Abstract
The crystallization behavior of avobenzone in cosmetic formulations has been investigated with a focus on its interaction with titanium dioxide and zinc oxide particles. Characterization studies using SEM, powder X-ray diffraction (PXRD), Raman spectroscopy, and energy-dispersive X-ray spectroscopy (EDS) reveal that avobenzone undergoes crystallization facilitated by nucleation on the surfaces of these metal oxide grains. The presence of wax and titanium oxide within the crystalline structures further suggests a complex formation, potentially involving catalytic effects on avobenzone nucleation and isomerization. Notably, the addition of ascorbyl palmitate inhibits unwanted crystallization, possibly through competitive complexation with exposed metal ions. These findings underscore the significance of formulation modifications in stabilizing avobenzone against crystallization, ensuring enhanced product stability in cosmetic applications. Future structural studies are anticipated to elucidate the precise nature of these co-crystalline phases, offering insights into optimizing sunscreen formulations for improved performance and longevity.
1. Introduction
Color cosmetics containing sun filters, as any thermodynamic system, may experience stability problems resulting from photodegradation, oxidation, phase separation, and recrystallization. For most of these problems, solutions have been proposed that can be found in the available literature, but the recrystallization of organic UV filters in finished products is still a significant problem. Due to the scarcity of information on this topic, this issue still requires further research. If, during the shelf life of the product or when the preparation is applied to the skin, the organic filter in the cosmetic recrystallizes, the assumed sun protection factor will not be provided, and therefore the product will not work as expected. Research related to the recrystallization of filters confirmed changes in the absorbance value and SPF level as a result of this problem [1,2,3]. UV filters are complex structures with many functional groups capable of interacting with molecules of other components of the composition [4,5]. Molecular interactions between molecules of organic UV-filters and substances forming co-crystals or with solvent molecules can, respectively, contribute to the formation of polymorphic crystals, co-crystals, or solvates. Crystal structures arise from a series of weak, sometimes unexpected, directional molecular interactions [6]. At room temperature, a solid material tends to re-crystallize after melting because the free energy of the solid form is lower than that of the liquid state below the melting point [7]. The formation of transiently ordered aggregates of liquid components occurs in systems with high solute concentrations, where molecules eventually collide and combine. When the clusters become stable, the nucleation process begins—the first crystal nuclei are formed, which can dissolve or grow into crystals. Important factors controlling the crystallization rate in melts and supersaturated solutions are interfacial energy, kinetic effects, viscosity, and temperature [7,8]. Impurities and crystals of other substances present in the system, including inorganic substances (pigments, inorganic filters), can also accelerate the crystallization of organic filters. Crystal growth can be inhibited by additives (e.g., solvents, surfactants, polymers) because such particles can slow down molecular collisions by increasing the solubility of the substance, disturbing the solid–liquid interface, or increasing the viscosity of the liquid phase [7,9]. Sunscreens are designed for different shelf lives. After production, they are subject to various environmental factors such as temperature fluctuations, humidity, sunlight, and mechanical forces (shaking, squeezing, rubbing on the skin). These factors may influence thermodynamic processes and, consequently, contribute to recrystallization [10]. It is important to confirm the stability of the filter system used to ensure their appropriate effectiveness throughout the product’s shelf life [6]. Even in the same system, the interaction between different UV filters can change recrystallization tendencies. Endo et al. conducted research on the solubility and supersaturation stability of UVA absorbers in a UVB absorber in the absence and presence of cosmetic oil. By analysis of the effect of the coexistence of UVA absorbers in order to dissolve them in high concentrations [11], they showed that in the presence of two UVA radiation absorbers (avobenzone and benzoate diethylamino-hydroxybenzoyl hexyl), the use of a hydrophobic ester can improve the supersaturation stability and solubility of these compounds in the UVB absorber (ethylhexyl methoxycinnamate) [9].
Ivan D. da Silva Souza and his colleagues investigated the effectiveness of hydrophobic solubilizers in preventing the recrystallization of solid hydrophobic UV filters in solutions and sunscreen preparations and during simulated human use [3]. Recrystallization was induced by temperature changes, ultrasound, or simulated human use. In the theoretical part of their work, in order to predict whether a given substance would dissolve another, they calculated its solubility parameters (or cohesion energy) defined by Hansen [12,13]. Two substances will be more miscible if the Hansen solubility parameters are similar. Solubilizers are treated here as solvents that have the ability to increase the solubility of UV filters through the presence of functional groups with high affinity to the solvent and inhibit the filter nucleation processes [6]. Unfortunately, expectations regarding selected solubilizers dedicated to selected filters did not confirm this theory. Some of the solubilizer–filter mixtures performed worse and some performed better than the calculations indicated. Scientists suggest that the lack of convergence for solubility and recrystallization is due to different forces that determine when the UV filter crystal will dissolve or form [3]. In their study, they concluded that the best solubilizer is not always the best solvent that can prevent recrystallization, thus suggesting that dissolution and nucleation depend on different factors. The recrystallization of organic sunscreens in cosmetic masses presents a significant challenge, as it can lead to an uneven distribution, reduced efficacy, and undesirable texture in the final product, which in fact is very complicated, and every modification in the base recipe may lead to faster deterioration. This issue compromises the stability and performance of sunscreens, potentially diminishing their protective qualities and negatively affecting consumer satisfaction. Consequently, it is essential to seek innovative solutions to prevent recrystallization, ensuring consistent sunscreen application and optimal skin protection while maintaining the desired cosmetic properties.
The aim of our work was to identify the mechanism of recrystallization of organic sunscreens in anhydrous fluid foundation and find the solutions enabling the effective inhibition/elimination of this problem in cosmetic masses.
2. Materials
The subject of this research was an anhydrous cosmetic fluid showing destabilization in the form of crystalline precipitations in the volume and on the surface of the product. Table 1 shows the composition of the recipe of the product in which the crystallization occurred during stability tests. In an anhydrous cosmetic fluid which is a mixture of waxes and emollients with pigments, fillers, and active ingredients such as organic UV filters (homosalate, ethylhexyl salicylate, avobenzone, bemotrizinol) and inorganic filters (surface coated zinc oxide and titanium oxide (in the form of pigment)), during aging tests (under specified temperature conditions and time), after about 2 months, whitish crystalline precipitations appeared both on the surface and in the volume of the product (Figure 1).

Table 1.
Example composition of the anhydrous fluid (in which crystallization occurred after 60 days).
