Time-Dependent Study of Inclusions in Bearing Steel Subjected to Rare Earth Treatment with Secondary Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Simulation
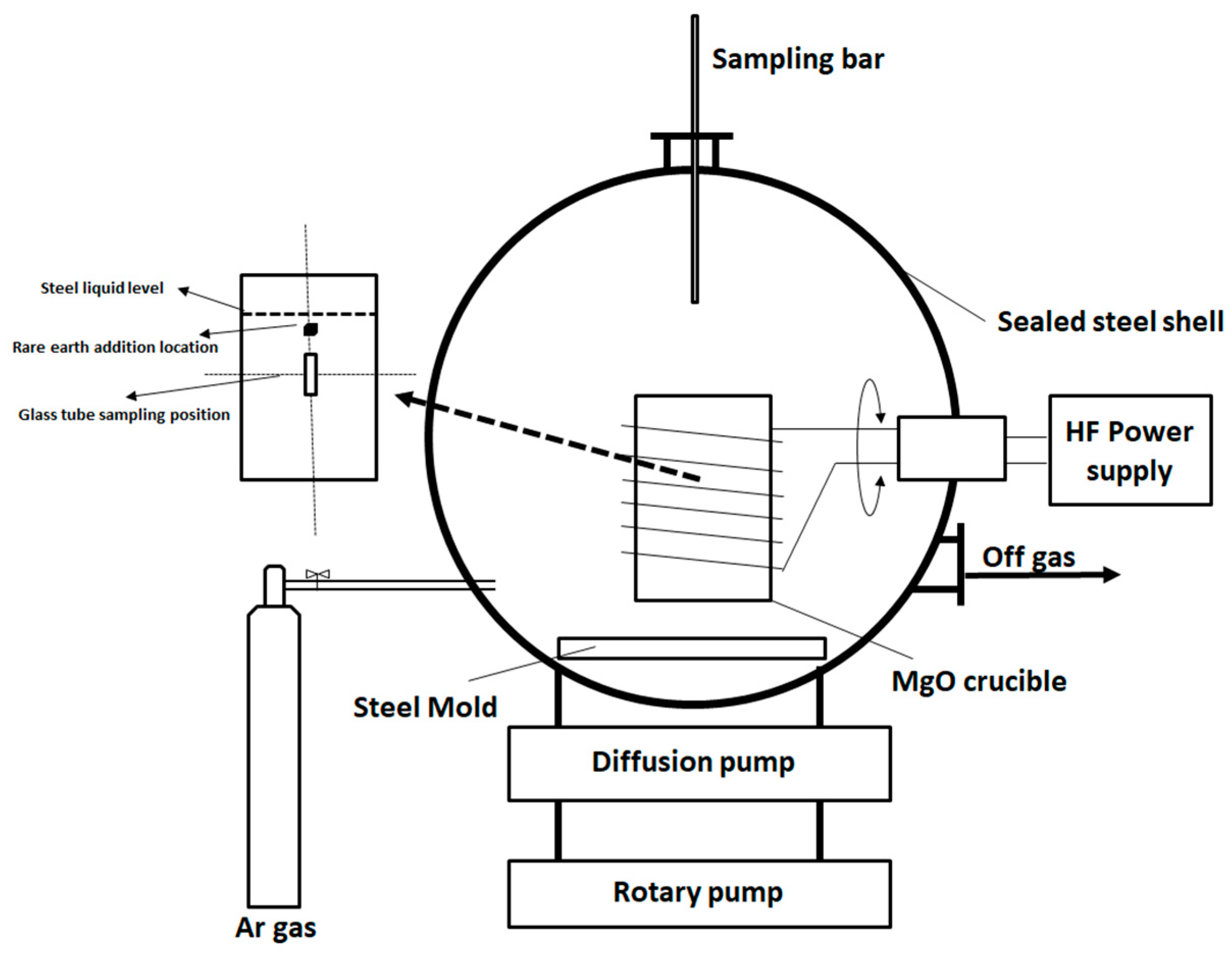
2.2. Vacuum Induction Furnace Experiment
2.3. Testing Methods
3. Results and Discussion
3.1. Rare Earth Treatment of Bearing Steel
3.1.1. Thermodynamic Simulation of Rare Earth Treatments
3.1.2. Rare Earth Vacuum Induction Furnace Trials
3.1.3. Enrichment Zones Exist in Rare Earth Processing
- (1)
- Rare earth Ce alloy dissolution and uniform distribution process
- (2)
- Number density, average size, and area density
3.2. Secondary Oxidation
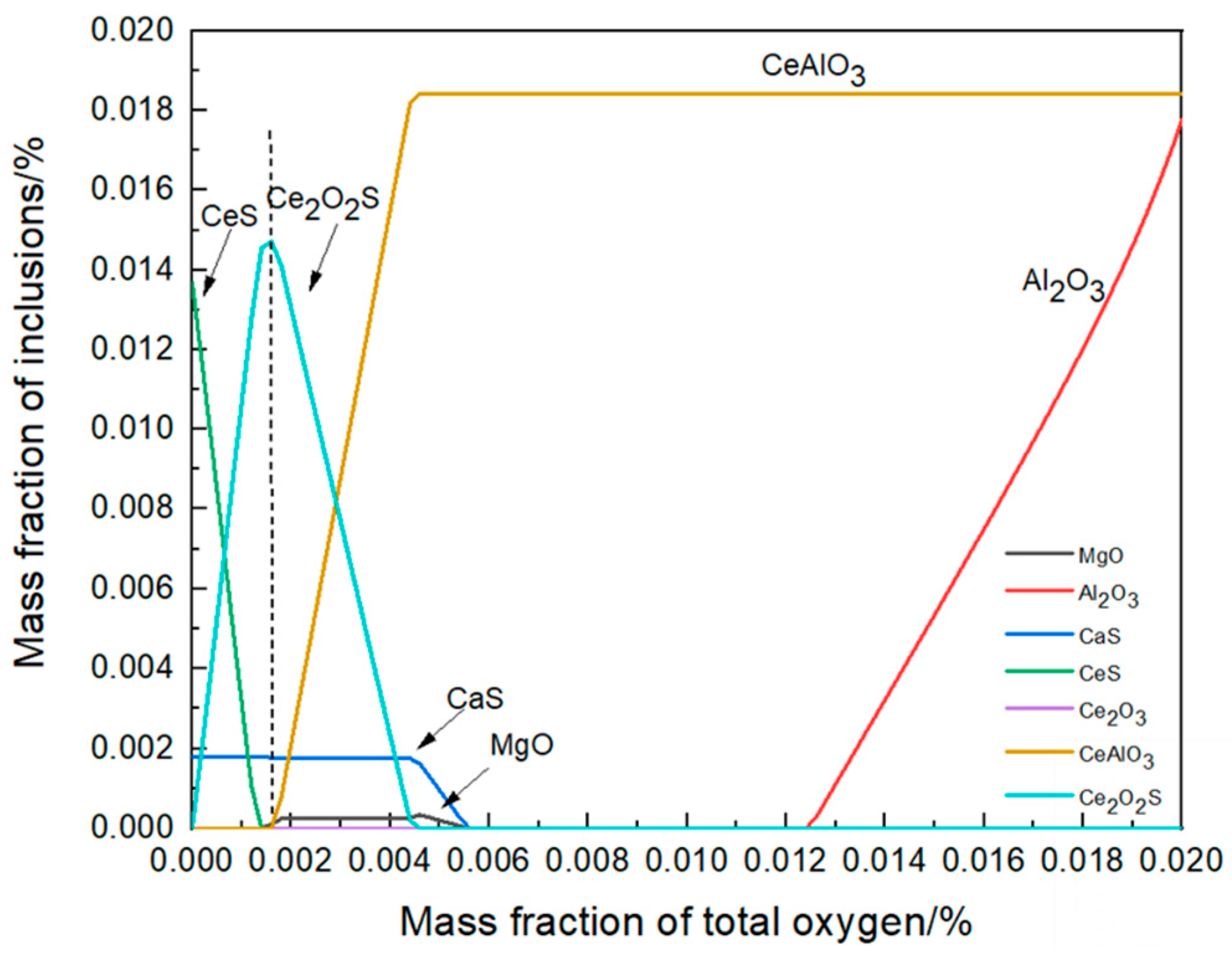
3.2.1. Thermodynamic Simulation of Secondary Oxidation
3.2.2. Secondary Oxidation Vacuum Induction Furnace Experiments
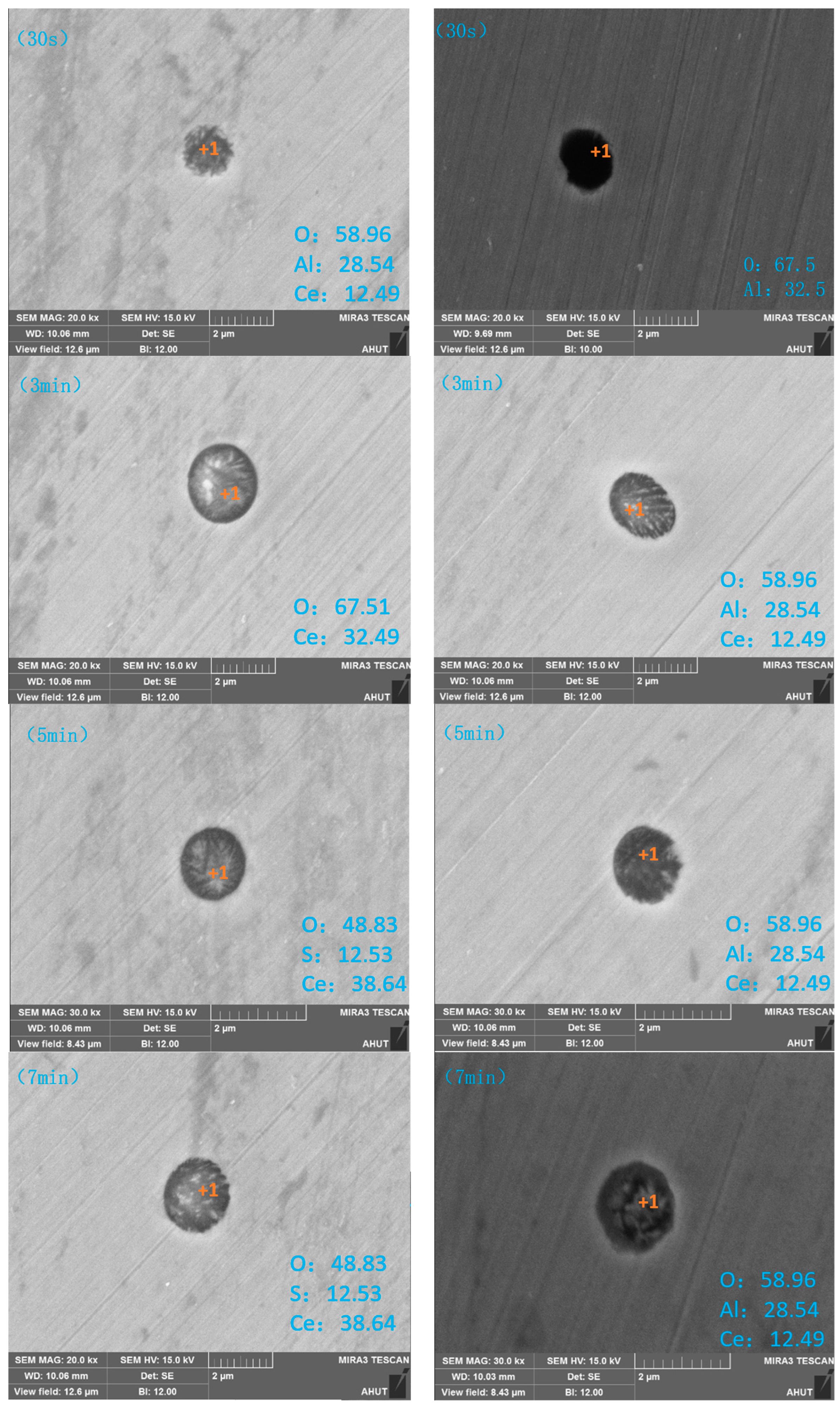
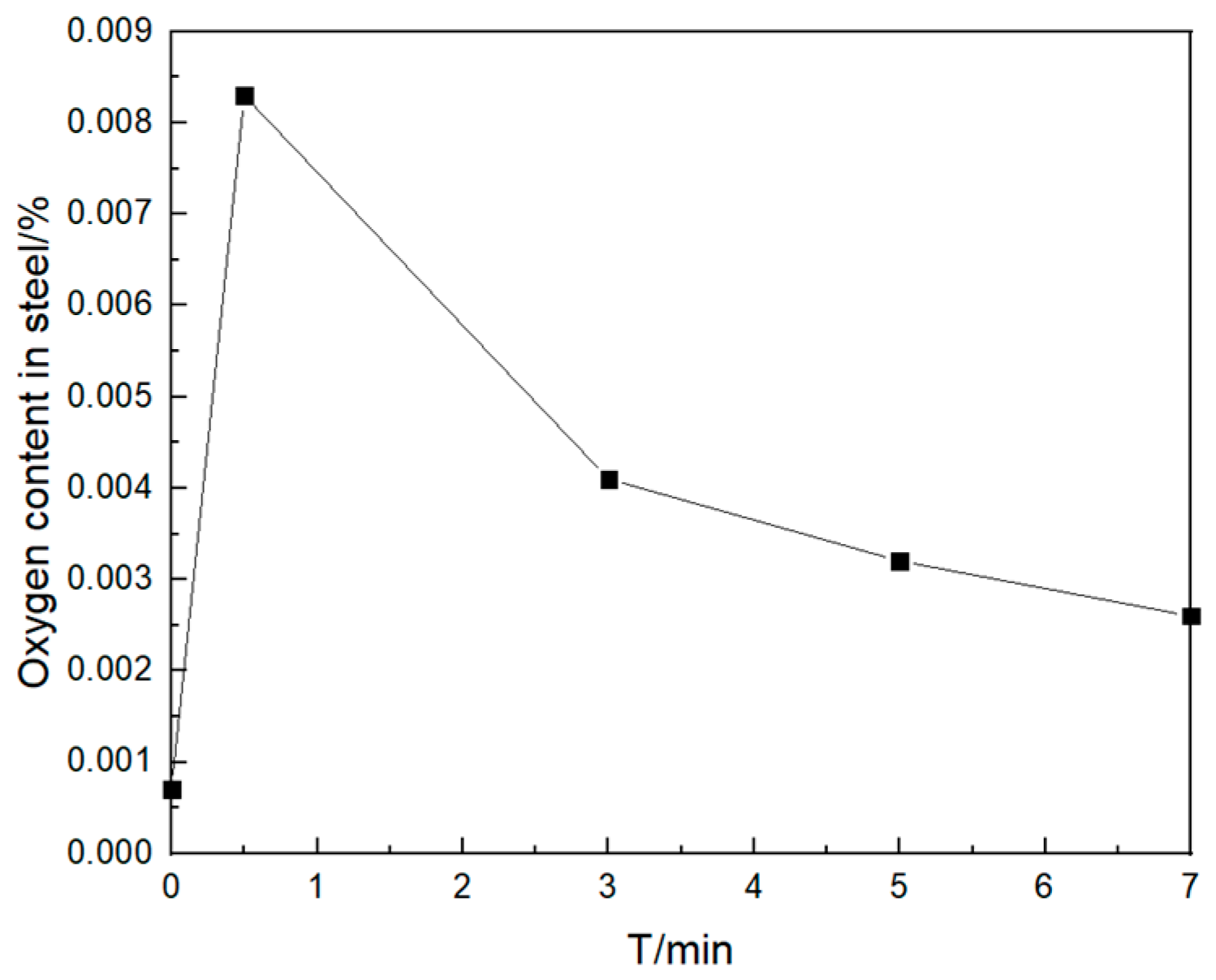
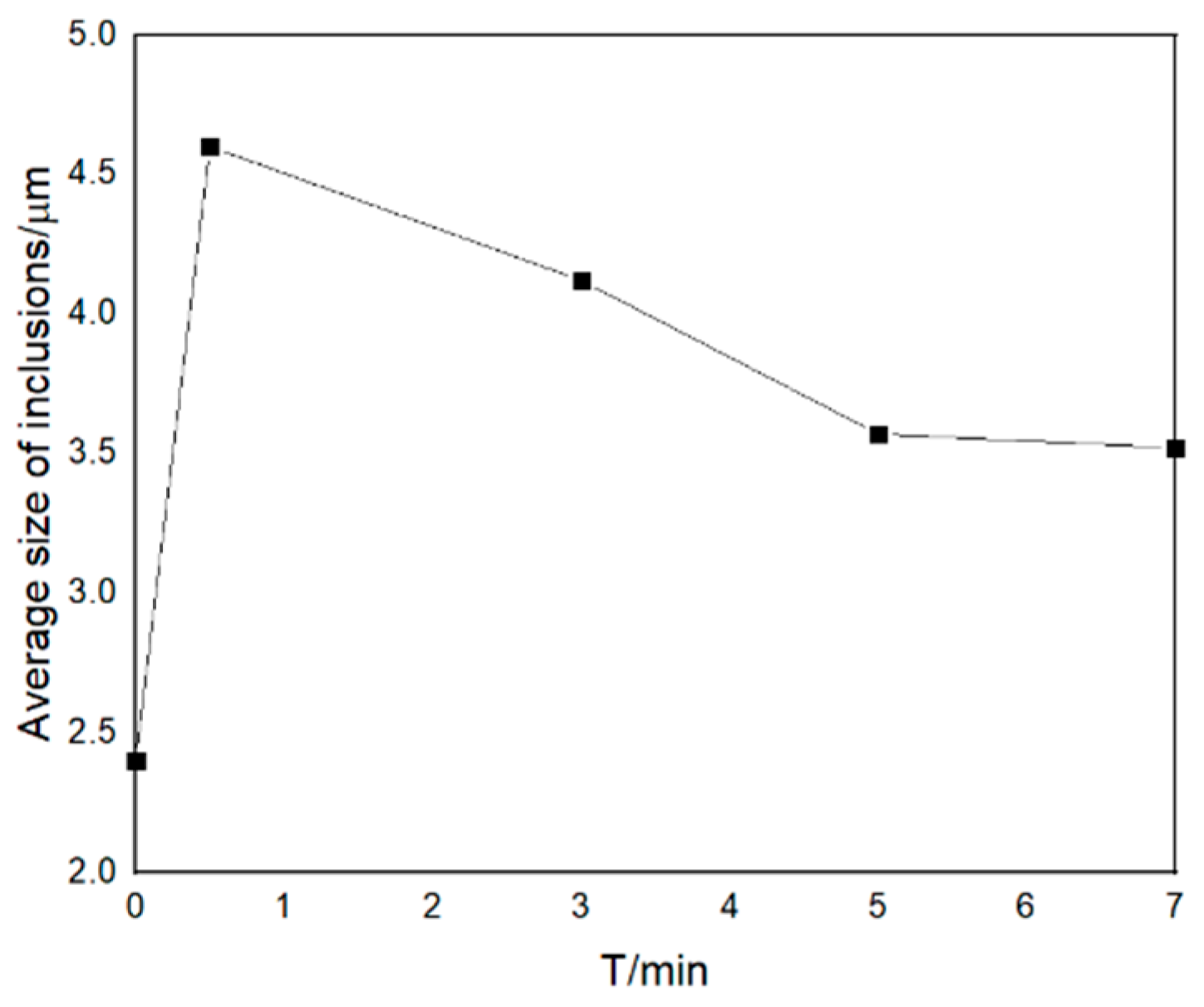
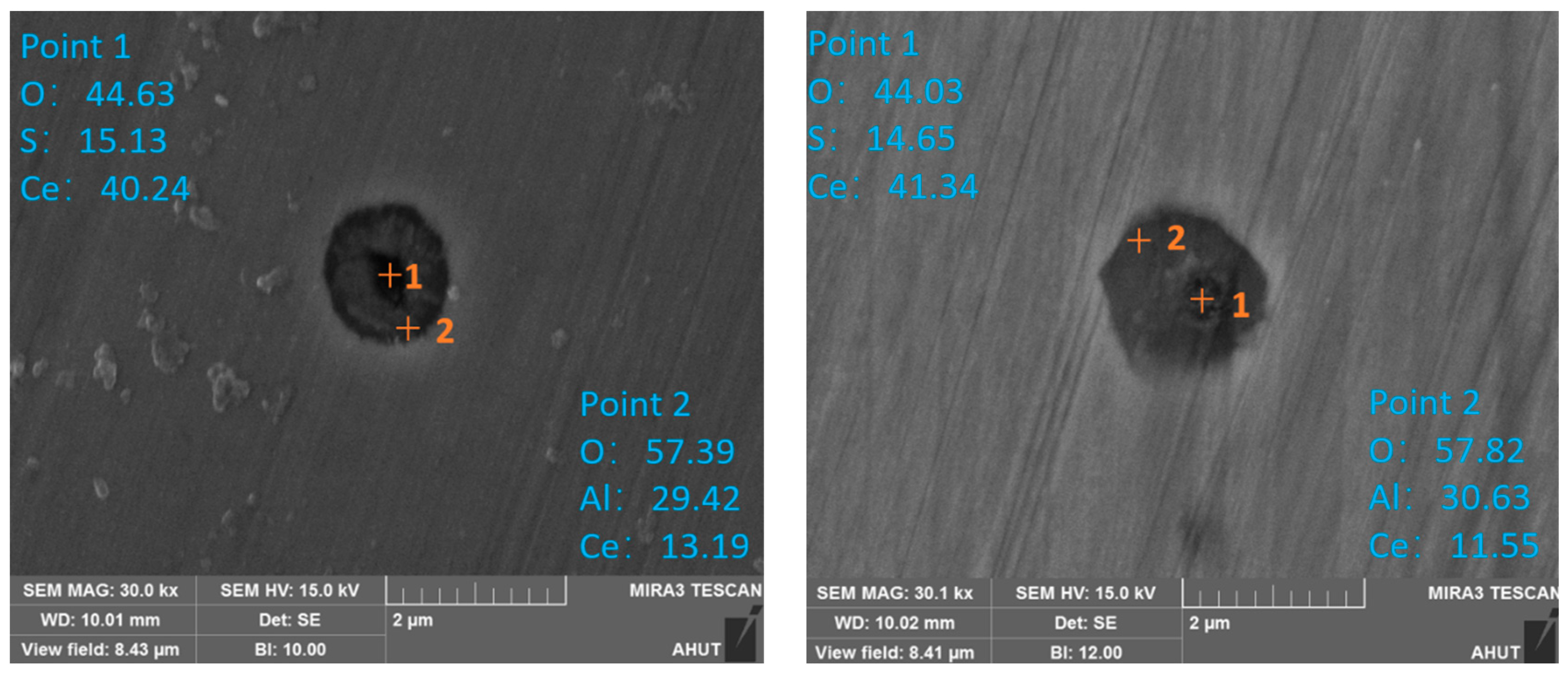
3.2.3. Enrichment Zones Exist during Secondary Oxidation
- (1)
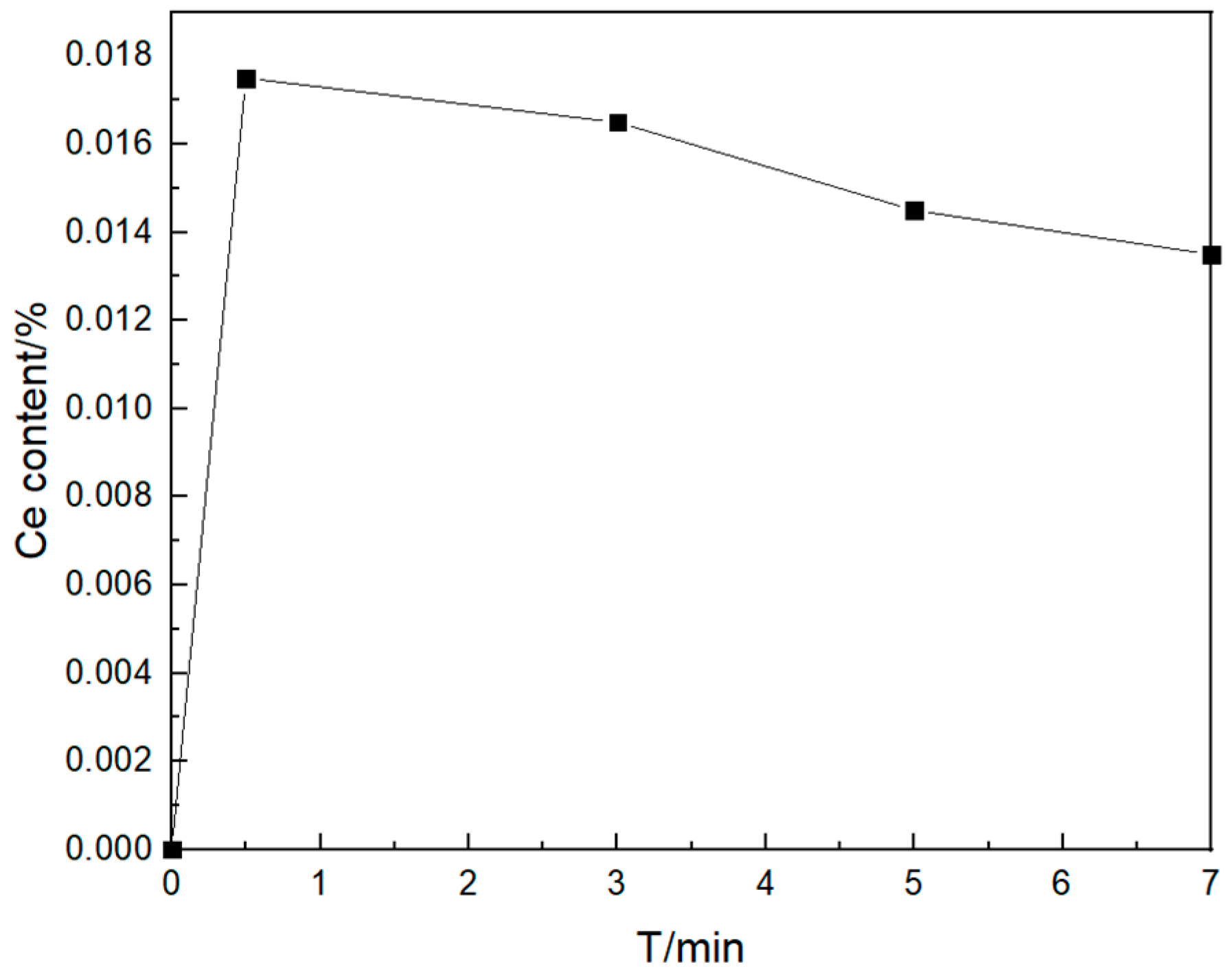
- Change the curve of oxygen content
- (2)
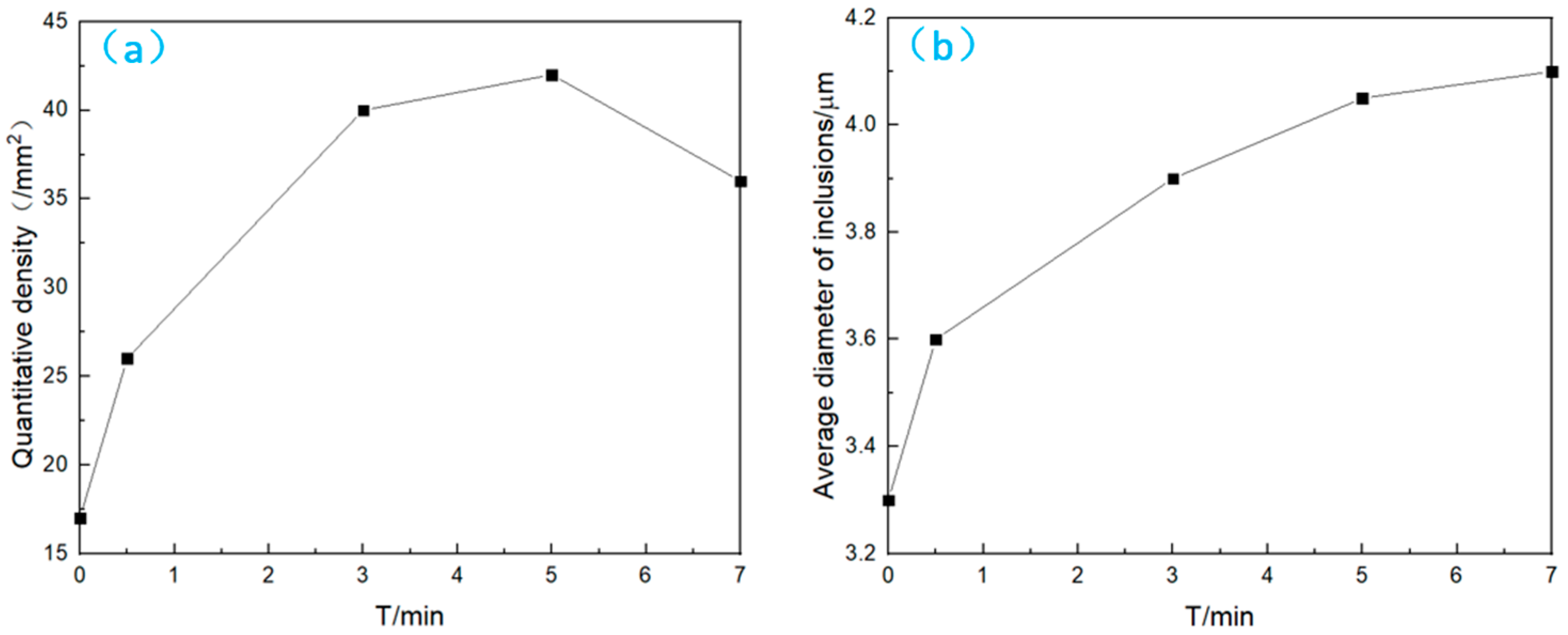
- Number and area density
- (3)
- Average size and parcel-type inclusions
4. Conclusions
- (1)
