furnace. The method’s main advantage is the minimal amount of source materials required, which results in economic benefits. Moreover, the reduced nucleation density might facilitate the formation of large, high-quality 2D crystals.
Using variable parameters of this method, the type of transition metals source and the seeding method of the source substrate, crystalline 2D TMDs were obtained. Almost all published reports used a transition metal oxide precursor as the transition metals source. In these cases, the microreactor method is a variant of the chemical vapor deposition (CVD) method. The chalcogen source is positioned outside the microreactor, in the upstream heating zone of the furnace. The methods used for seeding the source substrate were drop casting of the colloidal dispersion [7,8], sputtering [7], spin coating of metal salts (such as Na2WO4 and Na2MoO4) [9], oxide powders spreading [10], and electron beam evaporation [11,12,13]. High quality results have also been achieved when the oxide source is outside the microreactor [14,15,16].
There is only one report that uses the TMD as a transition metal source, which was deposited onto source substrate by pulsed laser deposition (PLD) at room temperature [17].
Lateral (WS2/MoS2 [9]) and vertical (MoS2/WSe2 [18]) heterostructures have been also produced by the confined-space method.
In this study, we sought to produce crystalline 2D MoS2 flakes using the space-confined vapor deposition method. We started with MoS2 nanoparticles deposition on the source substrate by drop casting the colloidal dispersion of MoS2. While there have been reports of large crystalline monolayer MoS2 domains produced by physical MoS2 vapor transport [19,20,21,22], our experiments showed that the MoS2 nanoparticles did not sublimate up to a temperature of 1050 °C. Because some salts (e.g., NaCl) can dramatically decrease the melting point of metal oxide precursors [2], NaCl was added in the colloidal dispersion of MoS2, and thus, we obtained 2D MoS2 flakes at 980 °C. This paper investigates their properties.
2. Materials and Methods
The source of transition metal used in this study was MoS2 powder (99.0% purity) with a particle size of less than 2 μm, purchased from Aldrich (Darmstadt, Germany). The key ingredient, NaCl (99.5% purity), was acquired from Fluka (Gillingham, UK). The deionized (DI) water used for colloidal dispersion was produced using a Barnstead Smart2Pure 3 UV Water Purification System from Thermo Scientific (Waltham, MA, USA).
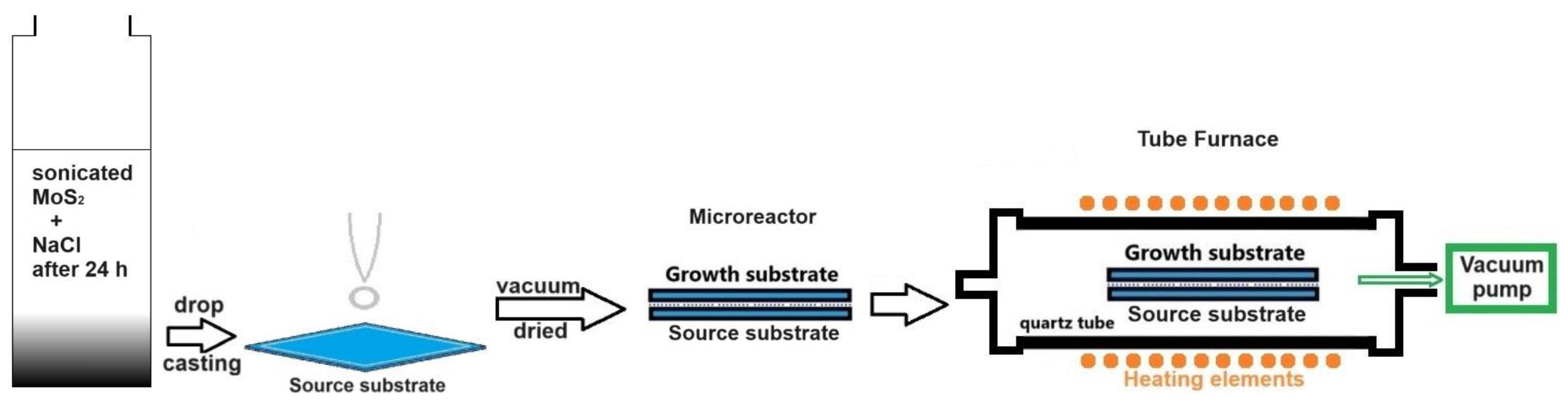
Firstly, a colloidal dispersion of MoS2 was prepared. A mixture of 57.7 mg of MoS2 powder and 14.1 mg of NaCl were added to 15.8 mL of deionized water. This mixture was subjected to ultrasonication for 40 min, after which the obtained black suspension was left undisturbed for 24 h to allow particles to settle. Following this period, all heavy particles had settled at the bottom of the container, resulting in a transparent, clear MoS2 colloidal dispersion (Figure 1). An ultra-thin gray film of hydrophobic MoS2 particles was observed floating on the liquid surface, likely comprising non-monolayer nanometric particles [23].
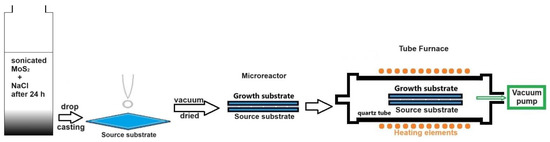
Figure 1.
Schematic diagram of the process for the loading and preparation of the microreactor (sandwich-structured) configuration to produce MoS2 nanosheets in a CVD furnace using the microreactor method (the extreme case of the space-confined chemical vapor deposition method).
The two substrates used were SiO2 (300 nm)/Si pieces, each with a size of 1 × 1 cm2. These were cleaned with acetone, isopropyl alcohol (IPA), and DI water. Two drops (2 × 0.2 mL) of the clear MoS2 colloidal dispersion in the saline solution were spread onto the source substrate using a graduated pipette (Figure 1). This substrate was then vacuum-dried to expedite water evaporation. Following this, the growth substrate was placed over the source substrate, creating the confined space of a microreactor (Figure 1).
The synthesis of 2D MoS2 was carried out in a tube furnace setup (model CY-1200X-III-50IC from Zhengzhou CY Scientific Instrument, Zhengzhou, China), equipped with a 2” quartz tube, three controlled heating zones and a gas mixing system. The microreactor was placed horizontally in the quartz tube, which was sealed at both ends with flanges, and positioned in the center of one of heated zones (Figure 1). The oxygen was evacuated from the quartz tube through three vacuum/purging cycles using nitrogen (99.999% purity). Then, the quartz tube was evacuated to a pressure of 1.3 Pa, which was maintained until the samples’ removal. The furnace was heated to 980 °C in 38 min, maintained at this temperature for 30 min, and then allowed to cool naturally to room temperature before the samples were extracted for investigation.
