Synthesis and Characterization of Carboxymethylcellulose-Functionalized Magnetite Nanoparticles as Contrast Agents for THz Spectroscopy with Applications in Oncology
Abstract
:1. Introduction
2. Materials and Preparation Method
3. Results and Discussion
3.1. Characterization Equipment
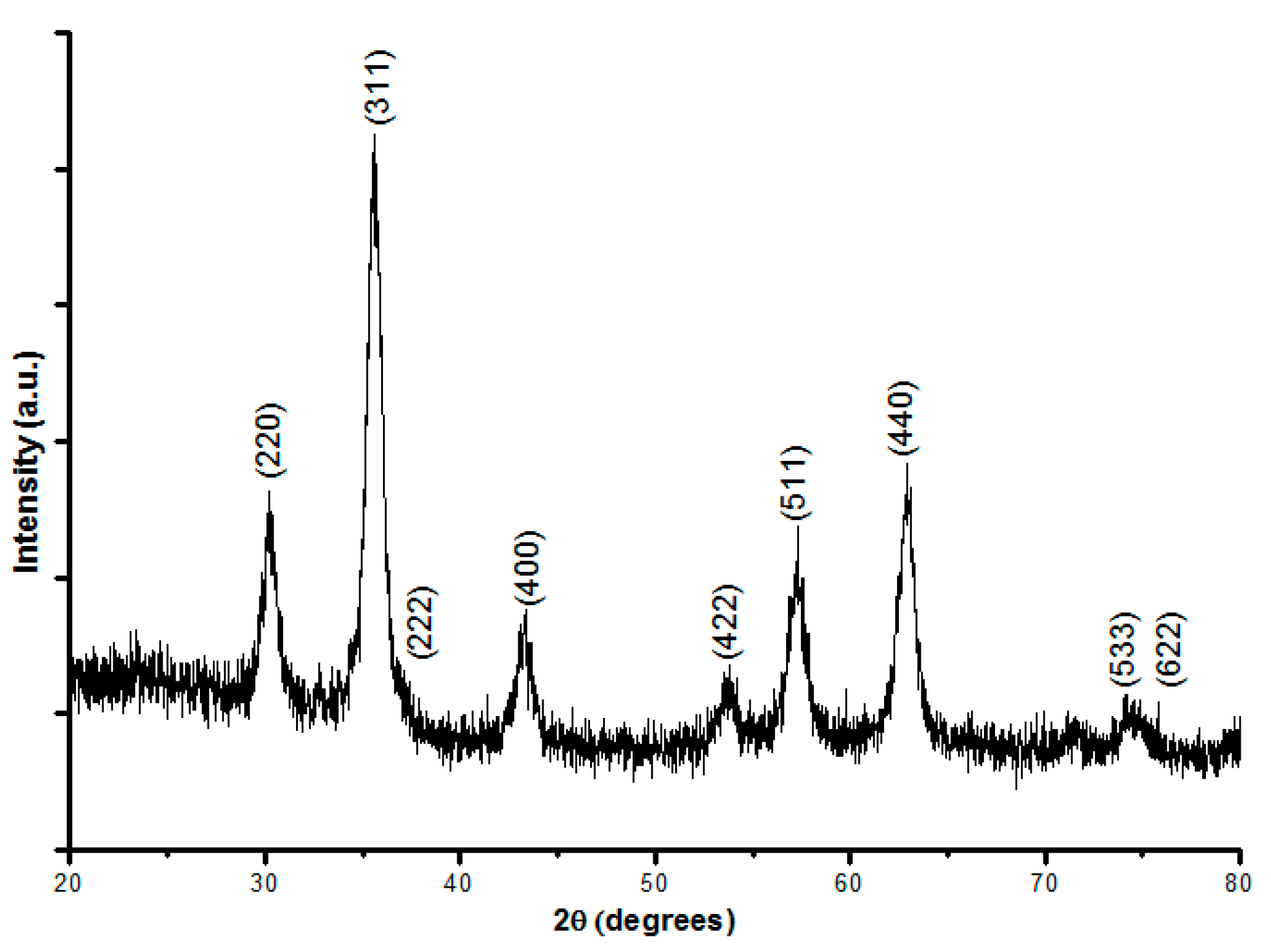
3.2. X-ray Diffraction Results
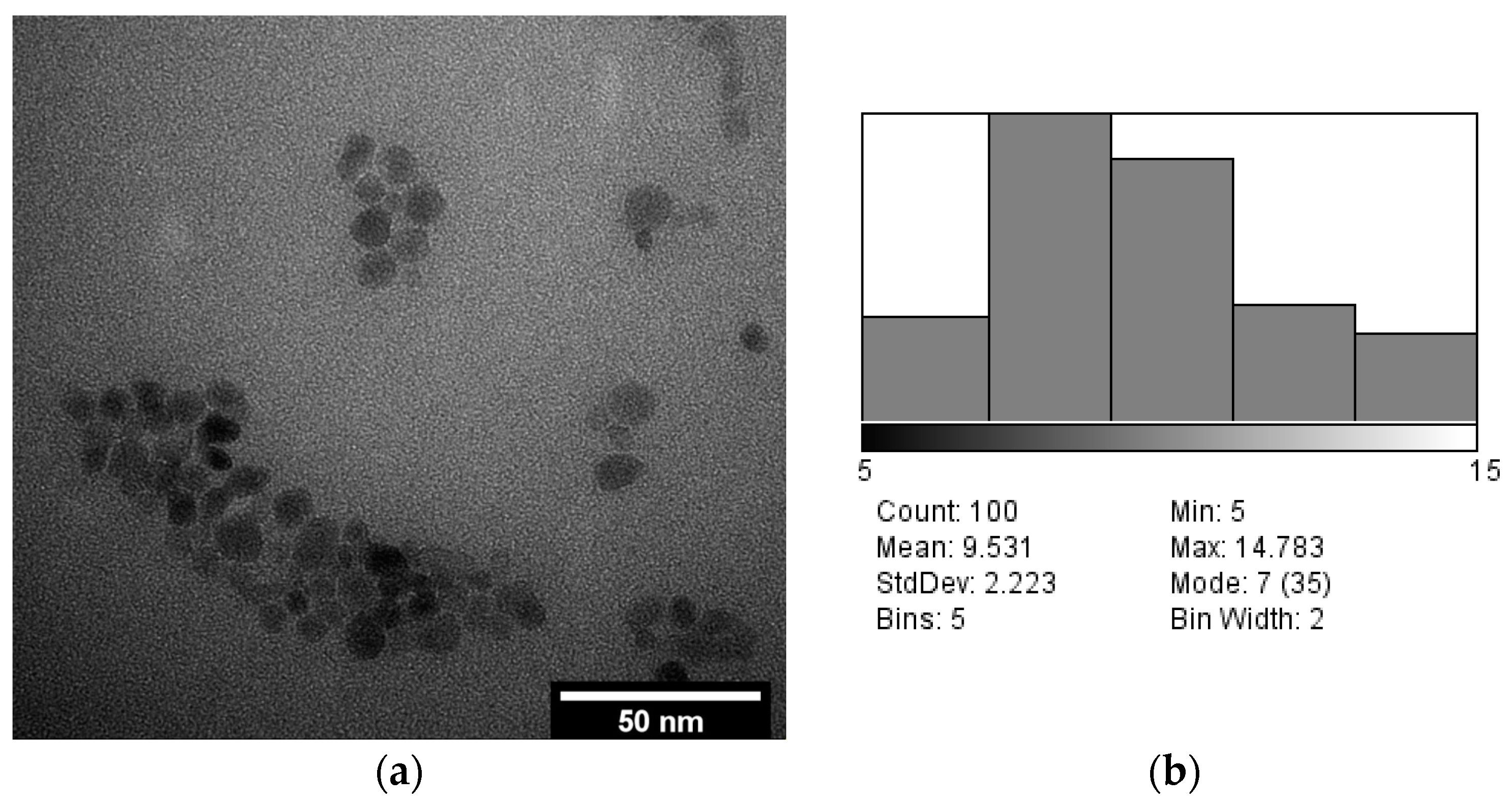
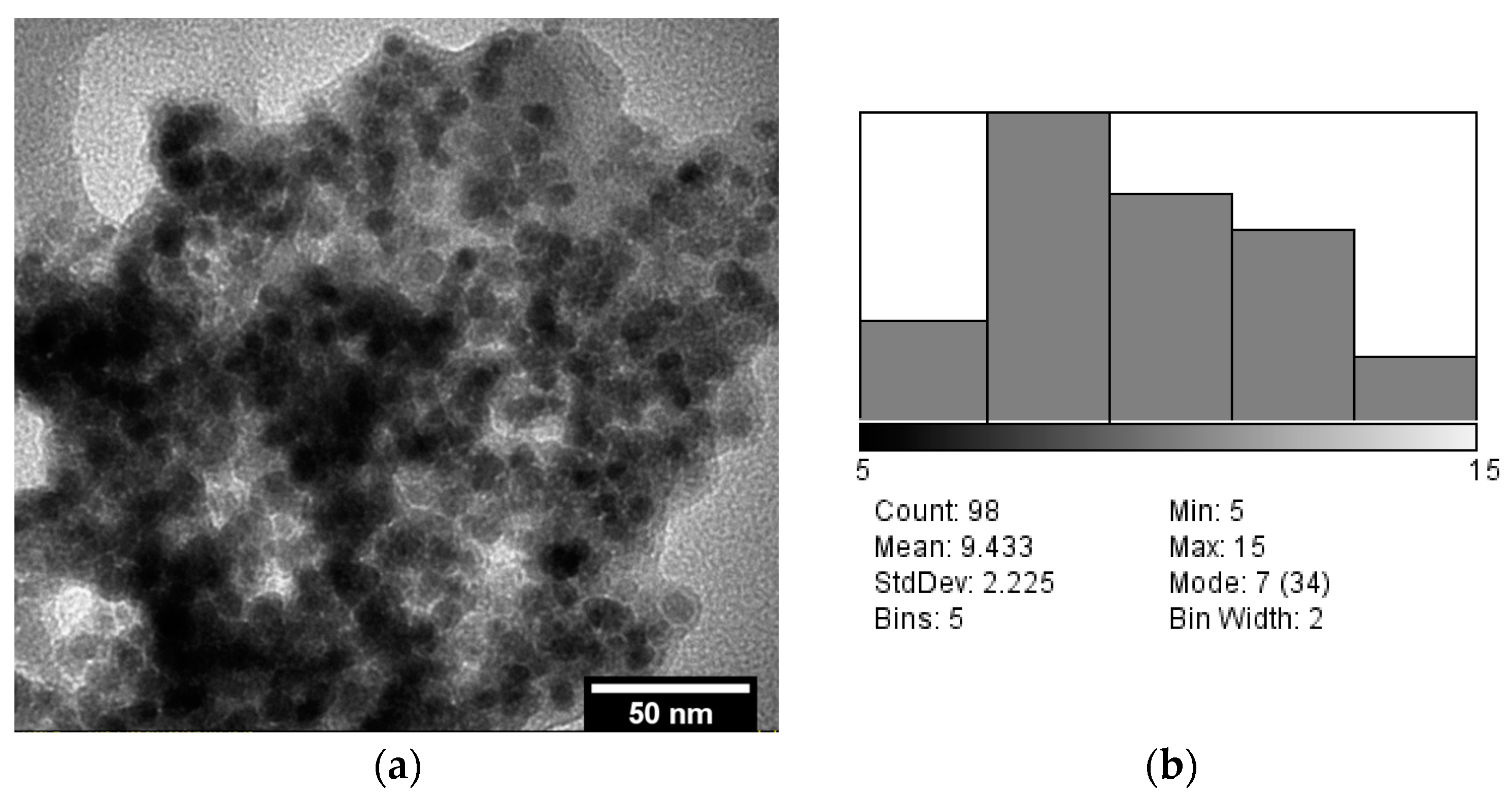