- When the bearing steel is subjected to rare earth Ce treatment, an enriched layer of Ce exists around the rare earth alloy. At a Ce content of 0.012%, the transformation path of inclusions over time is Al2O3 → CeS + Ce2O2S → Ce2O2S. After a certain period, the intermediate sample of inclusions in the vacuum induction furnace is finally in a state consistent with the thermodynamic phase diagram. With time, the number density increases and then decreases, the inclusion size gradually increases, and the area density gradually increases;
- (2)
- In transient secondary oxidation, local steel has an oxygen-rich layer. When the amount of secondary oxidation is 0.0025%, the transformation path of inclusions is Ce2O3 + CeAlO3 + Al2O3 → Ce2O3 + CeAlO3 → Ce2O2S + CeAlO3. After a certain period, the intermediate samples of inclusions in the vacuum induction furnace are finally in a consistent state with the thermodynamic phase diagram. With time, the number density and size of the inclusions increase and then decrease and the area density gradually increases;
- (3)
- In the actual production process of bearing steel, when rare earth alloys are added for rare earth treatment, measures should be taken to reduce the influence of rare earth enrichment layers and oxygen enrichment layers.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Feng, X.; Luo, D.; Zhang, Z.; Lai, C. Effect of Rare Earth Ce Addition on Inclusions in Offshore Engineering Steel Containing Arsenic. Mater. Res. Express 2023, 10, 116512. [Google Scholar] [CrossRef]
- Huang, F.; Li, J.; Geng, R.; Wang, C. Effect of Rare Earth on Inclusion Evolution and Corrosion Resistance of HRB400E Steel. Mater. Corros. 2023, 74, 53–67. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, W.; Yang, F.; Zeng, J.; Liang, Z.; He, J. Effect of Cerium Element on Non-Metallic Inclusions Formation During Molten and Solidification Processes of Q355 Microalloy Structural Steel. Metall. Mater. Trans. B 2024, 55, 1244–1260. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, R.; Lu, Y.; Yang, Y.; Wang, Y.; Yang, X. Effect of Trace Rare-Earth Element Ce on the Microstructure and Properties of Cold-Rolled Medium Manganese Steel. Metals 2023, 13, 116. [Google Scholar] [CrossRef]
- Ye, K.; Cai, X.; Sun, B.; Zhou, L.; Ma, S.; Yue, Y.; Xu, F.; Zheng, D.; Fu, X. Effect of Rare Earth Ce on the Microstructure and Mechanical Properties of Cast Al–7Si Alloys. J. Sci. Adv. Mater. Devices 2023, 8, 100634. [Google Scholar] [CrossRef]
