The Diversity of MOF Structures and Their Impact on Photoelectrochemical Sensors for Monitoring Environmental Pollution
Abstract
1. Introduction
- -
- PEC sensing is more sensitive than conventional electrochemical methods;
- -
- PEC sensors use light to stimulate photoactive materials;
- -
- PEC sensors have simpler instrumentation;
- -
- PEC sensors have low-cost production.
1.1. MOF Definition
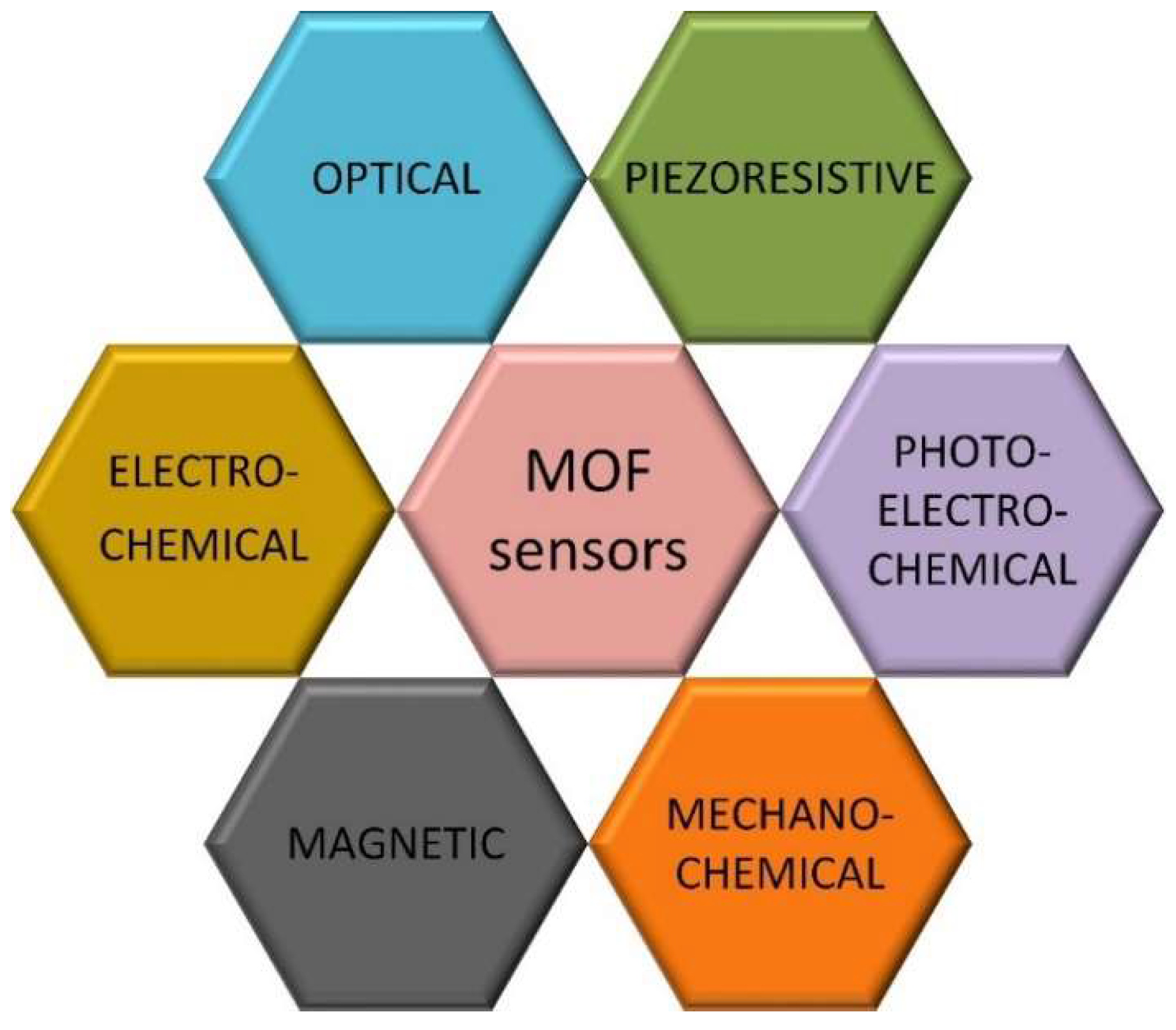
1.2. The Classification of MOF-Based Sensors
1.2.1. MOF Optical Sensors
1.2.2. MOF Piezoresistive Sensors
1.2.3. MOF Mechanochemical Sensors
1.2.4. MOF Magnetic Sensors
1.2.5. MOF Electrochemical Sensors
1.2.6. MOF Photoelectrochemical Sensors
2. Photoelectrochemistry of MOF Materials
2.1. Modification of MOF Structure
2.2. Surface Modifications of MOFs
2.3. Multicomponent Heterostructures Formations
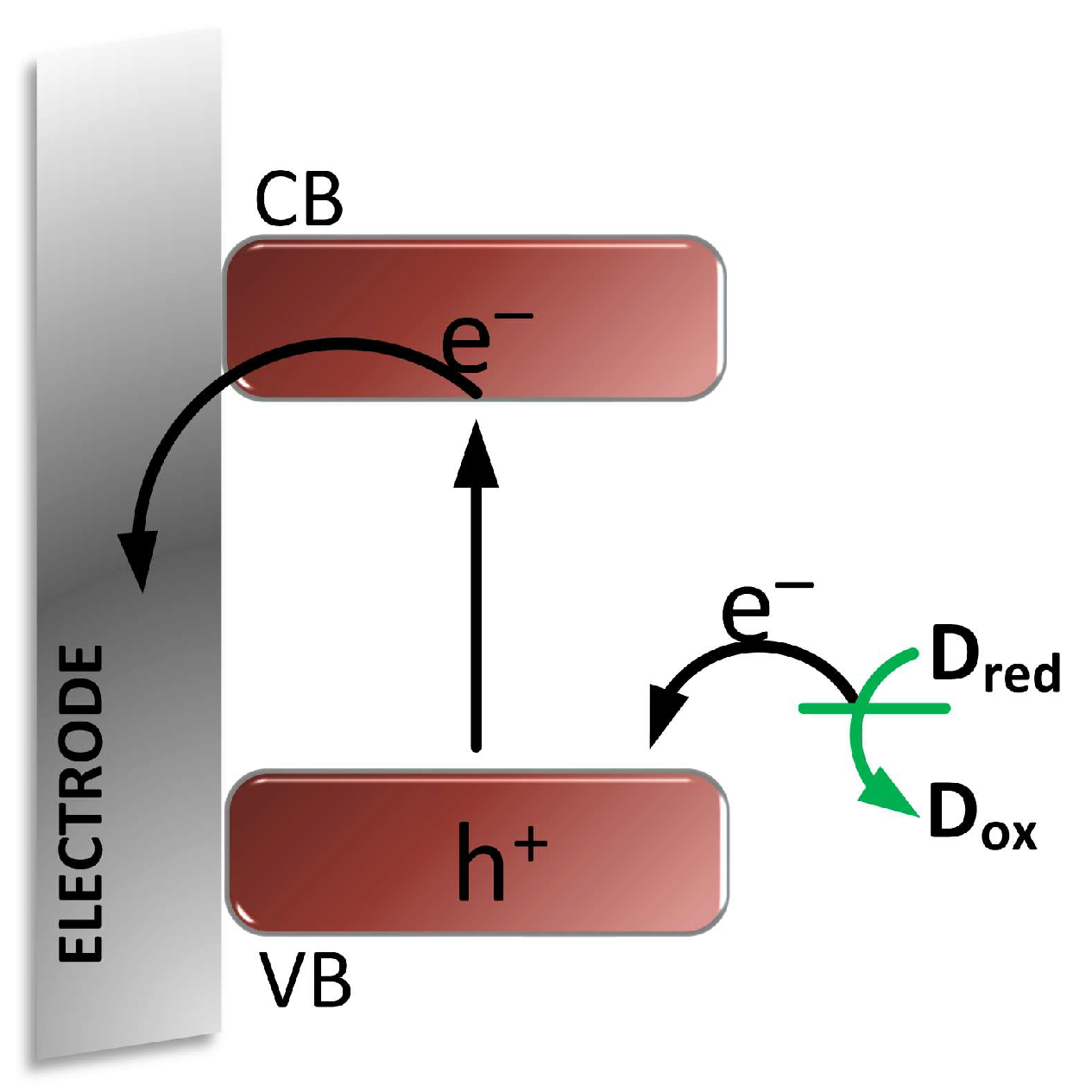
3. Mechanisms of Photocurrent Generation in Photoelectrochemical Sensors
4. MOF-Photoelectrochemical Sensors for Environmental Pollution Monitoring
4.1. Heavy Metal Compounds
4.1.1. Mercury
4.1.2. Arsenic Compound
4.1.3. Lead
4.2. Organic Pollutants—Generated Mainly by Pharmaceutical, Paper, and Textile Industry
4.2.1. Pesticides
| Sensing Materials | Analyte | Linear Detection Range | Sensitivity/Detection Limit | Reference |
|---|---|---|---|---|
| Cu-BTC MOF/g-C3N4 | glyphosate | 1.0 × 10−12–1.0 × 10−8 M | 1.3 × 10−13 M | [128] |
| FeMOF@PEDOT | malathion | 0.1–10 µg/L | 0.03 ng L−1 | [102] |
| NH2-MIL-125(Ti)@TiO2 | clethodim | 0.2–25 µM | 0.01 µM | [127] |
