Influence of Amino Acids on Calcium Oxalate Precipitation in Systems of Different Chemical Complexity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spontaneous Precipitation of Calcium Oxalate
- (a)
- Simple system: composed of only the constituent ions (calcium and oxalate) and the respective counter ions (salts: CaCl2 and Na2C2O4).
- (b)
- NaCl system: composed of the constituent ions (calcium and oxalate) and the respective counter ions and the sodium and chloride ions used to adjust the ionic strength to approximately 0.3 mol dm−3 (salts: CaCl2, Na2C2O4 and NaCl).
- (c)
- Artificial urine system: composed of the constituent ions (calcium and oxalate) and the respective counter ions and other components that mimic the composition of the urine (Table S1). The artificial urine components were selected according to the protocol suggested by Burns and Finlayson [31] (salts: CaCl2, Na2C2O4, Na2SO4, KCl, NH4Cl, NH4OH, MgSO4·7H2O and NaCl). However, Na2HPO4 was excluded from the original protocol because phosphate salts can coprecipitate under the applied experimental conditions. In addition, the citrate ions, in the form of sodium citrate, have also been omitted, since it is known that they promote the formation of COD [20].
2.2. Experimental Methods
3. Results
3.1. Spontaneous Precipitation of Calcium Oxalate in the Systems with the Addition of Amino Acids
3.1.1. FTIR and PXRD Characterization of Precipitates
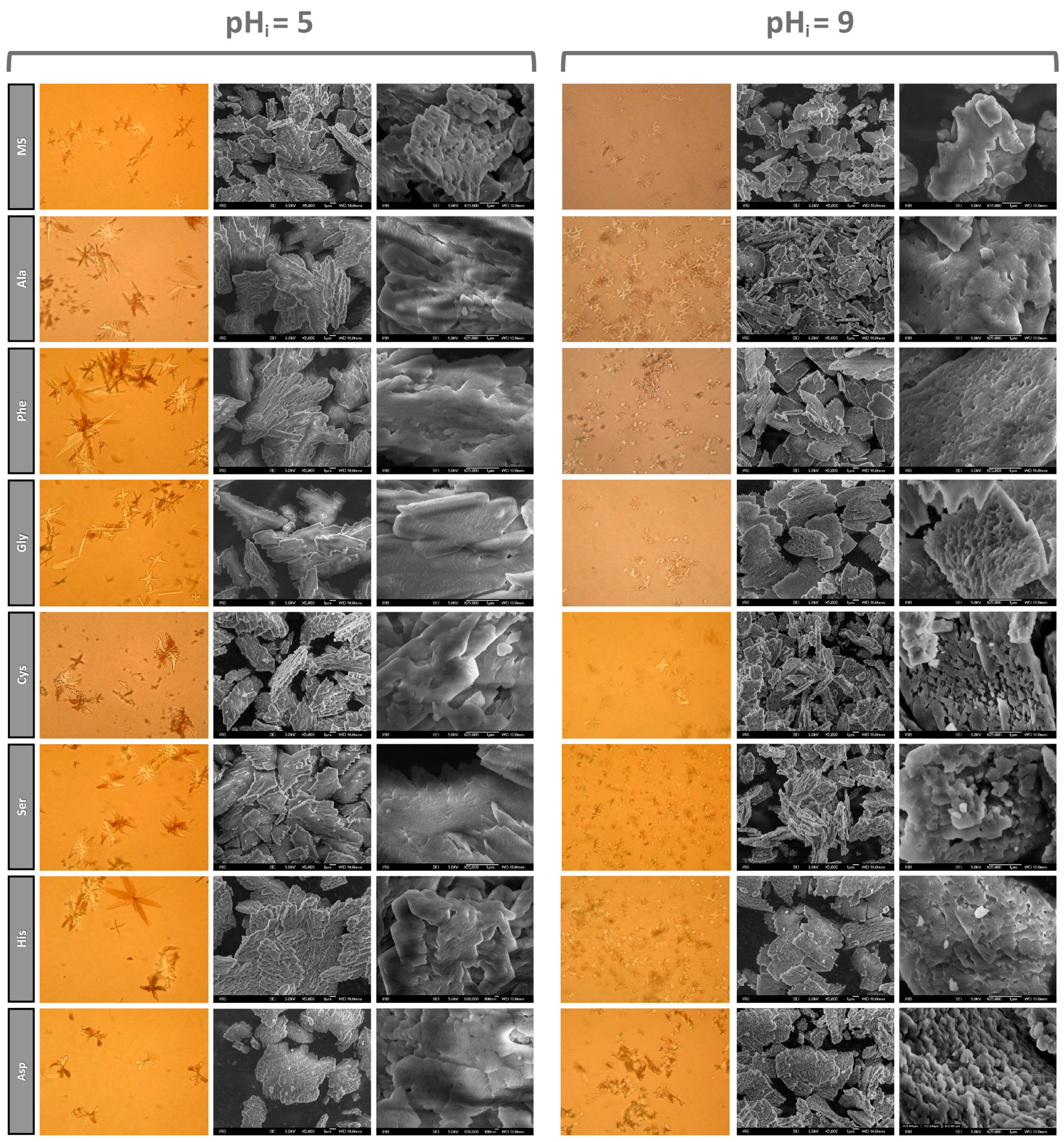
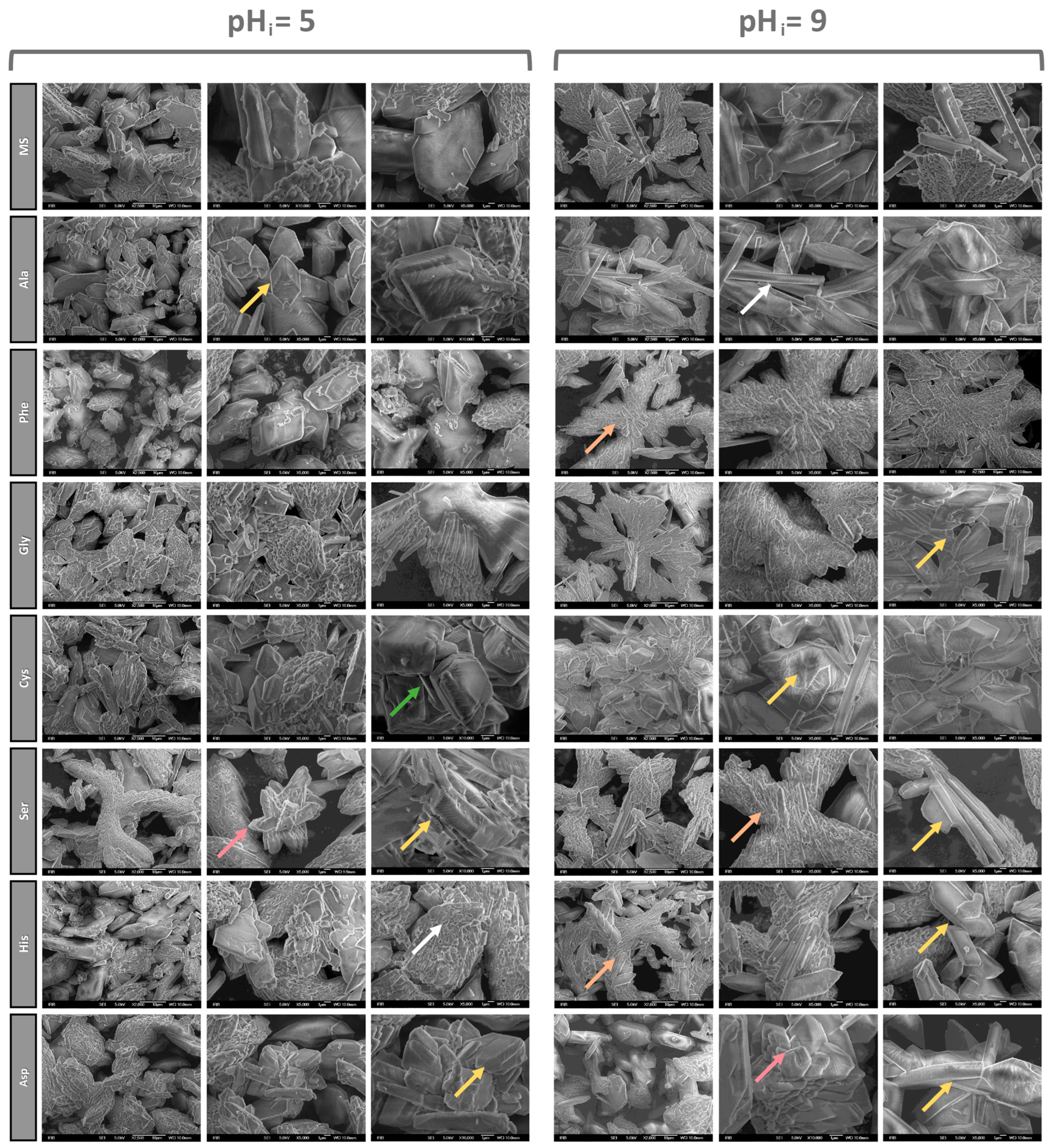
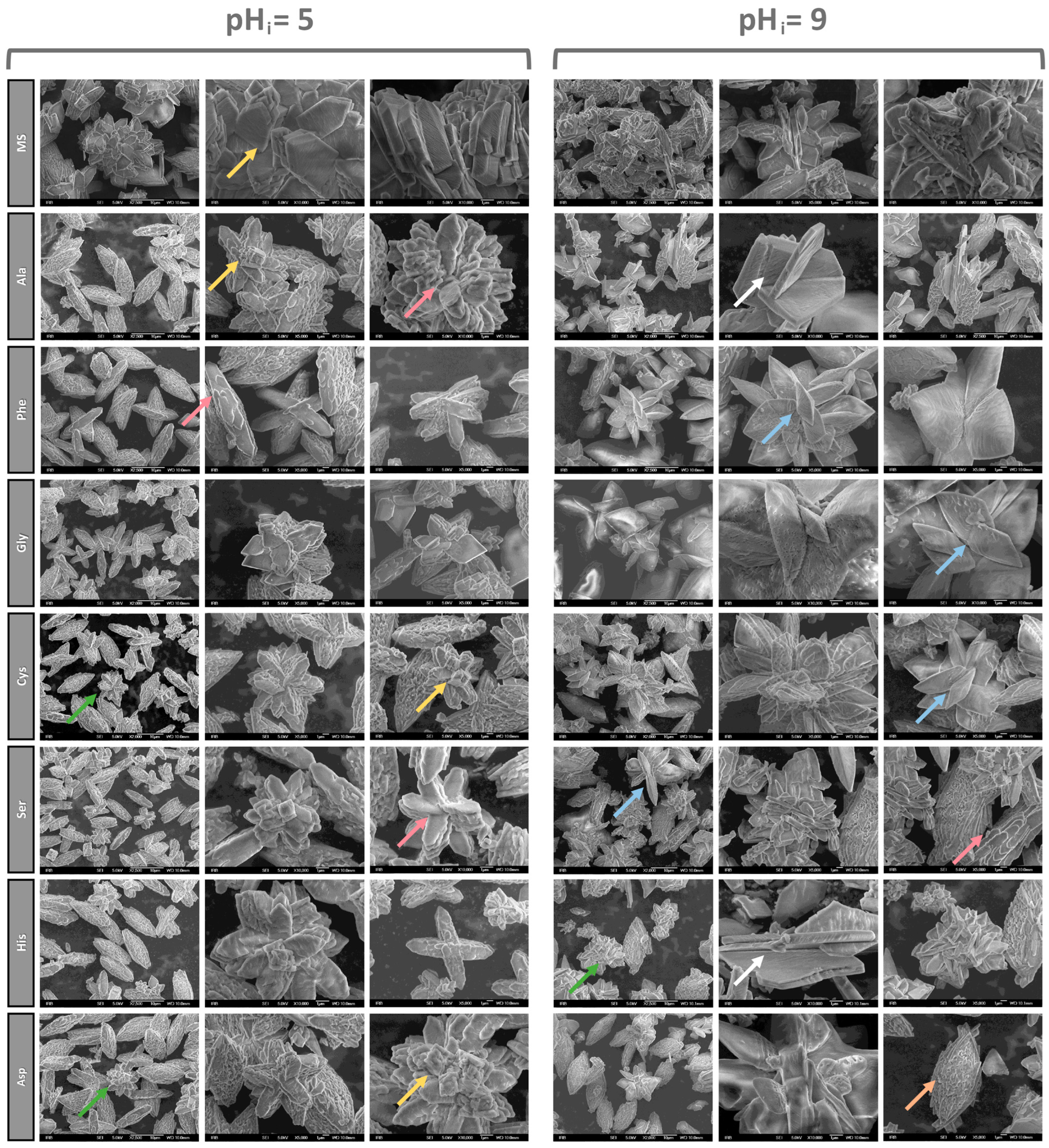
3.1.2. SEM Characterization of Precipitates
3.1.3. Effect of Amino Acids on the Habitus of COM Crystals
4. Conclusions
- The addition of AAs does not affect the composition of the solid phase in the simple and NaCl systems but shows an effect in the artificial urine. In the simple and NaCl systems at both pHi, and the artificial urine at pHi = 5.0, only COM precipitated. In the artificial urine at pHi = 9.0, the formation of COD was observed. The maximum content of COD was obtained in the systems with the addition of polar AAs and Phe (w(COD): Gly 65.2 % > Phe 59.2 % > Cys 28.7 % > Ser 23.5%). The effect can be explained by the fact that all the AAs at pHi = 9.0 were in the anionic form, so the electrostatic interactions of the AAs with the COM crystals, complemented by the additional hydrogen bonding of the side chains, inhibit its growth and consequently promote COD formation.