- Bai, G.J.; Yang, J.C.; Liang, W.J. Effect of Lanthanum–Cerium Mixed Rare Earth on Inclusions in U76CrRe Eavy Rail Steel. Can. Metall. Q. 2023, 62, 720–733. [Google Scholar] [CrossRef]
- Bai, G.; Yang, J.; Liang, W. Evaluation and Analysis of the Influence of Rare-Earth Ce on Inclusions in Heavy Rail Steel. Metals 2023, 13, 614. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Tang, H.; Liu, J.; Cai, S.; Zhang, J. Modification of Inclusions by Rare Earth Elements in a High-Strength Oil Casing Steel for Improved Sulfur Resistance. Materials 2023, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Feng, G.; Zhi, J.; Zhao, B.; Wu, W. The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals 2022, 12, 1358. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, S.; Liu, N.; Liu, Y.; Wang, X.; Qiu, L.; Gong, A. The Role of Rare Earths on Steel and Rare Earth Steel Corrosion Mechanism of Research Progress. Coatings 2024, 14, 465. [Google Scholar] [CrossRef]
- Hao, C.; Wen, J.; Liu, Z.; Yan, J.; Sang, B.; Luan, Y. Effect of Rare-Earth Elements on Fatigue Properties of Spring Steel. J. Mater. Eng. Perform. 2024, 1–10. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhi, J.; Lian, X.; Guo, L.; Dong, H. Corrosion Behavior of Weathering Steels with Different Contents of Rare Earth Elements La and Ce. Corrosion 2022, 78, 437–448. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, L.; Zhao, R.; Guo, Z.; Qiu, G. Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel. Metals 2023, 13, 1680. [Google Scholar] [CrossRef]
- Cheng, W.; Song, B.; Yang, Z.; Mao, J. Effect of Rare Earth Ce on Modifying Inclusions in Al-Killed X80 Pipeline Steel. Trans. Indian Inst. Met. 2022, 75, 2837–2846. [Google Scholar] [CrossRef]
- Ren, Q.; Hu, Z.; Cheng, L.; Zhang, L. Effect of Rare Earth Elements on Magnetic Properties of Non-Oriented Electrical Steels. J. Magn. Magn. Mater. 2022, 560, 169624. [Google Scholar] [CrossRef]
- Zhao, Q.; Luo, H.; Pan, Z.; Wang, X.; Cheng, H. Study on Mechanical Properties of Rare Earth Elements Modified High Carbon Chromium Bearing Steel. Mater. Today Commun. 2023, 34, 105329. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Zhang, F.; Li, Y.; Zhang, X.; An, S. Effects of Ce-Modified TiN Inclusions on the Fatigue Properties of Gear Steel 20CrMnTi. Crystals 2023, 13, 1071. [Google Scholar] [CrossRef]
- Zhuo, C.; Liu, R.; Zhao, Z.; Zhang, Y.; Hao, X.; Wu, H.; Sun, Y. Effect of Rare Earth Cerium Content on Manganese Sulfide in U75V Heavy Rail Steel. Metals 2022, 12, 1012. [Google Scholar] [CrossRef]
