Effect of Silver Vanadate Nanowires Addition on Structural and Morphological Properties of Dental Porcelain Prepared from Economic Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silver Vanadate Synthesis
2.2. Dental Porcelain Preparation
2.3. Characterization Methods
3. Results and Discussion
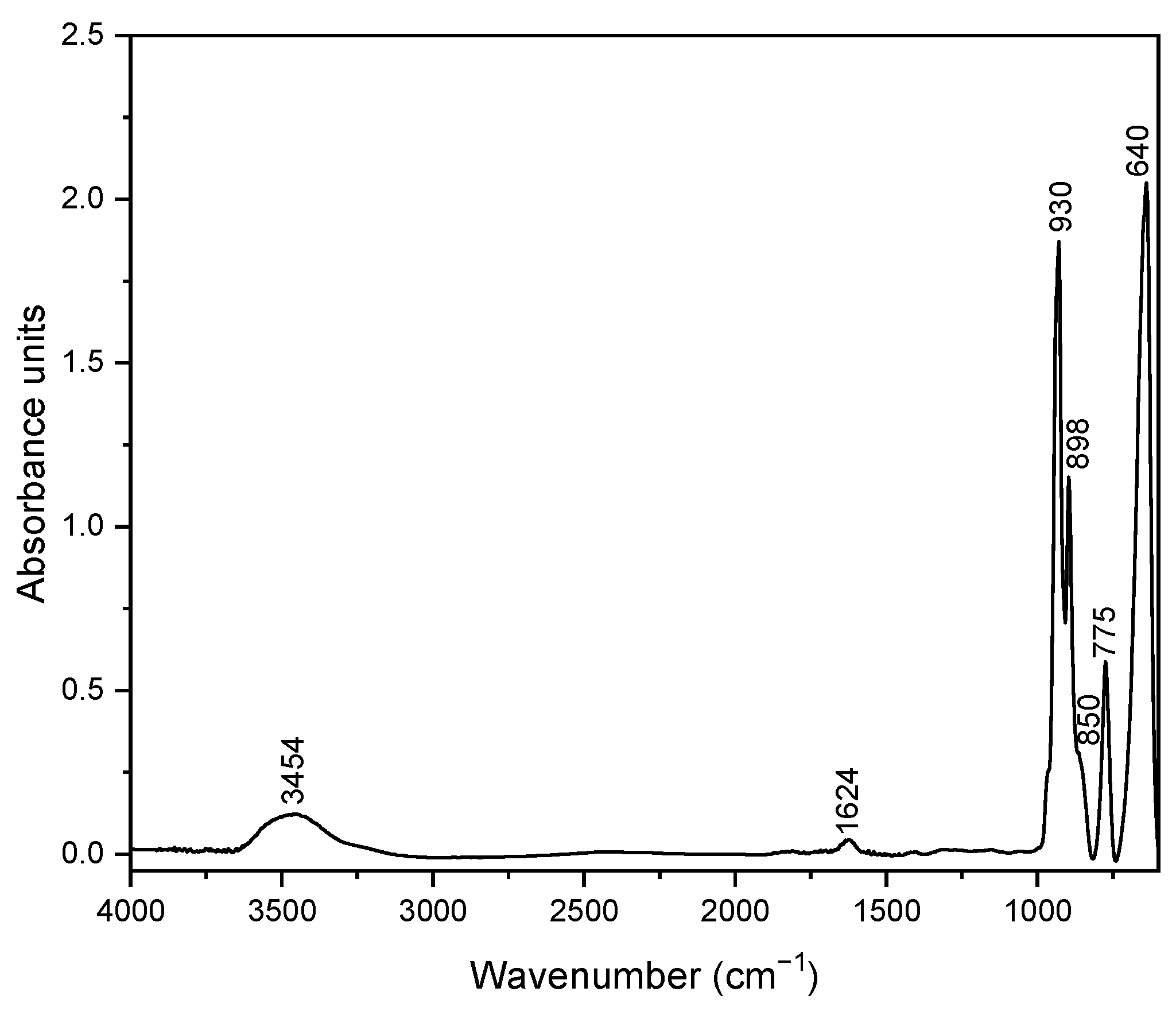
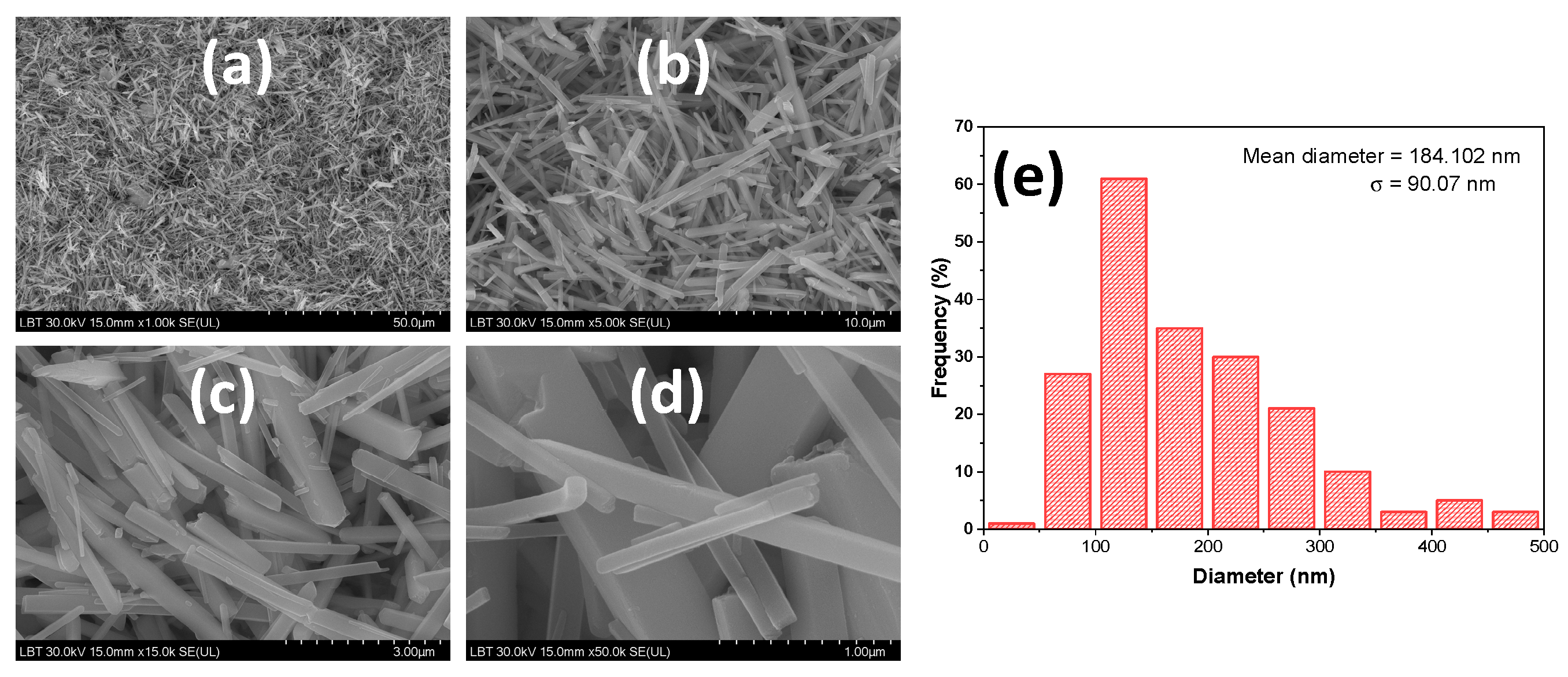
3.1. Silver Vanadate Characterization
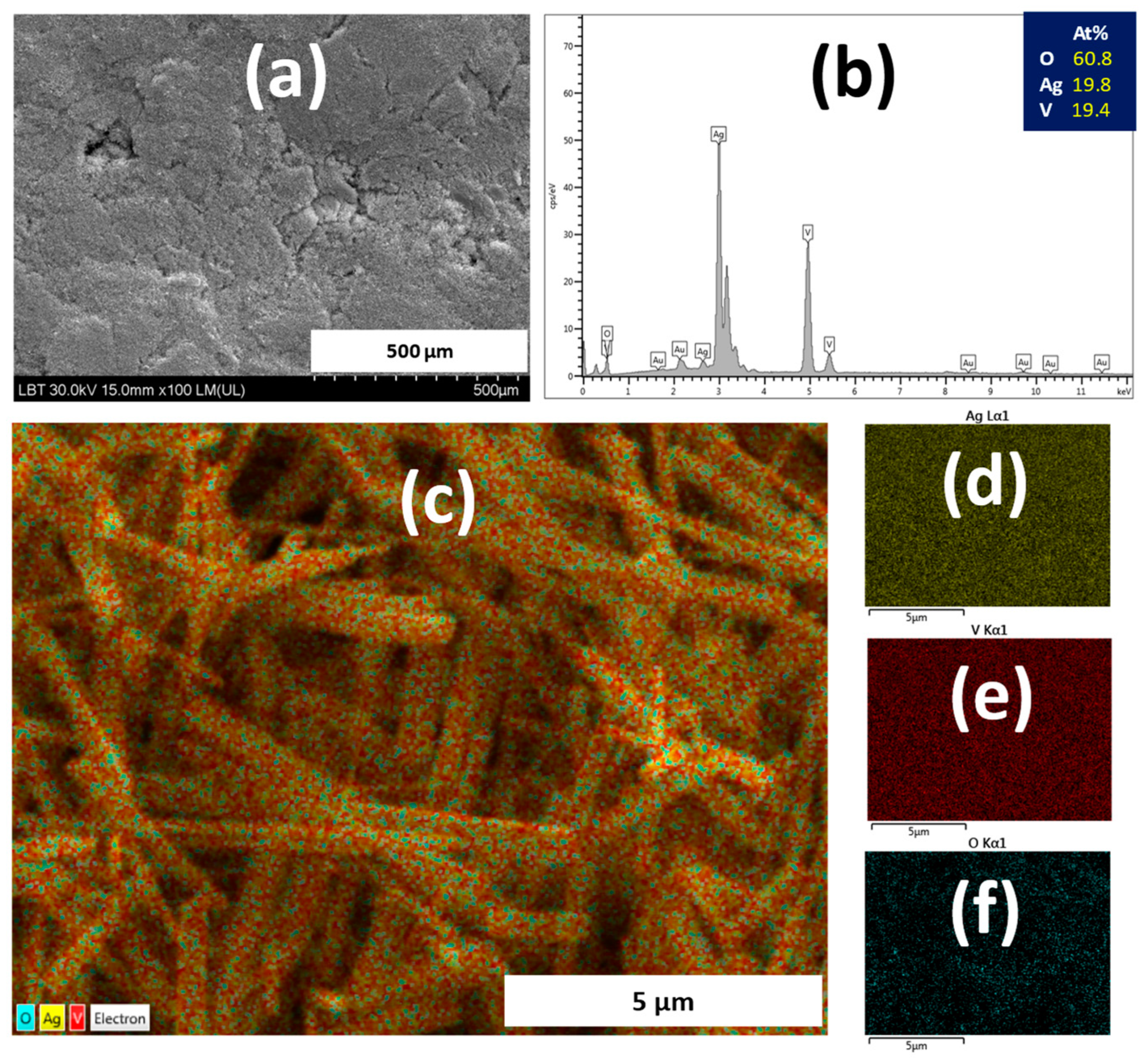
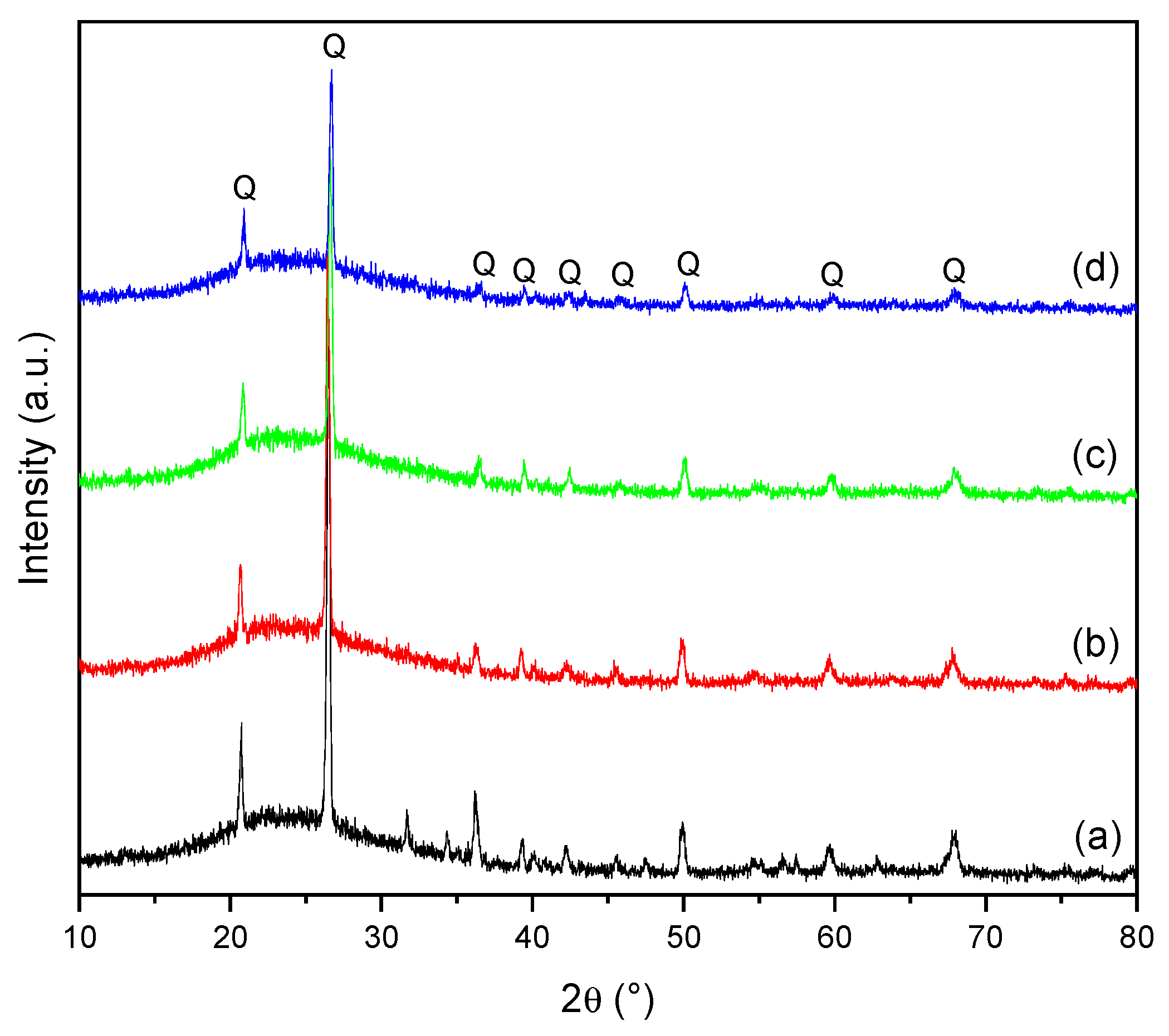
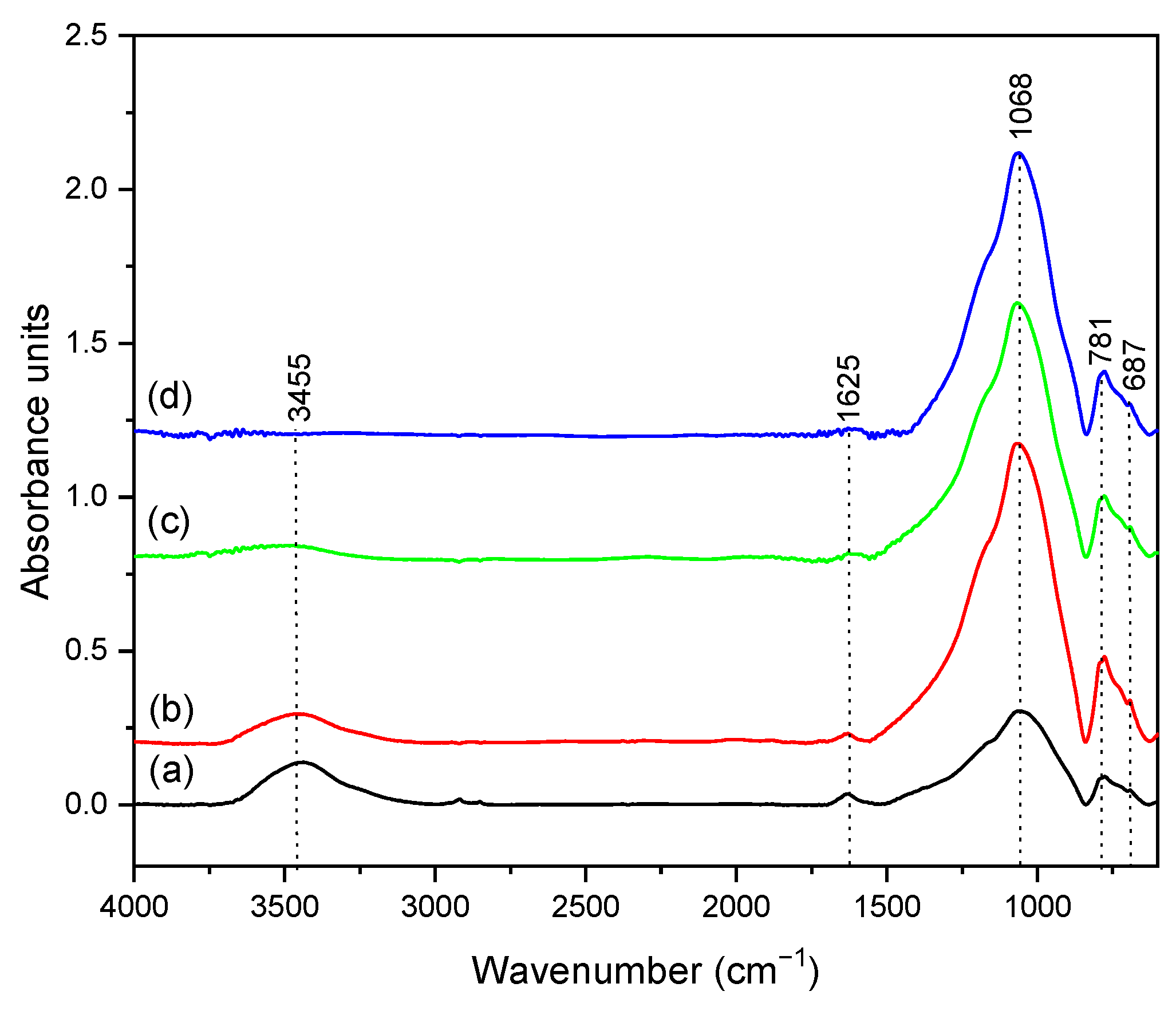
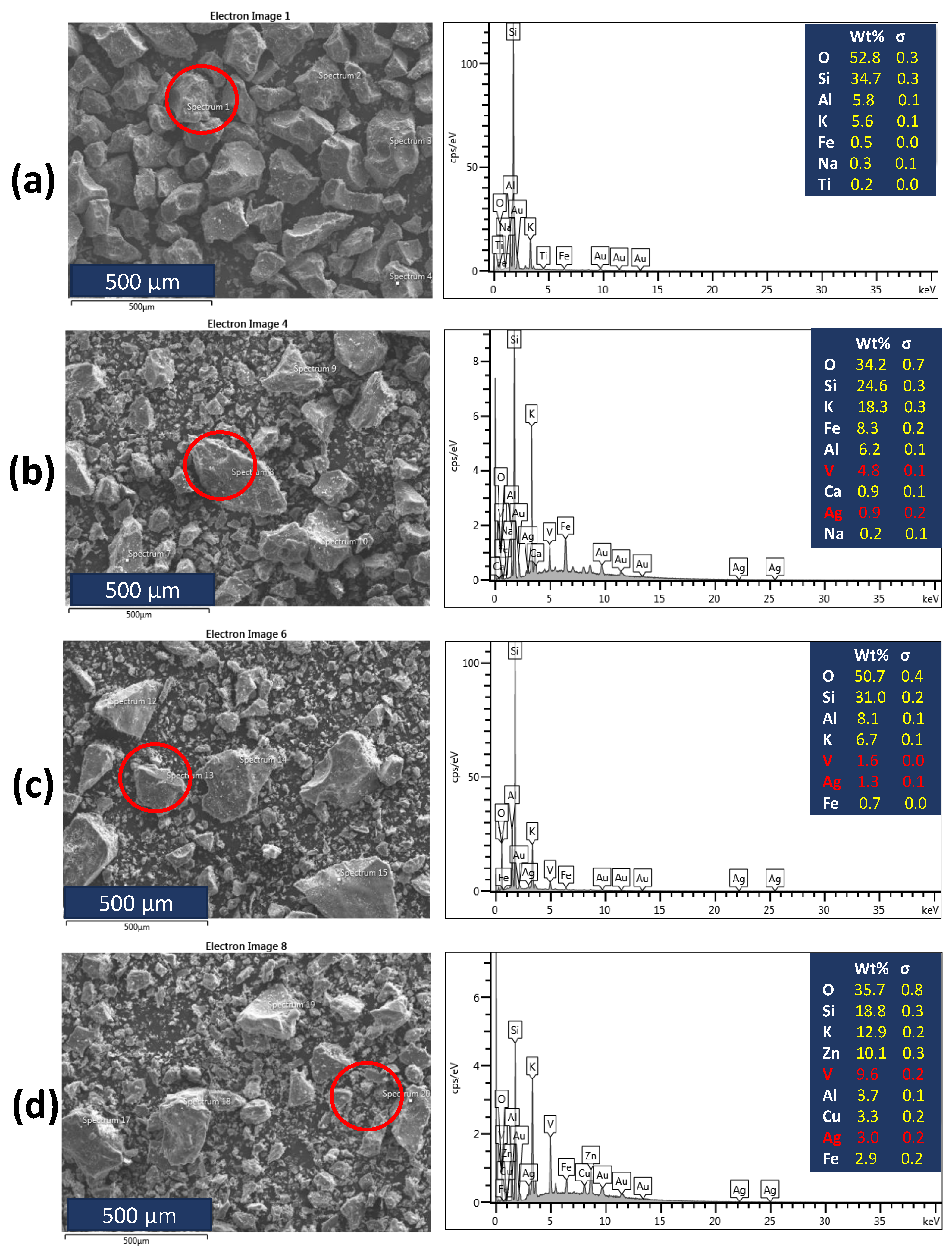
3.2. Characterization of Dental Porcelain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute of Biomedical Imaging and Bioengineering. Available online: https://www.nibib.nih.gov/science-education/science-topics/biomaterials (accessed on 27 May 2024).
- Kaur, G. Clinical Applications of Biomaterials. State-of-the-Art Progress, Trends, and Novel Approaches, 1st ed.; Springer: Cham, Switzerland, 2017; pp. 1–26. [Google Scholar]
- Wong, J.Y.; Bronzino, J.D. Biomaterials; CRC Press: Boca Raton, FL, USA, 2016; pp. 38–71. [Google Scholar]
- Bharadwaj, A. An Overview on Biomaterials and Its Applications in Medical Science. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012178. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Buxbaum, E. Bond Properties. In Biophysical Chemistry of Proteins; Springer: Boston, MA, USA, 2011; p. 453. [Google Scholar]