- The addition of AAs affects the size of the COM crystallites and the most significant impact was in the simple system. In the artificial urine system, the addition of AAs did not significantly affect the crystallite size.
- The addition of AAs affects the growth of COM crystals in all the investigated systems (simple, NaCl and artificial urine) at both pHi. The most pronounced effect in the simple and NaCl systems was observed in the case of Asp, which can be attributed to the presence of a side-charged group and stronger interaction with the COM surface. In the artificial urine, the most pronounced effects were in the presence of the polar AAs and Phe, where the growth of the (020) plane was promoted.
- In the NaCl and artificial urine systems, crystal aggregation was observed. The AAs affect the morphology of the COM particles, so at pHi = 5.0 in all the systems, the COM crystals have relatively smooth surfaces but their edges are rounded, stacked and regularly distributed. The combination of AAs and pHi = 9.0 gave rise to a porous structure on the COM crystal surfaces that is visualized as a slide of elongated primary crystals with roughly “protruding” crystallites.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campodoni, E.; Montanari, M.; Artusi, C.; Bassi, G.; Furlani, F.; Montesi, M.; Panseri, S.; Sandri, M.; Tampieri, A. Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials. J. Compos. Sci. 2021, 5, 278. [Google Scholar] [CrossRef]
- Scotland, K.B.; Wong, G.C.; Matusik, K.; Lun, M.C.; Gul, S.; Su, F.; Vine, D.; Gelb, J.; Yun, W. Understanding 3D Biomineralization in Human Kidney Stones with Correlative X-ray Micro-CT & X-ray Fluorescence Microscopy. Microsc. Microanal. 2022, 28, 288–289. [Google Scholar] [CrossRef]
- Tsolaki, E.; Bertazzo, S. Pathological Mineralization: The Potential of Mineralomics. Materials 2019, 12, 3126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraro, P.M.; Bargagli, M.; Trinchieri, A.; Gambaro, G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients 2020, 112, 779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coello, I.; Sanchis, P.; Pieras, E.C.; Grases, F. Diet in Different Calcium Oxalate Kidney Stones. Nutrients 2023, 15, 2607. [Google Scholar] [CrossRef]