3.3. TEM Analysis
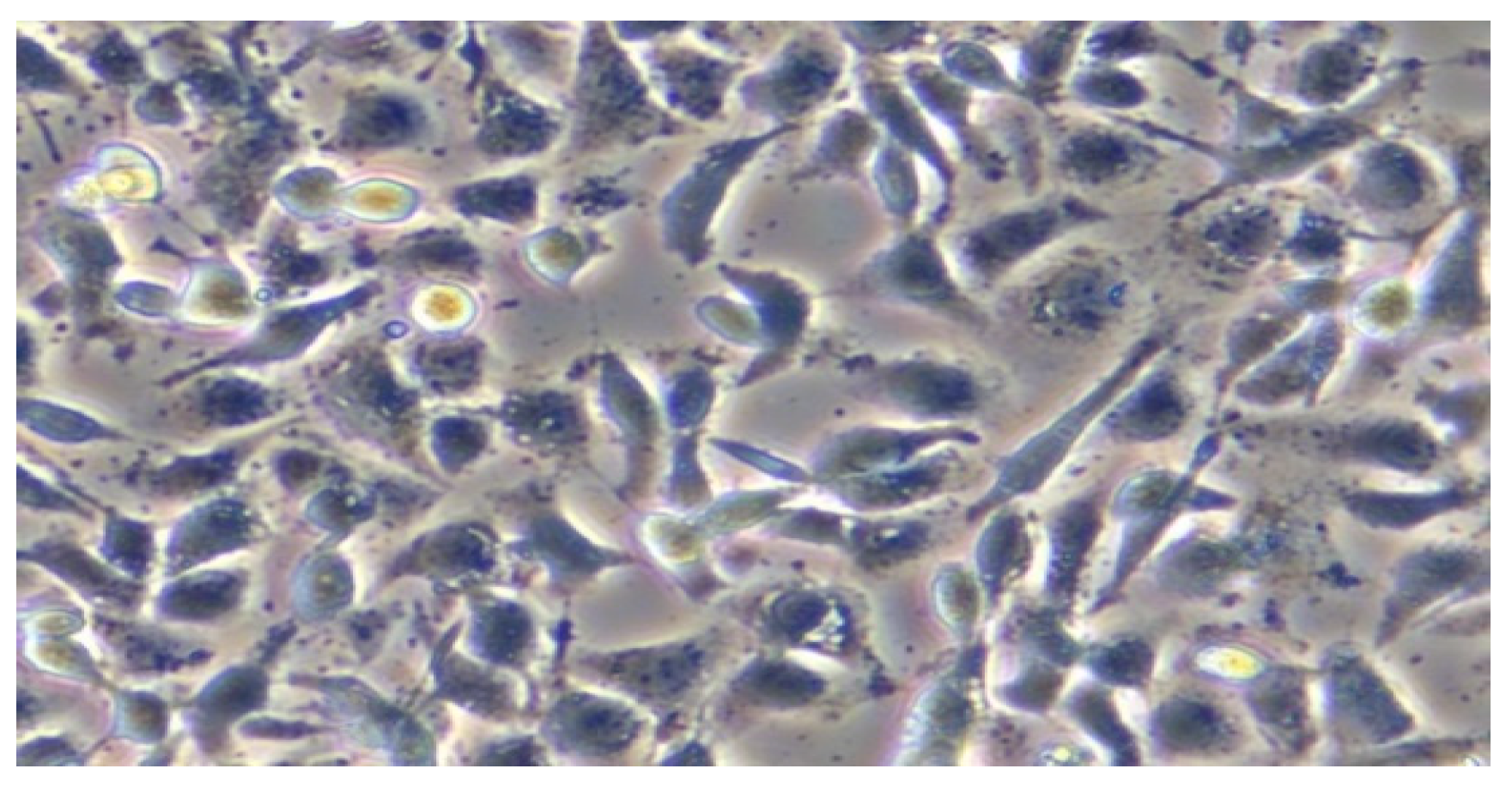
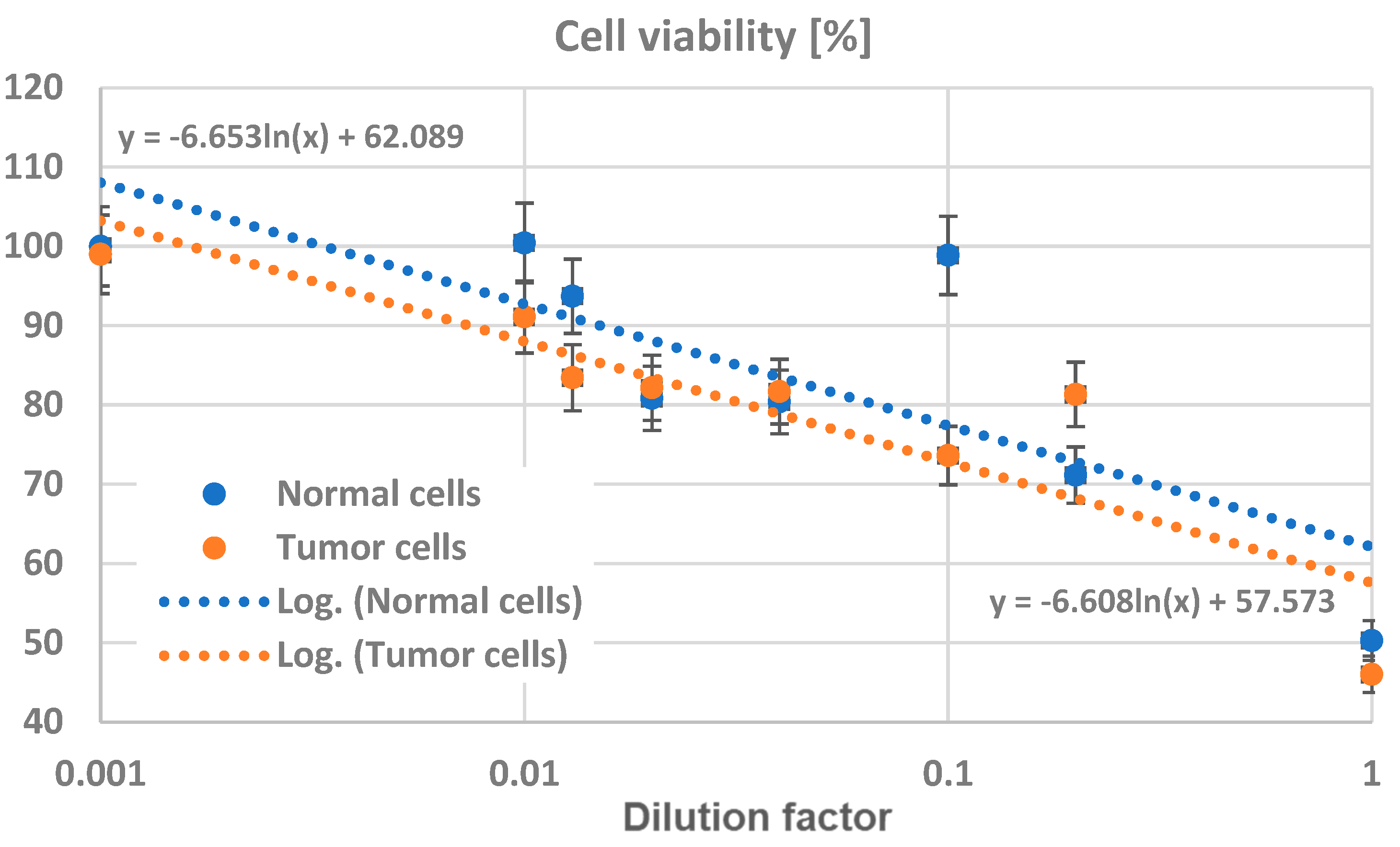
3.4. In Vitro Testing of Normal and Tumor Cells’ Survival and Viability in the Presence of Magnetite Nano-Assemblies