Optical microscopy images were captured using an OPTIKA (Budapest, Hungary) 50×–800× microscope, equipped with a trinocular, halogen light, and a polarizing kit.
Thickness measurements and atomic force microscopy (AFM) images were obtained utilizing an NT-MDT Aura Ntegra Prima (Amsterdam, The Netherlands) atomic force microscope in non-contact mode.
For morphological and elemental characterization, a Zeiss Gemini 500 scanning electron microscope (SEM) provided with an energy dispersive X-ray (EDX) spectrometer from Bruker (Billerica, MA, USA), was employed.
The micro-Raman spectra were recorded in the backscattering geometry using a LabRAM HR Evolution Raman spectrometer from HORIBA Jobin–Yvon (Palaiseau, France). The spectrometer was equipped with a confocal microscope and an He–Ne laser operating at an excitation wavelength of 633 nm. The laser radiation was focused onto the samples surface using an Olympus 100× objective (Tokyo, Japan). To avoid heating effects, the laser power was maintained at a suitable low level. The Raman shift was calibrated using the Si peak at 520 cm−1.
3. Results
3.1. Optical and Atomic Force Microscopy
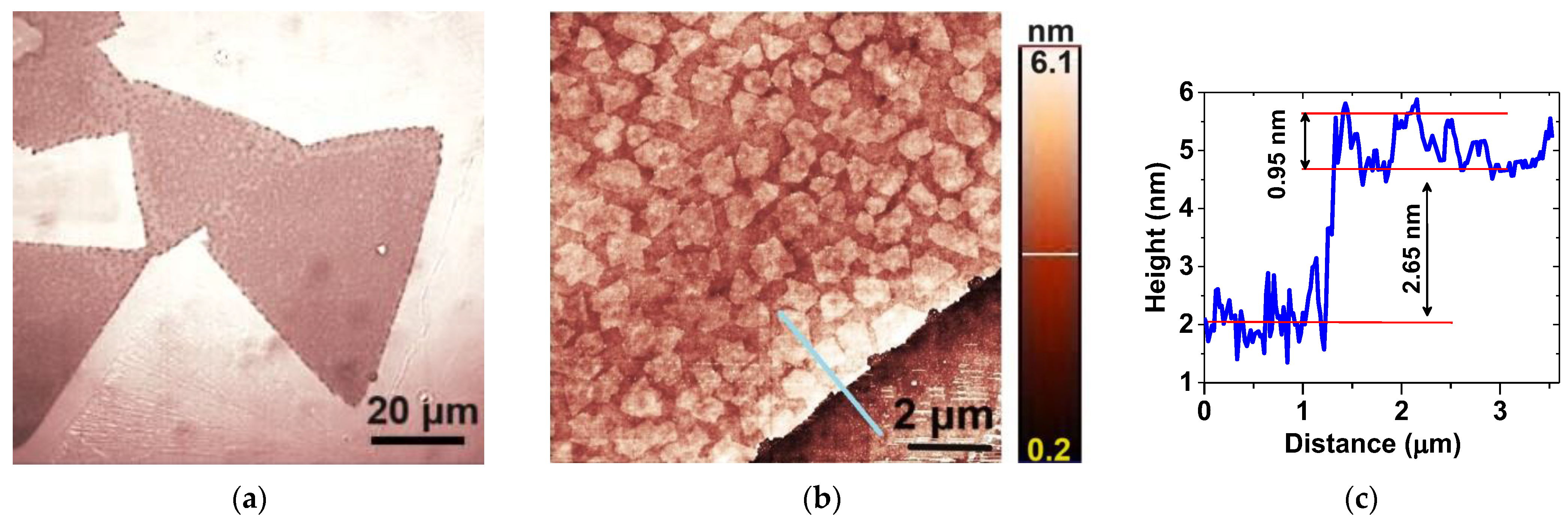
Figure 2a depicts the optical microscopy image of typical MoS2 nanosheets grown via space-confined chemical vapor deposition. The as-grown MoS2 nanosheets, which exhibit various shapes, including equilateral triangles, span sizes from 5 to 60 μm.
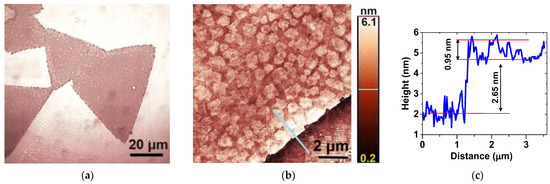
Figure 2.
Representative (a) optical microscopy and (b) AFM images of large MoS2 nanosheets; (c) the height profile obtained from the region indicated by the blue line in (b).
According to the AFM images, their surface is abundantly decorated with small, irregular flakes, approximately 0.9 μm in size, as can be seen in Figure 2b. Figure 2c shows that some of these flakes are monolayers with a thickness of ~0.8 nm [24], which have grown on top of the nanosheets. The nanosheets exhibit a thickness ranging between two and four layers. For instance, the one depicted in Figure 2b has a thickness of ~2.65 nm, which corresponds roughly to three layers.