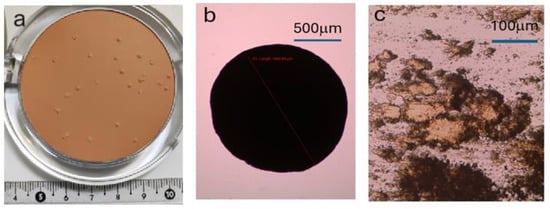
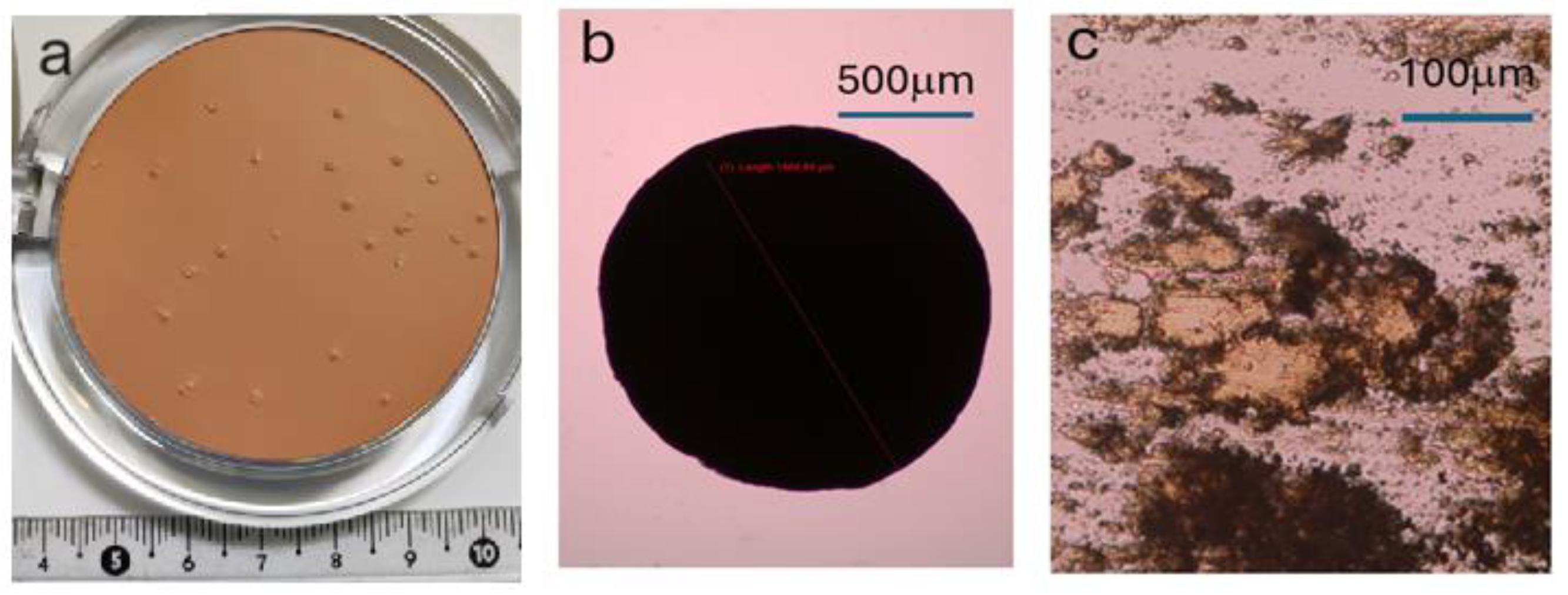
Figure 1.
(a) Photograph of cosmetic mass with precipitations visible on its surface. (b) Optical microscope image of crystallite isolated from cosmetic mass (red line indicates size of the crystallite: 1454μm). (c) Optical microscopy image of a crystallite showing its plate-like morphology (the observation sample was prepared by squeezing it between glass plates).
These crystalline precipitates were isolated from the cosmetic masses manually using tweezers, and repeatedly washed with acetone, dried in air, and further analyzed by means of optical microscopy to check their average size. Scanning electron microscopy (SEM) studies were performed with a PRISMA E scanning electron microscope (Thermo Fisher Scientific, Waltham, MA, USA) with an EDS X-ray microanalyzer (X-ray energy dispersion spectrometer) to determine the morphology of crystallites and their chemical composition. Prior to analysis, samples were sputtered with a thin layer of gold to avoid charging the sample surface. Room-temperature phase analysis was performed by means of powder X-ray diffraction measurements, using the BRUKER D8 ADVANCE diffractometer (Bragg–Brentano geometry; CuKα radiation; step size, 0.02°; 1 s/step; measurements performed in the 2Θ range of 10–60°). FT-IR ATR and Raman spectroscopies were used to confirm the chemical nature of the investigated materials. The FT-IR spectra were recorded at room temperature using a Thermo Scientific™ Fourier Transform Spectrometer, which utilizes the Attenuated Total Reflectance (ATR) phenomenon of IR radiation, in the range from 4000 to 500 cm−1 with a spectral resolution of 2 cm−1. Each spectrum was averaged from 32 scans using Nicolet Omnic 9 software. Raman spectra were collected at room temperature with the Raman Nicolet Almega XR Spectrometer (laser with a wavelength of 532 nm). To assess potential interactions, approximate models of the electronic structures of the molecules in question were created using the WinMOPAC 22.1.1 software and the PM3 semiempirical model for calculations.
3. Results and Discussion
Optical microscope images, taken with an optical microscope equipped with a digital camera (shown in Figure 1), revealed that the size of the crystallites reaches even the millimeter range, which makes them suitable for further studies.
Further examination of the morphology and chemical composition of crystalline precipitations isolated from masses was performed with SEM combined with EDS microanalysis. To prevent the surface from charging during the measurement, a thin layer of gold was evaporated onto the samples of the tested crystallites. The measurement was carried out in the backscattered electron (BSE) and secondary electron (SE) detection modes. The images are shown in Figure 2.

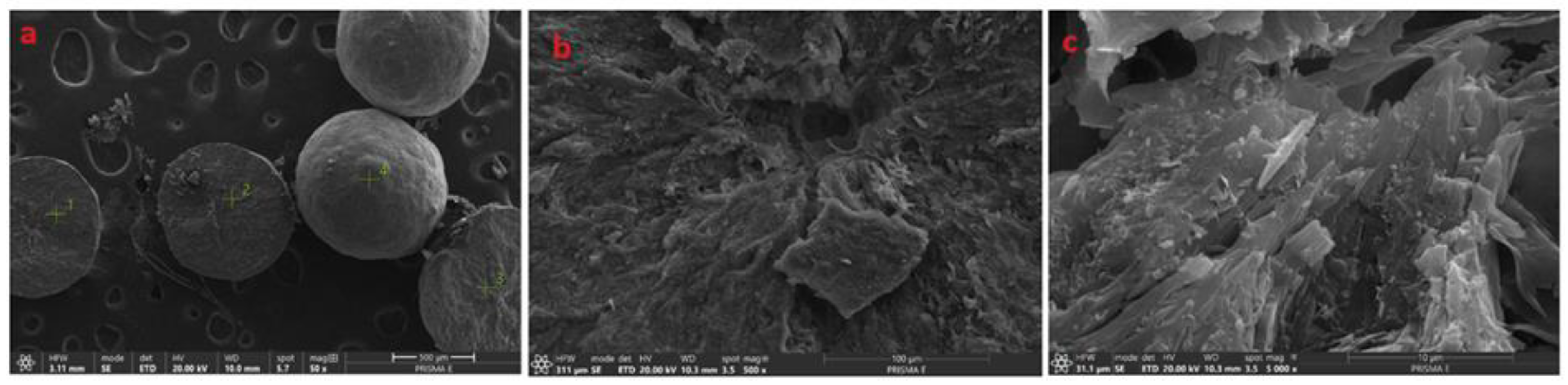
Figure 2.