3.5. In Vivo Testing of the Toxicity and Action of Nano-Assemblies on Animal Model
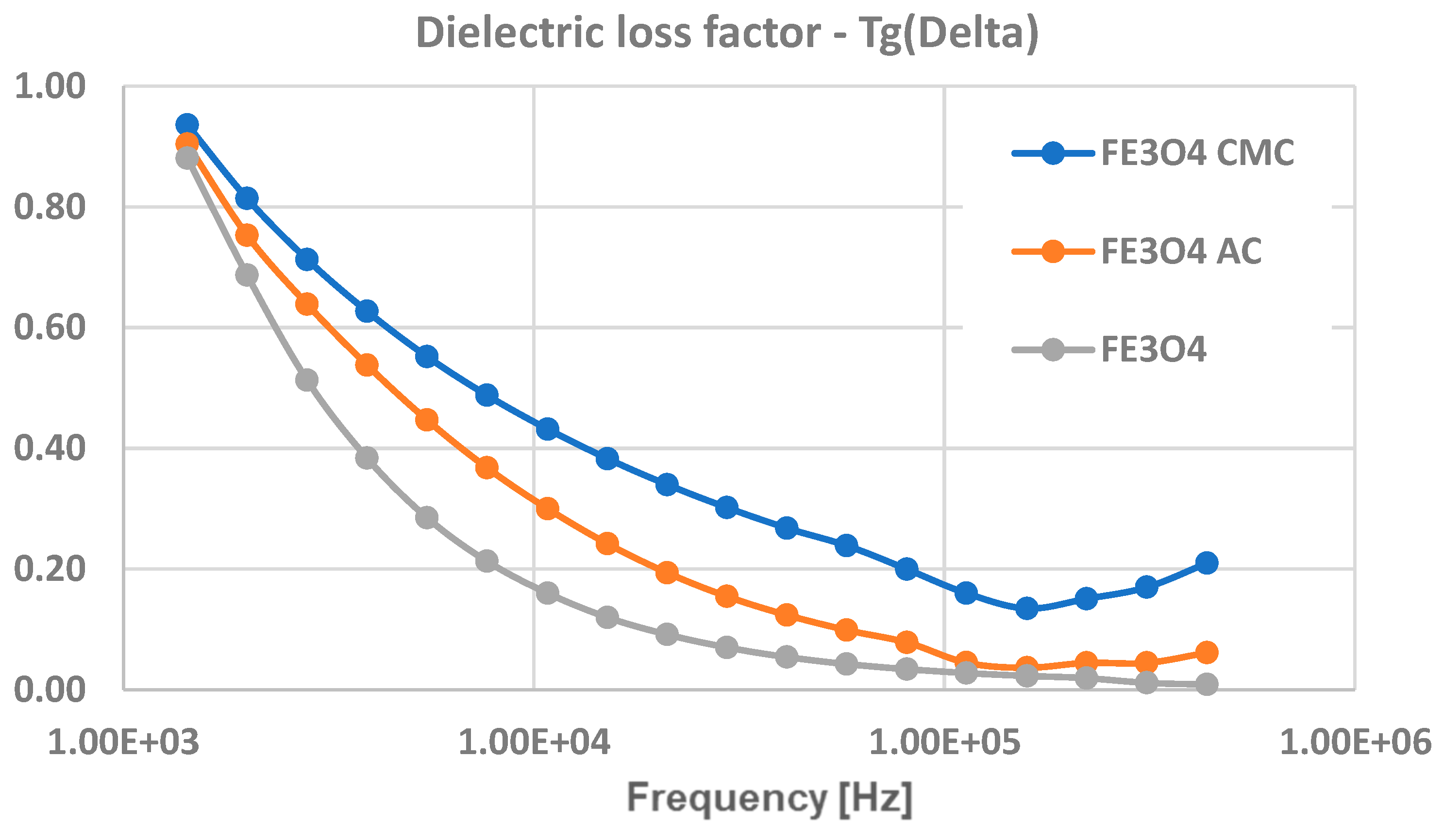
3.6. Dielectric Tests
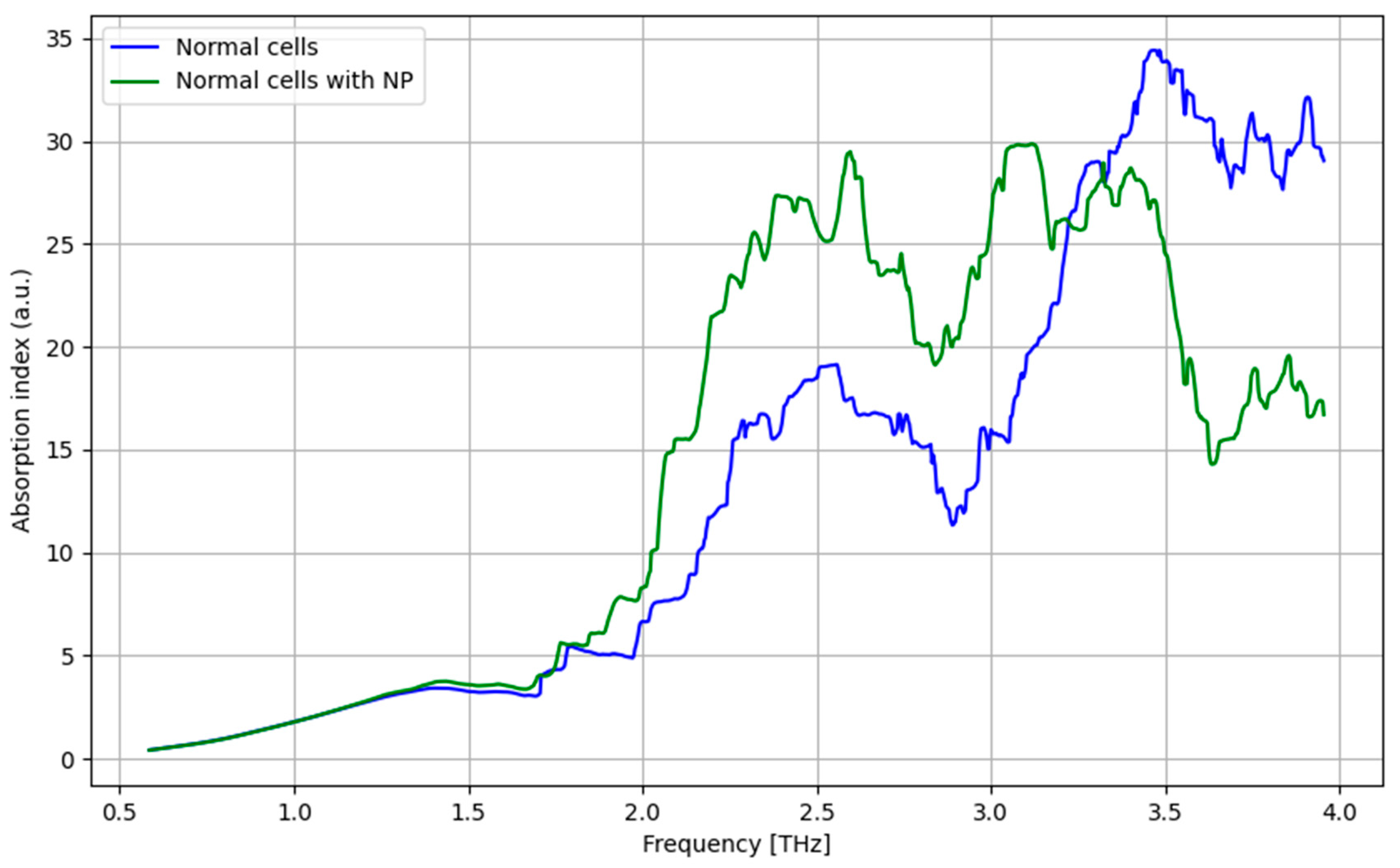