4.2.2. Drugs and Biomolecules
| Sensing Materials | Anayte | Linear Detection Range | Sensitivity/ Detection Limit | Reference |
|---|---|---|---|---|
| CdS@PCN-224 | Doxorubicin hydrochloride | 10 nM and 1 µM | 3.57 nM | [31] |
| CdS@PCN-224 | Gentamicin sulfate | 10 nM and 1 µM | 0.158 nM | [31] |
| Eu-MOF | Ampicillin | 0.1–200 nM | 0.01 nM | [108] |
| MOF-derived In2O3@g-C3N4 | Tetracycline | 0.01–500 nM | 3.3 pM | [97] |
| Mg-PTCA MOF | miRNA 21 | 10 aM to 10 pM | 2.8 aM | [128] |
| Au NPs/Yb-TCPP | SARS-CoV 2 spike glycoprotein | 0.5–8 μg/mL | 72 ng/mL | [115] |
| Cu-MOF@CuPc-TA-COF | HIV-1 DNA | 1 fM to 1 nM | 0.07 fM | [130] |
| NiPc-Cu MOF | N-acetyl-L-cysteine | 0.0125 to 42.5 μM | 5.2 nM | [129] |
| Eu-MOF@AuNPs | α-fetoprotein | 0.002–15.0 ng/mL | 0.16 pg/mL | [109] |
| AuNPs@Zn-MOF | α-fetoprotein | 0.005–15.0 ng/mL | 1.88 pg/L | [110] |
| Ce-Por-MOFs/ AgNWs | ronidazole | 0.1–104 nM | 0.038 nM | [131] |
5. Advantages/Disadvantages and Challenges in Photoelectrochemical MOF-Based Sensors
- (1)
- Narrow range of light absorbance: surface modification to increase the range of light, introduction of nanoparticles, and modification of linkers;
- (2)
- Low conductivity: introduction of nanoparticles, modification of linkers, creation of bimetallic systems, or creation of composites with semiconductors or carbon nanostructures;
- (3)
- Poor solubility: synthesis of materials directly on the electrode;
- (4)
- Low selectivity: enhanced using specific biomolecules (DNA, enzymes, and aptamers).