SEM images of crystallites isolated from cosmetic mass with different magnifications. (a) 50×, (b) 500×, (c) 5000×.
The SEM micrographs of crystallites isolated from the cosmetic mass (Figure 2a) revealed their spherical shapes with sizes in the range of 300–900 μm. Larger magnifications (500× and 5000×), taken from cross-sections of these crystallites, presented in Figure 2b,c, more clearly show the layered morphology of the crystallite, which grows spherulitically from the nucleus located in the center of the spheroid. To identify the chemical composition and the nature of the material initiating the crystallization, chemical analyses using an EDS microprobe were performed and their results are presented in Figure 3.
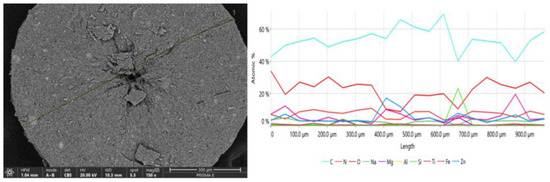
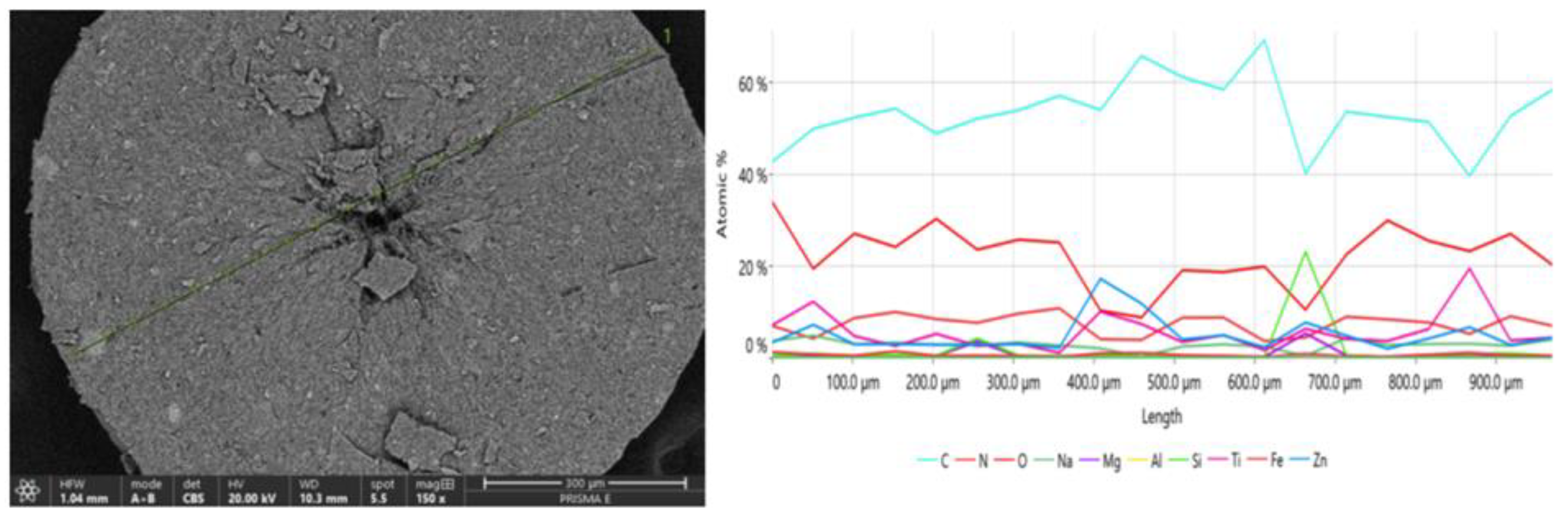
Figure 3.
SEM image of cross-section of the crystallite (magnification 150×); analysis of chemical composition was performed along the green line marked in the image. Concentration profiles (in at.%) for elements found in the measured sample of crystallized phase are shown in right panel.
The analysis of the chemical composition of the crystallite indicates some fluctuations along the cross-section of the crystal. A lower proportion of carbon and higher concentrations of titanium and zinc are observed on the crystallite surface, with a peak in the middle of the spheroid. The elemental composition determined at the crystallite surface differs from the analysis of the chemical composition made from a cross-section. This suggests that the product most likely separates part of the phase containing organic filter/filters and waxes around the grains of physical filters (TiO2 and ZnO). This is indeed confirmed by the analysis of chemical composition with the EDS microprobe, revealing that near the center of the crystallite, there is an increase in the amount of titanium (by about 10 at.%) and zinc (by about 20 at.%) compared to other areas of the sample, which may indicate that crystallization is induced by the interaction between avobenzone molecules (organic sunscreen) and inorganic oxide grains. An increased concentration of zinc was also observed in the near-surface area, most likely due to the shifting of the avobenzone crystallization front along with grain growth. This causes the zinc oxide nanosized grains to move to the near-surface area of the crystallite. Nitrogen that was also found in the chemical composition of the investigated crystallites can be assigned to the second organic filter TINOSORB S, which may show a tendency for co-crystallization with avobenzone.
To identify the crystallizing phases, powder X-ray diffraction measurements were performed. Diffraction data for crystals isolated from the cosmetic mass were recorded and compared to diffractograms of raw materials (organic UV filters, waxes, emulsifiers, etc.). The corresponding diffraction data are shown in Figure 4.