- Debelian, G.; Trope, M. The Use of Premixed Bioceramic Materials in Endodontics. G. Ital. Endod. 2016, 30, 70–80. [Google Scholar] [CrossRef]
- Keane, T.J.; Badylak, S.F. Biomaterials for Tissue Engineering Applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Wang, J.Y.; Yu, R.Q. Biomaterials in Dentistry. Encycl. Biomed. Eng. 2019, 1–3, 278–288. [Google Scholar]
- Sarikaya, I.; Güler, A.U. Effects of Different Polishing Techniques on the Surface Roughness of Dental Porcelains. J. Appl. Oral Sci. 2010, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.W. Ceramic Biomaterials. Key Eng. Mater. 1996, 122–124, 345–386. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials; Elsevier: St. Louis, MO, USA, 2013; pp. 1–538. [Google Scholar]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials, 13th ed.; Elsevier: Philadelphia, PA, USA, 2012; pp. 135–146. [Google Scholar]
- Naert, I. 7.24 Materials in Fixed Prosthodontics for Indirect Dental Restorations. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: London, UK, 2017; pp. 467–481. [Google Scholar]
- Van Noort, R.; Barbour, M.E. Introduction to Dental Materials, 5th ed.; Elsevier: London, UK, 2013; pp. 1–15. [Google Scholar]