3.2. SEM and EDX
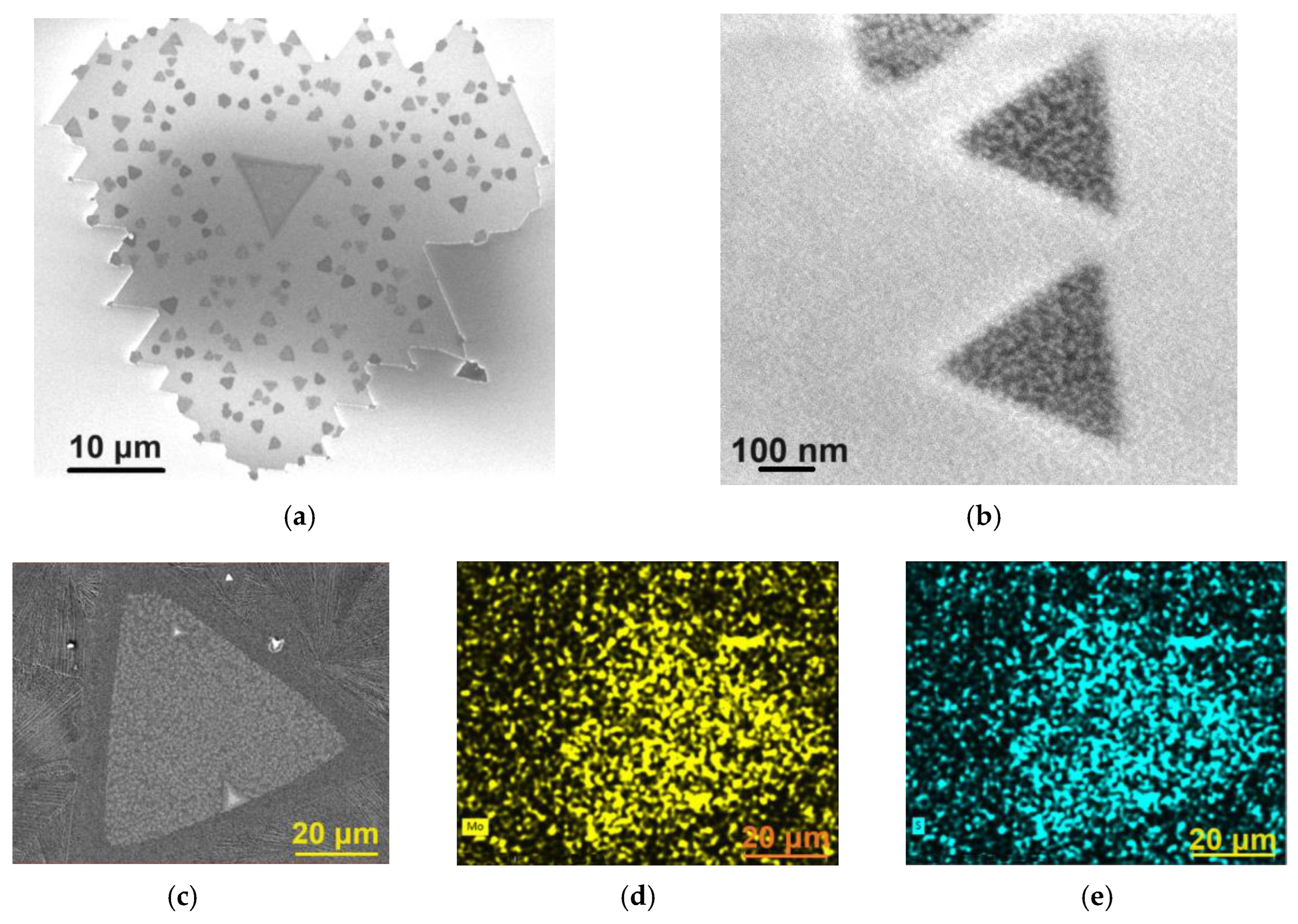
The SEM image depicted in Figure 3a shows one of the nanosheets with sharp edges, proving the crystalline nature of the MoS2 nanosheets.
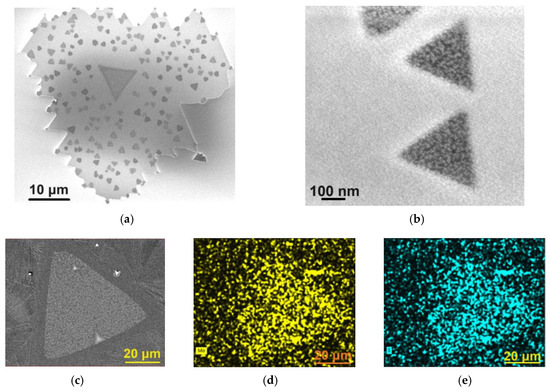
Figure 3.
SEM images of (a) MoS2 nanosheet and (b) MoS2 flakes grown on the surface of the MoS2 nanosheet. SEM image of (c) MoS2 nanosheet used in EDX mapping of (d) molybdenum and (e) sulfur.
When the MoS2 flakes, grown on top of the MoS2 nanosheets, are sparse, as seen in Figure 3a, they exhibit more regular shapes, often equilateral triangles, as shown in Figure 3b. These additional flakes become more irregular (Figure 2b) when the number of these flakes increases and the space between them decreases. Many of them are monolayers or bilayers (multilayer flakes are exceptions, as the Raman spectra will prove).
The analysis of the EDX spectra revealed that, within the limits of experimental errors, the nanosheets exhibited a sulfur deficiency in their composition, an observation consistent with MoS2 nanosheets prepared by other bottom-up methods [25,26]. For example, the nanosheet shown in the Figure 2b has an average composition of MoS1.33. Furthermore, within the limits of experimental errors, no traces of Cl or Na were detected in the nanosheets. Figure 3c shows the SEM image of a MoS2 nanosheet used in EDX mapping, while Figure 3d,e present the corresponding EDX maps of molybdenum and sulfur, respectively.
3.3. Raman Spectroscopy
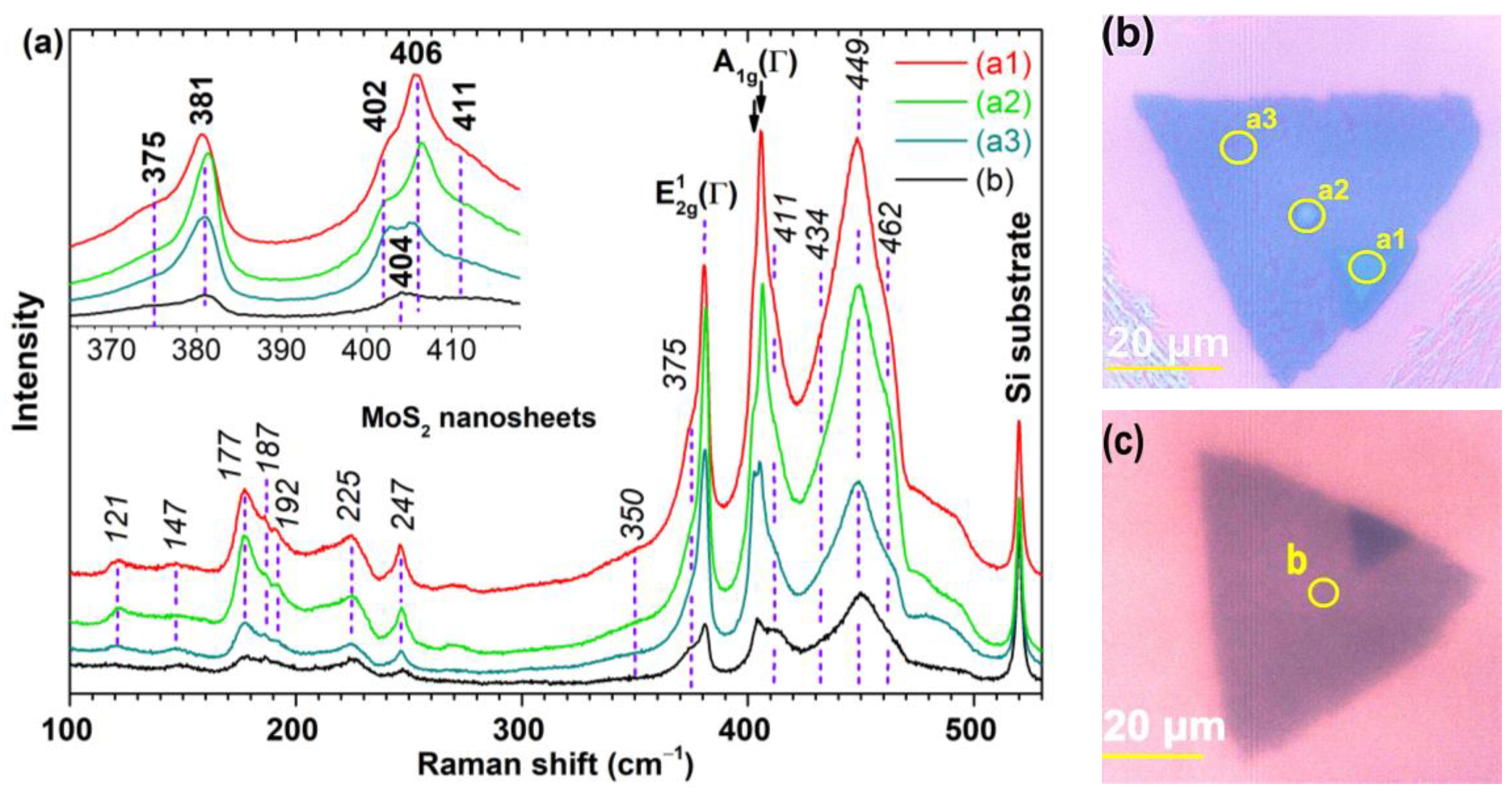
The micro-Raman spectra were recorded in the range 100÷530 cm−1, using a 632.8 nm excitation line across different MoS2 nanosheets in an ambient air environment. Figure 4a depicts the spectra for several microzones (a1, a2, a3, and b) on two distinct nanosheets, see Figure 4b,c. The morphology of the microzone (a3) is typical for most nanosheets, while that of the microzones (a1), (a2), and (b) are exceptions.
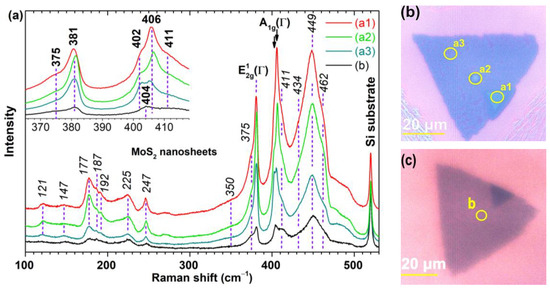
Figure 4.
Raman scattering of MoS2 nanosheets. (a) Raman spectra of selected microzones (a1, a2, a3, and b) on two MoS2 nanosheets. All spectra are normalized relative to the Raman peak of the silicon substrate and have been vertically offset for enhanced visual clarity. (b,c) The corresponding optical images of the nanosheets.
The intensity of the spectra decreases from (a1) to (b) relative to the silicon peak, indicating a decrease in the average thickness of the microzones. There are more Raman peaks than the two zone-center first-order Raman active modes, E12g(Γ) and A1g(Γ), expected for hexagonal 2H-MoS2, bulk or nanosheets with at least 5 layers, at 382 cm−1 and 407 cm−1, respectively, in backscattering geometry and without monochromators [27,28,29].
The A1g(Γ) peak frequency is thickness-dependent, red-shifted as the number of layers decreases below 5, while E12g(Γ) is less blue-shifted [27]. For such thinner MoS2 nanosheets, the frequency difference (δ) of the two Raman modes, with values of 19, 21, 22, 23, and 25 cm−1, serves as an indicator of the number (nm) of MoS2 layers (nm is 1, 2, 3, 4, and ≥5, respectively) [27]. However, this correlation, nm = f(δ), may become inaccurate in nanosheets presenting strain, doping, defects, and ionic substitution or adsorption [30].