- Chen, T.; Qian, B.; Zou, J.; Luo, P.; Zou, J.; Li, W.; Chen, Q.; Zheng, L. Oxalate as a potent promoter of kidney stone formation. Front. Med. 2023, 10, 1159616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Molec. Medic. 2021, 48, 149. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Burgos-Cara, A.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Cölfen, H.; Rodriguez-Navarro, C. A non-classical view on calcium oxalate precipitation and the role of citrate. Nat. Commun. 2017, 8, 768. [Google Scholar] [CrossRef]
- Kumar, V.; Lieske, J.C. Protein regulation of intrarenal crystallization. Curr. Opin. Nephrol. Hypertens. 2006, 15, 374–380. [Google Scholar] [CrossRef]
- Schubert, G. Stone analysis. Urol. Res. 2006, 34, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Olinger, E.G.; Bargagli, M.; Geraghty, R.; Taylor, L.; Nater, A.; Bruggmann, R.; Sayer, J.A.; Vogt, B.; Schaller, A.; et al. Prevalence and characteristics of genetic disease in adult kidney stone formers. Nephrol. Dial. Transplant. 2024, gfae074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, N.N.; Chandra Gupta, P.; Verma, R.; Yadav, V. An Update on Kidney Stones: Types, Mechanism and Treatment Approaches. Res. J. Pharmacogn. Phytochem. 2023, 15, 53–62. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Thongboonkerd, V. Prospects for proteomics in kidney stone disease. Expert. Rev. Proteom. 2017, 14, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Bramley, A.S.; Hounslow, M.J.; Ryall, R.L. Aggregation during precipitation from solution. Kinetics for calcium oxalate monohydrate. Chem. Eng. Sci. 1997, 52, 747–757. [Google Scholar] [CrossRef]
- Pitt, K.; Mitchell, G.P.; Ray, A.; Heywood, B.R.; Hounslow, M.J. Micro-mechanical model of calcium oxalate monohydrate aggregation in supersaturated solutions: Effect of crystal form and seed concentration. J. Cryst. Growth 2012, 361, 176–188. [Google Scholar] [CrossRef]
- Conti, C.; Casati, M.; Colombo, C.; Realini, M.; Brambilla, L.; Zerbi, G. Phase transformation of calcium oxalate dihydrate—Monohydrate: Effects of relative humidity and new spectroscopic data. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Ackermann, D.; Finlayson, B. Calcium oxalate dihydrate (weddellite) precipitation. J. Cryst. Growth 1989, 98, 285–292. [Google Scholar] [CrossRef]
- Šter, A.; Šafranko, S.; Bilić, K.; Marković, B.; Kralj, D. The effect of hydrodynamic and thermodynamic factors and the addition of citric acid on the precipitation of calcium oxalate dihydrate. Urolithiasis 2018, 46, 243–256. [Google Scholar] [CrossRef]
- Stanković, A.; Kontrec, J.; Džakula, B.N.; Kovačević, D.; Marković, B.; Kralj, D. Preparation and characterization of calcium oxalate dihydrate seeds suitable for crystal growth kinetic analyses. J. Cryst. Growth 2018, 500, 91–97. [Google Scholar] [CrossRef]