- Xiaoyu, Z.; Ruifeng, D.; Bo, G.; Jiquan, C.; Zhipeng, M.; Weizhe, Z.; Xiong, Y. Effect of Trace Rare Earth Elements (Ce) on Corrosion Resistance of High Strength Steel Used for Offshore Platform. Mater. Res. Express 2023, 10, 036514. [Google Scholar] [CrossRef]
- Xiao, J.; Yan, L.; Zhang, P.; Li, G.; Li, B.; Zhao, T.; Wang, H.; Chen, L.; Wang, D. Effects of Minor Ce Doping on the Microstructure and Mechanical Performances of an EH47 Grade HSLA Steel for Ship and Ocean Engineering. Mater. Charact. 2023, 201, 112931. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, Y.; Li, D.; Chen, Y.; Fu, P.; Liu, H.; Kang, X.; Liu, H.; Sun, C. On the Mechanism of Steel Homogenization via Rare Earth Addition: Experimental Characterization and Numerical Simulation. Metall. Mater. Trans. B 2022, 53, 1858–1874. [Google Scholar] [CrossRef]
- Duan, R.; Bai, P.; Yang, J.; Zhang, W.; Ding, H. Influence of Rare Earth Modification and Homogenization on the Microstructure and Mechanical Properties of Recycled Can 3004 Aluminum. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 264–268. [Google Scholar] [CrossRef]