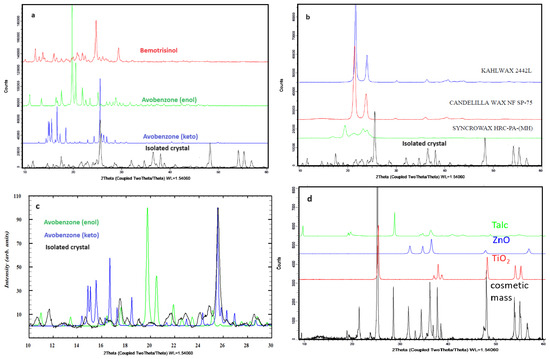
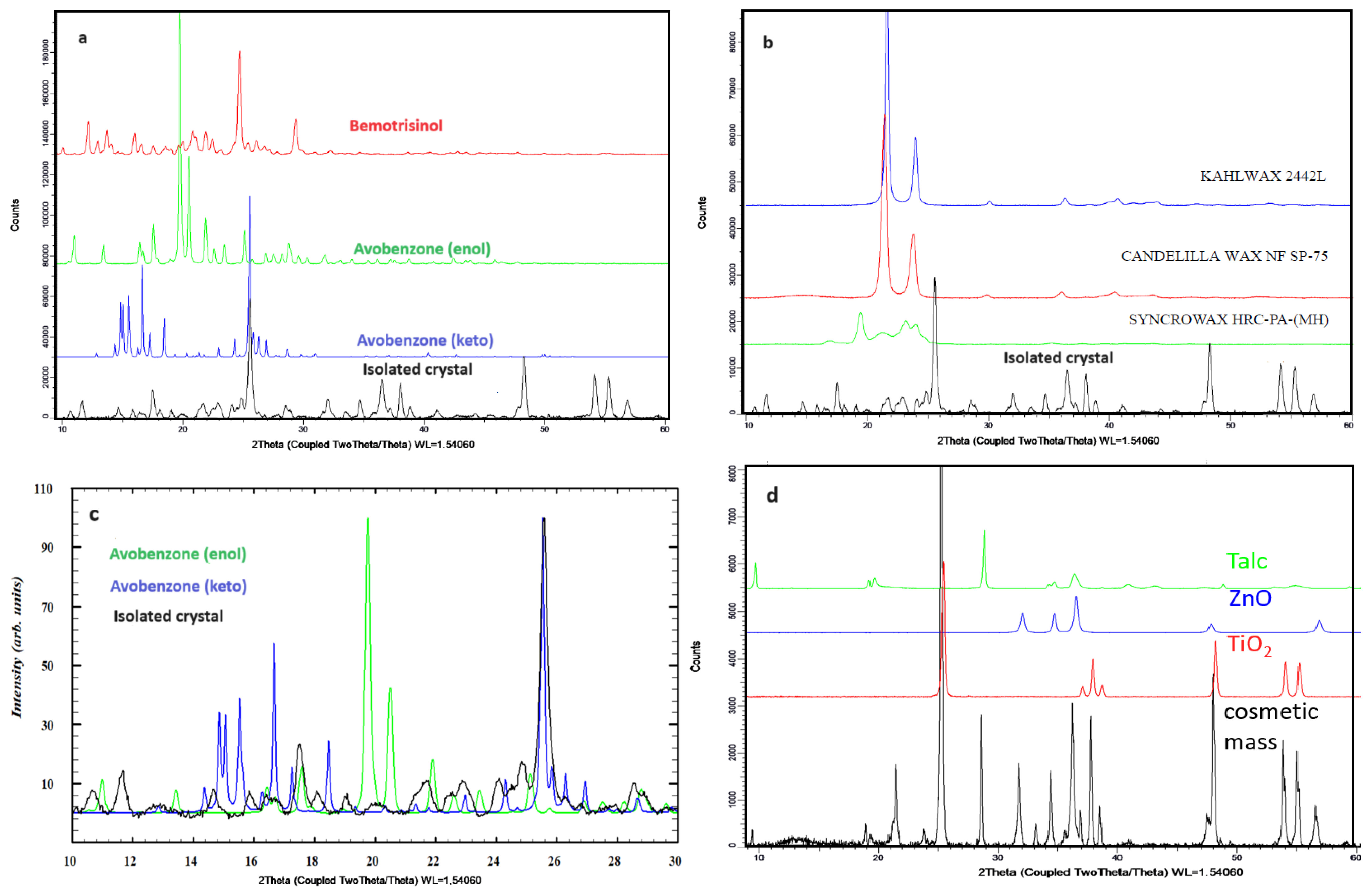
Figure 4.
Powder X-ray diffraction patterns for (a) organic filters and isolated crystals, (b) waxes and isolated crystals, (c) enlarged 2Θ region with the comparison of diffraction patterns of isolated crystals and two forms of avobenzone (normalized for better visibility), (d) inorganic crystalline raw materials and cosmetic mass.
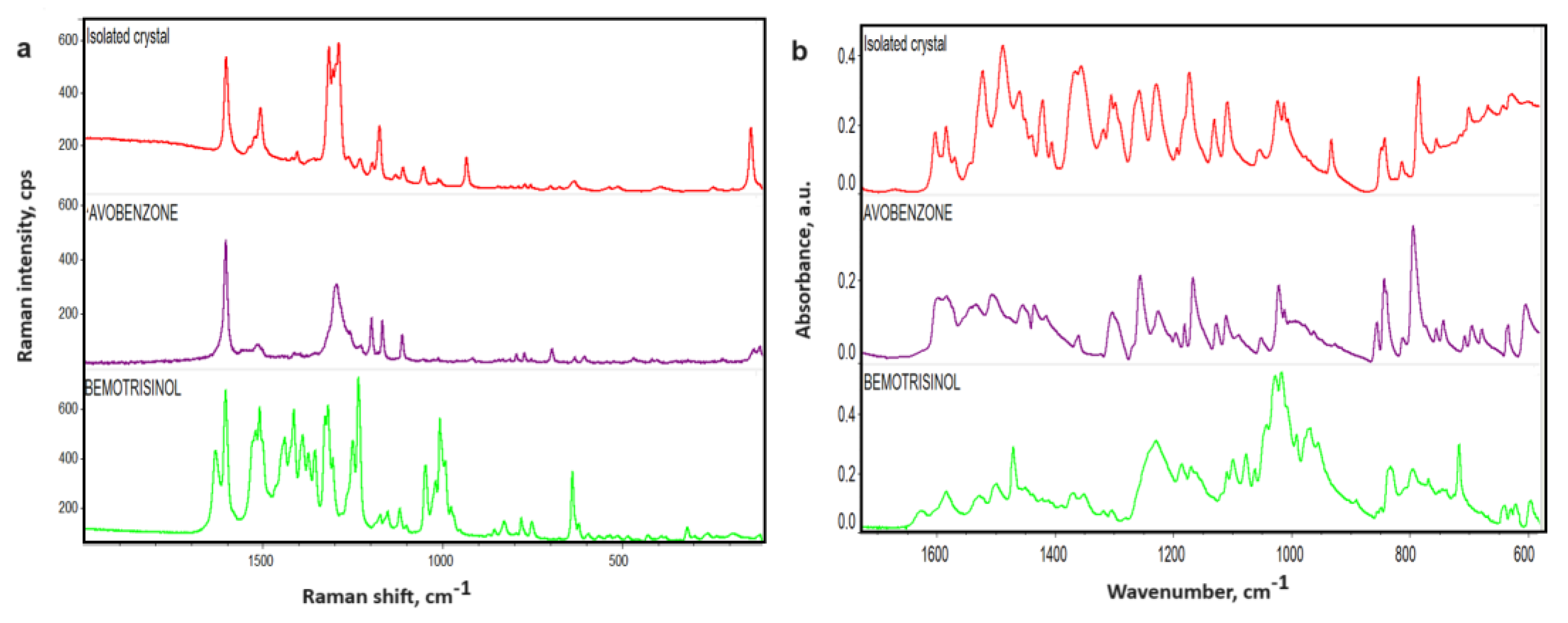
On the diffractograms recorded for the studied crystals, in addition to the reflections originating from the new phase, characteristic diffraction signals (in the higher-angle region, above 2Θ = 47°) are also present, resulting from the presence of small amounts of crystalline raw materials such as TALK EP7 (Talc), ZANO 10 PLUS (ZnO), B. UNIPURE WHITE LC 981 AS-EM (TiO2, anatase form) (shown in Figure 4d), and, to a lesser extent, other pigments and waxes. In the low-angle region (below 2Θ = 20°), additional reflections are observed on the diffractogram for the isolated crystals. The reflections on the diffractograms for two tautomeric forms of avobenzone (enol and keto-form) and the isolated crystals do not perfectly overlap. Figure 4c shows that there are slight shifts in reflections corresponding to the keto-form of avobenzone to lower angles, suggesting the incorporation of other components to the structure, most probably waxes, as the characteristic weak reflections for SYNCROWAX HRC-PA (MH) visible in the 2Θ range between 18 and 25° correspond to this material. This indicates the simultaneous crystallization of both forms of avobenzone and the co-crystallization of this raw material with other recipe components. No common reflections with the second filter, bemotrizinol, were observed, which might suggest that this raw material does not participate in the crystallization. Small signals in the 2Θ region 20–25° may be attributed to presence of waxes (KAHLWAX 2442L and CANDELILIA WAX). This is confirmed by the results of studies on crystals isolated from the defective cosmetic mass by means of Raman spectroscopy. The Raman spectra for the crystal and organic filters are presented in Figure 5a. The characteristic signal appearing for the isolated crystallites includes vibrations originating from the carbonyl group vibrations (1604 cm−1) and the aromatic ring (1550–1500 cm−1). Common signals for the crystal and avobenzone can be observed in the spectra. Additionally, common signals of the studied crystal with anatase (a crystalline form of TiO2) added as a pigment and inorganic filter were observed in the spectra in the region below 650 cm−1. Complementary tests were performed with FT-IR ATR spectroscopy, and the results are shown in Figure 5b.