The spectrum (a1) in Figure 4a exhibits δ as 25 cm−1 (likely representing 5 layers) but it also has a shoulder at 402 cm−1 (δ = 21 cm−1), corresponding to 2 layers. The intensity of the A1g(Γ) peak at 406 cm−1 surpasses that of E12g(Γ), an aspect also observed in thick nanosheets obtained by mechanical exfoliation under the resonance condition [27]. Therefore, it can be inferred that submicron holes exist within that thicker region of the nanosheet. A similar δ value was found for spectrum (a2), but the intensities of the two Raman modes, E12g(Γ) and A1g(Γ), are almost equal, indicative of a small number of layers (up to 4) [27]. Due to the overall lower intensity of the spectrum (a2) compared to (a1), it is possible that the (a2) microzone consists of four layers, with sparse holes of two layers. The spectrum of the (a3) microzone on the same nanosheet, shows that the two A1g(Γ) peaks at 403 and 405 cm−1, have the same intensity, suggesting a similar proportion of holes (2 layers) and plateaus (4 layers). The spectrum (b) exhibits a single A1g(Γ) peak at 404 cm−1 and, considering its smallest overall intensity, it is suggested that the microzone (b) mainly consists of 3 layers (δ = 23 cm−1). All these assumptions are validated by the details observed in the regions highlighted on the optical images in Figure 4b,c, but also in Figure 2c.
In order to assign vibrational modes to the other Raman peaks, we rely on findings of other research groups. They propose that these peaks result from two primary factors: double resonant Raman scattering and the presence of defects in MoS2 crystallites.
Let us first discuss how double resonant Raman scattering occurs. The calculated indirect band gap for bulk MoS2 is 1.23 eV [31] and it goes from Γ point to ~Q point in the Brillouin zone [32]. As the number of layers in MoS2 flakes decreases below 4 [33], the bandgap begins to widen up to 1.87–1.92 eV [31] (these are experimentally obtained direct band gaps at the K point of the Brillouin zone for MoS2 monolayers). When laser energy (1.96 eV for 632.8 nm in our case) has values close to the absorption edge of bulk MoS2, between 1.833 and 2.410 eV (676.4 to 514.5 nm), both the indirect and direct band gaps are strongly and mediumly, respectively, modulated by A1g mode displacements. Thus, periodically over time, bulk MoS2 becomes a material with a direct band gap and the excitation laser line resonates with the vertical electronic transitions in the K point [29]. In other words, there is a strong coupling between the electronic band structure and some lattice vibrational modes (the E12g mode couples only very weakly to the electronic structure [29]). As a result, the A1g peak is enhanced in relation to the E12g peak [27]. Such an enhancement disappears for ultrathin MoS2 nanosheets [27], as can also be seen in Figure 4a, indicating that the coupling between electronic transition at the K point with the A1g mode is weaker than in bulk.
