Ferroelectric and Structural Properties of Cobalt-Doped Lead Ferrite Thin Films Formed by Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
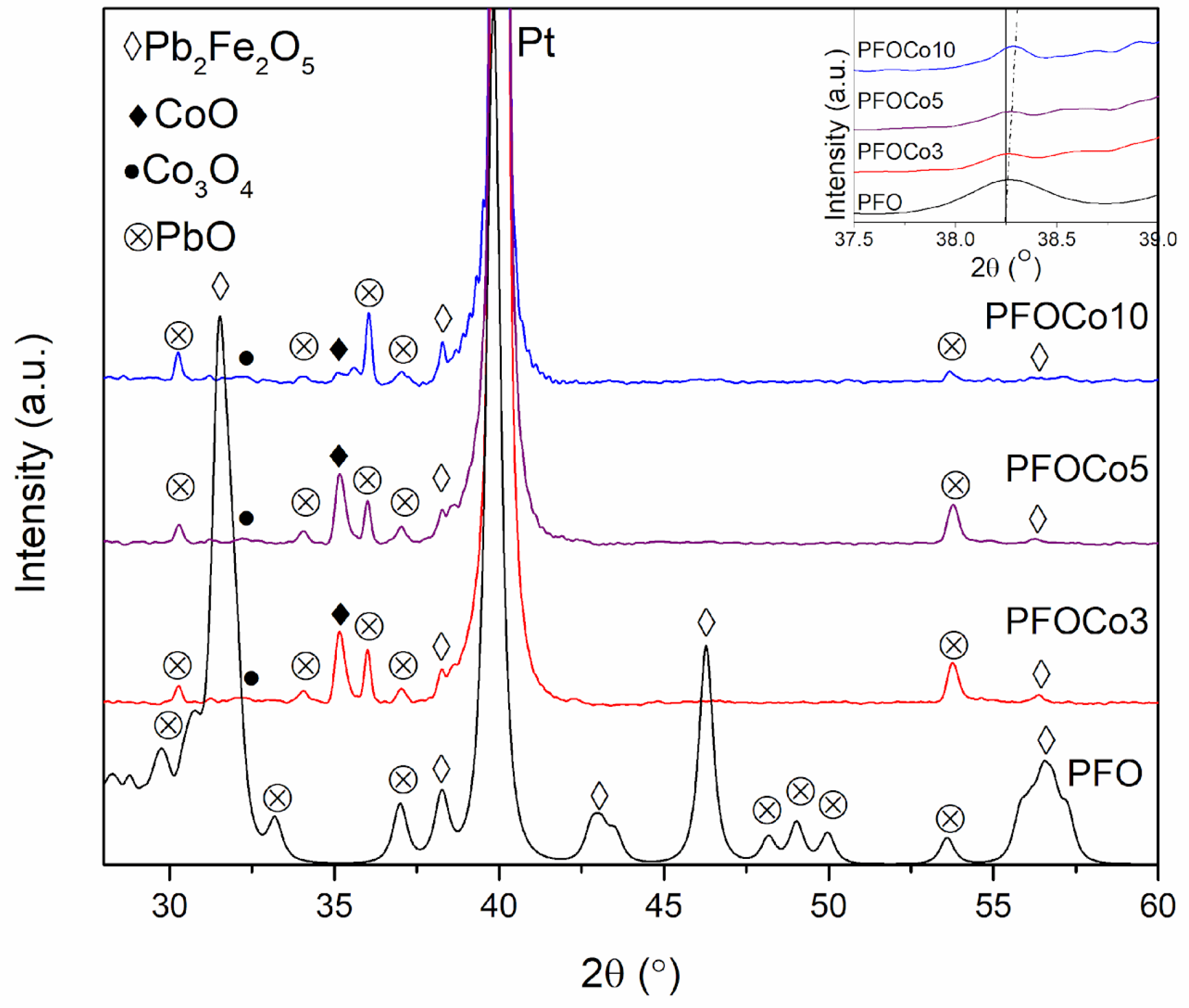
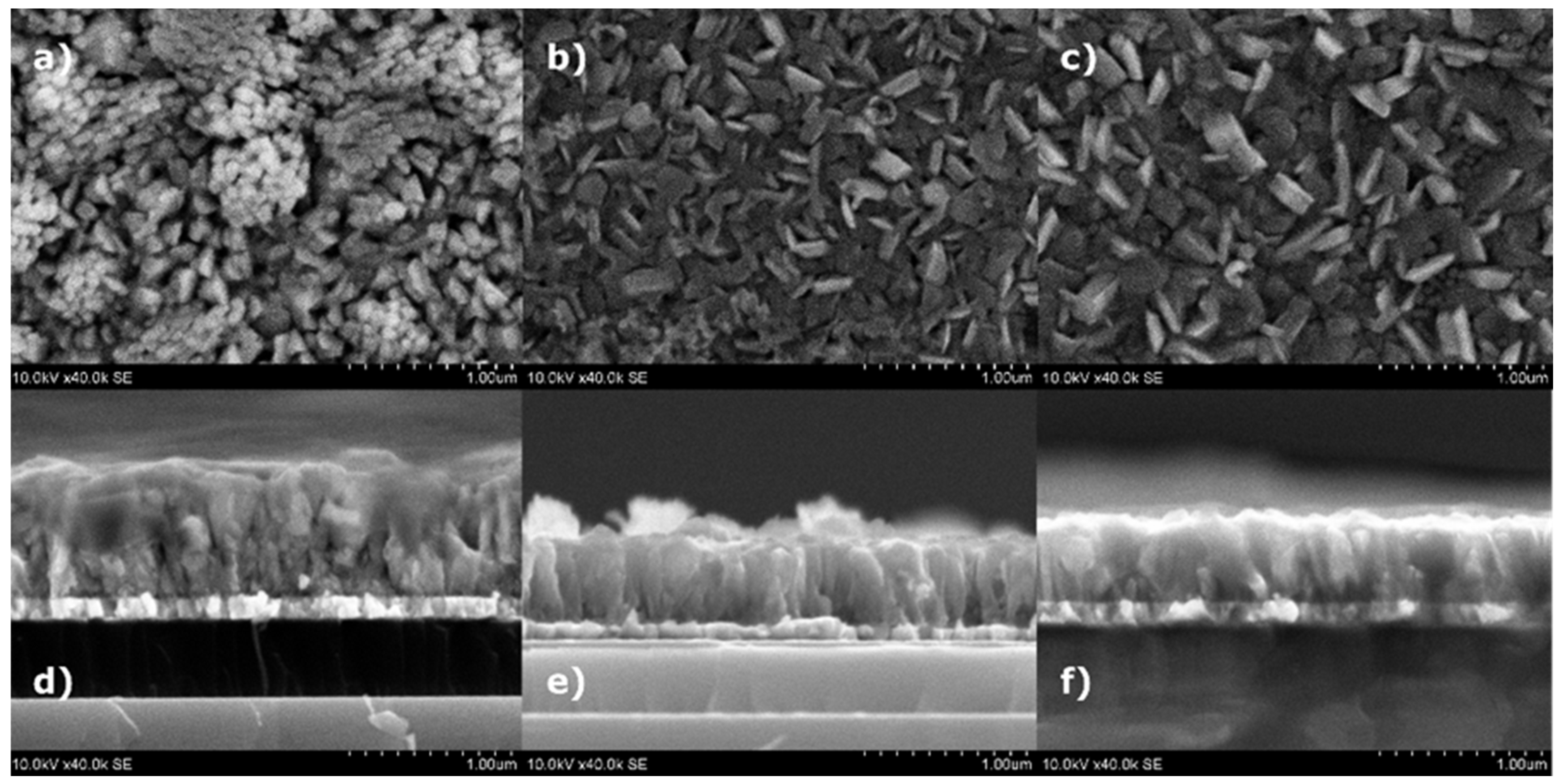
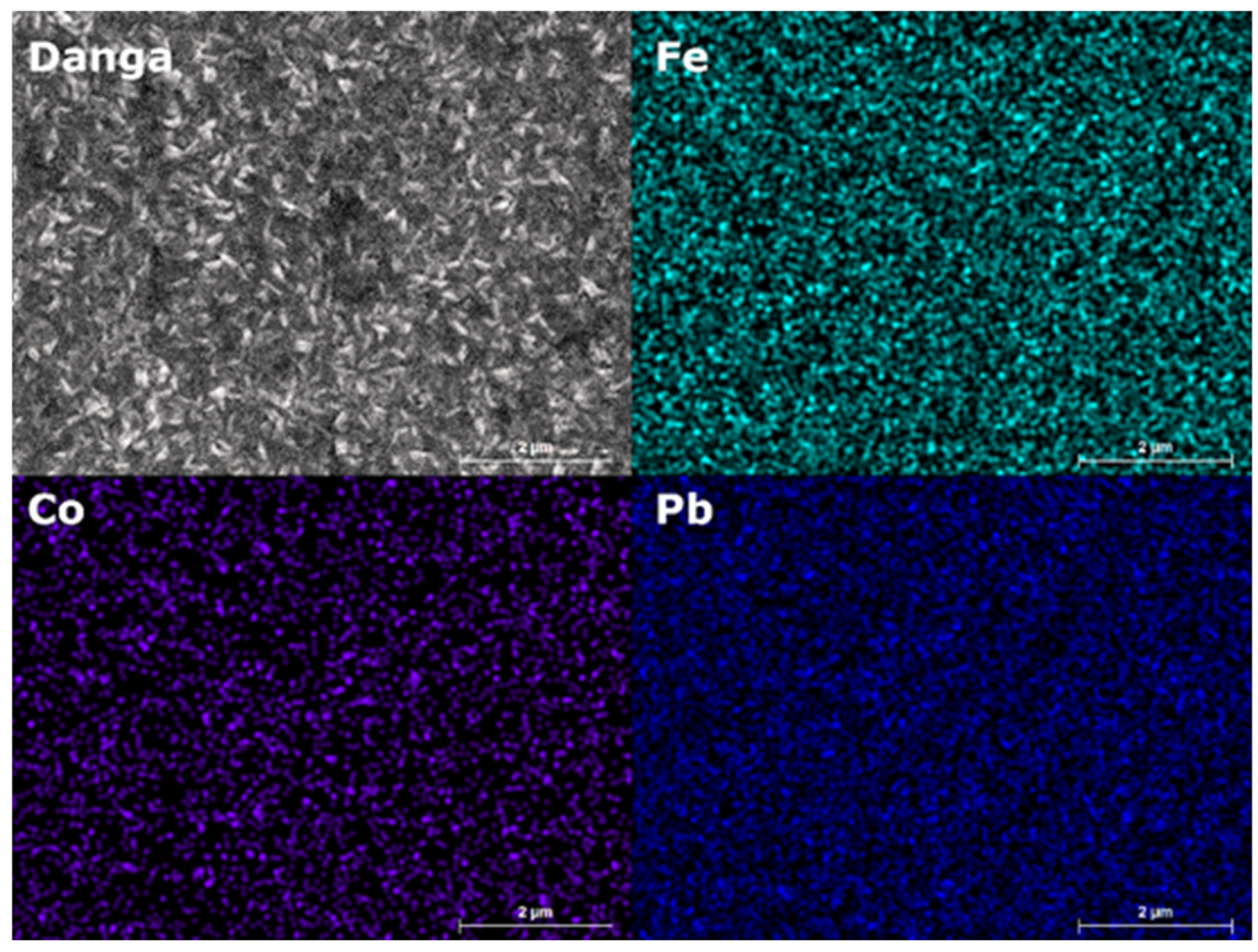
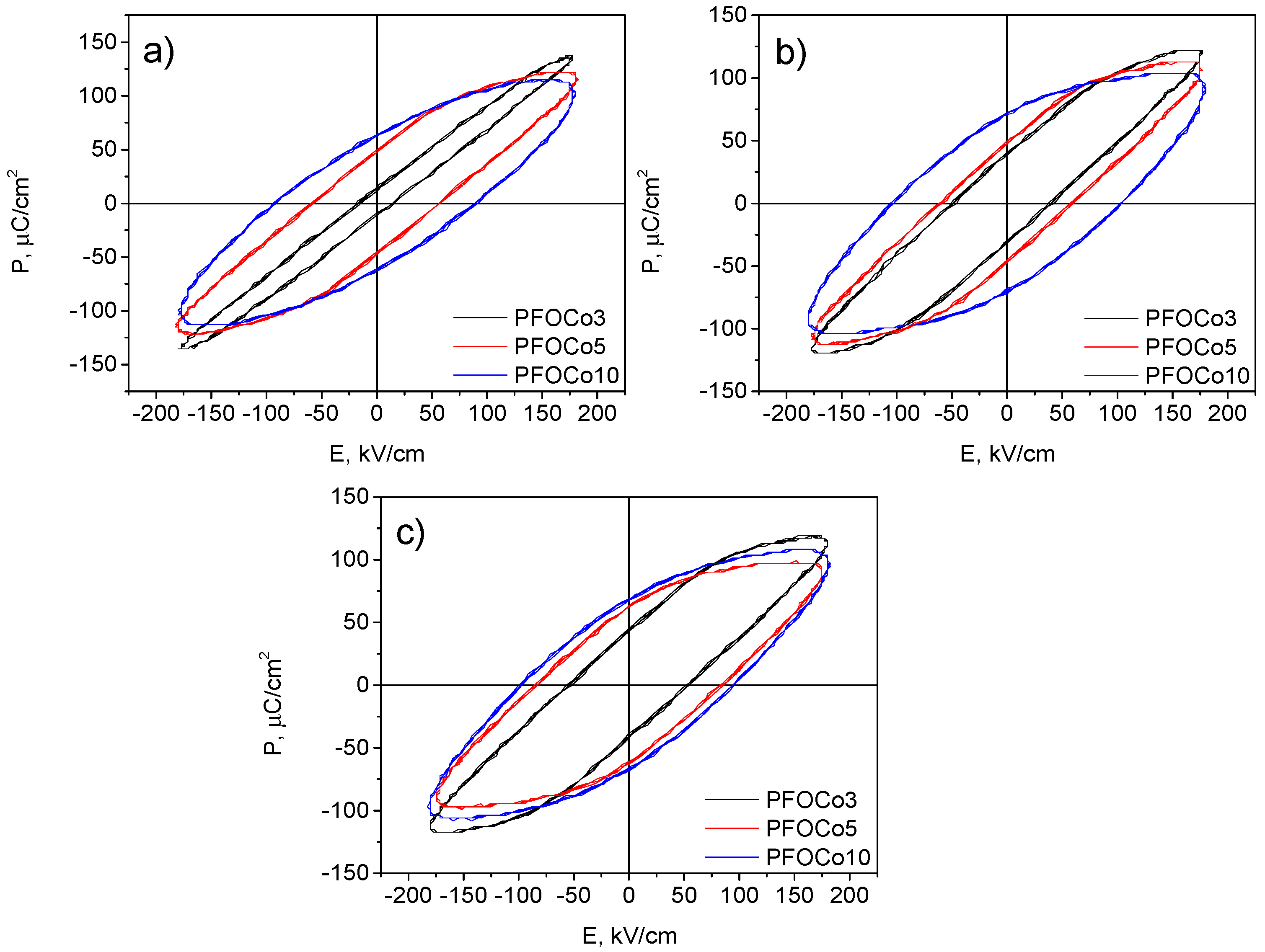
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Yang, S.; Li, X. Multiferroic heterostructures and tunneling junctions. J. Mater. 2015, 1, 263–284. [Google Scholar] [CrossRef]
- Khomskii, D.I. Multiferroics: Different ways to combine magnetism and ferroelectricity. J. Magn. Magn. Mater. 2006, 306, 1–8. [Google Scholar] [CrossRef]
- Roy, S.; Majumder, S.B. Recent advances in multiferroic thin films and composites. J. Alloys Compd. 2012, 538, 153–159. [Google Scholar] [CrossRef]
- Sekine, Y.; Akiyoshi, R.; Hayami, S. Recent advances in ferroelectric metal complexes. Coord. Chem. Rev. 2022, 469, 214663. [Google Scholar] [CrossRef]
- Kadomtseva, A.M.; Popov, Y.F.; Pyatakov, A.P.; Vorob’ev, G.P.; Zvezdin, A.K.; Viehland, D. Phase transitions in multiferroic BiFeO3 crystals, thin-layers, and ceramics: Enduring potential for a single phase, room-temperature magnetoelectric ‘holy grail’. Phase Transit. 2006, 79, 1019–1042. [Google Scholar] [CrossRef]
- Amirov, A. Chapter 15—Multiferroic, magnetic, and magnetoelectric nanomaterials for medical applications. In Magnetic Materials and Technologies for Medical Applications; Tishin, A.M., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 469–484. [Google Scholar] [CrossRef]