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Full Name |
| AA | ascorbic acid |
| AFP | α-fetoprotein |
| APTU | 1,3-bis(4-aminophenyl)thiourea |
| Asp | aspartic acid |
| bbim | 5-bromobenzimidazol |
| BDC | 1,4-benzenedicarboxylic acid |
| BIB | 1,4-bisimidazolebenzene |
| BPDC | biphenyl-4,4′-dicarboxylate |
| BTC | benzene tricarboxylate |
| CB | conduction band |
| COF | covalent organic framework |
| CN | gentamicin sulfate |
| DIA | diazinon |
| DBP | dibutyl phthalate |
| DMF | dimetyloformamid |
| DOT | 2,5-dioxido-1,4- benzenedicarboxylate |
| Dox | doxorubicin hydrochloride |
| EA | electron affinity |
| ECL | electrochemiluminescence |
| EDI | edifenphos |
| GCE | glassy carbon electrode |
| HKUST | Hong Kong University of Science and Technology |
| HMA | hydrophobically modified alginate |
| IP | ionization potential |
| IRMOF | isoreticular metal–organic frameworks |
| LOD | limit of detection |
| LSPR | localized surface plasmon resonance |
| MAL | malathion |
| mim | 2-methylimidazole |
| MIL | Materials of Institut Lavoisier |
| MOF | metal–organic framework |
| NAC | N-acetyl-L-cysteine |
| NIT | nitarsone |
| OECT | organic electrochemical transistor |
| OMT | omethoate |
| PEC | photoelectrochemical system |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PRO | profenofos |
| QCM | quartz crystal microbalance |
| ROX | roxarsone |
| SAW | surface acoustic wave |
| SPR | surface plasmonic resonance |
| TFPB | 1,3,5-Tris(4-formylphenyl)benzene |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| TCPP | tetracarboxy-phenylporphyrin |
| TPDC | [1,1′:4′,1″-terphenyl]-4,4″-dicarboxylate |
| UiO | Universitet in Oslo |
| VB | valence band |
| WHO | World Health Organization |
| ZIF | zeolite imidazolate framework |
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Dashtian, K.; Shahbazi, S.; Tayebi, M.; Masoumi, Z. A review on metal-organic frameworks photoelectrochemistry: A headlight for future applications. Coord. Chem. Rev. 2021, 445, 214097. [Google Scholar] [CrossRef]
- Liu, S.; Zhan, J.; Cai, B. Recent advances in photoelectrochemical platforms based on porous materials for environmental pollutant detection. RSC Adv. 2024, 14, 7940–7963. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liu, X.; Bao, Y.; Niu, L. Recent Advances in Photoelectrochemical Sensors for Analysis of Toxins and Abused Drugs in the Environment. Chemosensors 2023, 11, 412. [Google Scholar] [CrossRef]
- Nabipour, H.; Mozafari, M.; Hu, Y. Chapter 1—Nomenclature of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Foo, K.Y.; Schneider, R.; Abdelhameed, R.M.; Sabar, S. The chemistry of MIL-125 based materials: Structure, synthesis, modification strategies and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 106883. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, C.S. MOF-74-type frameworks: Tunable pore environment and functionality through metal and ligand modification. CrystEngComm 2021, 23, 1377–1387. [Google Scholar] [CrossRef]
- Hou, C.; Peng, J.; Xu, Q.; Ji, Z.; Hu, X. Elaborate fabrication of MOF-5 thin films on a glassy carbon electrode (GCE) for photoelectrochemical sensors. RSC Adv. 2012, 2, 12696–12698. [Google Scholar] [CrossRef]
- Javed, A.; Strauss, I.; Bunzen, H.; Caro, J.; Tiemann, M. Humidity-Mediated Anisotropic Proton Conductivity through the 1D Channels of Co-MOF-74. Nanomaterials 2020, 10, 1263. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal–Organic Frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Fuentes-Cabrera, M.; Nicholson, D.M.; Sumpter, B.G.; Widom, M. Electronic structure and properties of isoreticular metal-organic frameworks: The case of M-IRMOF1 (M = Zn, Cd, Be, Mg, and Ca). J. Chem. Phys. 2005, 123, 124713. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.; Chae, H.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; McIntyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.; Arora, C. A Review on Metal-organic Framework: Synthesis, Properties and Application. Charact. Appl. Nanomater. 2019, 2, 1–20. [Google Scholar] [CrossRef]
- O’Neill, L.D.; Zhang, H.; Bradshaw, D. Macro-/microporous MOF composite beads. J. Mater. Chem. 2010, 20, 5720–5726. [Google Scholar] [CrossRef]
- Steenhaut, T.; Filinchuk, Y.; Hermans, S. Aluminium-based MIL-100(Al) and MIL-101(Al) metal–organic frameworks, derivative materials and composites: Synthesis, structure, properties and applications. J. Mater. Chem. A 2021, 9, 21483–21509. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. Int. Ed. 2011, 50, 672–675. [Google Scholar] [CrossRef]
- Abraha, Y.W.; Tsai, C.-W.; Niemantsverdriet, J.W.H.; Langner, E.H.G. Optimized CO2 Capture of the Zeolitic Imidazolate Framework ZIF-8 Modified by Solvent-Assisted Ligand Exchange. ACS Omega 2021, 6, 21850–21860. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Tao, H.; Wu, Y.; Chen, J.; Wang, X. An ultrasensitive label-free electrochemical aptasensing platform for thiamethoxam detection based on ZIF-67 derived Co-N doped porous carbon. Bioelectrochemistry 2023, 149, 108317. [Google Scholar] [CrossRef] [PubMed]
- Syzgantseva, M.A.; Ireland, C.P.; Ebrahim, F.M.; Smit, B.; Syzgantseva, O.A. Metal Substitution as the Method of Modifying Electronic Structure of Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 6271–6278. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Hirano, A.; Tanaka, Y.; Akiyoshi, R.; Yoshikawa, H.; Tanaka, D. Synthesis of Mixed-Metal MIL-68 under Mild Conditions by Controlling Nucleation Using a Microfluidic System. Cryst. Growth Des. 2022, 22, 4139–4145. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Xie, L.-H.; Xu, M.-M.; Liu, X.-M.; Zhao, M.-J.; Li, J.-R. Hydrophobic Metal–Organic Frameworks: Assessment, Construction, and Diverse Applications. Adv. Sci. 2020, 7, 1901758. [Google Scholar] [CrossRef] [PubMed]