However, not all TMDs exhibit additional Raman peaks [34] despite the laser energies being close to the absorption edges (first resonance: the optical excitation energy matches a vertical electronic transition). An additional requirement is the existence of excitons in the TMDs optical absorption spectrum, which are strongly coupled with specific phonon modes and resonate (the second resonance) with the electronic band structure. In MoS2, these excitons at 1.88 eV and 2.06 eV for bulk [29] and 1.85 and 1.97 eV for monolayer [27] are linked to the transitions at the K point of the Brillouin zone, between spin-orbit split states in valence band and a degenerate state in the conduction band [32]. Thus, double resonant Raman (DRR) scattering processes enhance the second-order (or multiphonon) Raman scattering modes [34,35,36].
For a wide range of materials, including TMDs [34], the observed multiphonon scattering peaks in Raman spectroscopy can generally be categorized into two types [36]:
- Overtone (for instance 2×), combination (sum) or difference bands of the phonons with zero momentum (at the Γ point of the Brillouin zone);
- Overtone, combination or difference bands of phonons with nonzero momentum (for example, at the M point of the Brillouin zone [35]).
Experimental data reveal that variation in the excitation energy within the A and B excitonic level range yield different DRR spectra [36,37,38]. Consequently, DRR processes select other electronic states, in the K and Q valleys, and pairs of phonons, which have opposite momenta in the case of overtone (2×). They can be located either within the interior or at the edge of the Brillouin zone [37,38]. However, some restrictions exist: at the M point, the phonon combinations with different inversion symmetry (g or u) are not allowed [36]. Another interesting aspect of the DRR process is that even if certain first-order phonons are not observed in the Raman spectrum, they can participate in the formation of multiphonon bands [37]. Also, it is worth noting that the intensities of these multiphonon bands vary with temperature.
Hexagonal 2H-MoS2 presents 18 branches of the phonon dispersion relation [36]. In the center of the Brillouin zone (Γ), their symmetries are as follows:
- E22g (degenerate), E1g (degenerate), E12g (degenerate), and A1g—four Raman-active modes;
- E11u (degenerate) and A12u—two IR-active modes;
- B22g, E2u (degenerate), B1u, and B12g—four silent modes;
- E21u (degenerate) and A22u—two acoustic modes.
At the M point of the Brillouin zone, all phonons (called zone-edge phonons) are nondegenerate [36]. For instance, the doubly degenerate Γ-point phonon with E1g symmetry, extends and splits into two distinct phonons, E1g (M1) and E1g (M2), at the M point. Here, there are also acoustic phonons (longitudinal LA, transverse TA, or out-of-plane transverse ZA) and quasi-acoustic optical phonons (LA’, TA’, or ZA’) [36].
At the K point, there are only two doubly degenerate phonons, Eug(K1) and Eug(K2), which originate from E1g(M1), E2u(M2), and E12g(M1), E11u(M2), respectively [36].
We have managed to assign all experimental Raman peaks in the (a1)–(a3), and (b) spectra, see Figure 4a and Table 1, using the frequencies of some first-order phonons in hexagonal 2H-MoS2 [36], as well as frequencies of second-order modes calculated based on the first-order phonon frequencies [36]. The frequencies of first-order phonons at Γ point were experimentally measured at 300 K [36]. The frequencies of first-order phonons at the M and K points, theoretically calculated at 0 K [36], were reduced by 2 cm−1 [36] to obtain their room temperature counterparts.

Table 1.
The experimental Raman shifts (Δῦexp) along with their corresponding symmetry assignments, as reported in previous studies [35,36,37], for the bands observed in the resonance Raman spectra of 2H-MoS2 nanosheets. The measurements were conducted at room temperature, in an air ambient environment, using the incident light wavelength of 632.8 nm. A van Hove singularity between K and M is denoted by “vHs”.
As can be seen in Table 1, most double resonant second-order Raman processes predominantly involve M-point phonons; those between K-point phonons are less common. This aligns with the widely accepted viewpoint that these bands originate from phonons with a wavevector q > 0. Interestingly, we note that in Table 1, some frequencies of the resonant second-order Raman processes occurring between Γ-point phonons (q0) align closely with the experimental values, mirroring observations in Ref. [36].
While the assignment of Raman bands is potentially error prone due to the accuracy of theoretical calculations, the Raman experiments carried out across different states of the same sample provide more reliable insights. Some studies indicate that the quality of the MoS2 nanosheets can be inferred from the DRR spectra. For instance, it has been demonstrated [37,39] that defects (such as vacancies, anti-site defects) and strain (whether tensile or compressive), which arise in high quality MoS2 monolayers obtained by mechanical exfoliation of a 2H-MoS2 crystal, trigger the emergence of the Raman bands (termed “defect-activated peaks”) in the frequencies range below E12g(Γ). In the absence of such defects, this spectrum region has no Raman peaks, such as in case of the pristine samples. Furthermore, the intensity of defect-activated peaks is inversely proportional to L2D (where LD denotes the average inter-defect distance [39]). These observations likely hold true for nanosheets with thickness of a few layers.
We can find similarities in the positions of the defect-activated peaks for monolayers with LD ~ 2.2 nm [39] with those in our Raman spectra, barring the difference in intensity and full width at half maxima. In the defective monolayers, the DRR process involves a single phonon, the momentum conservation being achieved by elastic scattering of the excited electron on a defect [39]. Our nanosheets exhibit a few Raman peaks (at 147, 177, 187, 225, 247, and 375 cm−1) to which a single phonon can be assigned. However, as indicated in Table 1, two-phonon assignment is also possible. Additionally, certain Raman peaks (at 121 and 350 cm−1) can be exclusively explained by a two-phonon assignment. While these findings add complexity to the process of quantifying the crystallinity of the MoS2 nanosheets, they also distinctly point to the presence of defects within their structure.