- Liang, W.; Geng, R.; Zhi, J.; Li, J.; Huang, F. Oxide Metallurgy Technology in High Strength Steel: A Review. Materials 2022, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Q.; Kang, X.; Gao, J.; Zhang, L. Transformation of LaAlO3 Inclusions During Heating in a Solid Non-Oriented Electrical Steel. Metall. Mater. Trans. B 2022, 53, 637–649. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.; Hu, Z.; Cheng, L. Transient Influence of Cerium on Inclusions in an Al-Killed Non-Oriented Electrical Steel. Ironmak. Steelmak. 2021, 48, 191–199. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L. Effect of Cerium Content on Inclusions in an Ultra-Low-Carbon Aluminum-Killed Steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Li, B.; Zhu, H.; Zhao, J.; Song, M.; Li, J.; Xue, Z. Effect of Rare-Earth La on Inclusion Evolution in High-Al Steel. Steel Res. Int. 2022, 93, 2100347. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C. Effect of Reoxidation on Inclusions Characteristic During Casting in Al-Killed Steel Containing Rare Earth. Steel Res. Int. 2022, 93, 2200263. [Google Scholar] [CrossRef]
- GB/T 30834-2022; Rating and Classifying of Inclusions in Steel-Scanning Electron Microscrope Method. China Standard Press: Beijing, China, 2022.







| Heat Number | C | Si | Mn | P | S | O | Cr | Ni | Al | Ce |
|---|---|---|---|---|---|---|---|---|---|---|