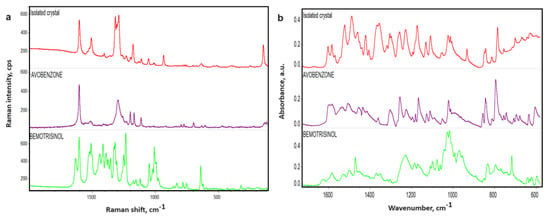
Figure 5.
Raman (a) and FTIR (b) spectra of organic filters and isolated crystals.
FTIR spectra recorded for crystallites show signals indicating the presence of certain raw materials from the formulation. However, there is no perfect matching of the crystal signals with a single raw material, as some peaks are shifted or their intensities differ from those of the pure raw materials. In the spectra of the crystallites, signals from PARSOL 1789 (avobenzone) and waxes can be observed, and additionally, pigments, talc, and zinc oxide influence the lowering of the baseline at lower wavenumbers. Analysis of the crystal spectrum indicates that it contains signals from aromatic rings and aliphatic functionalities, as well as carbonyl and ether groups originating from avobenzone (PARSOL 1789), waxes, and liquid raw materials. Comparing the spectrum of the crystal and the PARSOL 1789 raw material in different ranges of wavenumbers, we observe characteristic signals from carbonyl group vibrations around 1600 cm−1. In the case of the crystal, two distinct peaks are clearly noticeable in the same range as for the PARSOL 1789 raw material. In the 1600–1400 cm−1 range, bands characteristic of aromatic ring vibrations are also observed. Around 1110 cm−1, signals from C-O bond stretching vibrations can be seen, and at 1020 cm−1, from ether group vibrations. Below 900 cm−1, common vibrations from the aromatic ring are present.
Inhibition of Crystallization
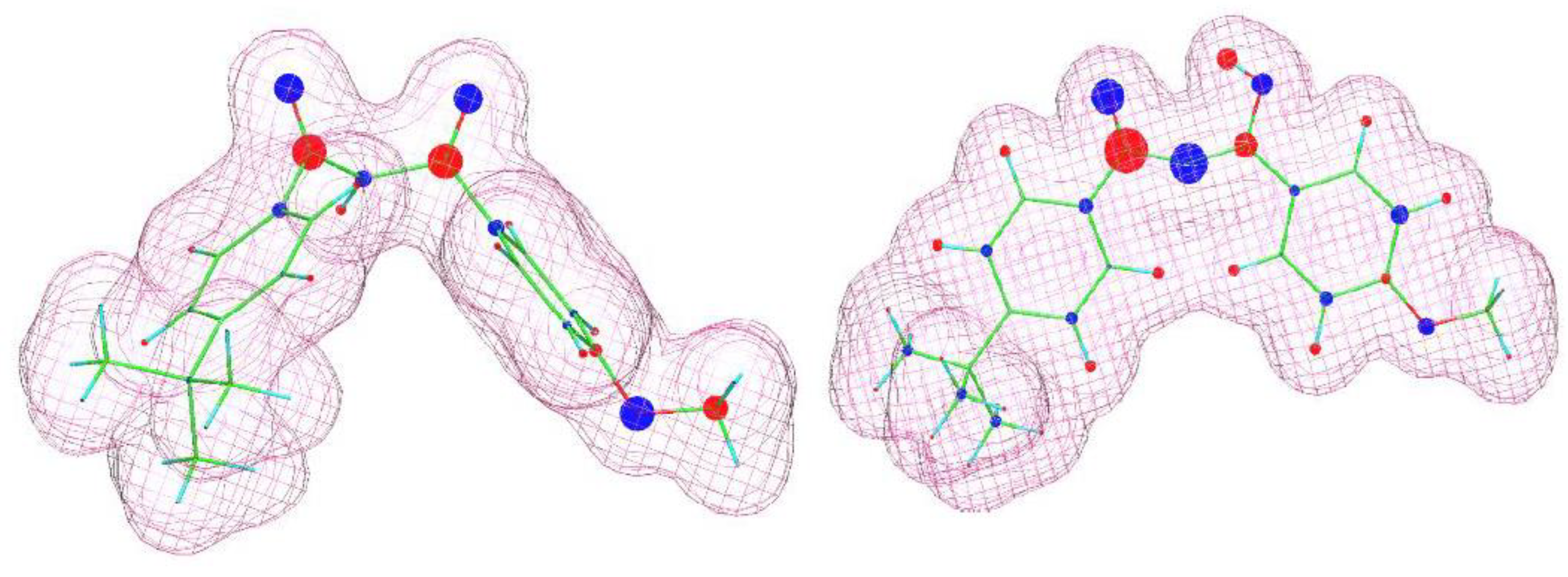
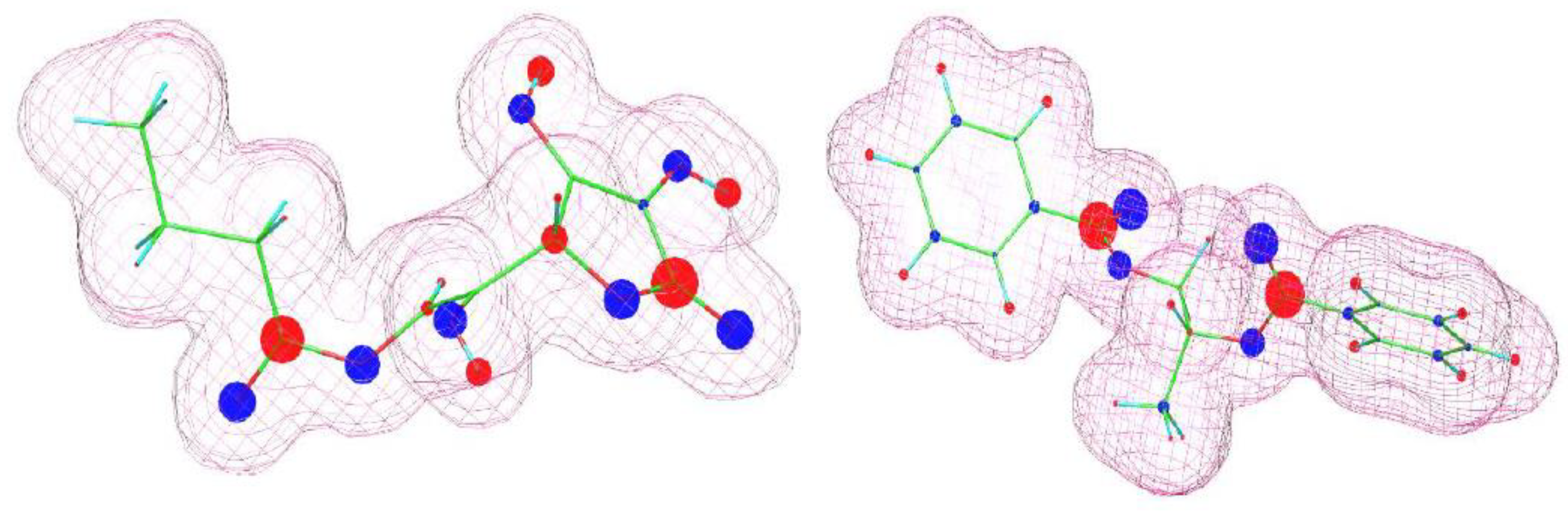
Gaining knowledge on the chemical and structural characteristic of the crystallizing substance led us to propose formulation modifications enabling the elimination/delay of avobenzone crystallization, thereby solving the problem of the stability of cosmetic masses. From the literature, the phenomenon of initiating the crystallization process of this compound (β-diketone form) by metal cations is known [14,15]. This mechanism has been at least partially confirmed in this workthrough SEM and Raman spectroscopy studies, which indicated that the crystallization nuclei form directly on the active centers created by transition metal ions on the surface of titanium oxide