- Pati, D.K.; Das, P.R.; Parida, B.N.; Padhee, R. Multifunctional characterization of multiferroic [Pb(Fe0.5Nb0.5)O3]0.5–[(Ca0.2Sr0.8)TiO3]0.5 for storage and photocatalytic applications. Ceram. Int. 2022, 48, 19344–19357. [Google Scholar] [CrossRef]
- Rahul, M.T.; Chacko, S.K.; Vinodan, K.; Raneesh, B.; Philip, K.A.; Bhadrapriya, B.C.; Bose, B.A.; Kalarikkal, N.; Rouxel, D.; Viswanathan, P.; et al. Multiferroic and energy harvesting characteristics of P(VDF-TrFE)-CuFe2O4 flexible films. Polymer 2022, 252, 124910. [Google Scholar] [CrossRef]
- Shah, J.; Verma, K.C.; Agarwal, A.; Kotnala, R.K. Novel application of multiferroic compound for green electricity generation fabricated as hydroelectric cell. Mater. Chem. Phys. 2020, 239, 122068. [Google Scholar] [CrossRef]
- Singh Pawar, M.; Raj, A.; Kumar Singh, A.; Tuli, V.; Anshul, A.; Kumar, M. Lead-free ‘Ca’ doped Bi0.80La0.20FeO3 multiferroic material for solar cell applications. Mater. Today Proc. 2022, 67, 713–718. [Google Scholar] [CrossRef]
- Vopson, M.M. Fundamentals of Multiferroic Materials and Their Possible Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 223–250. [Google Scholar] [CrossRef]
- Alkathy, M.S.; Rahman, A.; Zabotto, F.L.; Milton, F.P.; Raju, K.C.J.; Eiras, J.A. Room-temperature multiferroic behaviour in Co/Fe co-substituted layer-structured Aurivillius phase ceramics. Ceram. Int. 2022, 48, 30041–30051. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Chen, X.; Fu, Y.; Wang, F.; Hu, K.; Sun, Z.; Zhang, K. Room-temperature multiferroicity and magnetoelectric couplings in (Co0.75Al0.25)2(Fe0.75Mg0.25)O4 spinel films. J. Alloys Compd. 2022, 920, 165918. [Google Scholar] [CrossRef]