- Škrtić, D.; Marković, M.; Komunjer, L.; Füredi-Milhofer, H. Precipitation of calcium oxalates from high ionic strength solutions: I. Kinetics of spontaneous precipitation of calcium oxalate trihydrate. J. Cryst. Growth 1984, 66, 431–440. [Google Scholar] [CrossRef]
- Škrtić, D.; Füredi-Milhofer, H.; Marković, M. Precipitation of calcium oxalates from high ionic strength solutions: V. The influence of precipitation conditions and some additives on the nucleating phase. J. Cryst. Growth 1987, 80, 113–120. [Google Scholar] [CrossRef]
- Werner, H.; Bapat, S.; Schobesberger, M.; Segets, D.; Schwaminger, S.P. Calcium Oxalate Crystallization: Influence of pH, Energy Input, and Supersaturation Ratio on the Synthesis of Artificial Kidney Stones. ACS Omega 2021, 6, 26566–26574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brečević, L.; Škrtić, D.; Garside, J. Transformation of calcium oxalate hydrates. J. Cryst. Growth 1986, 74, 399–408. [Google Scholar] [CrossRef]
- Ibis, F.; Dhand, P.; Suleymanli, S.; van der Heijden, A.E.D.M.; Kramer, H.J.M.; Eral, H.B. A Combined Experimental and Modelling Study on Solubility of Calcium Oxalate Monohydrate at Physiologically Relevant pH and Temperatures. Crystals 2020, 10, 924. [Google Scholar] [CrossRef]
- Brečević, L.; Kralj, D.; Garside, J. Factors influencing the distribution of hydrates in calcium oxalate precipitation. J. Cryst. Growth 1989, 97, 460–468. [Google Scholar] [CrossRef]
- Walton, R.C.; Kavanagh, J.P.; Heywood, B.R.; Rao, P.N. Calcium oxalates grown in human urine under different batch conditions. J. Cryst. Growth 2005, 284, 517–529. [Google Scholar] [CrossRef]
- Baumann, J.M.; Affolter, B.; Siegrist, H.-P. Measurement of metastability, growth and aggregation of calcium oxalate in native urine. A new approach for clinical and experimental stone research. Urol. Int. 1997, 59, 214–220. [Google Scholar] [CrossRef]
- Robert, M.; Boularan, A.M.; Colette, C.; Averous, M.; Monnier, M. Urinary calcium oxalate saturation in ‘stone formers’ and normal subjects: An application of the Equil2 program. British J. Urol. 1994, 73, 358–361. [Google Scholar] [CrossRef]
- Christmas, K.G.; Gower, L.B.; Khan, S.R.; El-Shall, H. Aggregation and dispersion characteristics of calcium oxalate monohydrate: Effect of urinary species. J. Colloid. Interface Sci. 2002, 256, 168–174. [Google Scholar] [CrossRef]