- Fasna, F.P.H.; Sasi, S. A Comprehensive Overview on Advanced Sensing Applications of Functional Metal Organic Frameworks (MOFs). Chem. Sel. 2021, 6, 6365–6379. [Google Scholar]
- Assen, A.H.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. MOFs for the Sensitive Detection of Ammonia: Deployment of fcu-MOF Thin Films as Effective Chemical Capacitive Sensors. ACS Sens. 2017, 2, 1294–1301. [Google Scholar] [CrossRef]
- Dong, W.-D.; Li, Z.; Wen, W.; Liu, B.; Wen, G. Novel CdS/MOF Cathodic Photoelectrochemical (PEC) Platform for the Detection of Doxorubicin Hydrochloride and Gentamicin Sulfate. ACS Appl. Mater. Interfaces 2021, 13, 57497–57504. [Google Scholar] [CrossRef]
- Li, Y.; Jin, J.; Wang, D.; Lv, J.; Hou, K.; Liu, Y.; Chen, C.; Tang, Z. Coordination-responsive drug release inside gold nanorod@metal-organic framework core–shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy. Nano Res. 2018, 11, 3294–3305. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Hu, H.; Zhou, X.; You, F.; Yao, C.; Liu, F.-J.; Yu, P.; Wu, D.; Yao, J.; et al. Advanced Metal–Organic Frameworks-Based Catalysts in Electrochemical Sensors. Front. Chem. 2022, 10, 881172. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Wang, M.; Manickam, S.; Boczkaj, G.; Yoon, J.Y.; Sun, X. Metal-Organic Frameworks-Based Sensors for the Detection of Toxins in Food: A Critical Mini-Review on the Applications and Mechanisms. Front. Bioeng. Biotechnol. 2022, 10, 906374. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Velasco, E.; Li, J. Metal-organic frameworks as effective sensors and scavengers for toxic environmental pollutants. Natl. Sci. Rev. 2022, 9, nwac091. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Ahmad, N.; Nawaz, S.; Sher, F. Photocatalytic and adsorptive remediation of hazardous environmental pollutants by hybrid nanocomposites. Case Stud. Chem. Environ. Eng. 2020, 2, 100037. [Google Scholar] [CrossRef]
- Moret, S.; Scott, E.; Barone, A.; Liang, K.; Lennard, C.; Roux, C.; Spindler, X. Metal-Organic Frameworks for fingermark detection—A feasibility study. Forensic Sci. Int. 2018, 291, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
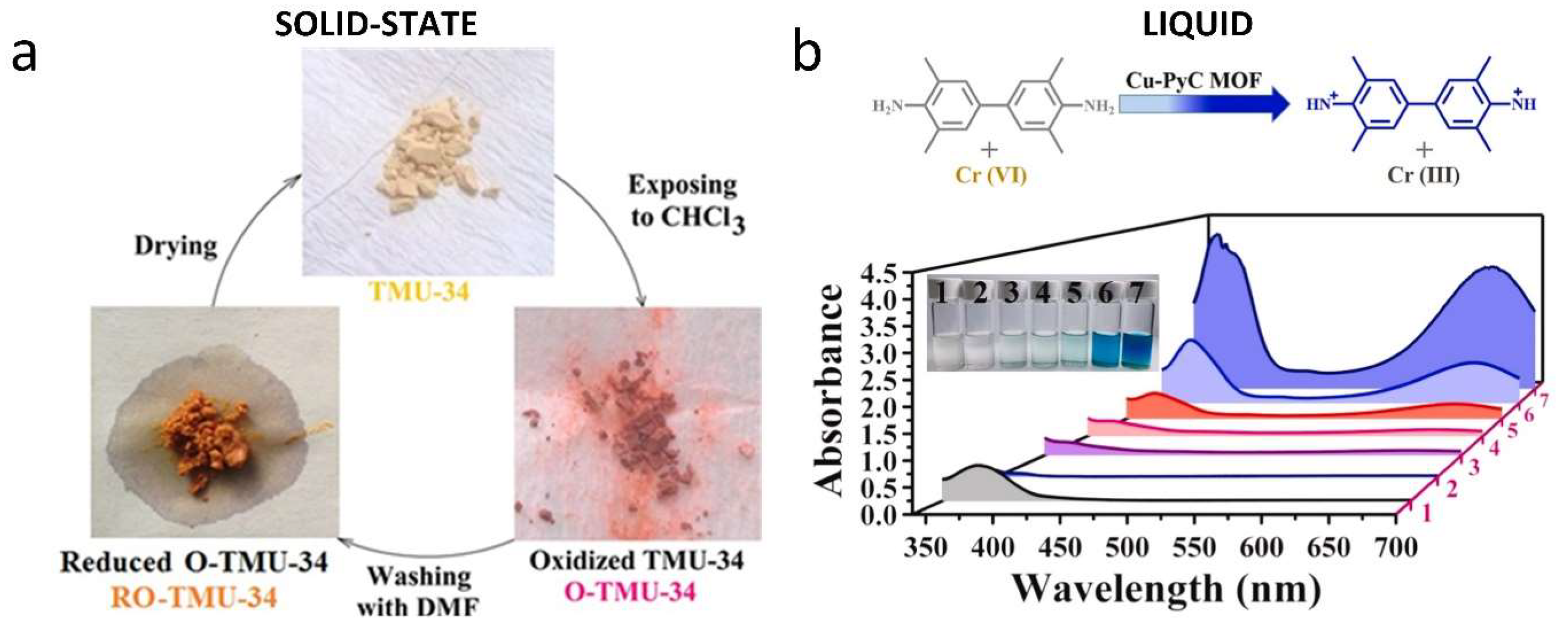
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Stimuli-Responsive Metal–Organic Framework (MOF) with Chemo-Switchable Properties for Colorimetric Detection of CHCl3. Chem. Eur. J. 2017, 23, 12559–12564. [Google Scholar] [CrossRef]
- Ullman, A.M.; Jones, C.G.; Doty, F.P.; Stavila, V.; Talin, A.A.; Allendorf, M.D. Hybrid Polymer/Metal–Organic Framework Films for Colorimetric Water Sensing over a Wide Concentration Range. ACS Appl. Mater. Interfaces 2018, 10, 24201–24208. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuan, J.; Guo, X.-q.; Ma, L.-j.; Wu, G.-q.; Cheng, G.J.; Liu, Y.; Liu, F. Ultrahigh Sensitive Flexible Piezoresistive Sensor with Carbonized Metal–Organic Framework Fe3O4@MIL-100(Fe). ACS Appl. Electron. Mater. 2022, 4, 1723–1731. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Hasanzadeh, M.; Simchi, A. Self-Powered Wearable Piezoelectric Sensors Based on Polymer Nanofiber–Metal–Organic Framework Nanoparticle Composites for Arterial Pulse Monitoring. ACS Appl. Nano Mater. 2020, 3, 8742–8752. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Pérez-Yáñez, S.; García, J.A.; Fernández, B.; Cepeda, J.; Rodríguez-Diéguez, A. Magnetic and Photoluminescent Sensors Based on Metal-Organic Frameworks Built up from 2-aminoisonicotinate. Sci. Rep. 2020, 10, 8843. [Google Scholar] [CrossRef] [PubMed]
- Ricco, R.; Styles, M.J.; Falcaro, P. 12—MOF-based devices for detection and removal of environmental pollutants. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–426. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, T.; Fan, Y.; Wang, L.; Duan, Z.; Du, W.; Zhang, D.; Xu, J. A benzene vapor sensor based on a metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2020, 311, 127365. [Google Scholar] [CrossRef]
- Si, X.; Jiao, C.; Li, F.; Zhang, J.; Wang, S.; Liu, S.; Li, Z.; Sun, L.; Xu, F.; Gabelica, Z.; et al. High and selective CO2 uptake, H2storage and methanol sensing on the amine-decorated 12-connected MOF CAU-1. Energy Environ. Sci. 2011, 4, 4522–4527. [Google Scholar] [CrossRef]
- Zhan, X.-Q.; Yu, X.-Y.; Tsai, F.-C.; Ma, N.; Liu, H.-L.; Han, Y.; Xie, L.; Jiang, T.; Shi, D.; Xiong, Y. Magnetic MOF for AO7 Removal and Targeted Delivery. Crystals 2018, 8, 250. [Google Scholar] [CrossRef]
- Ashrafzadeh Afshar, E.; Taher, M.A.; Karimi-Maleh, H.; Karaman, C.; Joo, S.-W.; Vasseghian, Y. Magnetic nanoparticles based on cerium MOF supported on the MWCNT as a fluorescence quenching sensor for determination of 6-mercaptopurine. Environ. Pollut. 2022, 305, 119230. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Kim, R.K.; Kim, S.; Schütz, G.; Choi, K.M.; Oh, H. Metal Organic Frameworks as Tunable Linear Magnets. Phys. Status Solidi (A) 2020, 217, 1901000. [Google Scholar] [CrossRef]
- Nath, A.; Asha, K.S.; Mandal, S. Conductive Metal-Organic Frameworks: Electronic Structure and Electrochemical Applications. Chem. Eur. J. 2021, 27, 11482–11538. [Google Scholar] [CrossRef]
- Ye, B.; Gheorghe, A.; van Hal, R.; Zevenbergen, M.; Tanase, S. CO2 sensing under ambient conditions using metal–organic frameworks. Mol. Syst. Des. Eng. 2020, 5, 1071–1076. [Google Scholar] [CrossRef]