- Beklešovas, B.; Stankus, V.; Link, J.; Stern, R. Structural, ferroelectric and magnetic properties of lead ferrite (Pb2Fe2O5) thin films synthesized by reactive magnetron deposition. Thin Solid Film. 2020, 708, 138124. [Google Scholar] [CrossRef]
- Hadermann, J.; Abakumov, A.M.; Nikolaev, I.V.; Antipov, E.V.; Van Tendeloo, G. Local structure of perovskite-based “Pb2Fe2O5”. Solid State Sci. 2008, 10, 382–389. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G. Multiferroic properties of Pb2Fe2O5 ceramics. Mater. Res. Bull. 2011, 46, 438–441. [Google Scholar] [CrossRef]
- Dawber, M.; Rabe, K.; Scott, J. Physics of thin-film ferroelectric oxides. Rev. Mod. Phys. 2005, 77, 1083–1130. [Google Scholar] [CrossRef]
- Rabe, K.M.; Dawber, M.; Lichtensteiger, C.; Ahn, C.H.; Triscone, J.-M. Modern Physics of Ferroelectrics: Essential Background; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Chauhan, S.; Kumar, M.; Yousuf, A.; Rathi, P.; Sahni, M.; Singh, S. Effect of Na/Co co-substituted on structural, magnetic, optical and photocatalytic properties of BiFeO3 nanoparticles. Mater. Chem. Phys. 2021, 263, 124402. [Google Scholar] [CrossRef]
- Makhdoom, A.R.; Akhtar, M.J.; Rafiq, M.A.; Siddique, M.; Iqbal, M.; Hasan, M.M. Enhancement in the multiferroic properties of BiFeO3 by charge compensated aliovalent substitution of Ba and Nb. AIP Adv. 2014, 4, 037113. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bhushan, B.; Jagannath; Sharma, R.K.; Sen, S.; Mandal, B.P.; Meena, S.S.; Bhatt, P.; Prajapat, C.L.; Priyam, A.; et al. Enhanced dielectric, magnetic and optical properties of Cr-doped BiFeO3 multiferroic nanoparticles synthesized by sol-gel route. Results Phys. 2019, 13, 102299. [Google Scholar] [CrossRef]
- Hoque, M.M.; Islam, M.T.; Islam, M.R.; Zubair, M.A. Effective bandgap tuning with non-trivial modulation in room temperature magnetic and electrical responses of low level Ba–Cr co-substituted BiFeO3 nanoparticles. Ceram. Int. 2022, 48, 19583–19596. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Irfan, M.; Naz, S.; Wu, D.; Gao, J. Magnetic properties of Ni/BiFeO3 hybrid nanostructures. J. Alloys Compd. 2022, 912, 165133. [Google Scholar] [CrossRef]
- Tefera Kebede, M.; Devi, S.; Dillu, V.; Chauhan, S. Effects of Sm and Cr co-doping on structural, magnetic, optical and photocatalytic properties of BiFeO3 nanoparticles. Mater. Sci. Eng. B 2022, 283, 115859. [Google Scholar] [CrossRef]
- Beklešovas, B.; Iljinas, A.; Stankus, V.; Čyvienė, J.; Andrulevičius, M.; Ivanov, M.; Banys, J. Structural, Morphologic, and Ferroelectric Properties of PZT Films Deposited through Layer-by-Layer Reactive DC Magnetron Sputtering. Coatings 2022, 12, 717. [Google Scholar] [CrossRef]
- Iljinas, A.; Stankus, V. Structural and ferroelectric properties of bismuth ferrite thin films deposited by direct current reactive magnetron sputtering. Thin Solid Film. 2016, 601, 106–110. [Google Scholar] [CrossRef]