An intriguing peak is the narrow one from 247 cm−1, exhibiting an intensity (I247) comparable to that of the peak at 225 cm−1 (IvHs). The ratios I2×vHs/I247 in the (a1)–(a3) and (b) spectra are 8.2, 7.4, 7.7, and 10.2, respectively. To the best of our knowledge, only one study reports this peak in Raman spectra of the MoS2 fullerene-like structures [29], where its intensity is considerably lower. Thus, this peak likely emerges due to a substantially higher concentration of topological defects in nanosheets compared to MoS2 fullerene-like structures. Such topological defects are identified as Stone–Wales type [40]. Such a defect appears through the topological transformation of four adjacent six-membered rings (each composed of three Mo and three S atoms) into two five-membered rings (FmR) and two seven-membered rings (SmR). One of the two FmR is composed of three Mo and two S atoms, and the other one is consisting of two Mo and three S atoms. One SmR includes an extra sulfur atom and the other SmR includes an additional Mo atom. In fullerene-like structures, the topological defects are five-membered rings, each of them being surrounded by five six-membered rings [41]. In Table 1, for the 247 cm−1 peak, a single phonon assignment could be E22g(K2) = LA’(K) (at 235 cm−1), while a two-phonon assignment, A1g(M) − E22g(M1) at 252 cm−1, presents a closer match. Despite this, we propose that the single phonon assignment is more likely. Consequently, microzone (b) appears to have the lowest concentration of topological defects.
Another focal point for discussion is the Raman band (2 × vHs) at 449 cm−1, which is a combination between the van Hove singularities (vHs) of two branches, between K and M, that fall around the same frequency (225 cm−1) [36]. According to other reports [37,39], its intensity (I2×vHs) decreases with increasing defect concentration in the MoS2 monolayer, whereas the intensity of the first-order peak (IvHs) increases. In spectra (a1)–(a3), and (b) in Figure 4a, the ratios I2×vHs/IvHs decrease as 9, 8.4, 7.3, and 4.6, respectively. This implies an increase in defect concentration (other than topological defects), peaking in zone (b).
4. Discussion
Two-dimensional crystalline materials, being inherently anisotropic, can grow, in relation to the substrate, with molecular layers aligned in two complementary directions: horizontal and vertical [42].
For applications in nanoelectronics [43] and optoelectronics [44], the horizontal orientation of molecular layers is often preferred due to the need for high crystallinity (very low defect density) and large surface areas. However, this configuration tends to make the surface of these 2D materials catalytically inactive, as only the sulfur atoms along their edges exhibit significant catalytic activity [45]. Therefore, maximizing the number of active sites is crucial for catalytic applications. Strategies such as creating large quantities of small nanosheets or engineering defective surfaces are considered [45,46,47,48].
In the case of the vertical morphology, the active edge sites are naturally and abundantly present at the edges of the MoS2 layers [42]. Kumara et al. [49] successfully grew vertically aligned MoS2 flakes on top of horizontally aligned monolayer/bilayer MoS2 sheets using conventional CVD methods.
These approaches demonstrate how different growth orientations of 2D materials can be strategically employed to enhance specific properties which are important in various technological applications.
In this study, using space-confined chemical vapor deposition, the objective to maximize the number of active edge sites in horizontally aligned 2H-MoS2 nanosheets is achieved due to the abundant presence on their surface of smaller, horizontally aligned MoS2 flakes. Undoubtedly, various point defect species [26], also catalytically active [50], are present in different concentrations within the surface of both MoS2 nanosheets and flakes, as Raman spectroscopy revealed. The edge sites are abundantly present in our samples and their catalytic activity has been proven [51,52].
The nanosheets consist of the 2H-MoS2 phase (according to the Raman spectra). The 1T-MoS2 phase appears to be absent, indicated by the lack of its characteristic peaks at 156 and 333 cm−1 [53]. The growth mechanism follows a layer-plus-island (Stranski–Krastanov) model: an initial multilayer structure comprising of two to three layers forms, on top of which additional flakes grow, varying in abundance and regularity. Two factors influence this growth process as follows: (i) the presence of chlorine vapors which react with the MoSx groups (x < 2) at the edges of the MoS2 source nanoparticles to form MoSyClz (0 < y ≤ x < 2) vapors. These vapors condense on the growth substrate, forming small nuclei with high mobility which continually attach to the edges of the growing MoS2 nanosheets [2]. The chlorine is then removed through a self-purification process; and (ii) the extremely low amount of source materials, specific to the microreactor method, causes a significant decrease in the MoSyClz vapor pressure towards the end of the deposition time. This reduction in vapor pressure leads to the decrease in the growth rate, resulting in the formation of MoS2 flakes on top of the previously formed nanosheets.
The entire surface of the substrate (or electrode) can be completely coated with a few-layer horizontally aligned MoS2 nanosheets decorated with MoS2 flakes, through the appropriate increase of the deposition parameters (deposition time and amount of source materials).
5. Conclusions
We report a route that uses a microreactor, the extreme case of space-confined chemical vapor deposition, that can coat a substrate (or an electrode) with few-layer horizontally aligned 2H-MoS2 nanosheets, decorated with a varying amount of regular or defective MoS2 flakes. This method significantly increases the concentration of catalytically active edge sites; more precisely, the undercoordinated atoms located at the edges of the MoS2 flakes. The growth mechanism follows the layer-plus-island (Stranski–Krastanov) model, as revealed by optical, AFM, and SEM images. EDX and double-resonance Raman (DRR) spectra indicate the presence of defects (vacancies, anti-site defects, and topological defects) and strain (tensile or compressive) in varying concentrations across all nanosheets and flakes, in addition to the undercoordinated edge atoms. Resonant second-order Raman processes predominantly involve M-point phonons, but K-point or Γ-point phonons also align well with the experimental values. DRR bands at 147, 177, 187, 225, 247, and 375 cm−1 can be assigned to a single phonon process facilitated by elastic scattering of excited electrons on defects, though two-phonon assignments are also feasible. Raman peaks at 121 and 350 cm−1 can only be explained by two-phonon assignments. The narrow peak at 247 cm−1 is postulated to be a topological defect-activated peak, which is a rare finding in the literature. These results are significant to develop efficient photocatalysts (photoanode) made by low-cost, environmentally friendly, stable materials, for hydrogen production by water splitting using only sunlight as an energy source.
6. Patents
Florinel Sava, Alin Velea, Angel Theodor Buruiana, Claudia Mihai, Elena Matei, Teddy Tite have patent pending to State Office for Inventions and Trademarks—Romania, resulting from the work reported in this manuscript.
Author Contributions
Conceptualization, F.S.; methodology, F.S. and A.V.; validation, F.S.; formal analysis, F.S.; investigation, A.-T.B., E.M., T.T. and F.S.; resources, A.V.; writing—original draft preparation, F.S., A.V. and C.M.; writing—review and editing, F.S., A.V. and C.M.; visualization, F.S.; project administration, A.V.; funding acquisition, F.S. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Core Program of the National Institute of Materials Physics, granted by the Romanian Ministry of Research, Innovation and Digitization through the Project PC3-PN23080303 and contract no. 21N/2019, additional act 11/2022. The authors kindly acknowledge the financial support of the Executive Unit for the Financing of Higher Education, Research, Development and Innovation (UEFISCDI) in the framework of the M-ERA.NET 109/2019 (ERANET-M.-2D-SPIN-MEM).
