Fast One-Step Microwave-Assisted Synthesis of Iron-Doped ZnS for Photocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Generation of Pure and Iron-Doped ZnS Nanoparticles
2.3. Characterization of Pure and Iron-Doped ZnS Nanoparticles
2.4. Photocatalytic Experiments of Quinoline Yellow
3. Results and Discussion
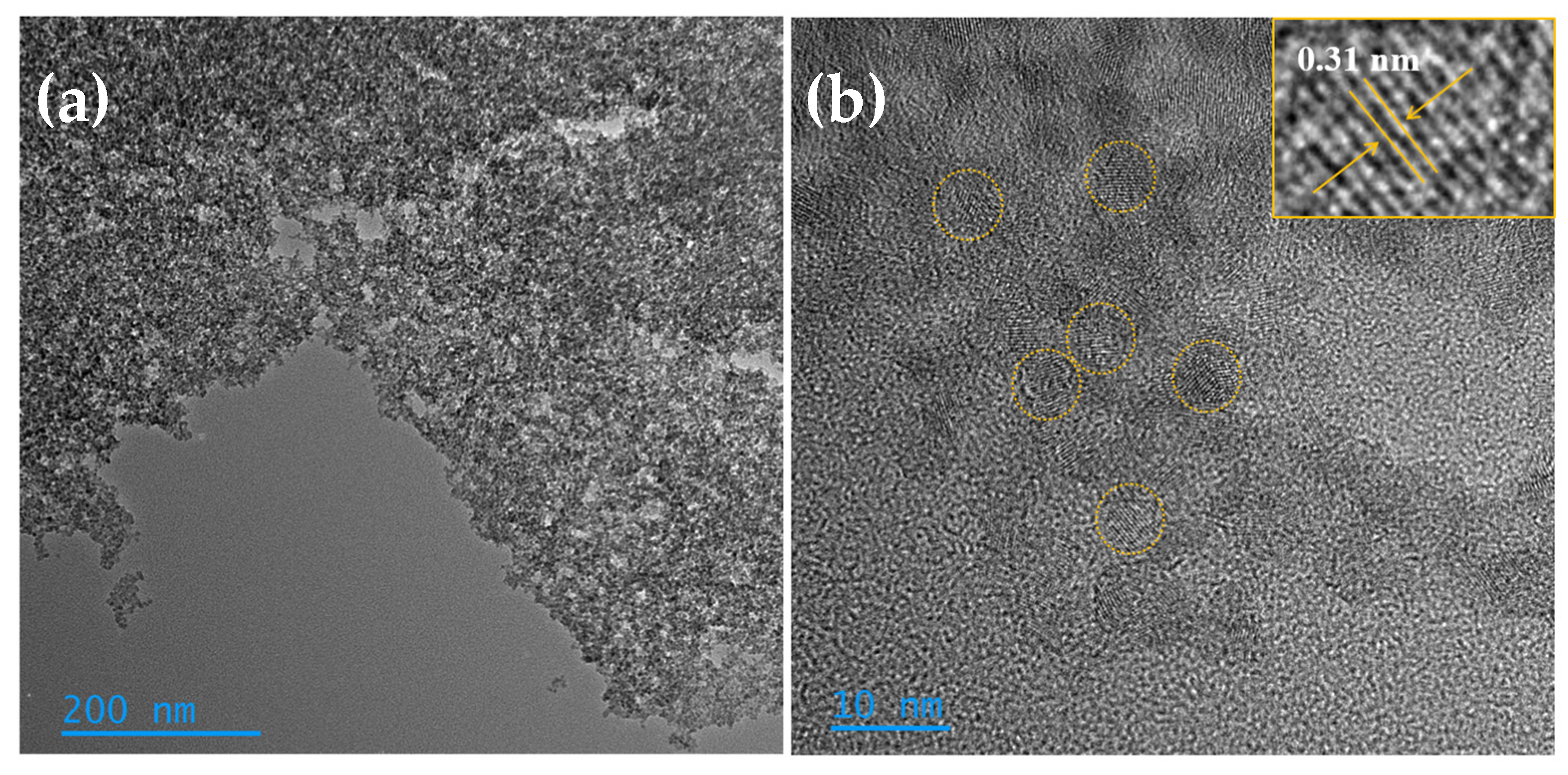
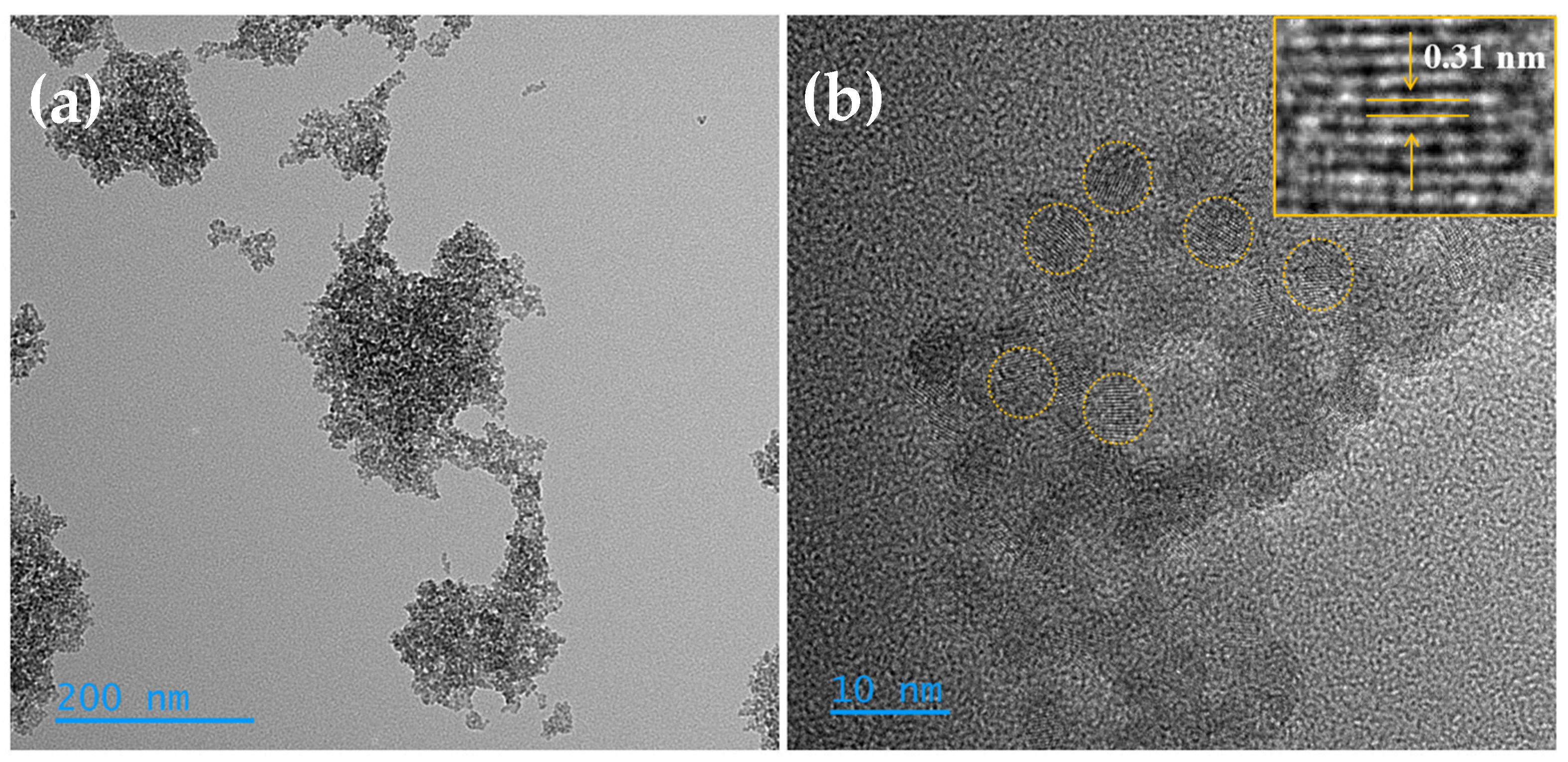
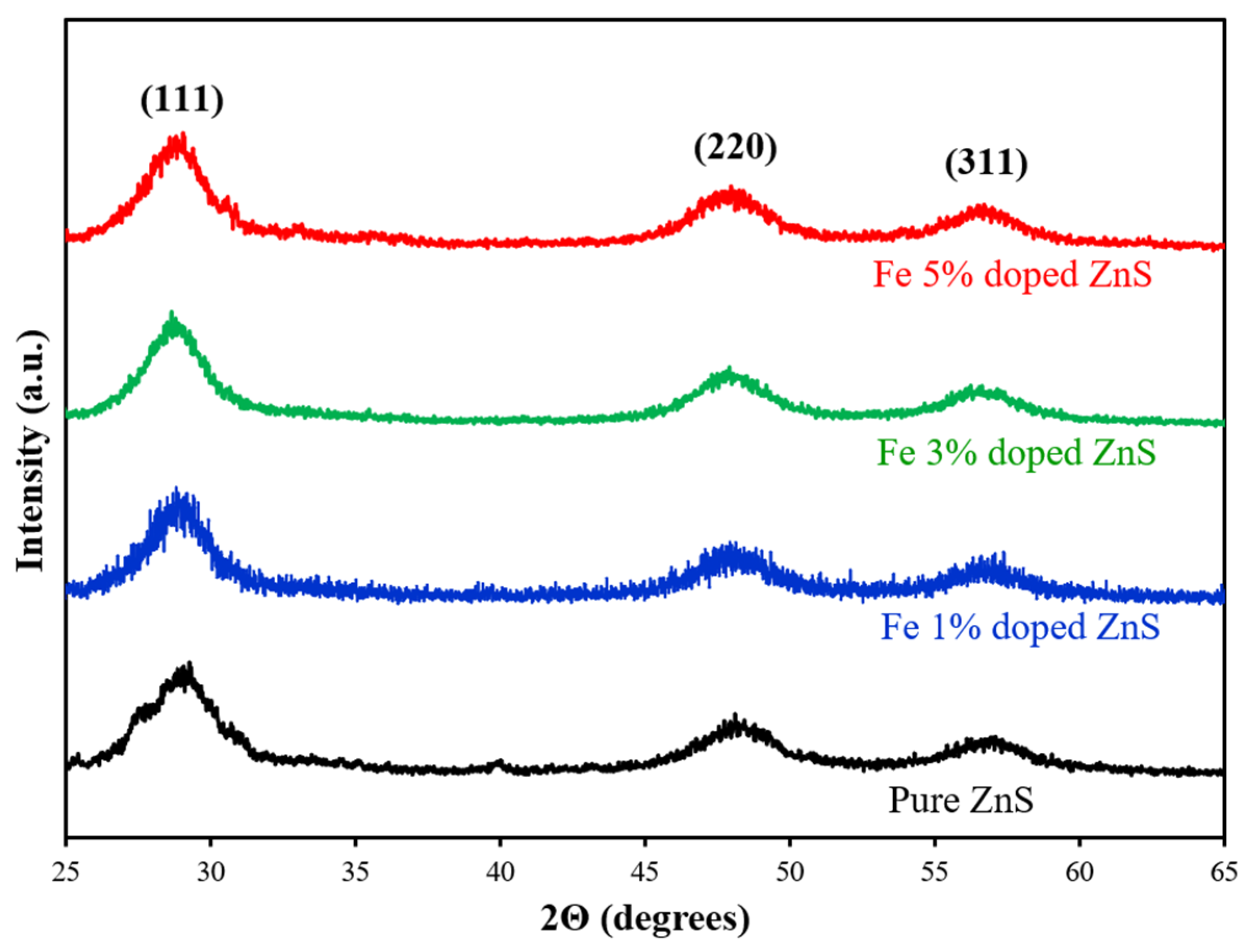
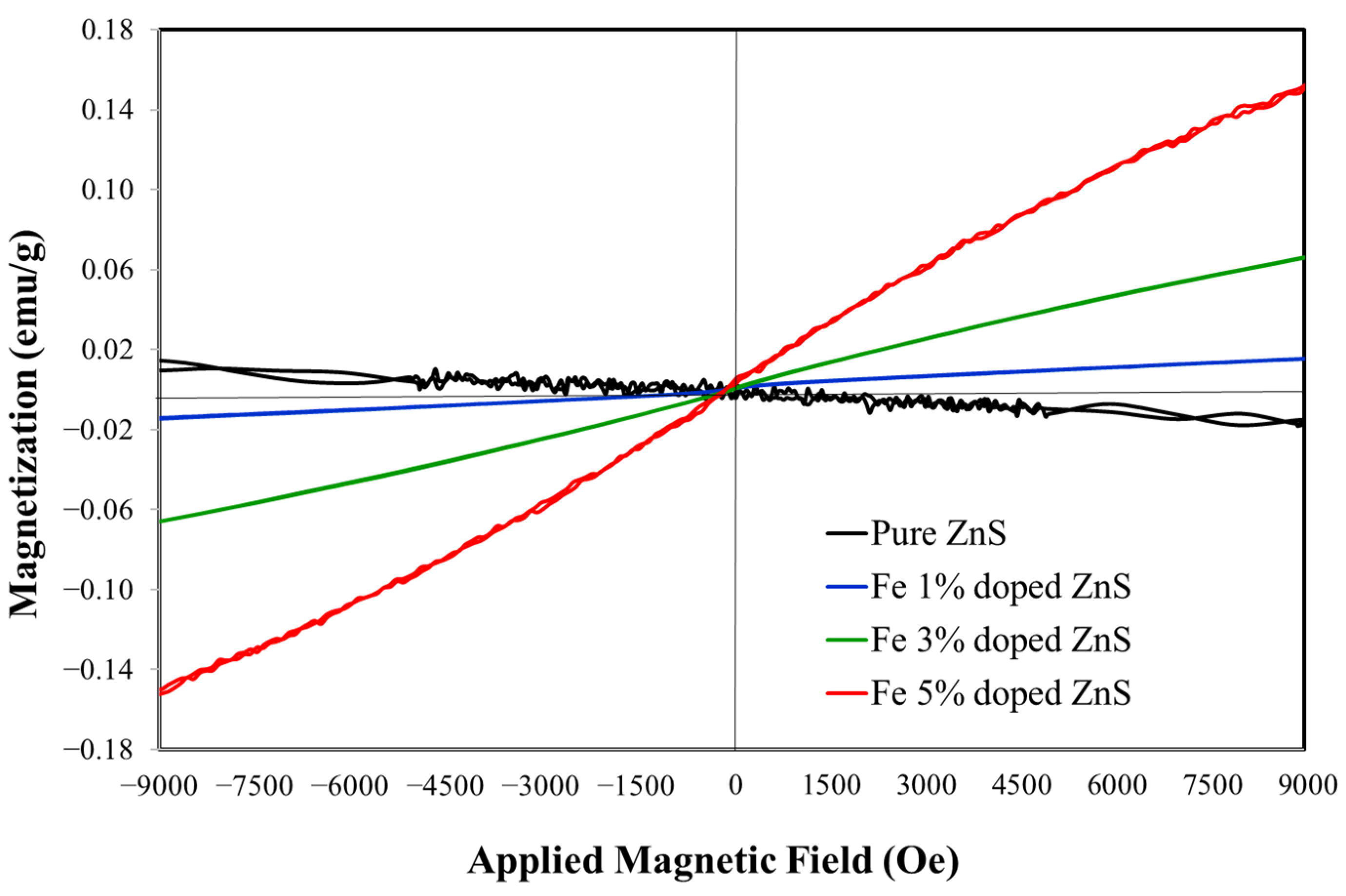
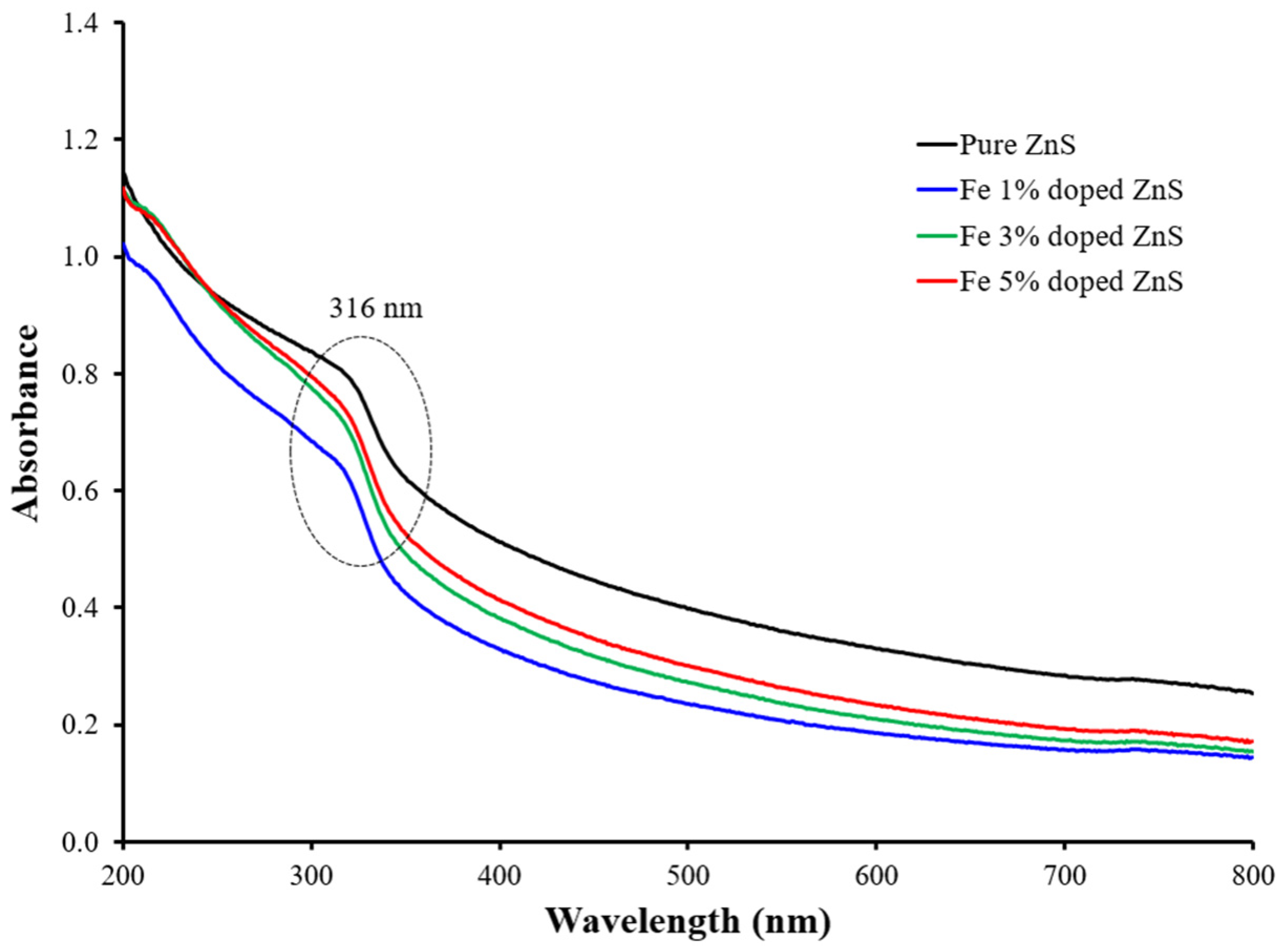
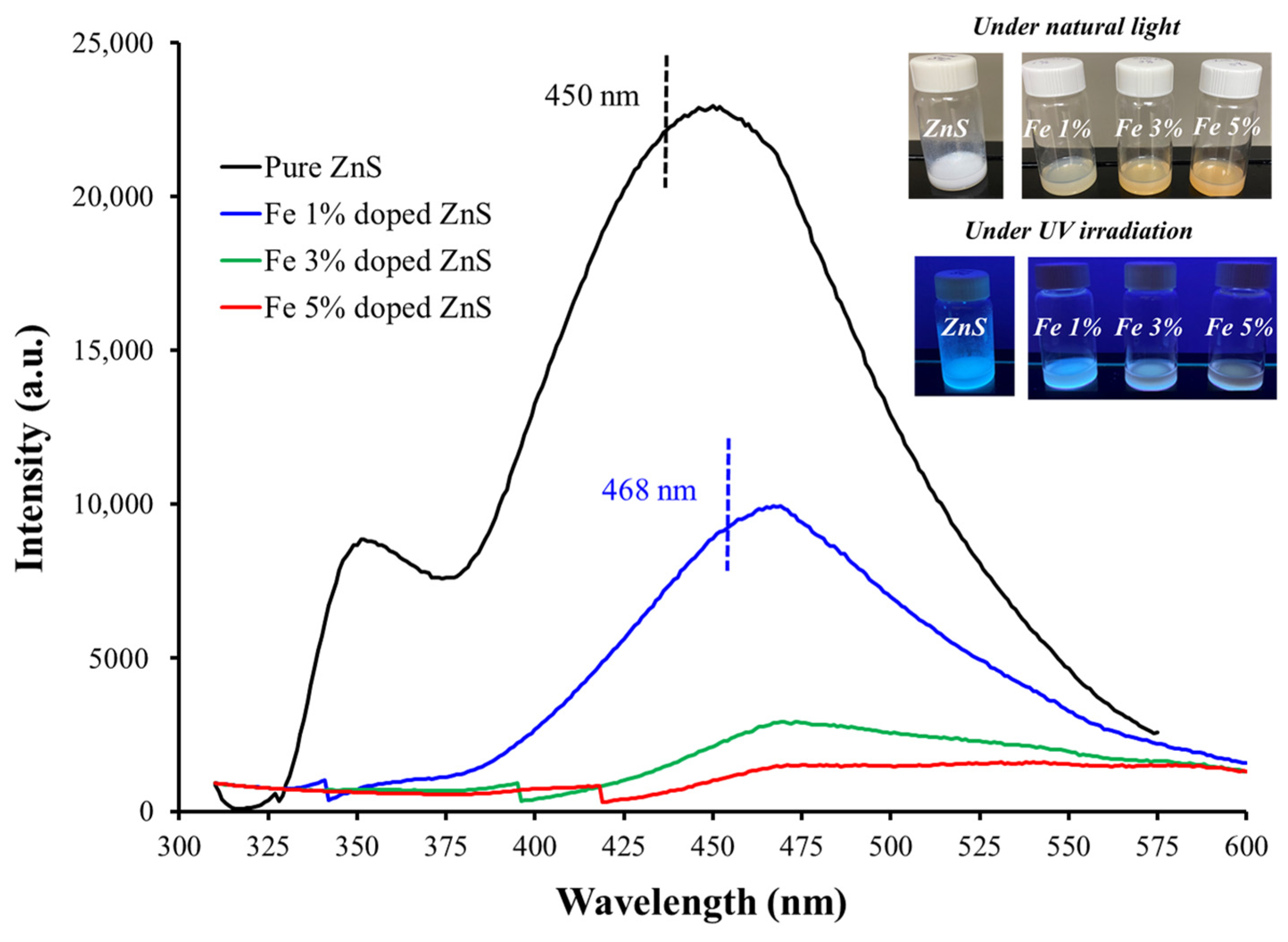
3.1. Morphological, Compositional, and Optical Characterization
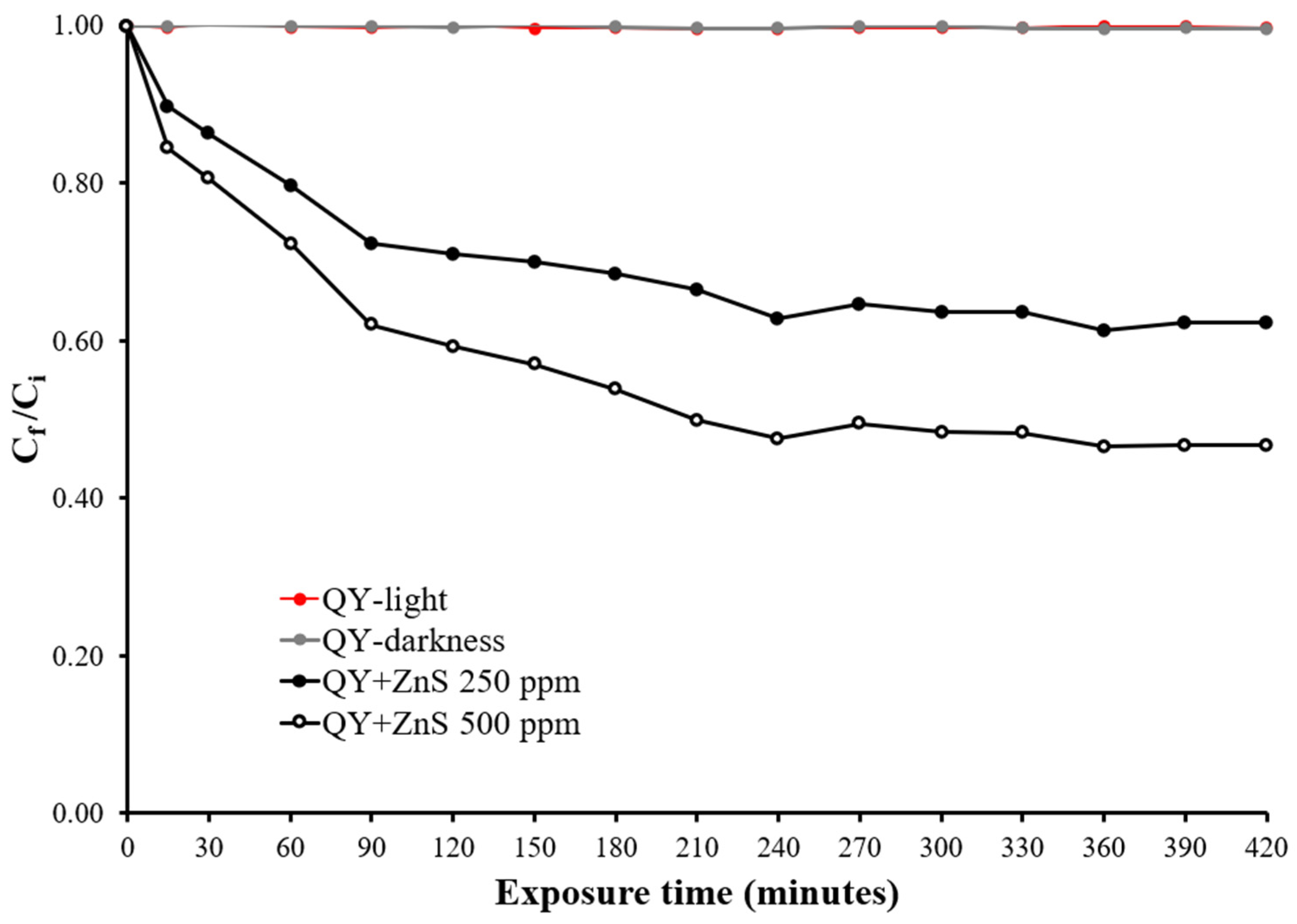
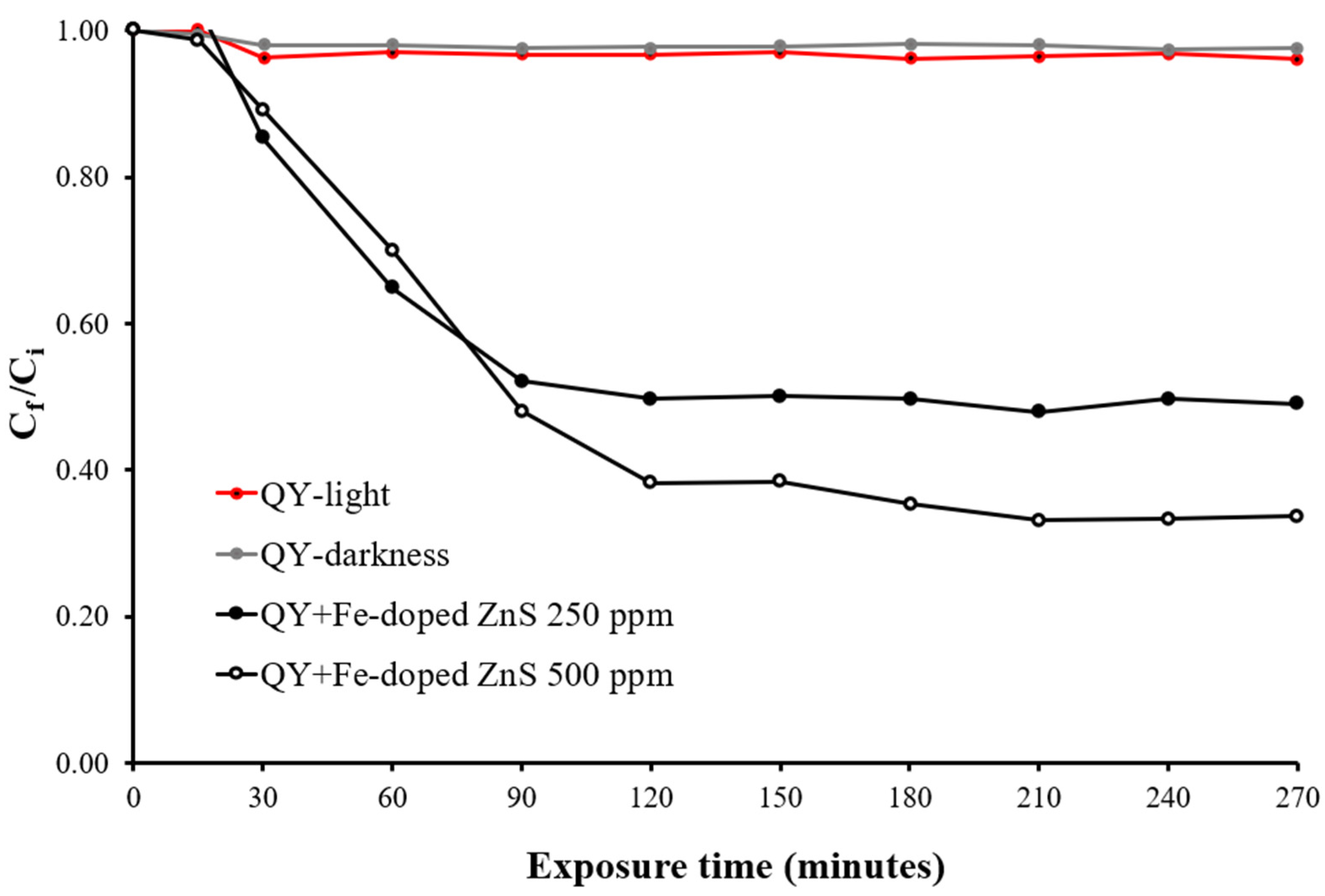
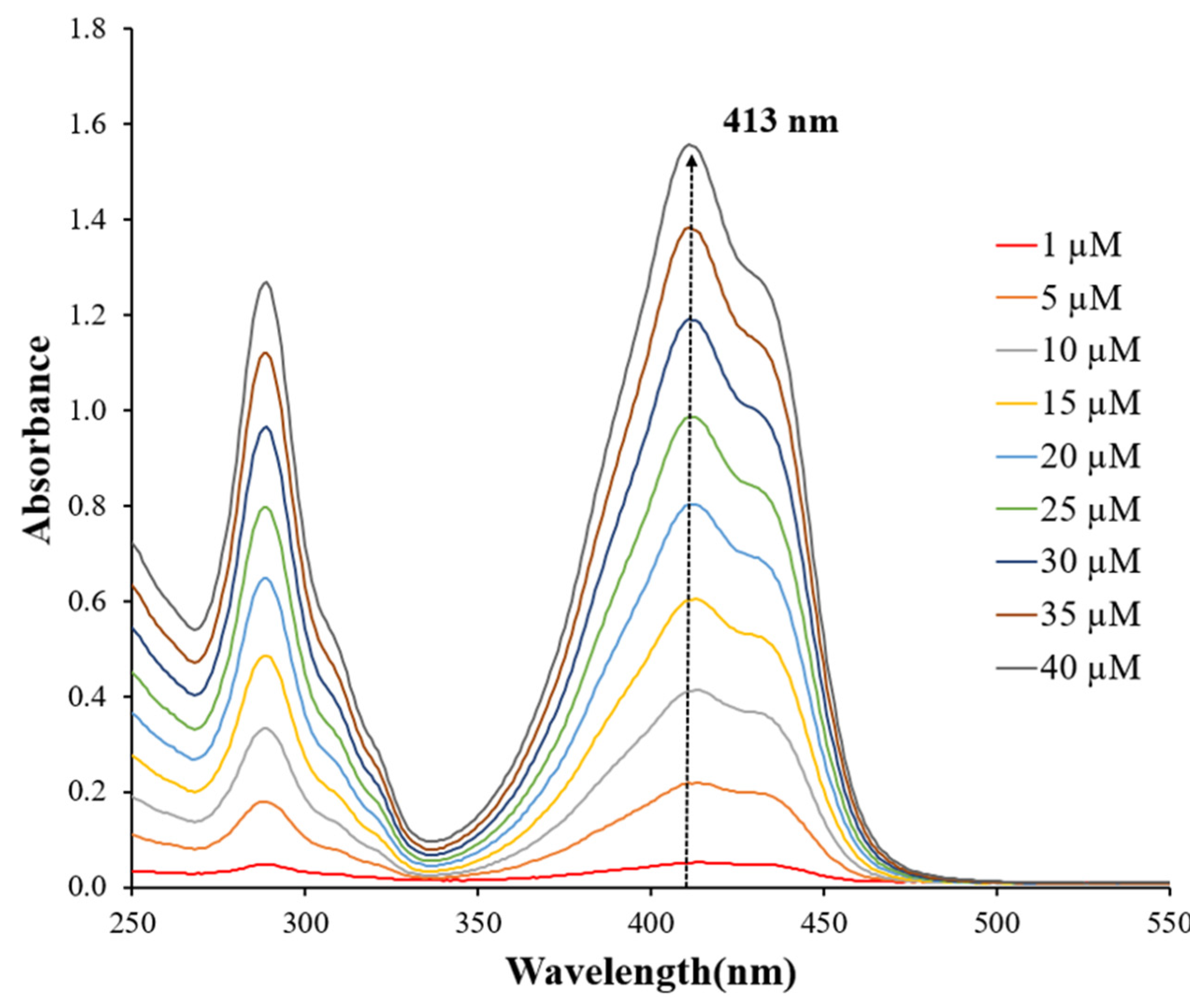
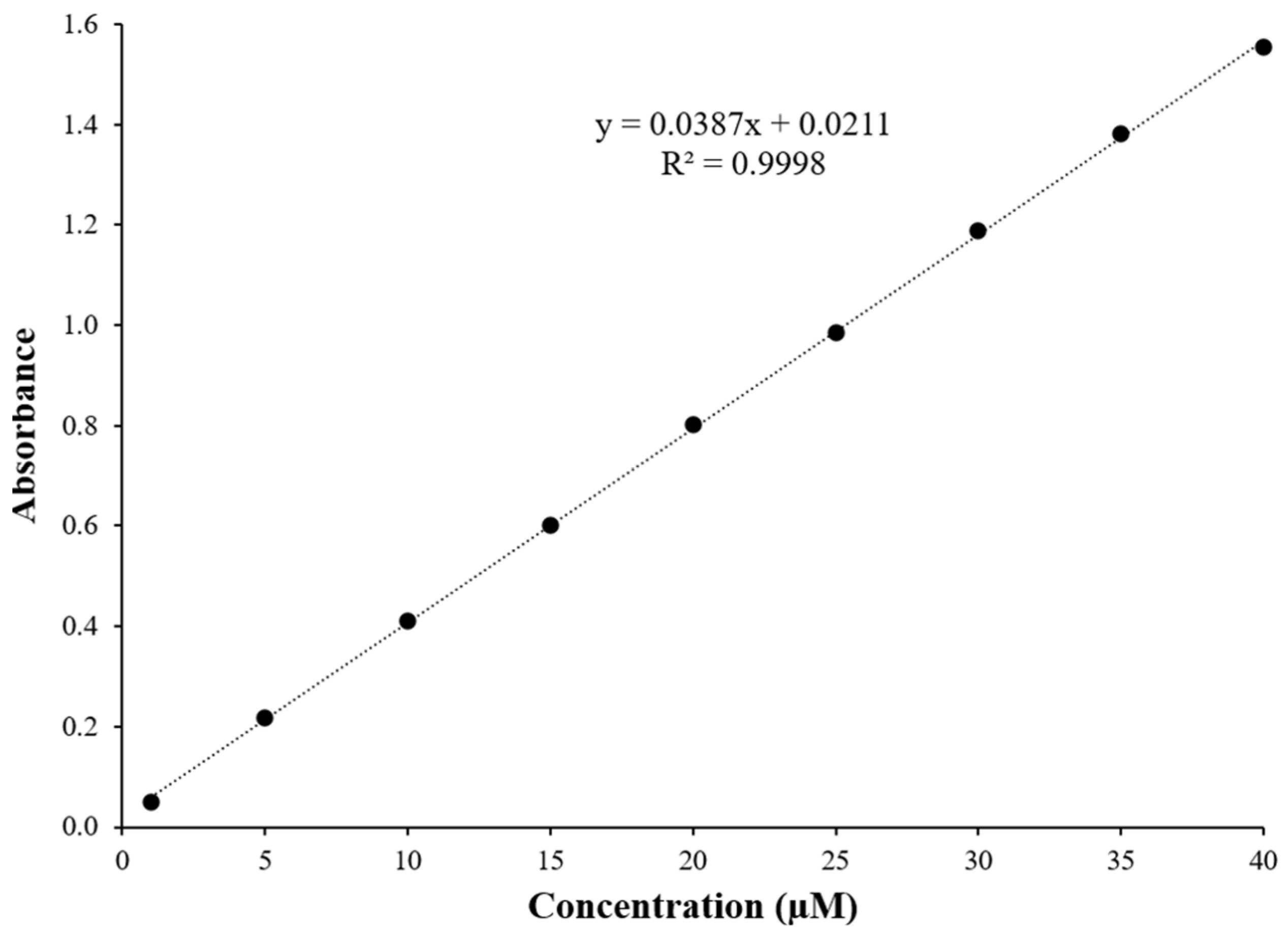
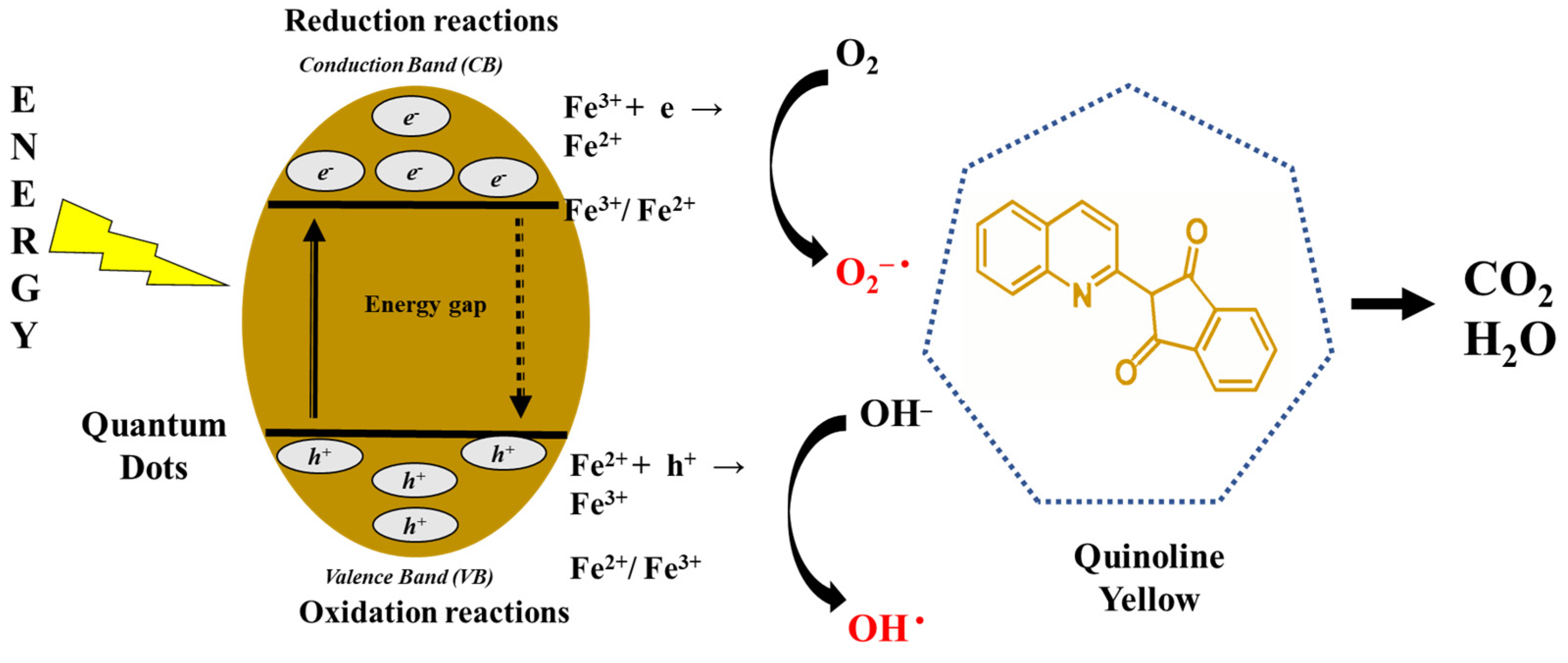
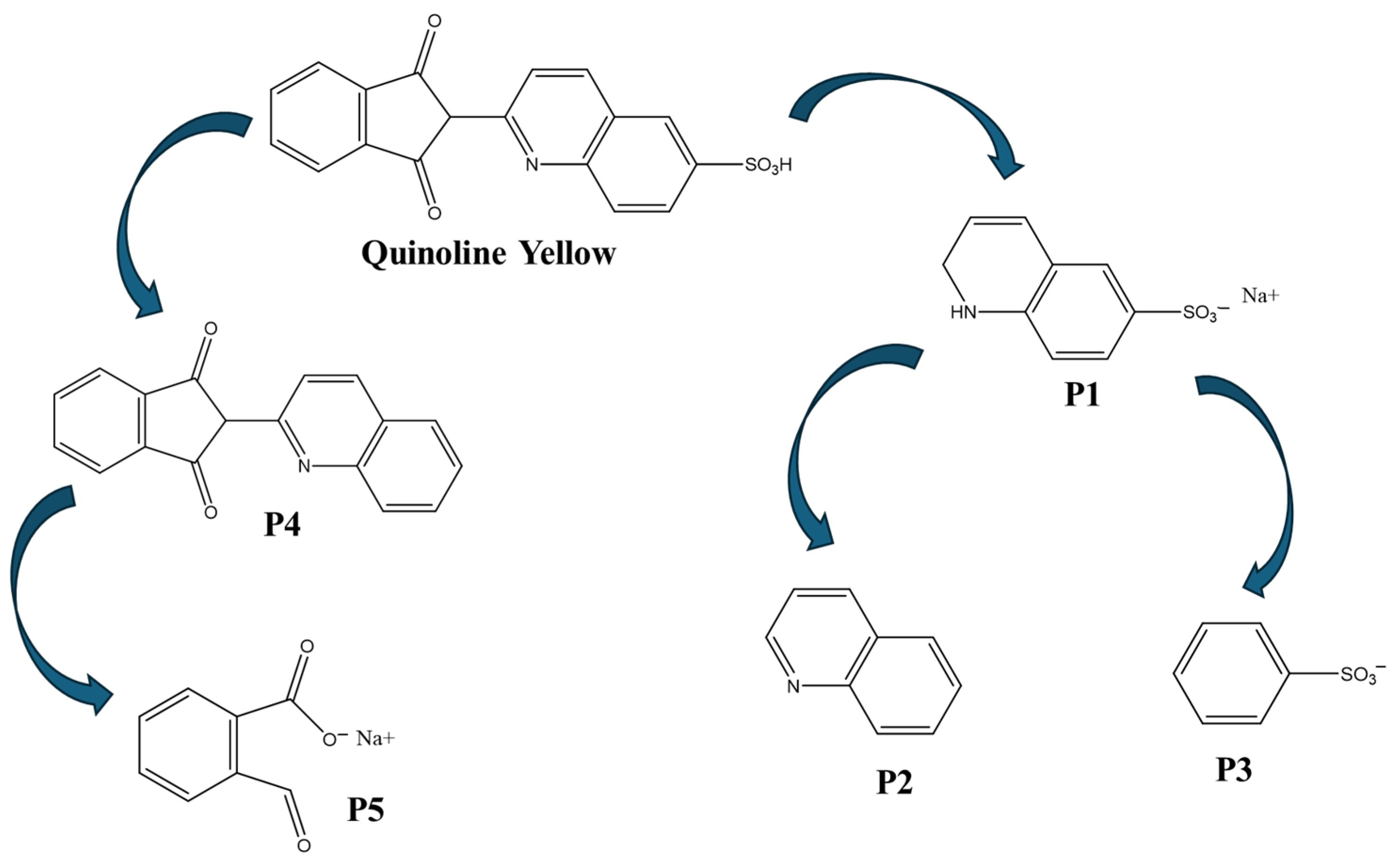
3.2. Photodegradation of Quinoline Yellow
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, P.; Srivastava, A.; Sharma, R.K.; Sharma, D.; Srivastava, S.K. Zinc Oxide: A Fascinating Material for Photovoltaic Applications. In Nanomaterials for Innovative Energy Systems and Devices; Khan, Z.H., Ed.; Springer Nature: Singapore, 2022; pp. 173–241. ISBN 978-981-19055-3-7. [Google Scholar]
- Ding, M.; Guo, Z.; Zhou, L.; Fang, X.; Zhang, L.; Zeng, L.; Xie, L.; Zhao, H. One-Dimensional Zinc Oxide Nanomaterials for Application in High-Performance Advanced Optoelectronic Devices. Crystals 2018, 8, 223. [Google Scholar] [CrossRef]
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Klink, M.J.; Laloo, N.; Leudjo Taka, A.; Pakade, V.E.; Monapathi, M.E.; Modise, J.S. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens. Molecules 2022, 27, 3532. [Google Scholar] [CrossRef] [PubMed]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-Based Antimicrobial Coatings for Biomedical Applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Li, Z.; Govindan, R.; Jayakumar, R.; Wang, C.; Long Gu, F. Zinc Oxide-Quercetin Nanocomposite as a Smart Nano-Drug Delivery System: Molecular-Level Interaction Studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Leone, F.; Cataldo, R.; Mohamed, S.S.Y.; Manna, L.; Banchero, M.; Ronchetti, S.; Mandras, N.; Tullio, V.; Cavalli, R.; Onida, B. Nanostructured ZnO as Multifunctional Carrier for a Green Antibacterial Drug Delivery System—A Feasibility Study. Nanomaterials 2019, 9, 407. [Google Scholar] [CrossRef]