- Xiong, J.; Tan, W.; Cai, J.; Lu, K.; Mo, X. Photoelectrochemical sensor based on nickel phthalocyanine based metal organic framework for sensitive detection of Curcumin. Int. J. Electrochem. Sci. 2022, 17, 22048. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, K.; Yang, X.; Nie, G. Photoelectrochemical sensor for detection Hg2+ based on in situ generated MOFs-like structures. Anal. Chim. Acta 2022, 1233, 340496. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, J.; Wu, Y.; Pang, C.; Li, S.; Li, J.; Xiong, Y.; Luo, J.; Wang, M.; Xu, Z. Recent advances in metal/covalent organic framework-based materials for photoelectrochemical sensing applications. Trends Anal. Chem. 2022, 157, 116793. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Podborska, A.; Luty-Błocho, M. Molecular structure of methyl orange and its role in the process of [Pd(Azo)] compound and MOF formation. J. Mol. Struct. 2023, 1273, 134312. [Google Scholar] [CrossRef]
- Farrugia, K.N.; Makuc, D.; Podborska, A.; Szaciłowski, K.; Plavec, J.; Magri, D.C. Colorimetric Naphthalene-Based Thiosemicarbazide Anion Chemosensors with an Internal Charge Transfer Mechanism. Eur. J. Org. Chem. 2016, 2016, 4415–4422. [Google Scholar] [CrossRef]
- Kulandaivel, S.; Lo, W.-C.; Lin, C.-H.; Yeh, Y.-C. Cu-PyC MOF with oxidoreductase-like catalytic activity boosting colorimetric detection of Cr(VI) on paper. Anal. Chim. Acta 2022, 1227, 340335. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Gao, X.-j.; Ge, F.; Zheng, H.-g. Metal–organic frameworks (MOFs) as fluorescence sensors: Principles, development and prospects. CrystEngComm 2022, 24, 7881–7901. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Reddy, A.S.; Panda, A.; Sarkar, D.; Son, Y.; Yoon, M. Reversible Fluorescence Switching of Metal–Organic Framework Nanoparticles for Use as Security Ink and Detection of Pb2+ Ions in Aqueous Media. ACS Appl. Nano Mater. 2020, 3, 3684–3692. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Zhang, J.; Hu, H.; Wu, X.; Long, Z.; Hou, X. Facile colorimetric sensing of Pb2+ using bimetallic lanthanide metal-organic frameworks as luminescent probe for field screen analysis of lead-polluted environmental water. Microchem. J. 2017, 134, 140–145. [Google Scholar] [CrossRef]
- Vaid, K.; Dhiman, J.; Kumar, S.; Kim, K.-H.; Kumar, V. Mixed metal (cobalt/molybdenum) based metal-organic frameworks for highly sensitive and specific sensing of arsenic (V): Spectroscopic versus paper-based approaches. Chem. Eng. J. 2021, 426, 131243. [Google Scholar] [CrossRef]
- Liu, S.; Liu, M.; Guo, M.; Wang, Z.; Wang, X.; Cui, W.; Tian, Z. Development of Eu-based metal-organic frameworks (MOFs) for luminescence sensing and entrapping of arsenate ion. J. Lumin. 2021, 236, 118102. [Google Scholar] [CrossRef]
- Lv, J.; Wang, B.; Xie, Y.; Wang, P.; Shu, L.; Su, X.; Li, J.-R. Selective detection of two representative organic arsenic compounds in aqueous medium with metal–organic frameworks. Environ. Sci. Nano 2019, 6, 2759–2766. [Google Scholar] [CrossRef]
- Dutta, S.; Let, S.; Shirolkar, M.M.; Desai, A.V.; Samanta, P.; Fajal, S.; More, Y.D.; Ghosh, S.K. A luminescent cationic MOF for bimodal recognition of chromium and arsenic based oxo-anions in water. Dalton Trans. 2021, 50, 10133–10141. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.-S.; Hu, H.-C.; Xu, H.; Qiao, W.-Z.; Zhao, B. Two solvent-stable MOFs as a recyclable luminescent probe for detecting dichromate or chromate anions. CrystEngComm 2016, 18, 4445–4451. [Google Scholar] [CrossRef]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Laiya, R.; Suresh, E. Mechanochemical and Conventional Synthesis of Zn(II)/Cd(II) Luminescent Coordination Polymers: Dual Sensing Probe for Selective Detection of Chromate Anions and TNP in Aqueous Phase. Inorg. Chem. 2017, 56, 2627–2638. [Google Scholar] [CrossRef]
- Kaur, H.; Sinha, S.; Krishnan, V.; Koner, R.R. Photocatalytic Reduction and Recognition of Cr(VI): New Zn(II)-Based Metal–Organic Framework as Catalytic Surface. Ind. Eng. Chem. Res. 2020, 59, 8538–8550. [Google Scholar] [CrossRef]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Suresh, E. Mixed-Ligand LMOF Fluorosensors for Detection of Cr(VI) Oxyanions and Fe3+/Pd2+ Cations in Aqueous Media. Inorg. Chem. 2017, 56, 10939–10949. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, X.-M.; Zhang, Q.; Jiang, H.-B.; Feng, R. A luminescent Zn-MOF exhibiting high water stability: Selective detection of Cr(VI) ion and treatment activity on sepsis. Des. Monomers Polym. 2021, 24, 218–225. [Google Scholar] [CrossRef]
- Adotey, E.K.; Amouei Torkmahalleh, M.; Balanay, M.P. Zinc metal–organic framework with 3-pyridinecarboxaldehyde and trimesic acid as co-ligands for selective detection of Cr (VI) ions in aqueous solution. Methods Appl. Fluoresc. 2020, 8, 045007. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. Hydrolytically Stable Luminescent Cationic Metal Organic Framework for Highly Sensitive and Selective Sensing of Chromate Anions in Natural Water Systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.-J.; Ju, Y.; Li, X.; Qian, J.; He, M.-Y.; Wang, J.-Q.; Zhang, Z.-H.; Lin, J. A MOF-based luminometric sensor for ultra-sensitive and highly selective detection of chromium oxyanions. Talanta 2023, 252, 123894. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Guo, G.; Ding, W.-M.; Han, W.-Y.; Li, J.; Deng, Z.-P. A highly stable Eu-MOF multifunctional luminescent sensor for the effective detection of Fe3+, Cr2O72−/CrO42− and aspartic acid in aqueous systems. CrystEngComm 2022, 24, 1358–1367. [Google Scholar] [CrossRef]