Data Availability Statement
All data generated or analyzed during this study are contained in this article.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Florinel Sava, Alin Velea, Angel Theodor Buruiana, Claudia Mihai, Elena Matei, and Teddy Tite have patent application to the State Office for Inventions and Trademarks—Romania.
References
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lin, J.; Huang, X.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.; Lei, J.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Yoffe, A.D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ji, H.; Chou, H.; Li, Q.; Li, H.; Suk, J.W.; Piner, R.; Liao, L.; Cai, W.; Ruoff, R.S. Millimeter-Size Single-Crystal Graphene by Suppressing Evaporative Loss of Cu During Low Pressure Chemical Vapor Deposition. Adv. Mater. 2013, 25, 2062–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gan, L.; Wang, D.; Li, H.; Zhai, T. Space-confined vapor deposition synthesis of two dimensional materials. Nano Res. 2018, 11, 2909–2931. [Google Scholar] [CrossRef]
- O’Brien, M.; McEvoy, N.; Hallam, T.; Kim, H.-Y.; Berner, N.C.; Hanlon, D.; Lee, K.; Coleman, J.N.; Duesberg, G.S. Transition Metal Dichalcogenide Growth via Close Proximity Precursor Supply. Sci. Rep. 2014, 4, 7374. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; McEvoy, N.; Hanlon, D.; Hallam, T.; Coleman, J.N.; Duesberg, G.S. Mapping of Low-Frequency Raman Modes in CVD-Grown Transition Metal Dichalcogenides: Layer Number, Stacking Orientation and Resonant Effects. Sci. Rep. 2016, 6, 19476. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hashimoto, R.; Misawa, M.; Liu, Y.; Kishibuchi, M.; Ishimura, K.; Tsuruta, K.; Miyata, Y.; Hayashi, Y. Surface diffusion-limited growth of large and high-quality monolayer transition metal dichalcogenides in confined space of microreactor. ACS Nano 2022, 16, 11360–11373. [Google Scholar] [CrossRef]
- Cong, C.; Shang, J.; Wu, X.; Cao, B.; Peimyoo, N.; Qiu, C.; Sun, L.; Yu, T. Synthesis and Optical Properties of Large-Area Single-Crystalline 2D Semiconductor WS2 Monolayer from Chemical Vapor Deposition. Adv. Opt. Mater. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Kang, K.; Godin, K.; Kim, Y.D.; Fu, S.; Cha, W.; Hone, J.; Yang, E.-H. Graphene-Assisted Antioxidation of Tungsten Disulfide Monolayers: Substrate and Electric-Field Effect. Adv. Mater. 2017, 29, 1603898. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kang, K.; Chen, S.; Du, R.; Yang, E.-H. Location-specific growth and transfer of arrayed MoS2 monolayers with controllable size. 2D Mater. 2017, 4, 025093. [Google Scholar] [CrossRef]
- Fu, S.; Kang, K.; Shayan, K.; Yoshimura, A.; Dadras, S.; Wang, X.; Zhang, L.; Chen, S.; Liu, N.; Jindal, A.; et al. Enabling room temperature ferromagnetism in monolayer MoS2 via in situ iron-doping. Nat. Commun. 2020, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, H.; Xiao, S.; Wan, X.; Ni, Z.; Gu, X.; Ostrikov, K. Shape-Uniform, High-Quality Monolayered MoS2 Crystals for Gate-Tunable Photoluminescence. ACS Appl. Mater. Interfaces 2017, 9, 42121–42130. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.K.; Deb, S.; Singh, B.P.; Vasa, P.; Dhar, S. Strictly monolayer large continuous MoS2 films on diverse substrates and their luminescence properties. Appl. Phys. Lett. 2016, 108, 042101. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Shi, L.; Nan, H.; Wan, X.; Gu, X.; Ni, Z.; Ostrikov, K. Large-size Mo1-xWxS2 and W1-xMoxS2 (x = 0–0.5) monolayers by confined-space chemical vapor deposition. Appl. Surf. Sci. 2018, 457, 591–597. [Google Scholar] [CrossRef]
- Mahjouri-Samani, M.; Tian, M.; Wang, K.; Boulesbaa, A.M.; Rouleau, C.; Puretzky, A.A.; McGuire, M.A.; Srijanto, B.R.; Xiao, K.; Eres, G.; et al. Digital Transfer Growth of Patterned 2D Metal Chalcogenides by Confined Nanoparticle Evaporation. ACS Nano 2014, 8, 11567–11575. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, D.; Chang, C.S.; Seo, S.; Hu, Y.; Cha, S.; Kim, H.; Shin, J.; Lee, J.-H.; Lee, S.; et al. Non-epitaxial single-crystal 2D material growth by geometric confinement. Nature 2023, 614, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Stoica, T.; Stoica, M.; Duchamp, M.; Tiedemann, A.; Mantl, S.; Grützmacher, D.; Buca, D.; Kardynał, B.E. Vapor transport growth of MoS2 nucleated on SiO2 patterns and graphene flakes. Nano Res. 2016, 9, 3504–3514. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, Y.; Hong, J.; Zhang, M.; Duan, W.; Mao, N.; Wu, J.; Xu, H.; Dong, F.; Lin, F.; et al. Growth of Large-Area 2D MoS2(1−x)Se2x Semiconductor Alloys. Adv. Mater. 2014, 26, 2648–2653. [Google Scholar] [CrossRef]
- Wu, S.; Huang, C.; Aivazian, G.; Ross, J.S.; Cobden, D.H.; Xu, X. Vapor-Solid Growth of High Optical Quality MoS2 Monolayers with Near-Unity Valley Polarization. ACS Nano 2013, 7, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Catalán-Gómez, S.; Garg, S.; Redondo-Cubero, A.; Gordillo, N.; de Andrés, A.; Nucciarelli, F.; Kim, S.; Kung, P.; Pau, J.L. Photoluminescence enhancement of monolayer MoS2 using plasmonic gallium nanoparticles. Nanoscale Adv. 2019, 1, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.K.; Singh, E.; Viana, B.C.; Gao, J.; Luo, J.; Li, J.; Lin, Z.; Elías, A.L.; Shi, Y.; Wang, Z.; et al. Wetting of mono and few-layered WS2 and MoS2 films supported on Si/SiO2 substrates. ACS Nano 2015, 9, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Venkata Subbaiah, Y.P.; Saji, K.J.; Tiwari, A. Atomically Thin MoS2: A Versatile Nongraphene 2D Material. Adv. Funct. Mater. 2016, 26, 2046–2069. [Google Scholar] [CrossRef]