- Lahariya, V.; Ramrakhiani, M. Luminescence Study on Mn, Ni Co-Doped Zinc Sulfide Nanocrystals. Luminescence 2020, 35, 924–933. [Google Scholar] [CrossRef]
- Tajoli, F.; Dengo, N.; Mognato, M.; Dolcet, P.; Lucchini, G.; Faresin, A.; Grunwaldt, J.-D.; Huang, X.; Badocco, D.; Maggini, M.; et al. Microfluidic Crystallization of Surfactant-Free Doped Zinc Sulfide Nanoparticles for Optical Bioimaging Applications. ACS Appl. Mater. Interfaces 2020, 12, 44074–44087. [Google Scholar] [CrossRef]
- Mostafa, M.; El Nady, J.; Ebrahim, S.M.; Elshaer, A.M. Synthesis, Structural, and Optical Properties of Mn2+ Doped ZnS Quantum Dots for Biosensor Application. Opt. Mater. 2021, 112, 110732. [Google Scholar] [CrossRef]
- Ghasemi, H.; Mozaffari, M.H.; Moradian, R. Effects of Deposition Time on Structural and Optical Properties of ZnS and ZnS/Au Thin Films Grown by Thermal Evaporation. Phys. B Condens. Matter 2022, 627, 413616. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Chen, J.; Cai, S.; Long, Z.; Fang, X. Design Principles and Material Engineering of ZnS for Optoelectronic Devices and Catalysis. Adv. Funct. Mater. 2018, 28, 1802029. [Google Scholar] [CrossRef]
- Sun, C.; Gu, Y.; Wen, W.; Zhao, L. ZnSe Based Semiconductor Core-Shell Structures: From Preparation to Application. Opt. Mater. 2018, 81, 12–22. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Ma, Y.; Zhai, T. ZnSe Nanostructures: Synthesis, Properties and Applications. Prog. Mater. Sci. 2016, 83, 472–535. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yan, T.; Peng, Y.; Xu, D.; Deng, D. InP/ZnSe/ZnS Quantum Dots with Strong Dual Emissions: Visible Excitonic Emission and near-Infrared Surface Defect Emission and Their Application In Vitro and In Vivo Bioimaging. J. Mater. Chem. B 2017, 5, 8152–8160. [Google Scholar] [CrossRef]