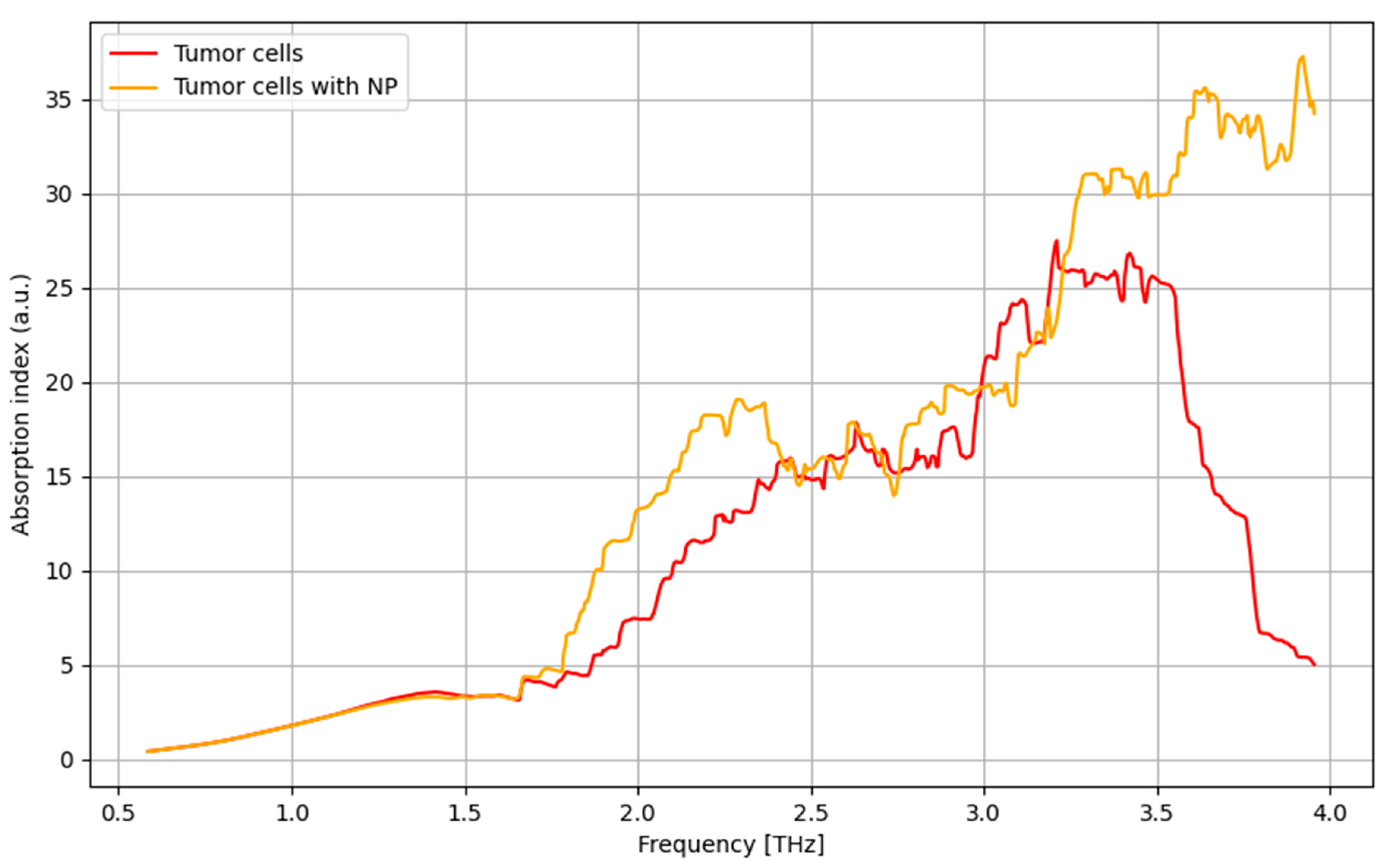
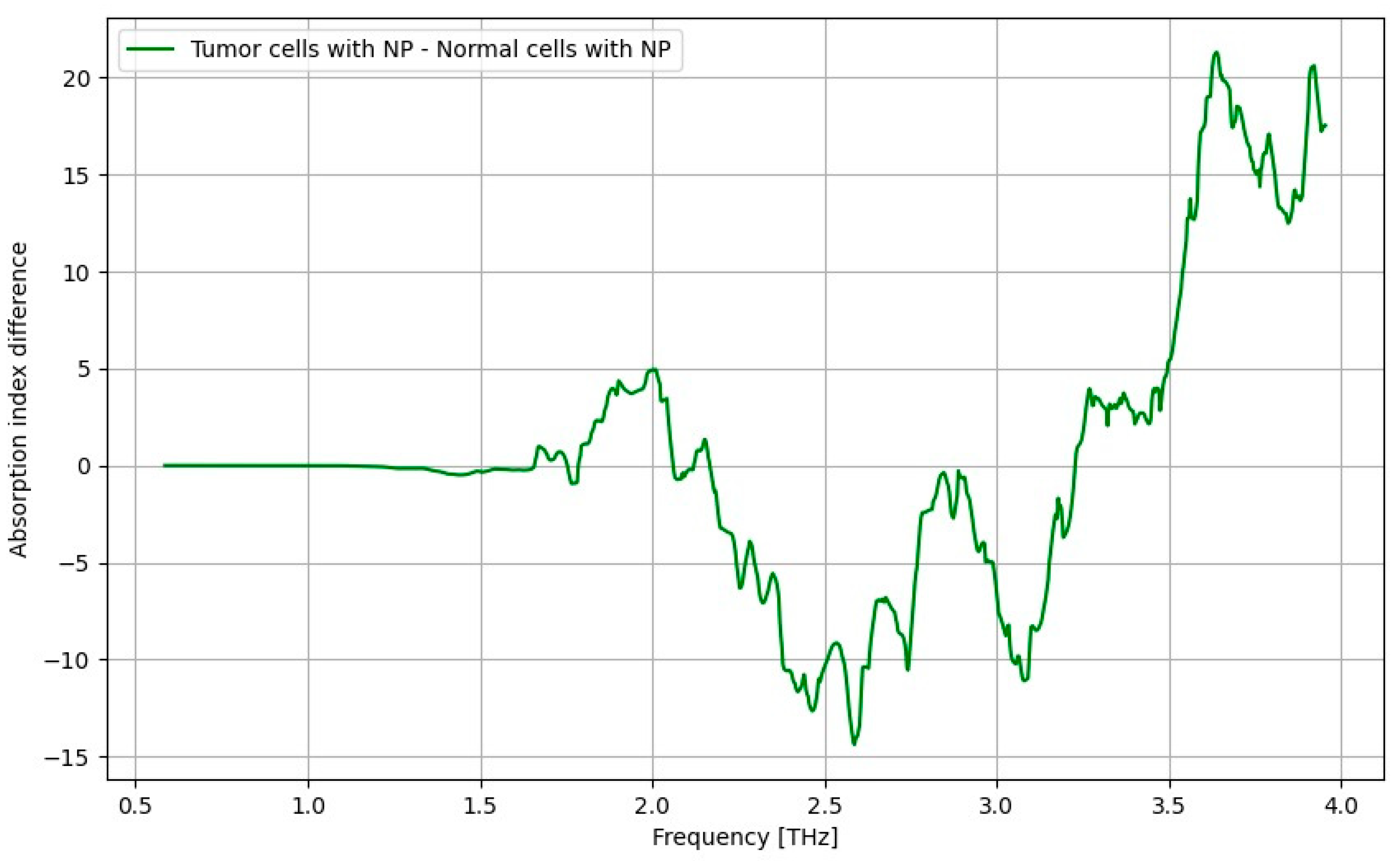
3.7. THz Spectroscopy Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, K.S.; Suh, M. Screening for gastric cancer: The usefulness of endoscopy. Clin. Endosc. 2014, 47, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Stomach Cancer Screening, 2023. Available online: https://www.cancer.gov/types/stomach/screening (accessed on 25 April 2024).