- El-Meliegy, E.; van Noort, R. History, Market and Classification of Bioceramics. In Glasses and Glass Ceramics for Medical Applications; Springer: New York, NY, USA, 2012; pp. 3–17. [Google Scholar]
- Durán, N.; Marcato, P.; De Conti, R.; Alves, O.; Costa, F.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, Their Toxicity and Possible Mechanisms of Action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Uehara, L.M.; Ferreira, I.; Botelho, A.L.; Valente, M.L.D.C.; Reis, A.C.D. Influence of β-AgVO3 Nanomaterial Incorporation on Mechanical and Microbiological Properties of Dental Porcelain. Dent. Mater. 2022, 38, e174–e180. [Google Scholar] [CrossRef]
- Ferreira, I.; Vidal, C.L.; Botelho, A.L.; Ferreira, P.S.; Valente, M.L.C.; Schiavon, M.A.; Alves, O.L.; dos Reis, A.C. Effect of Nanomaterial Incorporation on the Mechanical and Microbiological Properties of Dental Porcelain. J. Prosthet. Dent. 2020, 123, 529.e1–529.e5. [Google Scholar] [CrossRef]
- Vidal, L.; Ferreira, I.; Ferreira, P.; Valente, M.; Teixeira, M.; Reis, A. Incorporation of Hybrid Nanomaterial in Dental Porcelains: Antimicrobial, Chemical, and Mechanical Properties. Antibiotics 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Holtz, R.D.; Filho, A.G.S.; Brocchi, M.; Durán, N.; Alves, O.L. Development of Nanostructured Silver Vanadates Decorated with Silver Nanoparticles as a Novel Antibacterial Agent. Nanotechnology 2010, 21, 185102. [Google Scholar] [CrossRef] [PubMed]