| a | 0.995 | 0.23 | 0.344 | 0.005 | 0.003 | 0.0010 | 1.418 | 0.018 | 0.014 | 0.012 |
| b | 0.991 | 0.25 | 0.331 | 0.003 | 0.003 | 0.0025 | 1.431 | 0.018 | 0.014 | 0.012 |
| Time | 0 | 30 s | 3, 5 min | 7 min |
|---|---|---|---|---|
| Typical inclusions | Al2O3 | Ce2O2S, CeS | Ce2O2S, CeS | Ce2O2S |
| Time | 30 s | 3 min | 5, 7 min |
|---|---|---|---|
| Typical inclusions | Ce2O3, CeAlO3, Al2O3 | Ce2O3, CeAlO3 | Ce2O2S, CeAlO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xia, W.; Zhou, Y.; Deng, A.; Bao, G.; Liao, Z.; Wang, H. Time-Dependent Study of Inclusions in Bearing Steel Subjected to Rare Earth Treatment with Secondary Oxidation. Crystals 2024, 14, 697. https://doi.org/10.3390/cryst14080697
Wang W, Xia W, Zhou Y, Deng A, Bao G, Liao Z, Wang H. Time-Dependent Study of Inclusions in Bearing Steel Subjected to Rare Earth Treatment with Secondary Oxidation. Crystals. 2024; 14(8):697. https://doi.org/10.3390/cryst14080697
Chicago/Turabian StyleWang, Weining, Wenzhi Xia, Yun Zhou, Aijun Deng, Guangda Bao, Zhiyou Liao, and Haichuan Wang. 2024. "Time-Dependent Study of Inclusions in Bearing Steel Subjected to Rare Earth Treatment with Secondary Oxidation" Crystals 14, no. 8: 697. https://doi.org/10.3390/cryst14080697