- Hibino, M.; Hamashima, C.; Iwata, M.; Terasawa, T. Radiographic and endoscopic screening to reduce gastric cancer mortality: A systematic review and meta-analysis. Lancet Reg. Health 2023, 35, 100741. [Google Scholar] [CrossRef] [PubMed]
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig. Endosc. 2022, 34, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-H. Terahertz electromagnetic interactions with biological matter and their applications. J. Appl. Phys. 2009, 105, 102033. [Google Scholar] [CrossRef]
- Parrott, E.P.; Sy, S.M.Y.; Blu, T.; Wallace, V.P.; Pickwell-Macpherson, E. Terahertz pulsed imaging in vivo: Measurements and processing methods. J. Biomed. Opt. 2011, 16, 106010. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical Applications of Terahertz Spectroscopy and Imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef]
- Yu, C.; Fan, S.; Sun, Y.; Pickwell-MacPherson, E. The potential of terahertz imaging for cancer diagnosis: A review of investigations to date. Quant. Imaging Med. Surg. 2012, 2, 33. [Google Scholar]
- Oh, S.J.; Huh, Y.M.; Suh, J.S.; Choi, J.; Haam, S.; Son, J.H. Cancer Diagnosis by Terahertz Molecular Imaging Technique. J. Infrared Millim. Terahertz Waves 2012, 33, 74–81. [Google Scholar] [CrossRef]
- Li, H.; Zhang, K.; Zhou, Q.; Mao, W.; Dong, P.; Tian, F. Case Report: Terahertz spectroscopy for cancer diagnosis. Int. J. Clin. Exp. Med. 2017, 10, 3766–3773. [Google Scholar]
- Oh, S.J.; Choi, J.; Maeng, I.; Park, J.Y.; Lee, K.; Huh, Y.-M.; Suh, J.-S.; Haam, S.; Son, J.-H. Molecular imaging with terahertz waves. Opt. Express 2011, 19, 4009–4016. [Google Scholar] [CrossRef]
- Doradla, P.; Alavi, K.; Joseph, C.; Giles, R. Detection of colon cancer by continuous-wave terahertz polarization imaging technique. J. Biomed. Opt. 2013, 18, 090504. [Google Scholar] [CrossRef]
- Chen, H.; Ma, S.; Wu, X.; Yang, W.; Zhao, T. Diagnose human colonic tissues by terahertz near-field imaging. J. Biomed. Opt. 2015, 20, 036017. [Google Scholar] [CrossRef] [PubMed]
- Terahertz Imaging Advances Toward Medical Diagnostics, 2022. Available online: https://www.laserfocusworld.com/test-measurement/article/14235398/terahertz-imaging-advances-toward-medical-diagnostics (accessed on 25 April 2024).
- Shi, S.; Yuan, S.; Zhou, J.; Jiang, P. Terahertz technology and its applications in head and neck diseases. iScience 2023, 26, 107060. [Google Scholar] [CrossRef] [PubMed]
- Gezimati, M.; Singh, G. Terahertz imaging technology for localization of cancer tumors: A technical review. Multimed. Tools Appl. 2024, 83, 33675–33711. [Google Scholar] [CrossRef]
- Hellebust, A.; Richards-Kortum, R. Advances in molecular imaging: Targeted optical contrast agents for cancer diagnostics. Nanomedicine 2012, 7, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Maeng, I.; Shin, H.J.; Lee, J.; Kang, J.; Haam, S.; Huh, Y.M.; Suh, J.; Son, J.H. Nanoparticle contrast agents for Terahertz medical imaging. In Proceedings of the 33rd International Conference on Millimeter and Terahertz Waves, Pasadena, CA, USA, 15–19 September 2008. [Google Scholar]
- Oh, S.J.; Kang, J.; Maeng, I.; Suh, J.-S.; Huh, Y.-M.; Haam, S.; Son, J.-H. Nanoparticle-enabled Terahertz Imaging for Cancer Diagnosis. Opt. Express 2009, 17, 3469–3475. [Google Scholar] [CrossRef]
- Stylianou, A.; Talias, M.A. Nanotechnology-supported THz medical imaging. F1000Research 2013, 2, 100. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.Z.; Yu, J.; Hou, Y.L.; Gao, S. Magnetic nanoparticle-based cancer nanodiagnostics. Chin. Phys. B 2013, 22, 058702. [Google Scholar]
- Lee, J.; Yang, J.; Ko, H.; Oh, S.; Kang, J.; Son, J.; Lee, K.; Lee, S.W.; Yoon, H.G.; Suh, J.S.; et al. Multifunctional magnetic gold nanocomposites: Human epithelial cancer detection via magnetic resonance imaging and localized synchronous therapy. Adv. Funct. Mater. 2008, 18, 258–264. [Google Scholar] [CrossRef]
- Sadeghi, A.; Naghavi, S.M.H.; Mozafari, M.; Afshari, E. Nanoscale biomaterials for terahertz imaging: A non-invasive approach for early cancer detection. Transl. Oncol. 2023, 27, 101565. [Google Scholar] [CrossRef]
- Sadeghi, L.; Espanani, H.R. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Lek. Listy 2015, 116, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.-C.; Costache, M.; Galateanu, B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng. C 2020, 110, 110714. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Yu, J.; Liu, Y.; Dai, W.; Li, J.; Tao, J.; Tian, W. Optical tuning of absorption coefficient and dielectric properties of amorphous Cu doped α-Fe2O3 in the THz range. Mater. Res. Express 2019, 6, 105204. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Ayoub, N.; Mir, J.F.; Alanazi, A.M.; Shah, M.A.; Gulzar, A. In vitro and in vivo MRI imaging and photothermal therapeutic properties of Hematite (α-Fe2O3) Nanorods. J. Mater. Sci. Mater. Med. 2022, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Akakuru, O.U.; Yao, C.; Yang, F.; Wu, A. Transition metal ion-doped ferrites nanoparticles for bioimaging and cancer therapy. Transl. Oncol. 2022, 15, 101264. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.W.; Ababneh, B.; Lemine, O.M.; Alsadig, A.; Omri, K.; El Mir, L.; Sulieman, A.; Mattar, E. The significant effect of size and concentrations of iron oxide nanoparticles on magnetic resonance imaging contrast enhancement. Results Phys. 2019, 15, 102651. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zhao, G.F.; Jin, B.B.; Hou, Y.Y.; Jia, H.H.; Chen, J.; Wu, P.H. Terahertz Imaging on Subcutaneous Tissues and Liver Inflamed by Liver Cancer Cells. Terahertz Sci. Technol. 2012, 5, 114–123. [Google Scholar]