- Iljinas, A.; Stankus, V.; Čyvienė, J.; Abakevičienė, B. Formation of PbTiO3 thin films on seed layers using DC magnetron layer-by-layer deposition. Vacuum 2015, 122, 310–313. [Google Scholar] [CrossRef]
- Beklešovas, B.; Stankus, V.; Abakevičienė, B.; Link, J.; Stern, R.; Plyushch, A.; Banys, J.; Čyvienė, J.; Girčys, R.; Bašinskas, M.; et al. Synthesis and Characterization of Cr-Doped Pb2Fe2O5 Thin Films by Reactive Magnetron Sputtering. ECS J. Solid State Sci. Technol. 2023, 12, 103014. [Google Scholar] [CrossRef]
- Wang, X.; Ge, H.; Ye, Q.; Si, P.; Chen, H. Weak Ferromagnetism and Exchange Bias in Antiferromagnetic Cobalt Oxide Nanoparticles. J. Magn. 2018, 23, 487–490. [Google Scholar] [CrossRef]
- Gil, D.M.; Nieva, G.; Franco, D.G.; Gómez, M.I.; Carbonio, R.E. Lead nitroprusside: A new precursor for the synthesis of the multiferroic Pb2Fe2O5, an anion-deficient perovskite. Mater. Chem. Phys. 2013, 141, 355–361. [Google Scholar] [CrossRef]
- Bai, L.; Sun, M.; Ma, W.; Yang, J.; Zhang, J.; Liu, Y. Enhanced magnetic properties of co-doped BiFeO3 thin films via structural progression. Nanomaterials 2020, 10, 1798. [Google Scholar] [CrossRef]
- Sinha, A.; Bhushan, B.; Gupta, N.; Sen, S.; Prajapat, C.; Nuwad, J.; Bhatt, P.; Mishra, S.; Meena, S.; Priyam, A. Effect of cobalt-doping on dielectric, magnetic and optical properties of BiFeO3 nanocrystals synthesized by sol–gel technique. Solid State Sci. 2020, 102, 106168. [Google Scholar] [CrossRef]