- Balan, L.; Malval, J.P.; Schneider, R.; Burget, D. Silver Nanoparticles: New Synthesis, Characterization and Photophysical Properties. Mater. Chem. Phys. 2007, 104, 417–421. [Google Scholar] [CrossRef]
- Baptista, I.O.; Alves, M.F.R.P.; Ferreira, S.; Santos, C.; Vieira, S.I.; Fernandes, M.H.V. Antibacterial activity improvement of dental glass-ceramic by incorporation of AgVO3 nanoparticles. Dent. Mater. 2022, 38, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- De Castro, D.T.; Valente, M.L.C.; Da Silva, C.H.L.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A.; Alves, O.L.; dos Reis, A.C. Evaluation of Antibiofilm and Mechanical Properties of New Nanocomposites Based on Acrylic Resins and Silver Vanadate Nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.; da Silva, J.S.; Gonçalves, M.O.; de Almeida Rodolpho, J.M.; de Lima Fragelli, B.D.; Corte, A.B.P.; Ribeiro, L.K.; Teodoro, M.D.; de Freitas Anibal, F.; de Sousa, C.P.; et al. Bactericidal Activity of Ag4V2O7/β-AgVO3 Heterostructures against Antibiotic-Resistant Klebsiella Pneumoniae. Biomater. Adv. 2022, 141, 213097. [Google Scholar] [CrossRef] [PubMed]