- Fitzgerald, A.J.; Wallace, V.P.; Jimenez-Linan, M.; Bobrow, L.; Pye, R.J.; Purushotham, A.D.; Arnone, D.D. Terahertz pulsed imaging of human breast tumor. Radiology 2006, 2, 3533–3540. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.H.; Tseng, T.F.; Lu, J.T.; Kuo, C.C.; Fu, S.C.; Lee, W.J.; Tsai, Y.F.; Huang, Y.Y.; Chuang, E.Y.; et al. High-sensitivity in vivo THz transmission imaging of early human breast cancer in a subcutaneous xenograft mouse model. Opt. Express 2011, 19, 21552–21562. [Google Scholar] [CrossRef]
- Jung, E.A.; Lim, M.H.; Moon, K.W.; Do, Y.W.; Lee, S.S.; Han, H.W.; Choi, H.J.; Cho, K.S.; Kim, K.R. Terahertz Pulse Imaging of Micrometastatic Lymph Nodes in Early-stage Cervical Cancer Patients. J. Opt. Soc. Korea 2011, 15, 155–160. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, H.; Kim, T.; Cho, B.; Lee, K.; Son, J.H. Characteristics of Gadolinium Oxide Nanoparticles as Contrast Agents for Terahertz Imaging. J. Infrared Millim. Terahertz Waves 2011, 32, 506–512. [Google Scholar] [CrossRef]
- Habibi, N. Preparation of biocompatible magnetite-carboxymethyl cellulose nanocomposite: Characterization of nanocomposite by FTIR, XRD, FESEM and TEM. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Vijayasree, V.P.; Manan, N.S.A. Magnetite carboxymethylcellulose as biological macromolecule-based absorbent for cationic dyes removal from environmental samples. Int. J. Biol. Macromol. 2023, 242, 124723. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Berry, C.C.; West, R.M.; Gonzalez-Monterrubio, E.; Angulo-Molina, A.; Arias-Carrión, Ó.; Méndez-Rojas, M.Á. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood–brain barrier. Nanoscale Adv. 2019, 1, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagi, H.; Yazbik, V.; Abdelwahed, W.; Alchab, L. Magnetite Nanoparticle Co-precipitation Synthesis, Characterization, and Applications: Mini Review. Bionanoscience 2023, 13, 853–859. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards. Available online: https://pubs.acs.org/doi/10.1021/ac60293a779 (accessed on 6 July 2024).
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Souza, D.M.; Andrade, A.L.; Fabris, J.D.; Valério, P.; Góes, A.M.; Leite, M.F.; Domingues, R.Z. Synthesis and in vitro evaluation of toxicity of silica-coated magnetite nanoparticles. J. Non-Cryst. Solids 2008, 354, 4894–4897. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef]
- Schreiner, O.D.; Socotar, D.; Ciobanu, R.C.; Schreiner, T.G.; Tamba, B.I. Statistical Analysis of Gastric Cancer Cells Response to Broadband Terahertz Radiation with and without Contrast Nanoparticles. Cancers 2024, 16, 2454. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavenko, A.S.; Zhelnov, V.A.; Melikyants, D.G.; Chernomyrdin, N.V.; Lebedev, S.P.; Bukin, V.V.; Garnov, S.V.; Kurlov, V.N.; Zaytsev, K.I.; Katyba, G.M. Super-resolution THz endoscope based on a hollow-core sapphire waveguide and a solid immersion lens. Opt. Express 2023, 31, 13366–13373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Discussion on the Possibility of Using Terahertz Enhanced Endoscope to Detect Early-Stage Cancer. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4679748 (accessed on 6 July 2024).







| Reference Lot | Administrated Fe3O4/CMC Lot | ||||||
|---|---|---|---|---|---|---|---|
| Animal Weight (g) | Food (g) | Water (mL) | Animal Weight (g) | Food (g) | Water (mL) | ||
| Initial, p0 | Average | 41.83 | 110.01 | 50 | 34.17 | 105.84 | 139.17 |
| SD | 2.73 | 1.77 | 0 | 2.48 | 2.94 | 3.76 | |
| 24 h, p24 | Average | 40.68 | 105.53 | 40 | 33.04 | 102.81 | 133.17 |
| SD | 2.66 | 1.99 | 3.17 | 2.47 | 2.78 | 7.36 | |
| Difference p24-p0 | Average | 1.15 | 4.48 | 10.00 | 1.12 | 3.03 | 6.00 |
| SD | 0.50 | 1.37 | 3.16 | 0.68 | 1.94 | 4.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, O.D.; Samoila, P.; Schreiner, T.G.; Socotar, D.; Ciobanu, R.C. Synthesis and Characterization of Carboxymethylcellulose-Functionalized Magnetite Nanoparticles as Contrast Agents for THz Spectroscopy with Applications in Oncology. Crystals 2024, 14, 696. https://doi.org/10.3390/cryst14080696
Schreiner OD, Samoila P, Schreiner TG, Socotar D, Ciobanu RC. Synthesis and Characterization of Carboxymethylcellulose-Functionalized Magnetite Nanoparticles as Contrast Agents for THz Spectroscopy with Applications in Oncology. Crystals. 2024; 14(8):696. https://doi.org/10.3390/cryst14080696
Chicago/Turabian StyleSchreiner, Oliver Daniel, Petrisor Samoila, Thomas Gabriel Schreiner, Diana Socotar, and Romeo Cristian Ciobanu. 2024. "Synthesis and Characterization of Carboxymethylcellulose-Functionalized Magnetite Nanoparticles as Contrast Agents for THz Spectroscopy with Applications in Oncology" Crystals 14, no. 8: 696. https://doi.org/10.3390/cryst14080696