- Burns, J.R.; Finlayson, B. A proposal for a standard reference artificial urine in vitro urolithiasis experiments. Investig. Urol. 1980, 18, 167–169. [Google Scholar] [PubMed]
- Isaacson, L.C. Urinary composition in calcific nephrolithiasis. Investig. Urol. 1969, 6, 356–363. [Google Scholar] [PubMed]
- Doremus, R.H.; Teich, S.; Silvis, P.X. Crystallization of calcium oxalate from synthetic urine. Investig. Urol. 1978, 15, 469–472. [Google Scholar] [PubMed]
- Miller, J.D.; Randolph, A.D.; Drach, G.W. Observations upon calcium oxalate crystallization kinetics in simulated urine. J. Urol. 1977, 117, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.M.; Pallante, S.L.; Eisenberg, H.; Joule, J.A.; Becker, G.L.; Howard, J.E. Simple synthetic and natural urines have equivalent anticalcifying properties. Investig. Urol. 1974, 12, 79–81. [Google Scholar] [PubMed]
- Rose, M.B. Renal stone formation. The inhibitory effect of urine on calcium oxalate precipitation. Investig. Urol. 1975, 12, 428–433. [Google Scholar] [PubMed]
- Hassan, A.I.; Saleh, H.M. Production of Amino Acids and Nucleic Acids from Genetically Engineered Microbial Cells and their Relevance to Biodegradation. Green Energy Environ. Technol. 2023, 2, 1–47. [Google Scholar] [CrossRef]
- Primiano, A.; Persichilli, S.; Ferraro, P.M.; Calvani, R.; Biancolillo, A.; Marini, F.; Picca, A.; Marzetti, E.; Urbani, A.; Gervasoni, J. A Specific Urinary Amino Acid Profile Characterizes People with Kidney Stones. Dis. Markers. 2020, 2020, 8848225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, D.E.; van Bronswijk, W.; Ryall, R.L. A comparative study of the adsorption of amino acids on to calcium minerals found in renal calculi. Clinical Sci. 2001, 101, 159–168. [Google Scholar] [CrossRef]
- Guo, S.; Ward, M.D.; Wesson, J.A. Direct visualization of calcium oxalate monohydrate crystallization and dissolution with atomic force microscopy and the role of polymeric additives. Langmuir 2002, 18, 4284–4291. [Google Scholar] [CrossRef]
- Marković, M.; Komunjer, L.; Füredi-Milhofer, H.; Škrtić, D.; Sarig, S. Precipitation of calcium oxalate from high ionic strength solutions VII. The influence of glutamic acid. J. Cryst. Growth 1988, 88, 118–124. [Google Scholar] [CrossRef]
- Taranets, X.Y.; Dryhailo, M.K.; Bezkrovna, O.M.; Pritula, I.M. The role of amino acids in the processes of nucleation of pathogenic crystals of calcium oxalate monohydrate under human body imitating conditions. J. Cryst. Growth 2023, 602, 126973. [Google Scholar] [CrossRef]