- Qiu, L.; Ma, Z.; Li, P.; Hu, X.; Chen, C.; Zhu, X.; Liu, M.; Zhang, Y.; Li, H.; Yao, S. Sensitive and selective detection of chromium (VI) based on two-dimensional luminescence metal organic framework nanosheets via the mechanism integrating chemical oxidation-reduction and inner filter effect. J. Hazard. Mater. 2021, 419, 126443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, H.; Yang, Y.; Qian, G.; Cui, Y. Cationic Metal–Organic Framework-Based Mixed-Matrix Membranes for Fast Sensing and Removal of Cr2O72− Within Water. Front. Chem. 2022, 10, 852402. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Yan, T.; Li, Y.; Liu, H.; Zhang, Y.; Wu, D.; Du, B.; Wei, Q. Electrogenerated Chemiluminescence Behavior of Au nanoparticles-hybridized Pb (II) metal-organic framework and its application in selective sensing hexavalent chromium. Sci. Rep. 2016, 6, 22059. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Ho, P.H.; Vu, P.D.; Pham, T.L.D.; Trens, P.; Di Renzo, F.; Phan, N.T.S.; Le, H.V. Efficient Removal of Chromium(VI) Anionic Species and Dye Anions from Water Using MOF-808 Materials Synthesized with the Assistance of Formic Acid. Nanomaterials 2021, 11, 1398. [Google Scholar] [CrossRef]
- Serre, C.; Mellot-Draznieks, C.; Surblé, S.; Audebrand, N.; Filinchuk, Y.; Férey, G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef]
- Devkota, J.; Kim, K.-J.; Ohodnicki, P.R.; Culp, J.T.; Greve, D.W.; Lekse, J.W. Zeolitic imidazolate framework-coated acoustic sensors for room temperature detection of carbon dioxide and methane. Nanoscale 2018, 10, 8075–8087. [Google Scholar] [CrossRef]
- Jain, P.; Ramachandran, V.; Clark, R.J.; Zhou, H.D.; Toby, B.H.; Dalal, N.S.; Kroto, H.W.; Cheetham, A.K. Multiferroic Behavior Associated with an Order−Disorder Hydrogen Bonding Transition in Metal−Organic Frameworks (MOFs) with the Perovskite ABX3 Architecture. J. Am. Chem. Soc. 2009, 131, 13625–13627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, N.; Xu, Y.; Pang, H. Two-Dimensional MOF and COF Nanosheets: Synthesis and Applications in Electrochemistry. Chem. Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef] [PubMed]
- Sappia, L.D.; Tuninetti, J.S.; Ceolín, M.; Knoll, W.; Rafti, M.; Azzaroni, O. MOF@PEDOT Composite Films for Impedimetric Pesticide Sensors. Glob. Chall. 2020, 4, 1900076. [Google Scholar] [CrossRef] [PubMed]
- Chansi; Bhardwaj, R.; Rao, R.P.; Mukherjee, I.; Agrawal, P.K.; Basu, T.; Bharadwaj, L.M. Layered construction of nano immuno-hybrid embedded MOF as an electrochemical sensor for rapid quantification of total pesticides load in vegetable extract. J. Electroanal. Chem. 2020, 873, 114386. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical investigation of a new Cu-MOF and its electrocatalytic activity towards H2O2 oxidation in alkaline solution. Electrochem. Commun. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Xie, J.; Hu, X. Metal–organic framework modified carbon paste electrode for lead sensor. Sens. Actuators B Chem. 2013, 177, 1161–1166. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Li, J.; Ju, H. Electrochemical Sensor for Lead Cation Sensitized with a DNA Functionalized Porphyrinic Metal–Organic Framework. Anal. Chem. 2015, 87, 10635–10641. [Google Scholar] [CrossRef] [PubMed]
- Bodkhe, G.A.; Hedau, B.S.; Deshmukh, M.A.; Patil, H.K.; Shirsat, S.M.; Phase, D.M.; Pandey, K.K.; Shirsat, M.D. Selective and sensitive detection of lead Pb(II) ions: Au/SWNT nanocomposite-embedded MOF-199. J. Mater. Sci. 2021, 56, 474–487. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, G.; Shang, X.; Hu, B.; Zhang, B.; Tang, Z.; Yang, J.; Liu, J. An Fe-MOF/MXene-based ultra-sensitive electrochemical sensor for arsenic(III) measurement. J. Electroanal. Chem. 2022, 916, 116382. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B Chem. 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Saraf, M.; Rajak, R.; Mobin, S.M. A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J. Mater. Chem. A 2016, 4, 16432–16445. [Google Scholar] [CrossRef]
- Deep, A.; Bhardwaj, S.K.; Paul, A.K.; Kim, K.-H.; Kumar, P. Surface assembly of nano-metal organic framework on amine functionalized indium tin oxide substrate for impedimetric sensing of parathion. Biosens. Bioelectron. 2015, 65, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, M.; Li, Z.; He, L.; Hu, M.; He, L.; Zhang, Z.; Du, M. A bimetallic CoNi-based metal−organic framework as efficient platform for label-free impedimetric sensing toward hazardous substances. Sens. Actuators B Chem. 2020, 311, 127927. [Google Scholar] [CrossRef]
- Syzgantseva, M.A.; Stepanov, N.F.; Syzgantseva, O.A. Band Alignment as the Method for Modifying Electronic Structure of Metal−Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 17611–17619. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yan, T.; Wu, T.; Zhang, N.; Yang, Q.; Sun, M.; Yan, L.; Du, B.; Wei, Q. A label-free photoelectrochemical aptasensing platform base on plasmon Au coupling with MOF-derived In2O3@g-C3N4 nanoarchitectures for tetracycline detection. Sens. Actuators B Chem. 2019, 298, 126817. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Zhuang, Y.-H.; Shan, D.; Su, G.-F.; Cosnier, S.; Zhang, X.-J. Zirconium-Based Porphyrinic Metal–Organic Framework (PCN-222): Enhanced Photoelectrochemical Response and Its Application for Label-Free Phosphoprotein Detection. Anal. Chem. 2016, 88, 11207–11212. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Zhang, X.; Ding, Z.; Xu, X.; Cai, X.; Wang, Y.; Li, C.; Sun, D. Fabrication of a high-performance photoelectrochemical aptamer sensor based on Er-MOF nanoballs functionalized with ionic liquid and gold nanoparticles for aflatoxin B1 detection. Sens. Actuators B Chem. 2023, 378, 133153. [Google Scholar] [CrossRef]