- de Campos, M.R.; Botelho, A.L.; Dos Reis, A.C. Nanostructured silver vanadate decorated with silver particles and their applicability in dental materials: A scope review. Heliyon 2021, 7, e07168. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Dias Holtz, R.; Carneiro, F.; Zanini, M.; De Sousa, M. Nano Silver Vanadate AgVO3: Synthesis, New Functionalities and Applications. Chem. Rec. 2018, 18, 973–985. [Google Scholar] [CrossRef]
- Alves da Silva Pimentel, B.N.; de Foggi, C.C.; Barbugli, P.A.; de Oliveira, R.C.; de Avila, E.D.; Longo, E.; Vergani, C.E. Antifungal activity and biocompatibility of α-AgVO3 microcrystals: A promising material against oral Candida disease. Mater. Sci. Eng. C 2020, 108, 110405. [Google Scholar] [CrossRef]
- Ferreira, I.; Alves, O.L.; Schiavon, M.A.; dos Reis, A.C. Influence of incorporation of nanostructured silver vanadate decorated with silver nanoparticles on roughness, microhardness, and color change of pit and fissure sealants. Heliyon 2024, 10, e25525. [Google Scholar] [CrossRef] [PubMed]
- Alvim, G.C.; Oliveira, V.d.C.; dos Reis, A.C.; Schiavon, M.A.; Pinto, M.R.; da Silva, M.V.; Lepri, C.P.; de Castro, D.T. Effect of silver vanadate on the antibiofilm, adhesion and biocompatibility properties of denture adhesive. In Future Microbiology; Taylor and Francis: Oxfordshire, UK, 2024; pp. 1–11. [Google Scholar]