- Grases, F.; March, J.G.; Bibiloni, F.; Amat, E. The crystallization of calcium oxalate in the presence of aminoacids. J. Cryst. Growth 1988, 87, 299–304. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Punin, Y.U.O.; Vystoskiy, A.S.; Khannanov, V.R. Effect of Organic and Inorganic Impurities on the Nucleation of Calcium Oxalate Monohydrate. Chem. Sustain. Dev. 2011, 19, 463–470. [Google Scholar]
- Golovanova, O.A.; Achkasova, E.Y.; Punin, Y.O.; Zhelyaev, E.V. Main regularities of crystallization of calcium oxalate in the presence of amino acids. Crystallogr. Rep. 2006, 51, 348–354. [Google Scholar] [CrossRef]
- Shee, K.; Stoller, M.L. Perspectives in primary hyperoxaluria—Historical, current and future clinical interventions. Nat. Rev. Urol. 2022, 19, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Šafranko, S.; Kontrec, J.; Njegić Džakula, B.; Lyons, D.M.; Marković, B.; Kralj, D. Calcium oxalate precipitation in model systems mimicking the conditions of hyperoxaluria. Cryst. Res. Technol. 2019, 54, 1800210. [Google Scholar] [CrossRef]
- Pierratos, A.E.; Khalaff, H.; Cheng, P.T.; Psihramis, K.; Jewett, M.A. Clinical and biochemical differences in patients with pure calcium oxalate monohydrate and calcium oxalate dihydrate kidney stones. J. Urol. 1994, 151, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Matijaković Mlinarić, N.; Šafranko, S.; Vidas, B.; Goman, D.; Jokić, S.; Kontrec, J.; Stanković, A. Precipitation of Calcium Oxalate Monohydrate Under Nearly the Same Initial Supersaturation. Croat. Chem. Acta 2021, 94, 59–66. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0– new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Echigo, T.; Kimata, M.; Kyono, A.; Shimizu, M.; Hatta, T. Re-investigation of the crystal structure of whewellite [Ca(C2O4)·H2O] and the dehydration mechanism of caoxite [Ca(C2O4)·3H2O]. Mineral. Mag. 2005, 69, 77–88. [Google Scholar] [CrossRef]
- Shen, Y.; Li, S.; Xie, A.; Xu, W.; Qiu, L.; Yao, H.; Yu, X.; Chen, Z. Controlled growth of calcium oxalate crystal in bicontinuous microemulsions containing amino acids. Colloids Surf. B Biointerfaces 2007, 58, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yue, W.; Xie, A.; Li, S.; Qian, Z. Effects of amino acids on crystal growth of CaC2O4 in reverse microemulsion. Colloids Surf. B. Biointerfaces 2005, 45, 120–124. [Google Scholar] [CrossRef]