- Dave, S.; Jone Kirubavathy, S. Chapter 11—Biosensors based on metal-organic framework (MOF): Paving the way to point-of-care diagnosis. In Electrochemical Applications of Metal-Organic Frameworks; Dave, S., Sahu, R., Tripathy, B.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 255–267. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Shakib, S.; Majidi, M.R.; Razmjou, A.; Yoon, Y.; Khataee, A. Metal–Organic Framework-Based Biosensing Platforms for the Sensitive Determination of Trace Elements and Heavy Metals: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 4611–4627. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Lai, J.; Zhu, W.; Fan, C.; Hao, N.; Wei, J.; Qian, J.; Wang, K. Turning on High-Sensitive Organic Electrochemical Transistor-Based Photoelectrochemical-Type Sensor over Modulation of Fe-MOF by PEDOT. Adv. Funct. Mater. 2022, 32, 2202735. [Google Scholar] [CrossRef]
- Wang, L.; Wu, F.; Chen, X.; Ren, J.; Lu, X.; Yang, P.; Xie, J. Defective Metal–Organic Framework Assisted with Nitrogen Doping Enhances the Photoelectrochemical Performance of BiVO4. ACS Appl. Energy Mater. 2021, 4, 13199–13207. [Google Scholar] [CrossRef]
- Gu, J.; Ban, C.; Meng, J.; Li, Q.; Long, X.; Zhou, X.; Liu, N.; Li, Z. Construction of dual Z-scheme UNiMOF/BiVO4/S-C3N4 photocatalyst for visible-light photocatalytic tetracycline degradation and Cr(VI) reduction. Appl. Surf. Sci. 2023, 611, 155575. [Google Scholar] [CrossRef]
- Jiao, W.; Zhu, J.; Ling, Y.; Deng, M.; Zhou, Y.; Feng, P. Photoelectrochemical properties of MOF-induced surface-modified TiO2 photoelectrode. Nanoscale 2018, 10, 20339–20346. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Shariatinia, S.; Zargari, S.; Yaghoubi Berijani, M.; Ghaffarinejad, A.; Shojaie, Z.S. Synthesis, characterization, and photocurrent generation of a new nanocomposite based Cu–TCPP MOF and ZnO nanorod. RSC Adv. 2015, 5, 46624–46631. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Wu, S.; Wang, L.; Hu, J.; Ma, J.; Jiang, W.; Zhang, N.; Zheng, X.; Gao, C.; et al. Metal–Organic Framework Coating Enhances the Performance of Cu2O in Photoelectrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 10924–10929. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Y.; Ji, W.; Gao, Z.; Zhang, J. Synthesis of a CdS-decorated Eu-MOF nanocomposite for the construction of a self-powered photoelectrochemical aptasensor. Analyst 2019, 144, 6617–6624. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Zhang, X.; He, M.; Zhang, J.; Liu, P.; Tang, X.; Li, C.; Wang, Y. Eu-MOF nanorods functionalized with large heterocyclic ionic liquid for photoelectrochemical immunoassay of a-fetoprotein. Anal. Chim. Acta 2022, 1195, 339459. [Google Scholar] [CrossRef]
- Qin, X.; Pan, Y.; Zhang, J.; Shen, J.; Li, C. Ionic liquid functionalized trapezoidal Zn-MOF nanosheets integrated with gold nanoparticles for photoelectrochemical immunosensing alpha-fetoprotein. Talanta 2023, 253, 123684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, Q.; Zhang, H.; Yang, P.; You, T. A self-powered photoelectrochemical oxytetracycline aptasensor: An integrated heterojunction photoanode of metal-organic framework derived ZnO nanopolyhedra/graphitic carbon nitride with high carrier density. J. Colloid Interface Sci. 2023, 632, 35–43. [Google Scholar] [CrossRef]
- Gao, K.; Li, H.; Meng, Q.; Wu, J.; Hou, H. Efficient and Selective Visible-Light-Driven Oxidative Coupling of Amines to Imines in Air over CdS@Zr-MOFs. ACS Appl. Mater. Interfaces 2021, 13, 2779–2787. [Google Scholar] [CrossRef]
- Liu, X.; He, L.; Zheng, J.; Guo, J.; Bi, F.; Ma, X.; Zhao, K.; Liu, Y.; Song, R.; Tang, Z. Solar-Light-Driven Renewable Butanol Separation by Core–Shell Ag@ZIF-8 Nanowires. Adv. Mater. 2015, 27, 3273–3277. [Google Scholar] [CrossRef]
- Zheng, G.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Plasmonic metal-organic frameworks. SmartMat 2021, 2, 446–465. [Google Scholar] [CrossRef]
- Jian, Z.-W.; Zhao, T.-T.; Li, C.-M.; Li, Y.-F.; Huang, C.-Z. 2D MOF-Based Photoelectrochemical Aptasensor for SARS-CoV-2 Spike Glycoprotein Detection. ACS Appl. Mater. Interfaces 2021, 13, 49754–49761. [Google Scholar] [CrossRef] [PubMed]
- Hoener, B.S.; Kirchner, S.R.; Heiderscheit, T.S.; Collins, S.S.E.; Chang, W.-S.; Link, S.; Landes, C.F. Plasmonic Sensing and Control of Single-Nanoparticle Electrochemistry. Chem 2018, 4, 1560–1585. [Google Scholar] [CrossRef]
- Xiao, K.; Zhu, R.; Zhang, X.; Du, C.; Chen, J. Ultrasensitive detection and efficient removal of mercury ions based on covalent organic framework spheres with double active sites. Anal. Chim. Acta 2023, 1278, 341751. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Guan, G.; Deng, H.; Han, B.; Tian, C.; Zhuang, J.; Xu, Y.; Liu, W.; Lin, Z. PCN-224/rGO nanocomposite based photoelectrochemical sensor with intrinsic recognition ability for efficient p-arsanilic acid detection. Environ. Sci. Nano 2019, 6, 207–215. [Google Scholar] [CrossRef]
- Yu, Q.; Fu, Y.; Xiao, K.; Zhang, X.; Du, C.; Chen, J. A label-free photoelectrochemical biosensor with ultra-low-background noise for lead ion assay based on the Cu2O-CuO-TiO2 heterojunction. Anal. Chim. Acta 2022, 1195, 339456. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, C.; Zhang, P. Mercury Deposition, Climate Change and Anthropogenic Activities: A Review. Front. Earth Sci. 2020, 8, 316. [Google Scholar] [CrossRef]
- Díaz, J.A.; Serrano, J.; Leiva, E. Bioleaching of Arsenic-Bearing Copper Ores. Minerals 2018, 8, 215. [Google Scholar] [CrossRef]