- Kreve, S.; Oliveira, V.C.; Bachmann, L.; Alves, O.L. Influence of AgVO3 Incorporation on Antimicrobial Properties, Hardness, Roughness and Adhesion of a Soft Denture Liner. Sci. Rep. 2019, 9, 11889. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; de Foggi, C.C.; Mondego Teixeira, M.; Penha da Silva, M.D.; Assis, M.; Francisco, E.M.; Alves da Silva Pimentel, B.N.; dos Santos Pereira, P.F.; Vergani, C.E.; Machado, A.L.; et al. Mechanism of Antibacterial Activity via Morphology Change of α-AgVO3: Theoretical and Experimental Insights. ACS Appl. Mater. Interfaces 2017, 9, 11472–11481. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, S.; Matsuno, K.; Akashi, H. Crystal structure of alpha-AgVO3 and phase relation of AgVO3. J. Solid State Chem. 1999, 142, 360–367. [Google Scholar] [CrossRef]
- Esmaeili, E.; Salavati-Niasari, M.; Mohandes, F. Modified Single-Phase Hematite Nanoparticles via a Facile Approach for Large-Scale Synthesis. Chem. Eng. J. 2011, 170, 278–285. [Google Scholar] [CrossRef]
- Liu, H.; Tang, D. Synthesis of ZnV2O6 Powder and Its Cathodic Performance for Lithium Secondary Battery. Mater. Chem. Phys. 2009, 114, 656–659. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Li, X.; Min, X. A novel 2D graphene oxide modified α-AgVO3 nanorods: Design, fabrication, and enhanced visible-light photocatalytic performance. J. Adv. Ceram. 2022, 11, 308–320. [Google Scholar] [CrossRef]