- Aswathy, R.G.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Near-Infrared Quantum Dots for Deep Tissue Imaging. Anal. Bioanal. Chem. 2010, 397, 1417–1435. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent Advances in Photocatalytic Removal of Organic and Inorganic Pollutants in Air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Nguyen, T.N.; Van Tran, V.; Hur, J.; Kim, I.T.; Park, D.; Lee, Y.-C. Photocatalytic Materials for Indoor Air Purification Systems: An Updated Mini-Review. Environ. Technol. Innov. 2021, 22, 101471. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable Synthesis of Zinc Oxide Nanoparticles for Photocatalytic Degradation of Organic Pollutant and Generation of Hydroxyl Radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Som, I.; Roy, M.; Saha, R. Advances in Nanomaterial-Based Water Treatment Approaches for Photocatalytic Degradation of Water Pollutants. ChemCatChem 2020, 12, 3409–3433. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic Water Decontamination Using Graphene and ZnO Coupled Photocatalysts: A Review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Peter, A.; Al-Najar, B.; Vijaya, J.; Bououdina, M. Photocatalysis: Activity of Nanomaterials. In Nanotechnology in Environmental Science; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 209–292. ISBN 978-3-527-80885-4. [Google Scholar]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Bailon-Ruiz, S.J.; Cedeño-Mattei, Y.; Torres-Torres, K.; Alamo-Nole, L. Photodegradation of Tropaeolin O in the Presence of Ag-Doped ZnO Nanoparticles. Micro 2023, 3, 643–652. [Google Scholar] [CrossRef]
- Wagh, S.S.; Kadam, V.S.; Jagtap, C.V.; Salunkhe, D.B.; Patil, R.S.; Pathan, H.M.; Patole, S.P. Comparative Studies on Synthesis, Characterization and Photocatalytic Activity of Ag Doped ZnO Nanoparticles. ACS Omega 2023, 8, 7779–7790. [Google Scholar] [CrossRef]
- Ghorai, S.; Patra, N.; Pal, A.; Bhattacharyya, D.; Jha, S.N.; Ray, B.; Chatterjee, S.; Ghosh, A.K. Insights into Local Atomic Structure of Fe Alloyed ZnS Nano Crystals: Correlation with Structural, Optical, Magnetic and Photocatalyst Properties. J. Alloys Compd. 2019, 805, 363–378. [Google Scholar] [CrossRef]