- You, S.; Zhang, B. Enhanced magnetic properties of cobalt-doped bismuth ferrite nanofibers. Mater. Res. Express 2020, 7, 046102. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.-J.; Li, Y.; Cao, W.-Q.; Fang, X.-Y.; Yuan, J.; Cao, M.-S. Cobalt doping of bismuth ferrite for matched dielectric and magnetic loss. Appl. Phys. Lett. 2019, 115, 212902. [Google Scholar] [CrossRef]
- Sales, J.N.B.d.; da Silva, R.; Lara, L.R.S.; Ramos, S.L.; Soares, J.d.S.; Soares, T.A.S.; Machado, G.; Manhabosco, S.M.; de Oliveira, A.; de Carvalho, H. Structural, optical, and magnetic evaluation of Co-, Ni-, and Mn-modified multiferroic BiFeO3 ceramics. Ceram. Int. 2021, 47, 24564–24573. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Bairagi, S.; Järrendahl, K.; Eriksson, F.; Hultman, L.; Birch, J.; Hsiao, C.-L. Glancing angle deposition and growth mechanism of inclined AlN nanostructures using reactive magnetron sputtering. Coatings 2020, 10, 768. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Escribano, B.; Piro, O.; Sainz-Diaz, C.I.; Sánchez, P.A.; Sintes, T. Ice Film Morphologies and the Structure Zone Model. In Proceedings of the AIP Conference Proceedings; American Institute of Physics: College Park, ML, USA, 2008; pp. 696–701. [Google Scholar]
- Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. 2002, 41, 3053–3060. [Google Scholar] [CrossRef]
- Chinchay-Espino, H.A.; Montes-Albino, G.M.; Morales-Cruz, C.M.; Dobbertin-Sanchez, S.E.; Rojas-Flores, S. Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders. Crystals 2022, 12, 1058. [Google Scholar] [CrossRef]