- Vilela Teixeira, A.B.; Larissa Vidal, C.; Albiasetti, T.; Tornavoi De Castro, D.; Cândido Dos Reis, A. Influence of Adding Nanoparticles of Silver Vanadate on Antibacterial Effect and Physicochemical Properties of Endodontic Sealers. Iran. Endod. J. 2019, 14, 7–13. [Google Scholar] [PubMed]
- da Silva, J.S.; Machado, T.R.; Trench, A.B.; Silva, A.D.; Teodoro, V.; Vieira, P.C.; Martins, T.A.; Longo, E. Enhanced photocatalytic and antifungal activity of hydroxyapatite/α-AgVO3 composites. Mat. Chem. Phys. 2020, 252, 123294. [Google Scholar] [CrossRef]
- de Menezes, B.R.C.; Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; Koga-Ito, C.Y.; Thim, G.P. Synthesis of β-AgVO3 nanowires by hydrothermal and precipitation routes: A comparative study. SN Appl. Sci. 2019, 1, 1327. [Google Scholar] [CrossRef]
- Harabi, A.; Guerfa, F.; Harabi, E.; Benhassine, M.T.; Foughali, L.; Zaiou, S. Preparation and Characterization of New Dental Porcelains, Using K-Feldspar and Quartz Raw Materials. Effect of B2O3 Additions on Sintering and Mechanical Properties. Mater. Sci. Eng. C 2016, 65, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, S. Thermodynamics and Lattice Vibrations of Minerals: 2. Vibrational Characteristics of Silicates. Rev. Geophys. Space Phys. 1979, 17, 20–34. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Tan, S.; Zheng, J. Organoclays from Alkaline-Treated Acid-Activated Clays: Properties and Thermal Stability. J. Therm. Anal. Calorim. 2014, 115, 1465–1475. [Google Scholar] [CrossRef]