and zinc oxide grains. On a molecular level, it can be inferred that such interactions are related to the formation of central d-metal ion–ligand structures involving the β-diketone group of avobenzone. Hence, it can be concluded that other components of the mass are capable of interacting with the same group of the avobenzone molecule. In this way, they can alter the equilibrium of the formation of the aforementioned structures and thus block the delayed recrystallization of avobenzone. This compound exists in two forms, enol and ketone, which are in dynamic equilibrium with each other. Therefore, it is necessary to consider potential interactions of additional mixture components with both of these forms. To assess potential interactions, approximate models of the electronic structures of the molecules in question were created using the WinMOPAC 22.1.1 software and semiempirical PM3 model. Electron density maps along with a graphical representation of partial charges for both forms of avobenzone are shown in Figure 6. They indicate the presence of a strongly electronegative β-diketone group in the ketone structure and the presence of an acidic proton in the enol structure. Similar calculations were also performed for ascorbyl palmitate (an approximation was used where the palmitic chain was replaced by a shorter (3-carbon) alkyl chain, which does not affect the electronic structure of the relevant fragment of the molecule) and propylene glycol dibenzoate (INCI: propylene glycol dibenzoate). A visualization of the structures is shown in Figure 7.
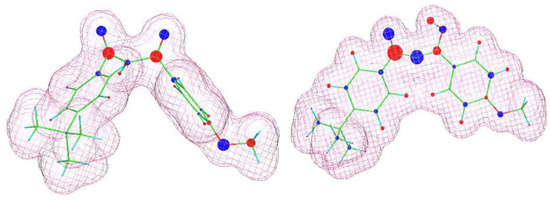
Figure 6.
Visualization of the molecular structures of avobenzone (keto form—left; enol form—right) estimated using the WinMOPAC 22.1.1software. The area covered by the “mesh” corresponds to an electron density of 0.99. Partial charges are depicted graphically, with blue representing negative charges and red representing positive charges. The size of the colored circles reflects the magnitude of the partial charge.
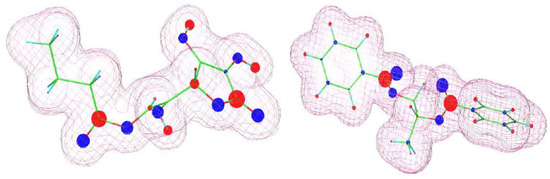
Figure 7.
Visualization of molecular structures (ascorbyl butyrate—left; dipropylene glycol dibenzoate ester—right) estimated using WinMOPAC 22.1.1 software. The area covered by the “grid” corresponds to an electron density of 0.99. Partial charges are depicted graphically: blue color indicates negative charges; red indicates positive charges. The size of the colored circles reflects the magnitude of the partial charge.