- Trainer, D.J.; Nieminen, J.; Bobba, F.; Wang, B.; Xi, X.; Bansil, A.; Iavarone, M. Visualization of defect induced in-gap states in monolayer MoS2. NPJ 2D Mater. Appl. 2022, 6, 13. [Google Scholar] [CrossRef]
- Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Gu, L.; Mao, N.; Feng, Q.; Xie, L.; et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Liang, F.; Xu, H.; Wu, X.; Wang, C.; Luo, C.; Zhan, J. Raman spectroscopy characterization of two-dimensional materials. Chin. Phys. B 2018, 27, 037802. [Google Scholar] [CrossRef]
- Frey, G.L.; Tenne, R.; Matthews, M.J.; Dresselhaus, M.S.; Dresselhaus, G. Raman and resonance Raman investigation of MoS2 nanoparticles. Phys. Rev. B 1999, 60, 2883. [Google Scholar] [CrossRef]
- Cortijo-Campos, S.; Prieto, C.; De Andrés, A. Size Effects in Single- and Few-Layer MoS2 Nanoflakes: Impact on Raman Phonons and Photoluminescence. Nanomaterials 2022, 12, 1330. [Google Scholar] [CrossRef]
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic properties of bulk and monolayer TMDs: Theoretical study within DFT framework (GVJ-2e method). Phys. Status Solidi A 2017, 12, 1700218. [Google Scholar] [CrossRef]
- Roldan, R.; Silva-Guillen, J.A.; Lopez-Sancho, M.P.; Guinea, F.; Cappelluti, E.; Ordejon, P. Electronic properties of single-layer and multilayer transition metal dichalcogenides MX2 (M = Mo, W and X = S, Se). Ann. Phys. 2014, 526, 347–357. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.M.; Hodul, D.T. Raman spectra of IVb and VIb transition metal disulfides using laser energies near the absorption edges. Phys. Chem. Solids 1985, 46, 405–409. [Google Scholar] [CrossRef]
- Xia, M.; Li, B.; Yin, K.; Capellini, G.; Niu, G.; Gong, Y.; Zhou, W.; Ajayan, P.M.; Xie, Y.-H. Spectroscopic Signatures of AA’ and AB Stacking of Chemical Vapor Deposited Bilayer MoS2. ACS Nano 2015, 9, 12246–12254. [Google Scholar] [CrossRef] [PubMed]
- Livneh, T.; Spanier, J.E. A comprehensive multiphonon spectral analysis in MoS2. 2D Mater. 2015, 2, 035003. [Google Scholar] [CrossRef]
- Carvalho, B.R.; Wang, Y.; Mignuzzi, S.; Roy, D.; Terrones, M.; Fantini, C.; Crespi, V.H.; Malard, L.M.; Pimenta, M.A. Intervalley scattering by acoustic phonons in two-dimensional MoS2 revealed by double-resonance Raman spectroscopy. Nat. Commun. 2017, 8, 14670. [Google Scholar] [CrossRef]
- Pimenta, M.A.; del Corro, E.; Carvalho, B.R.; Fantini, C.; Malard, L.M. Comparative Study of Raman Spectroscopy in Graphene and MoS2-type Transition Metal Dichalcogenides. Acc. Chem. Res. 2015, 48, 41–47. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef]
- Popescu, M.; Sava, F.; Lőrinczi, A.; Velea, A. Disorder in order: Silicon versus graphene. Phys. Status Solidi B 2013, 250, 1008–1010. [Google Scholar] [CrossRef]
- Popescu, M.; Sava, F.; Lőrinczi, A.; Velea, A.; Simandan, I.D. Nanocarbon embedded chalcogenides. Onion-like model. J. Optoelectron. Adv. Mater. 2011, 13, 1450–1453. [Google Scholar]
- Jung, Y.; Shen, J.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal seed layer thickness-induced transition from vertical to horizontal growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Li, Y.; Yun, T.; Li, Y.; Gui, T.; Yu, W.; Mu, H.; Cui, N.; Chen, W.; Lin, S. Triply degenerate semimetal PtBi2 as van der Waals contact interlayer in two-dimensional transistor. Mater. Futures 2024, 3, 025302. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, Z.-Q.; Li, D.; Zhang, J.; Aharonovich, I.; Zhang, Y. Two-Dimensional Hexagonal Boron Nitride for Building Next-Generation Energy-Efficient Devices. ACS Energy Lett. 2021, 6, 985–996. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotech 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Anbalagan, A.k.; Hu, F.-C.; Chan, W.K.; Gandhi, A.C.; Gupta, S.; Chaudhary, M.; Chuang, K.-W.; Ramesh, A.K.; Billo, T.; Sabbah, A.; et al. Gamma-Ray Irradiation Induced Ultrahigh Room-Temperature Ferromagnetism in MoS2 Sputtered Few-Layered Thin Films. ACS Nano 2023, 17, 6555–6564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Habib, M.R.; Khan, A.; Chen, P.-C.; Murugesan, T.; Gupta, S.; Anbalagan, A.k.; Tai, N.-H.; Lee, C.-H.; Lin, H.-N. Sulfur Monovacancies in Liquid-Exfoliated MoS2 Nanosheets for NO2 Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 9459–9470. [Google Scholar] [CrossRef]
- Kumara, P.; Viswanath, B. Horizontally and vertically aligned growth of strained MoS2 layers with dissimilar wetting and catalytic behaviors. CrystEngComm 2017, 19, 5068–5078. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Shao, G.; Xiang, H.; Li, Z.; Jin, Y.; Chen, Y.; Jiang, H.; Li, H.; Shui, J.; et al. Activating the Electrocatalysis of MoS2 Basal Plane for Hydrogen Evolution via Atomic Defect Configurations. Small 2022, 18, 2200601. [Google Scholar] [CrossRef]
- Bruix, A.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Andersen, M.; Porsgaard, S.; Besenbacher, F.; Hammer, B.; Lauritsen, J.V. In Situ Detection of Active Edge Sites in Single-Layer MoS2 Catalysts. ACS Nano 2015, 9, 9322–9330. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Feng, C.; Xu, G.; Xie, C.; Yuan, X.; Xiang, B. MoS2 nanosheet/MoS2 flake homostructures for efficient electrocatalytic hydrogen evolution. Mater. Res. Express 2019, 6, 085005. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of Intercalated Metals on the Electrocatalytic Activity of 1T-MoS2 for the Hydrogen Evolution Reaction. ACS Energy Lett. 2018, 3, 7–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).