- Zafar, S.; Zubair, M.; Shah, S.M.; Khan, M.I.; Khan, A.A.; Iqbal, M.F.; Hassan, A.; Din, M.F.U. Effect of Fe Doping on the Structural and Optical Properties of ZnS Macro-Spheres. Optik 2022, 262, 169342. [Google Scholar] [CrossRef]
- Jrad, A.; Naouai, M.; Abdallah, A.; Ammar, S.; Turki-Kamoun, N. Doping ZnS Films with Cobalt: Structural, Compositional, Morphological, Optical, Electrical, Magnetic and Photocatalytic Properties. Phys. B Condens. Matter 2021, 603, 412776. [Google Scholar] [CrossRef]
- Manivannan, N.; Chandar Shekar, B.; Senthil Kumaran, C.K.; Sugapriya, S. Structural, Morphological, Opto-Luminescence and Magnetic Behavioral Variations of Co–ZnS Hybrid Nanoparticles. Indian J. Phys. 2020, 94, 919–925. [Google Scholar] [CrossRef]
- Sheraz Khan, M.; Shi, L.; Zou, B.; Ali, S. Theoretical Investigation of Optoelectronic and Magnetic Properties of Co-Doped ZnS and (Al, Co) Co-Doped ZnS. Comput. Mater. Sci. 2020, 174, 109491. [Google Scholar] [CrossRef]
- Bhakta, N.; Chakrabarti, P.K. Defect Induced Room Temperature Ferromagnetism and Optical Properties of (Co, Y) Co-Doped ZnO Nanoparticles. J. Magn. Magn. Mater. 2019, 485, 419–426. [Google Scholar] [CrossRef]
- Singh, N.K.; Koutu, V.; Malik, M.M. Enhancement of Room Temperature Ferromagnetic Behavior of Co-Doped ZnO Nanoparticles Synthesized via Sol–Gel Technique. J. Sol-Gel Sci. Technol. 2019, 91, 324–334. [Google Scholar] [CrossRef]
- Ji, H.; Cai, C.; Zhou, S.; Liu, W. Structure, Photoluminescence, and Magnetic Properties of Co-Doped ZnO Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 12917–12926. [Google Scholar] [CrossRef]
- Basha, S.J.; Sarma, G.V.S.S.; Khidhirbrahmendra, V.; Rajyalakshmi, T.; Swetha, D.; Ravikumar, R.V.S.S.N. Enhanced Magnetic Properties of Fe3+ Doped ZnS Nanocrystals via Low Temperature Co-Precipitation: Spintronic and Nano-Device Applications. Phys. Scr. 2020, 95, 105802. [Google Scholar] [CrossRef]
- Krsmanović Whiffen, R.; Montone, A.; Pietrelli, L.; Pilloni, L. On Tailoring Co-Precipitation Synthesis to Maximize Production Yield of Nanocrystalline Wurtzite ZnS. Nanomaterials 2021, 11, 715. [Google Scholar] [CrossRef]