- He, D.; Xiong, Y.; Wang, L.; Sun, W.; Liu, R.; Yue, T. Arsenic (III) Removal from a High-Concentration Arsenic (III) Solution by Forming Ferric Arsenite on Red Mud Surface. Minerals 2020, 10, 583. [Google Scholar] [CrossRef]
- Cheriton, L.W.; Gupta, J.P. Building materials. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 304–314. [Google Scholar] [CrossRef]
- Hill, S.J. Chapter 9—Lead. In Techniques and Instrumentation in Analytical Chemistry; Stoeppler, M., Ed.; Elsevier: Oxford, UK, 1992; Volume 12, pp. 231–255. [Google Scholar]
- Yang, Y.; Wei, H.; Wang, X.; Sun, D.; Yu, L.; Bai, B.; Jing, X.; Qin, S.; Qian, H. MOF/COF heterostructure hybrid composite-based molecularly imprinted photoelectrochemical sensing platform for determination of dibutyl phthalate: A further expansion for MOF/COF application. Biosens. Bioelectron. 2023, 223, 115017. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Q.; Yu, L.; Hu, X. Photoelectrochemical detection of the herbicide clethodim by using the modified metal-organic framework amino-MIL-125(Ti)/TiO2. Microchim. Acta 2015, 182, 1885–1892. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Hu, X.; Liu, Y.; Wang, G. Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Zhuge, W.; Liu, Y.; Huang, W.; Zhang, C.; Wei, L.; Peng, J. Conductive 2D phthalocyanine-based metal-organic framework as a photoelectrochemical sensor for N-acetyl-L-cysteine detection. Sens. Actuators B Chem. 2022, 367, 132028. [Google Scholar] [CrossRef]
- Xu, M.; Chen, K.; Zhu, L.; Wang, M.; He, L.; Zhang, Z.; Du, M. MOF@COF Heterostructure Hybrid for Dual-Mode Photoelectrochemical−Electrochemical HIV-1 DNA Sensing. Langmuir 2021, 37, 13479–13492. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ma, X.; Wu, Y.; Pang, C.; Li, S.; Li, J.; Xiong, Y.; Luo, J.; Wang, M.; Xu, Z. Ligand-variable metal clusters charge transfer in Ce-Por-MOF/AgNWs and their application in photoelectrochemical sensing of ronidazole. Microchim. Acta 2022, 189, 383. [Google Scholar] [CrossRef]
- Hu, C.; Pan, P.; Huang, H.; Liu, H. Cr-MOF-Based Electrochemical Sensor for the Detection of P-Nitrophenol. Biosensors 2022, 12, 813. [Google Scholar] [CrossRef]




| Abbreviation and Its Interpretation | Examples | Chemical Formula: Cluster (Ligand) | Crystal Structure | Refs. |
|---|---|---|---|---|
| MOF Metal–Organic Frameworks | MOF-5 | Zn4O(BDC)3 (BDC = 1,4-benzenedicarboxylic acid) | 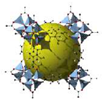 | [9,12] |
| MOF-74 | Zn2DOT (DOT = 2,5-dihydroxyterephthalate) | 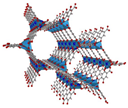 | [11,13] | |
| MOF-545 | Zr6O8(H2O)8(TCPP-H2)2 (TCPP = tetracarboxy- phenylporphyrin) | 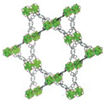 | [14] | |
| IRMOF Isoreticular Metal–Organic Framework | IRMOF-1 (MOF-5) | Zn4O(BDC)3·7DEF·3H2O (BDC = 1,4-benzenedicarboxylic acid) | 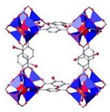 | [6] |
| UiO Universitet in Oslo | UiO-66 | Zr6O6(BDC)6 (BDC = 1,4-benzenedicarboxylic acid) | 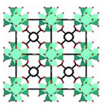 | [10,17,24] |
| UiO-67 | Zr6O6(BPDC)6 (BPDC = biphenyl-4,4′-dicarboxylate) |  | [10] | |
| UiO-68 | Zr6O6(TPDC)6 (TPDC = [1,1′:4′,1″-terphenyl]-4,4″-dicarboxylate) | 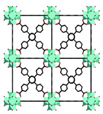 | [10] | |
| MIL Materials of Institut Lavoisier | MIL-125 | Ti8O8(OH)4–(BDC)6 (BDC = 1,4-benzenedicarboxylic acid) |  | [8] |
| MIL-68 | [Fe(OH)(COO)2]n (BDC = 1,4-benzenedicarboxylic acid) | 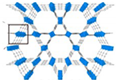 | [25] | |
| MIL-101 (Cr) | [Cr3(O)F(BDC)3(H2O)2] (BDC = 1,4-benzenedicarboxylic acid) | 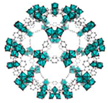 | [26] | |
| ZIF Zeolite Imidazolate Framework | ZIF-8 | Zn(mim)2 (mim = 2-methylimidazolate) |  | [21] |
| ZIF-67 | Co(Hmim)2, (Hmim = 2-methylimidazole) | 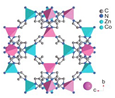 | [27] | |
| ZIF-300 | [Zn(2-mim)0.86(5-bbim)1.14] (mim = 2-methylimidazole bbim = 5-bromobenzimidazol) | 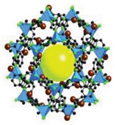 | [28] | |
| HKUST Hong Kong University of Science and Technology | HKUST-1 | [(Cu)3(BTC)2] (BTC—benzene tricarboxylate) |  | [19] |
| Sensing Materials | Analyte | Linear Detection Range | Sensitivity/ Detection Limit | Reference |
|---|---|---|---|---|
| HMA/Eu3+-CdS | Hg2+ | 0.1 pM to 1.0 μM | 0.067 pM | [55] |
| TFPB−APTU COF | Hg2+ | 10 pM to 100 μM | 0.006 nM | [117] |
| PCN-224/rGO | p-ASA | 10 ng/L to 10 mg/L | 5.47 ng/L | [118] |
| Cu-modified NH2-MIL-125 | Pb2+ | 10 fM–1 µM | 6.8 fM | [119] |
| Sensing Materials | Analyte | Linear Detection Range | Sensitivity/ Detection Limit | Reference |
|---|---|---|---|---|
| NH2-UiO-66/TpPa-1-COF | dibutyl phthalate (DBP) | 1.0 × 10−10–1.0 × 10−4 M | 3.0 × 10−11 M | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luty-Błocho, M.; Podborska, A. The Diversity of MOF Structures and Their Impact on Photoelectrochemical Sensors for Monitoring Environmental Pollution. Crystals 2024, 14, 626. https://doi.org/10.3390/cryst14070626
Luty-Błocho M, Podborska A. The Diversity of MOF Structures and Their Impact on Photoelectrochemical Sensors for Monitoring Environmental Pollution. Crystals. 2024; 14(7):626. https://doi.org/10.3390/cryst14070626
Chicago/Turabian StyleLuty-Błocho, Magdalena, and Agnieszka Podborska. 2024. "The Diversity of MOF Structures and Their Impact on Photoelectrochemical Sensors for Monitoring Environmental Pollution" Crystals 14, no. 7: 626. https://doi.org/10.3390/cryst14070626
APA StyleLuty-Błocho, M., & Podborska, A. (2024). The Diversity of MOF Structures and Their Impact on Photoelectrochemical Sensors for Monitoring Environmental Pollution. Crystals, 14(7), 626. https://doi.org/10.3390/cryst14070626