- Komadel, P. Chemically Modified Smectites. Clay Miner. 2003, 38, 127–138. [Google Scholar] [CrossRef]
- Lawson, N.C.; Burgess, J.O. Dental ceramics: A current review. Comp. Cont. Educ. Dent. 2014, 35, 161–166. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakhkhane, B.E.; Mureșan-Pop, M.; Barbu-Tudoran, L.; Lovász, T.; Bizo, L. Effect of Silver Vanadate Nanowires Addition on Structural and Morphological Properties of Dental Porcelain Prepared from Economic Raw Materials. Crystals 2024, 14, 616. https://doi.org/10.3390/cryst14070616
Sakhkhane BE, Mureșan-Pop M, Barbu-Tudoran L, Lovász T, Bizo L. Effect of Silver Vanadate Nanowires Addition on Structural and Morphological Properties of Dental Porcelain Prepared from Economic Raw Materials. Crystals. 2024; 14(7):616. https://doi.org/10.3390/cryst14070616
Chicago/Turabian StyleSakhkhane, Badr Eddine, Marieta Mureșan-Pop, Lucian Barbu-Tudoran, Tamás Lovász, and Liliana Bizo. 2024. "Effect of Silver Vanadate Nanowires Addition on Structural and Morphological Properties of Dental Porcelain Prepared from Economic Raw Materials" Crystals 14, no. 7: 616. https://doi.org/10.3390/cryst14070616