- Mandal, S.; Ali, S.I.; Mandal, A.C. Investigation of Structural, Optical and Photoluminescence Properties of the Sol–Gel Synthesized Powder ZnS Nanoparticles. Appl. Phys. A 2023, 129, 219. [Google Scholar] [CrossRef]
- Gupta, P.; Patel, P.; Sujata, K.; Litoriya, P.K.; Solanki, R.G. Facile Synthesis and Characterization of ZnSe Nanoparticles. Mater. Today Proc. 2023, 80, 1556–1561. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, L.; Mao, C.; Chu, Y. Solvothermal Synthesis of ZnO with Controllable Morphology. Mater. Lett. 2023, 341, 134161. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Gabała, E.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Controlled Microwave-Assisted and pH-Affected Growth of ZnO Structures and Their Photocatalytic Performance. Powder Technol. 2021, 386, 221–235. [Google Scholar] [CrossRef]
- Salah, N.; AL-Shawafi, W.M.; Alshahrie, A.; Baghdadi, N.; Soliman, Y.M.; Memic, A. Size Controlled, Antimicrobial ZnO Nanostructures Produced by the Microwave Assisted Route. Mater. Sci. Eng. C 2019, 99, 1164–1173. [Google Scholar] [CrossRef]
- Wang, W.; Lee, G.-J.; Wang, P.; Qiao, Z.; Liu, N.; Wu, J.J. Microwave Synthesis of Metal-Doped ZnS Photocatalysts and Applications on Degrading 4-Chlorophenol Using Heterogeneous Photocatalytic Ozonation Process. Sep. Purif. Technol. 2020, 237, 116469. [Google Scholar] [CrossRef]
- Jubeer, E.M.; Manthrammel, M.A.; Shkir, M.; Subha, P.A.; Yahia, I.S.; Alfaify, S.A. Microwave Assisted Synthesis of Quantum Dots like ZnS Nanoparticles for Optoelectronic Applications: An Effect of CTAB Concentrations. Optik 2021, 240, 166812. [Google Scholar] [CrossRef]
- Sanchez Tobon, C.; Ljubas, D.; Mandić, V.; Panžić, I.; Matijašić, G.; Ćurković, L. Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra. Nanomaterials 2022, 12, 1473. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kishihara, M.; Fukuoka, T.; Yamaguchi, A.; Utsumi, Y. On Chip Synthesis of Au Nanoparticles by Microwave Heating. Electron. Commun. Jpn. 2020, 103, 49–55. [Google Scholar] [CrossRef]