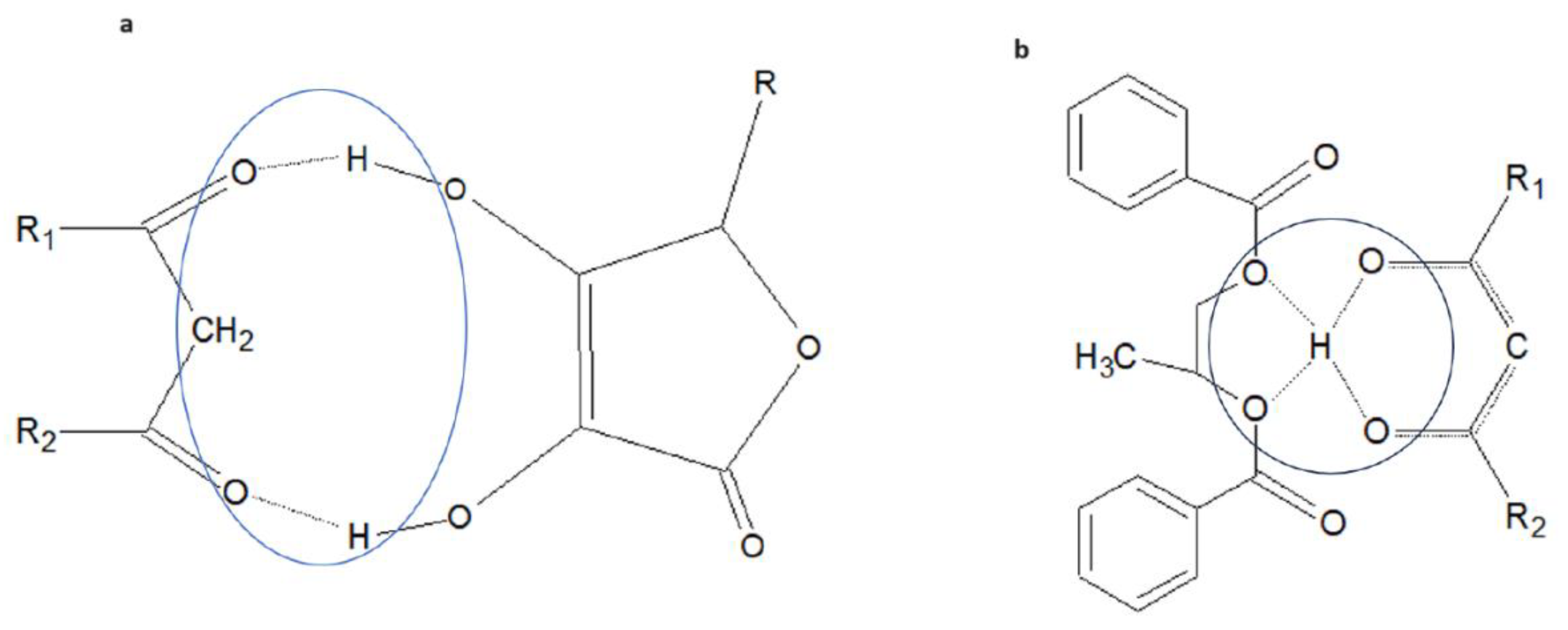
Consequently, it was observed that the ascorbyl group is able to form an adduct with the ketone form of avobenzone, by interacting with two acidic protons of the hydroxyl groups located at carbons 3 and 4 of the five-membered ring, adjacent to the carbonyl oxygens of the avobenzone ketone group. The resulting, likely cyclic structure composed of molecular fragments interconnected by two hydrogen bonds is depicted in Figure 8a.
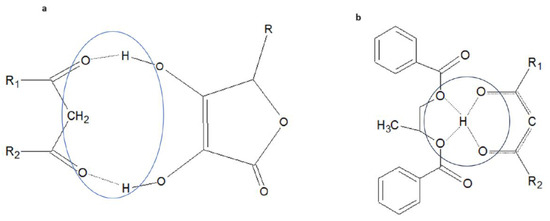
Figure 8.
(a) Hypothetical structure of the cyclic part of the adduct between the ketonic form of avobenzone and ascorbyl palmitate (R1, R2—residues of the avobenzone structure; R—alkyl residue of the ascorbyl palmitate structure). (b) Hypothetical structure of the dicyclic part of the adduct between the enolic form of avobenzone and the dibenzoate ester of propylene glycol (R1, R2—residues of the avobenzone structure). The circles mark the hydrogen bonding between interacting molecules.
The hypothetical adduct with this structure will exhibit two interesting functional characteristics relevant to the stability of the cosmetic mass. The first is the presence of a long aliphatic lipophilic chain in the molecule of ascorbyl palmitate, which should significantly increase the affinity of the adduct with avobenzone molecules to lipophilic components of the mass compared to the “free” molecule. The second is the steric hindrance around the diketone group, reducing the likelihood of complex formation with metal ions (the nine-membered ring is too “small” to accommodate ions of this ionic radius). This same characteristic will also be observed in the adduct of the enolic form of avobenzone with the ester of propylene glycol dibenzoate, where the acidic enolic proton can form hydrogen bonds with both ether oxygens and carbonyl oxygens of the ester groups (the structure of the interesting part of the adduct is illustrated in Figure 8b. In this case, however, it is not possible to justify the thesis of increased lipophilicity of the adduct; hence, the anticipated interaction of this component may practically turn out to be weaker than in the case of ascorbyl palmitate. From a practical point of view, however, this component did block the initiation of avobenzone crystallization and worked as an effective stabilizer for anhydrous cosmetic foundations. The calculated enthalpy of formation of the ascorbyl part of the ascorbyl butyrate molecule was −1134.0 kJ/mol, which is in good agreement with the literature data of −1160.3 ± 0.9 kJ/mol [16] and −1164.6 ± 1.0 kJ/mol [17], proving the proper attribution of the calculational model (PM3 semiempirical). The difference between the calculated formation of enthalpies of the keto and enol forms of avobenzone complexes with ascorbyl butyrate calculated in the same manner is equal to 29.6 kJ/mol in favor of the keto form.
4. Summary
The isolated crystals from the mass are characterized by a spherical shape and are on the order of millimeters in size. SEM studies of the crystal cross-section observed that crystallization is initiated from a nucleus located in the center of the formation, around which lamellae of the crystallizing filter grow. The use of an EDS microprobe allowed for the examination of the chemical composition and confirmed that the crystallization of avobenzone occurs with a small amount of wax being incorporated—the precise determination of the structure of such co-crystals requires further structural studies and will be the subject of future work. X-ray studies revealed the presence of additional reflections on the diffractograms in the low-angle region, where reflections from the two tautomeric forms of avobenzone are also located. These do not overlap precisely with the reflections for the crystals, indicating the formation of a new crystalline phase in the form of the aforementioned co-crystals of avobenzone with waxes. XRD analyses also show the presence of titanium oxide in the crystallites, supporting the hypothesis about their catalytic effect on the nucleation of avobenzone on the anatase surface. Raman studies indicate the co-crystallization of avobenzone along with other inorganic materials such as anatase. Avobenzone is a widely used chemical filter in sunscreens, known for its ability to absorb a broad spectrum of UVA radiation. However, this compound is prone to photodegradation and crystallization, especially under UV exposure. This problem is extremely important in the color cosmetic industry where the demand for multifunctional cosmetics capable of the protection of skin from UV radiation became highly appealing. The presence of metal centers, which appear on the surfaces of inorganic fillers added to cosmetic masses (i.e., TiO2, ZnO, iron oxides), can accelerate this process, leading to the formation of avobenzone crystals that reduce the sunscreen’s effectiveness.