- Ouyang, J.M.; Duan, L.; Tieke, B. Effects of carboxylic acids on the crystal growth of calcium oxalate nanoparticles in lecithin−water liposome systems. Langmuir 2003, 19, 8980–8985. [Google Scholar] [CrossRef]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zhang, C.-Y.; Bhadja, P.; Ouyang, J.-M. Preparation, properties, formation mechanisms, and cytotoxicity of calcium oxalate monohydrate with various morphologies CrystEngComm 2018, 20, 75–87. CrystEngComm 2018, 20, 75–87. [Google Scholar] [CrossRef]
- Tazzoli, V.; Domenegheti, C. The crystal structures of whewellite and weddellite: Re-examination and comparison. Am. Mineral. 1980, 65, 327–334. [Google Scholar]
- Deganello, S.; Piro, O.E. The crystal structure of calcium oxalate monohydrate (whewellite). N. Jb. Miner. Mh. H. 1981, 2, 81–88. [Google Scholar]
- Fischer, V.; Landfester, K.; Muñoz-Espí, R. Stabilization of calcium oxalate metastable phases by oligo (L-glutamic acid): Effect of peptide chain length. Cryst. Growth Des. 2011, 11, 1880–1890. [Google Scholar] [CrossRef]
- Sargut, S.T.; Sayan, P.; Kiran, B. Influence of essential and non-essential amino acids on calcium oxalate crystallization. Cryst. Res. Technol. 2010, 45, 31–38. [Google Scholar] [CrossRef]
- Sayan, P.; Titiz Sargut, S.; Kiran, B. Calcium oxalate crystallization in the presence of amino acids, proteins and carboxylic acids. Cryst. Res. Technol. 2009, 44, 807–817. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Punin, Y.O.; Izatulina, A.R.; Korol’kov, V.V. Crystallization of calcium oxalate monohydrate in the presence of amino acids: Features and regularities. J. Struct. Chem. 2014, 55, 1356–1370. [Google Scholar] [CrossRef]
- Wei, X.; Yang, J.; Li, Z.; Su, Y.; Wang, D. Comparison investigation of the effects of ionic surfactants on the crystallization behavior of calcium oxalate: From cationic to anionic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2014, 401, 107–115. [Google Scholar] [CrossRef]
- Sun, X.Y.; Xu, M.; Ouyang, J.M. Effect of Crystal Shape and Aggregation of Calcium Oxalate Monohydrate on Cellular Toxicity in Renal Epithelial Cells. ACS Omega 2017, 30, 6039–6052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, X.; Jung, T.; Wesson, J.A.; Ward, M.D. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc. Natl. Acad. Sci. USA 2005, 11, 267–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, S.R.; Wierzbicki, A.; Salter, E.A.; Zepeda, S.; Orme, C.A.; Hoyer, J.R.; Nancollas, G.H.; Cody, A.M.; De Yoreo, J.J. Modulation of calcium oxalate monohydrate crystallization by citrate through selective binding to atomic steps. J. Am. Chem. Soc. 2005, 127, 9036–9044. [Google Scholar] [CrossRef]