- Marinoiu, A.; Andrei, R.; Vagner, I.; Niculescu, V.; Bucura, F.; Constantinescu, M.; Carcadea, E. One Step Synthesis of Au Nanoparticles Supported on Graphene Oxide Using an Eco-Friendly Microwave-Assisted Process. Mater. Sci. 2020, 26, 249–254. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Siri, S.; Wadbua, P.; Thongtem, S.; Thongtem, T. Microwave-Assisted Synthesis, Photocatalysis and Antibacterial Activity of Ag Nanoparticles Supported on ZnO Flowers. J. Phys. Chem. Solids 2019, 126, 170–177. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Abdel-Galeil, M.M.; Matsuda, A. One-Pot Synthesis of Reduced Graphene Oxide Nanosheets Anchored ZnO Nanoparticles via Microwave Approach for Electrochemical Performance as Supercapacitor Electrode. J. Mater. Sci. Mater. Electron. 2020, 31, 15456–15465. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Y.; Huang, K. Advances in Microwave-Assisted Production of Reduced Graphene Oxide. Front. Chem. 2019, 7, 355. [Google Scholar] [CrossRef]
- Sathya, A.; Kalyani, S.; Ranoo, S.; Philip, J. One-Step Microwave-Assisted Synthesis of Water-Dispersible Fe3O4 Magnetic Nanoclusters for Hyperthermia Applications. J. Magn. Magn. Mater. 2017, 439, 107–113. [Google Scholar] [CrossRef]
- Banc, R.; Filip, L.; Cozma-Petrut, A.; Ciobarca, D.; Miere, D. Yellow and Red Synthetic Food Dyes and Potential Health Hazards: A Mini Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2024, 81, 1–17. [Google Scholar]
- Bailon-Ruiz, S.; Perales-Perez, O.J. Generation of singlet oxygen by water-stable CdSe(S) and ZnSe(S) quantum dots. Appl. Mater. Today 2017, 9, 161–166. [Google Scholar] [CrossRef]
- Lugo-Ruiz, A.A.; Paz-Ruiz, M.J.; Bailón-Ruiz, S.J. Degradation of Organic Dyes in the Presence of Activated Titanium-Based Nanoparticles. MRS Adv. 2022, 7, 289–294. [Google Scholar] [CrossRef]
- Nash-Montes, V.I.; Luciano-Velazquez, J.; Correa-Vargas, K.D.; Torres-Torres, K.N.; Bailón-Ruiz, S.J. Effect of the Capping Agent on the Photocatalytic Performance of ZnS Nanostructures. MRS Adv. 2022, 7, 203–207. [Google Scholar] [CrossRef]
- Lopés-Velasco, N.M.; Bailón-Ruiz, S.J. Effect of the Particle Size and pH on the Photocatalytic Performance of Cerium Oxide (CeO2) Nanoparticles. MRS Adv. 2021, 6, 769–773. [Google Scholar] [CrossRef]