It is likely that the crystallization of avobenzone is initiated on the surface of titanium dioxide and/or zinc oxide grains, whose triethoxyoctylsilane coatings have been disrupted due to friction caused by shear forces during the mass homogenization process. As a result, active sites on the titanium dioxide or zinc oxide grains act as nucleation points for crystallization and catalyze the isomerization and dimerization reactions of avobenzone molecules. Both forms of avobenzone, as well as dimers, chelate the exposed titanium and/or zinc ions; thus, the crystallizing material builds up with other materials (waxes), forming a layer on the surface of the grain. The ascorbyl palmitate used in this study plays a crucial role in inhibiting the crystallization of avobenzone, especially when the crystallization is initiated by d-metal centers. This compound is capable of effectively blocking these metal centers from catalyzing the crystallization of avobenzone on the surface of metal oxide via the chelation of transition metal ions. Through binding to these metal centers, ascorbyl palmitate reduces the catalytic activity that would otherwise promote the breakdown and crystallization of avobenzone. Upon exposure to UV light, TiO2 can generate reactive oxygen species, which can initiate the photodegradation of avobenzone. Ascorbyl palmitate, due to its antioxidative properties, also act as a scavenger of these active oxygen forms, preventing them from initiating the degradation process and protecting the avobenzone molecules from oxidative degradation to active forms that trigger the crystallization. The ascorbyl palmitate, studied here as an additive to anhydrous fluid foundation with SPF50, inhibits the crystallization of avobenzone initiated by metal centers on titanium dioxide through its antioxidant and metal-chelating properties. By scavenging reactive oxygen species and binding to metal ions on TiO2, ascorbyl palmitate stabilizes avobenzone and maintains its efficacy in sunscreen formulations. This synergistic action helps enhance the overall stability and performance of sunscreens containing both avobenzone and TiO2. This may be transferred to other compounds that may be applied in formulation in the color cosmetics industry to extend the shelf life of the product via simple modifications of the formulation using chelating agents for active metal centers or scavengers for active forms of oxygen, preventing not only the unwanted crystallization of sunscreens but also redox reactions, leading to the fading of product color when exposed to UV radiation. The introduction of ascorbyl palmitate to the cosmetic mass inhibits unwanted crystallization. It is likely that a competitive reaction occurs between the complexation of exposed d-block metal ions by ascorbyl ligands, which prevents the initiation of avobenzone isomerization and crystallization.
The proposed changes in formulations contributed to the inhibition/delay of the recrystallization of the organic filter (avobenzone) in the cosmetic mass, ensuring its appropriate stability for the required product life span.
Author Contributions
Conceptualization, A.K.-M.; Methodology, O.G., M.S. and A.K.-M.; Software, M.S.; Formal analysis, M.S. and A.K.-M.; Investigation, O.G., G.Z.Z. and E.Z.; Resources, A.K.-M.; Data curation, O.G., G.Z.Z. and A.K.-M.; Writing—original draft, O.G. and A.K.-M.; Writing—review & editing, A.K.-M. and M.S.; Supervision, A.K.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially financed by the statutory budget of the Faculty of Chemistry Warsaw University of Technology and Ministry of Science and Education of Poland under Implementation doctorate” project No. DWD/3/18/2019.
Data Availability Statement
The datasets presented in this article are not readily available because due to their partial confidentiality resulting from cooperation with the company (NUCO E. i G. Kosyl Sp. Jawna).
Acknowledgments
We thank NUCO E. i G. Kosyl Sp. Jawna for cooperation in this project.
Conflicts of Interest
Olga Goral was employed by the company NUCO E. i G. Kosyl Sp. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Levee, G.J.; Sayre, R.M.; Marlowe, E. P-aminobenzoic acid as a sunscreen and its behaviour on the skin. Int. J. Cosmet. Sci. 1981, 3, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Fletcher, P.D.I.; Johnson, A.J.; Marinopoulos, I.; Crowther, J.M.; Thompson, M.A. Evaporation of Particle-Stabilized Emulsion Sunscreen Films. ACS Appl. Mater. Interfaces 2016, 8, 21201–21213. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Jäger, E.; Jäger, A.; Štěpánek, P.; Cano, A.; Viseras, C.; de Melo Barbosa, R.; Chorilli, M.; Zielinska, A.; Severino, P.; et al. Lipid Nanomaterials for Targeted Delivery of Dermocosmetic Ingredients: Advances in Photoprotection and Skin Anti-Aging. Nanomaterials 2022, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A comprehensive review with the application of nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.; Cidade, H.; Lobo, L.; Almeida, I. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- da Silva Souza, I.D.; Berkowitz, E.; Chea, J.D.; McBride, N.; Sweet, K.; Torri, D.; Burgo, R.V.; Savelski, M.J.; Stanzione, J.F., 3rd. Efficient UV Filter Solubilizers Prevent Recrystallization Favoring Accurate and Safe Sun Protection. ACS Appl. Mater. Interfaces 2018, 10, 40411–40423. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef]
- Gholap, A.D.; Sayyad, S.F.; Hatvate, N.T.; Dhumal, V.V.; Pardeshi, S.R.; Chavda, V.P.; Vora, L.K. Drug delivery strategies for avobenzone: A case study of photostabilization. Pharmaceutics 2023, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Svärd, M.; Rasmuson, Å.C. Crystal Growth of Salicylic Acid in Organic Solvents. Cryst. Growth Des. 2017, 17, 2964–2974. [Google Scholar] [CrossRef]
- Sohn, M.; Prost-Dame, S.M.; Bayraktar, M.; Schäfer, A.; Herzog, B. Crystallization Velocity and UV Performance of Formulations With Oversaturated UV-Filter Content. J. Pharm. Sci. 2019, 108, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Mukawa, T.; Sato, N.; Maezawa, D.; Ohtsu, Y.; Kuroda, A.; Wakabayashi, M.; Asakura, K. Coexistence effect of UVA absorbers to increase their solubility and stability of supersaturation. Int. J. Cosmet. Sci. 2014, 36, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Fardi, T.; Stefanis, E.; Panayiotou, C.; Abbott, S.; van Loon, S. Artwork conservation materials and Hansen solubility parameters: A novel methodology towards critical solvent selection. J. Cult. Herit. 2014, 15, 583–594. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Rajbhoj, A.; Korde, N.S.; Gaikwad, S.T.; Korde, S.S. Efficient Ultrasound synthesis of β-diketone and its metal complexes. Der Pharma Chem. 2012, 4, 1868–1872. [Google Scholar]
- Pettinari, C.; Marchetti, F.; Drozdov, A. β-Diketones and Related Ligands. In Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2003; pp. 97–115. [Google Scholar] [CrossRef]
- Gerasimov, P.A.; Blokh, E.L.; Gubareva, A.I. Standard heats of formation of intermediates in the synthesis of vitamin C. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1985, 28, 54–56. [Google Scholar]
- Desai, P.; Wilhoit, R.C. Heats of combustion and enthalpies of formation of D-ribose, D-arabinose, and L-ascorbic acid. Thermochim. Acta 1970, 1, 61–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).