- Jung, T.; Sheng, X.; Choi, C.K.; Kim, W.S.; Wesson, J.A.; Ward, M.D. Probing crystallization of calcium oxalate monohydrate and the role of macromolecule additives with in situ atomic force microscopy. Langmuir 2004, 20, 8587–8596. [Google Scholar] [CrossRef]
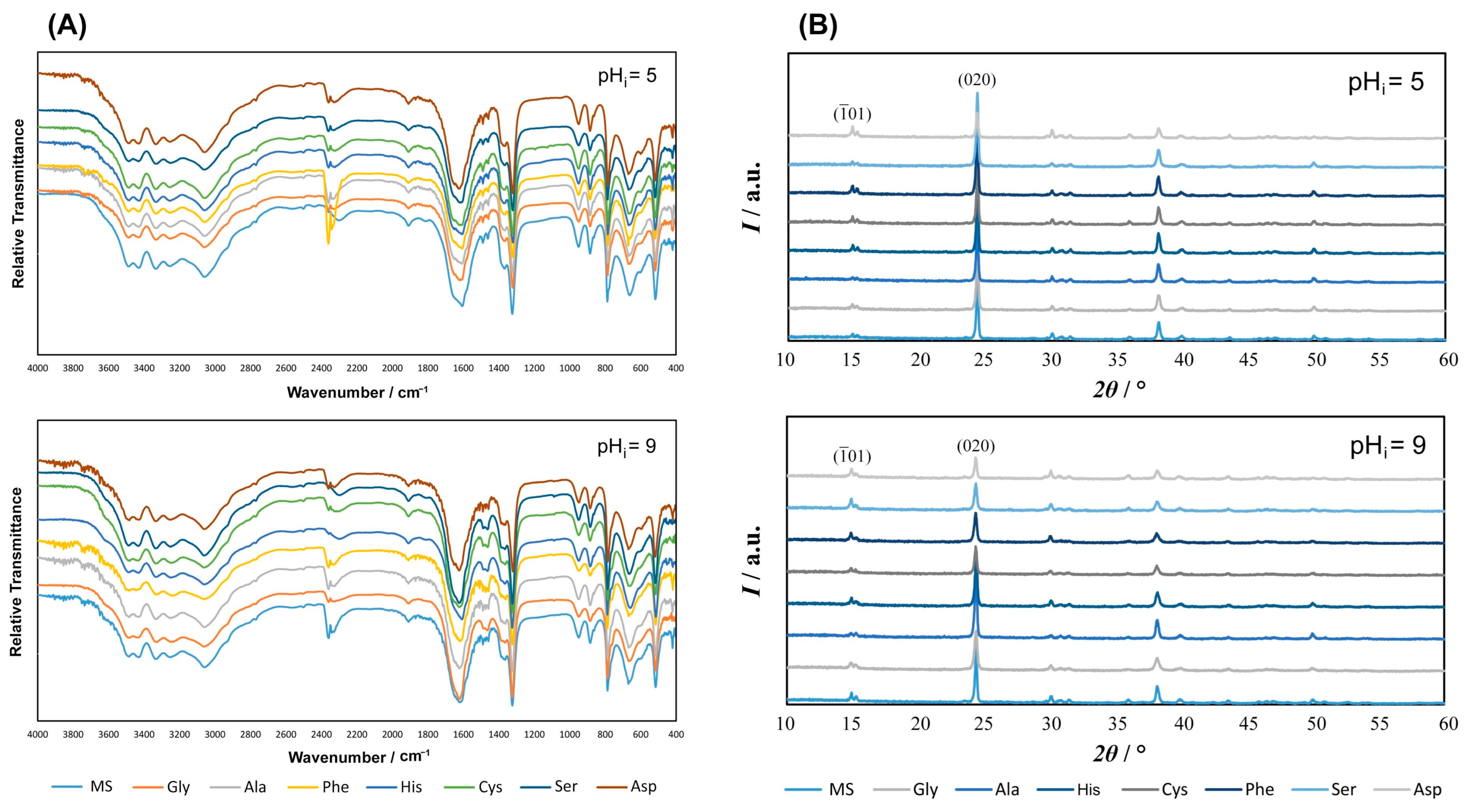
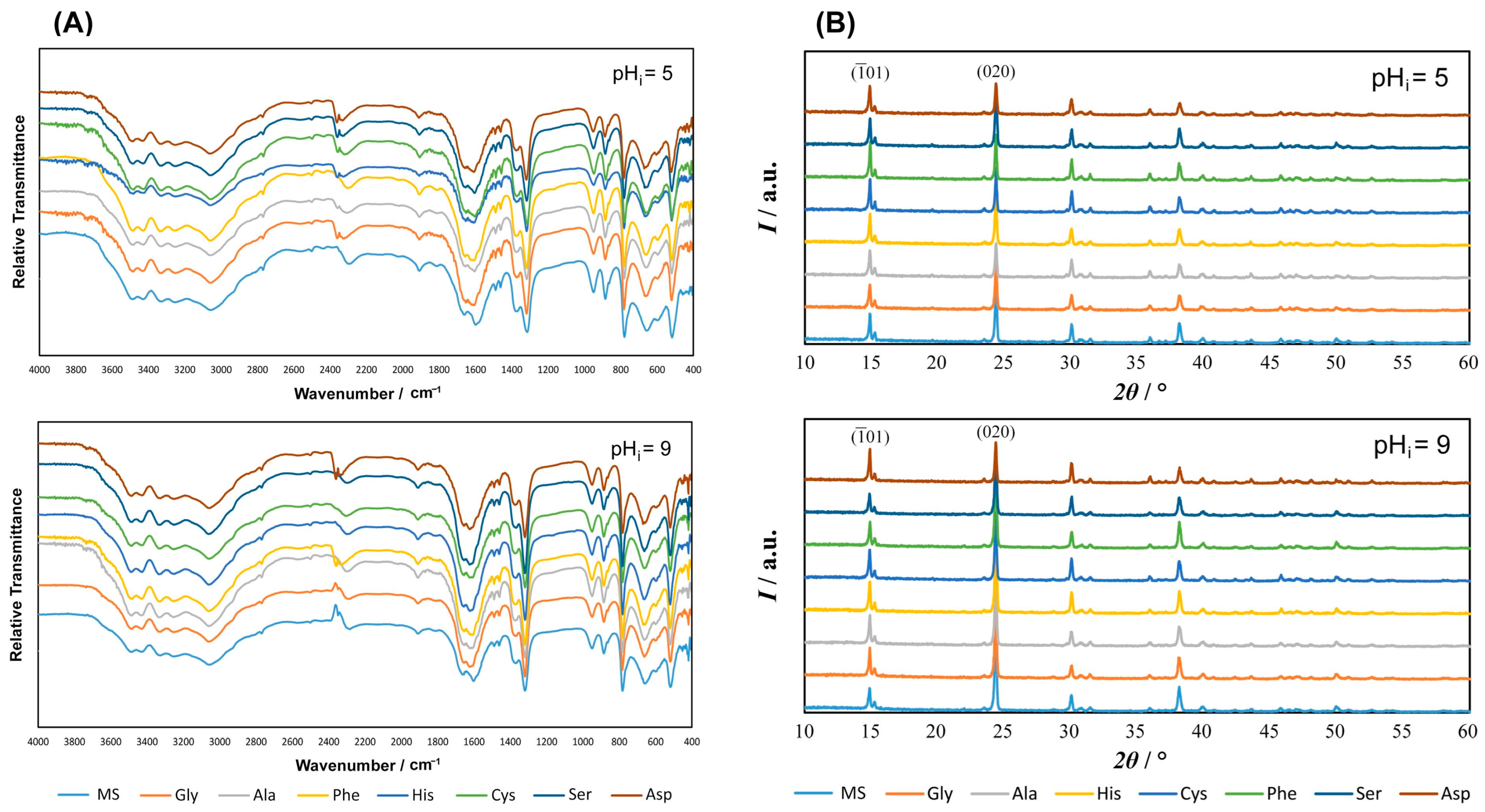
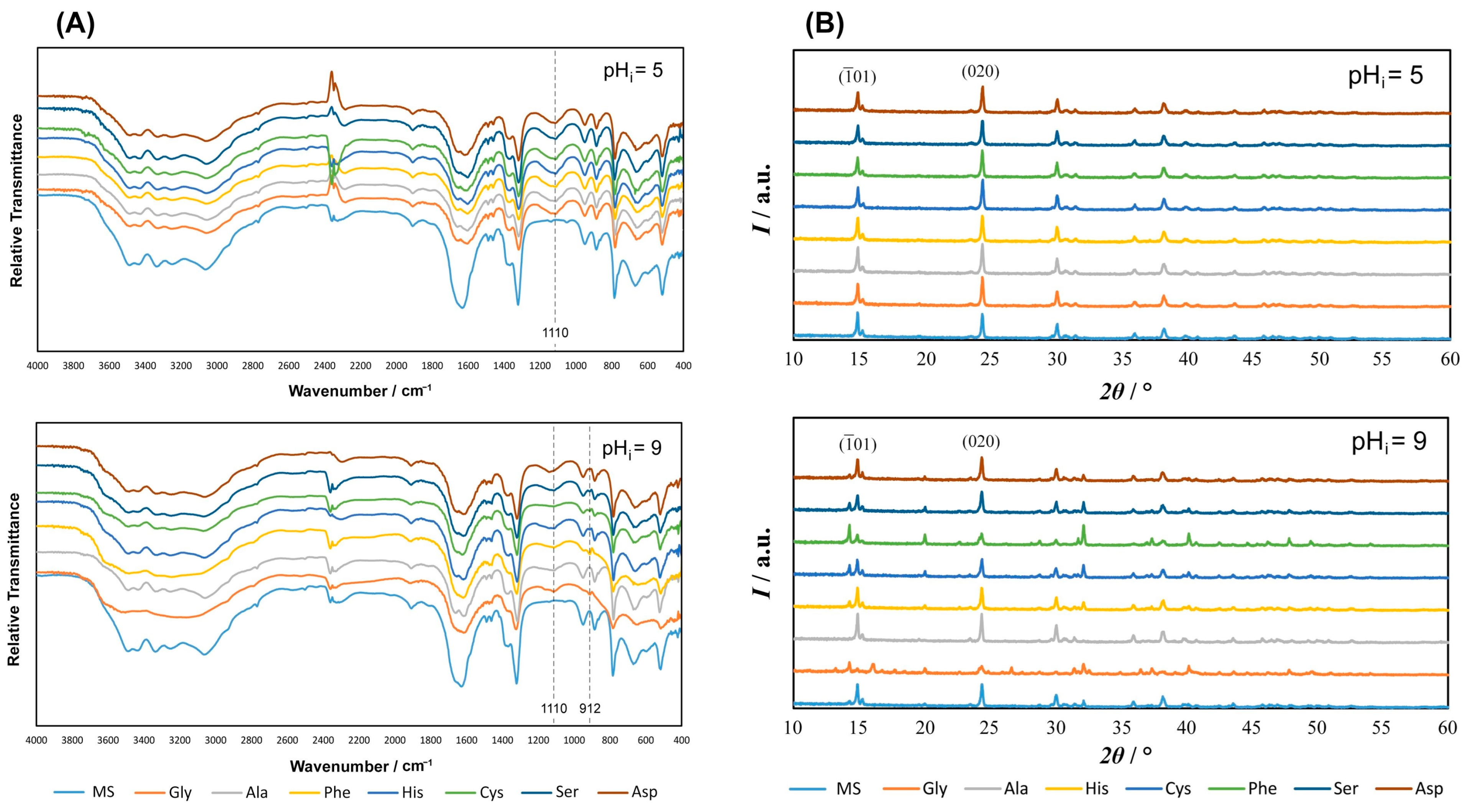

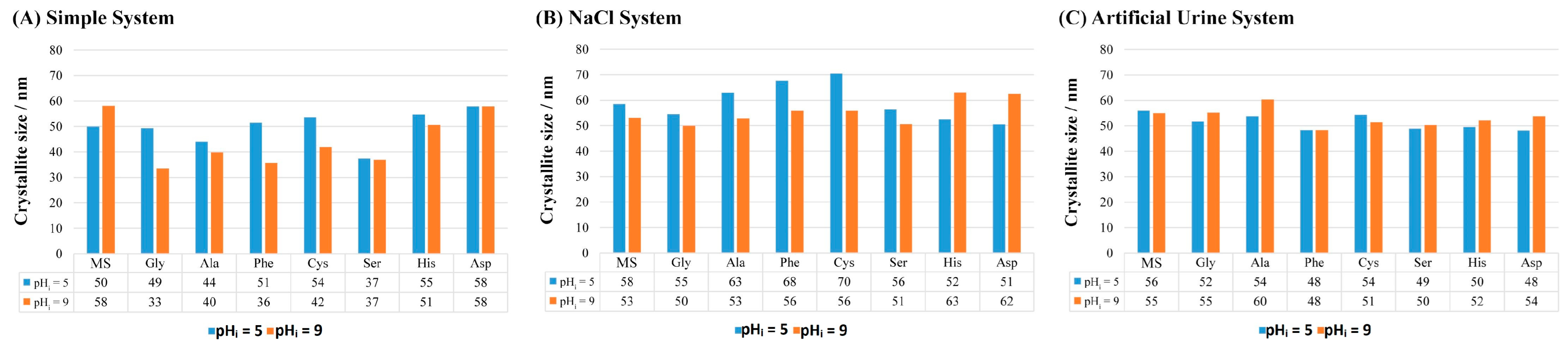
| pHi = 5 | pHi = 9 | ||||||
|---|---|---|---|---|---|---|---|
| Simple | NaCl | A. urine | Simple | NaCl | A. urine | ||
| Non-polar AA | Model | 0.1178 | 0.4474 | 1.0649 | 0.2078 | 0.2662 | 1.0041 |
| Ala | 0.0890 | 0.8042 | 0.8620 | 0.0885 | 0.4025 | 0.9759 | |
| Phe | 0.1349 | 0.6878 | 0.6708 | 0.3613 | 0.2219 | 0.9275 | |
| Polar AA | Gly | 0.0910 | 0.5625 | 0.7300 | 0.2218 | 0.3646 | 0.7365 |
| Ser | 0.0775 | 0.4726 | 0.7182 | 0.5370 | 0.3318 | 0.8276 | |
| Cys | 0.1061 | 0.7374 | 0.6341 | 0.3220 | 0.4628 | 0.9326 | |
| Charged AA | His | 0.1185 | 0.6063 | 0.9132 | 0.2176 | 0.3789 | 1.0212 |
| Asp | 0.4391 | 0.9248 | 0.7643 | 0.5425 | 0.7634 | 0.9126 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanković, A.; Matijaković Mlinarić, N.; Kontrec, J.; Njegić Džakula, B.; Lyons, D.M.; Marković, B.; Kralj, D. Influence of Amino Acids on Calcium Oxalate Precipitation in Systems of Different Chemical Complexity. Crystals 2024, 14, 599. https://doi.org/10.3390/cryst14070599
Stanković A, Matijaković Mlinarić N, Kontrec J, Njegić Džakula B, Lyons DM, Marković B, Kralj D. Influence of Amino Acids on Calcium Oxalate Precipitation in Systems of Different Chemical Complexity. Crystals. 2024; 14(7):599. https://doi.org/10.3390/cryst14070599
Chicago/Turabian StyleStanković, Anamarija, Nives Matijaković Mlinarić, Jasminka Kontrec, Branka Njegić Džakula, Daniel M. Lyons, Berislav Marković, and Damir Kralj. 2024. "Influence of Amino Acids on Calcium Oxalate Precipitation in Systems of Different Chemical Complexity" Crystals 14, no. 7: 599. https://doi.org/10.3390/cryst14070599
APA StyleStanković, A., Matijaković Mlinarić, N., Kontrec, J., Njegić Džakula, B., Lyons, D. M., Marković, B., & Kralj, D. (2024). Influence of Amino Acids on Calcium Oxalate Precipitation in Systems of Different Chemical Complexity. Crystals, 14(7), 599. https://doi.org/10.3390/cryst14070599