- Kustov, L.; Vikanova, K. Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy. Metals 2023, 13, 1714. [Google Scholar] [CrossRef]
- Shahi, A.K.; Pandey, B.K.; Singh, B.P.; Gupta, B.K.; Singh, S.; Gopal, R. Photo Physical Studies of PVP Arrested ZnS Quantum Dots. Electron. Mater. Lett. 2017, 13, 160–167. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.-J.; Choi, H.-J.; Gwag, J.-S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Al-Badaii, F.; Al-Khalidy, A.; Khalid, M.; Al-Jarfi, A.; Al-Ansi, F.; Al-Jarfi, A.; Al-Nujaimi, N.; Al-Haj, A.; Halim, A.; Alnehia, A.; et al. Green Synthesis of Zinc Oxide Nanoparticles Using Allium Sativum Extract: Evaluation of Antibacterial Activity Against Nosocomial Bacteria. Thamar Univ. J. Nat. Appl. Sci. 2024, 9, 14–19. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Wunderlich, W.; Kuchmizhak, A.A.; Kulinich, S.A. Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate. Materials 2019, 12, 3313. [Google Scholar] [CrossRef]
- Murugadoss, G. Synthesis and Optical Characterization of PVP and SHMP-Encapsulated Mn2+-Doped ZnS Nanocrystals. J. Lumin. 2010, 130, 2207–2214. [Google Scholar] [CrossRef]
- Cao, X.; Shen, F.; Zhang, M.; Bie, J.; Liu, X.; Luo, Y.; Guo, J.; Sun, C. Facile Synthesis of Chitosan-Capped ZnS Quantum Dots as an Eco-Friendly Fluorescence Sensor for Rapid Determination of Bisphenol A in Water and Plastic Samples. RSC Adv. 2014, 4, 16597–16606. [Google Scholar] [CrossRef]
- Bhushan, M.; Jha, R.; Bhardwaj, R. Reduced Band Gap and Diffusion Controlled Spherical N-Type ZnS Nanoparticles for Absorption of UV-Vis Region of Solar Spectrum. J. Phys. Chem. Solids 2019, 135, 109021. [Google Scholar] [CrossRef]
- Ciciliati, M.A.; Silva, M.F.; Fernandes, D.M.; de Melo, M.A.C.; Hechenleitner, A.A.W.; Pineda, E.A.G. Fe-Doped ZnO Nanoparticles: Synthesis by a Modified Sol–Gel Method and Characterization. Mater. Lett. 2015, 159, 84–86. [Google Scholar] [CrossRef]