- Abakumov, A.M.; Hadermann, J.; Bals, S.; Nikolaev, I.V.; Antipov, E.V.; Van Tendeloo, G. Crystallographic Shear Structures as a Route to Anion-Deficient Perovskites. Angew. Chem. Int. Ed. 2006, 45, 6697–6700. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Adeela, N.; Javed, K.; Riaz, S.; Ali, H.; Iqbal, M.; Han, X.; Naseem, S. Influence of cobalt doping on structural and magnetic properties of BiFeO3 nanoparticles. J. Nanoparticle Res. 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Coondoo, I.; Panwar, N.; Tomar, A.; Bdikin, I.; Kholkin, A.; Puli, V.S.; Katiyar, R.S. Improved magnetic and piezoresponse behavior of cobalt substituted BiFeO3 thin film. Thin Solid Film. 2012, 520, 6493–6498. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beklešovas, B.; Stankus, V.; Iljinas, A.; Marcinauskas, L. Ferroelectric and Structural Properties of Cobalt-Doped Lead Ferrite Thin Films Formed by Reactive Magnetron Sputtering. Crystals 2024, 14, 721. https://doi.org/10.3390/cryst14080721
Beklešovas B, Stankus V, Iljinas A, Marcinauskas L. Ferroelectric and Structural Properties of Cobalt-Doped Lead Ferrite Thin Films Formed by Reactive Magnetron Sputtering. Crystals. 2024; 14(8):721. https://doi.org/10.3390/cryst14080721
Chicago/Turabian StyleBeklešovas, Benas, Vytautas Stankus, Aleksandras Iljinas, and Liutauras Marcinauskas. 2024. "Ferroelectric and Structural Properties of Cobalt-Doped Lead Ferrite Thin Films Formed by Reactive Magnetron Sputtering" Crystals 14, no. 8: 721. https://doi.org/10.3390/cryst14080721