- Gudla, U.R.; Suryanarayana, B.; Raghavendra, V.; Emmanuel, K.A.; Murali, N.; Taddesse, P.; Parajuli, D.; Chandra Babu Naidu, K.; Ramakrishna, Y.; Chandramouli, K. Optical and Luminescence Properties of Pure, Iron-Doped, and Glucose Capped ZnO Nanoparticles. Results Phys. 2020, 19, 103508. [Google Scholar] [CrossRef]
- Ovhal, M.M.; Santhosh Kumar, A.; Khullar, P.; Kumar, M.; Abhyankar, A.C. Photoluminescence Quenching and Enhanced Spin Relaxation in Fe Doped ZnO Nanoparticles. Mater. Chem. Phys. 2017, 195, 58–66. [Google Scholar] [CrossRef]
- Carofiglio, M.; Conte, M.; Racca, L.; Cauda, V. Synergistic Phenomena between Iron-Doped ZnO Nanoparticles and Shock Waves Exploited against Pancreatic Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 17212–17225. [Google Scholar] [CrossRef]
- Tab, A.; Dahmane, M.; Chemseddin, B.; Bellal, B.; Trari, M.; Richard, C. Photocatalytic Degradation of Quinoline Yellow over Ag3PO4. Catalysts 2020, 10, 1461. [Google Scholar] [CrossRef]
- Kareem, M.; Bello, I.; Shittu, H.; Prakash, P.S.; Adedokun, O.; Arumugam, S. Synthesis, Characterization, and Photocatalytic Application of Silver Doped Zinc Oxide Nanoparticles. Clean. Mater. 2022, 3, 100041. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.-H.; Kim, D.-J.; Lee, Y.-J.; Jhee, K.-H.; Sohn, Y.; Jang, E.-S. Antimicrobial Activity of ZnO Nanoplates and Its Ag Nanocomposites: Insight into an ROS-Mediated Antibacterial Mechanism under UV Light. J. Solid State Chem. 2018, 267, 124–133. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailón-Ruiz, S.J.; Cedeño-Mattei, Y.; Núñez-Colón, A.M.; Torres-Torres, K. Fast One-Step Microwave-Assisted Synthesis of Iron-Doped ZnS for Photocatalytic Applications. Crystals 2024, 14, 699. https://doi.org/10.3390/cryst14080699
Bailón-Ruiz SJ, Cedeño-Mattei Y, Núñez-Colón AM, Torres-Torres K. Fast One-Step Microwave-Assisted Synthesis of Iron-Doped ZnS for Photocatalytic Applications. Crystals. 2024; 14(8):699. https://doi.org/10.3390/cryst14080699
Chicago/Turabian StyleBailón-Ruiz, Sonia J., Yarilyn Cedeño-Mattei, Angelie M. Núñez-Colón, and Kerianys Torres-Torres. 2024. "Fast One-Step Microwave-Assisted Synthesis of Iron-Doped ZnS for Photocatalytic Applications" Crystals 14, no. 8: 699. https://doi.org/10.3390/cryst14080699






