The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
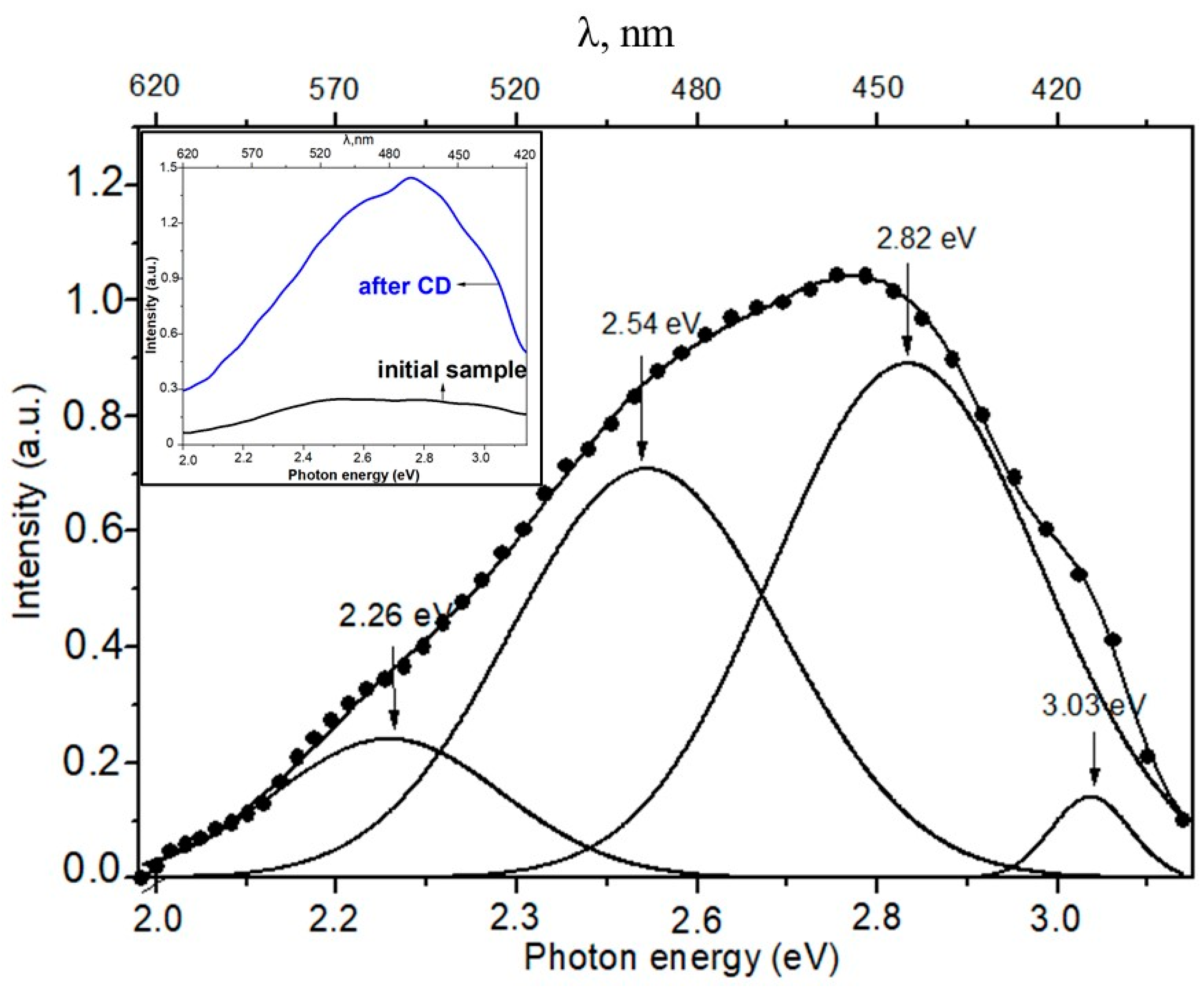
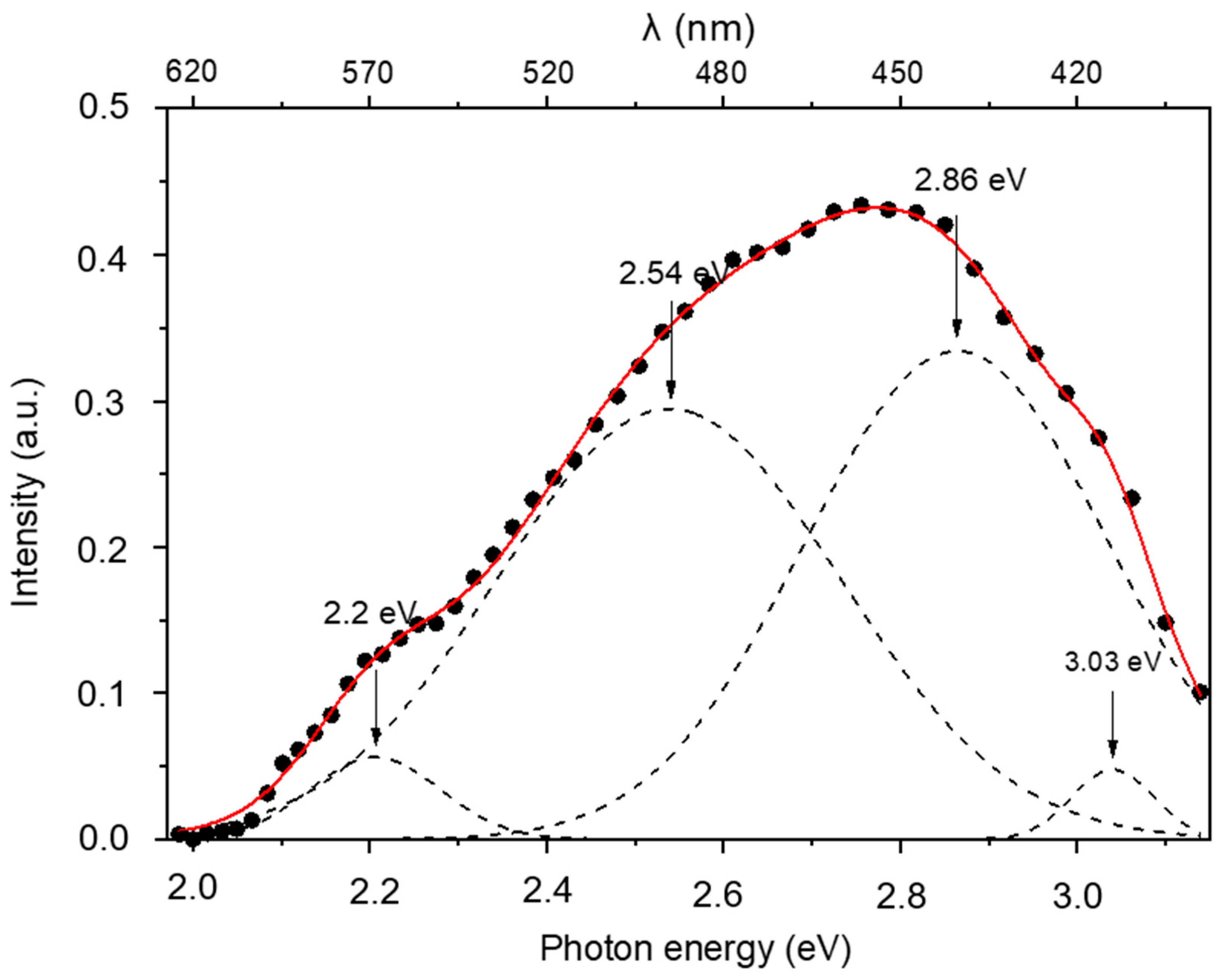
3.1. Photoluminescence
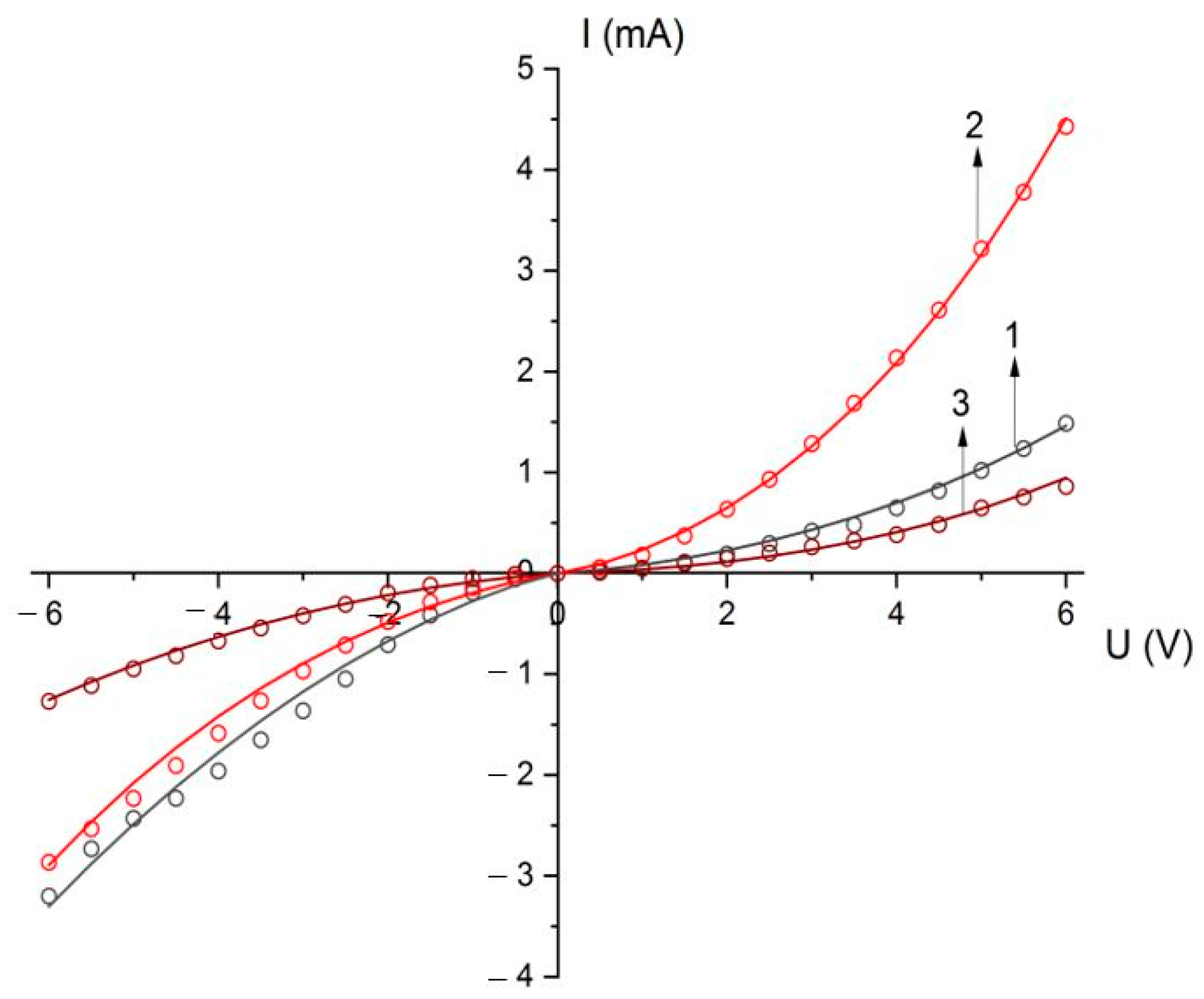
3.2. Electrical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-Dimensional (1D) Nanostructured Materials for Energy Applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qi, L. Light management with patterned micro-and nanostructure arrays for photocatalysis, photovoltaics, and optoelectronic and optical devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Wu, C.; Wang, K.; Batmunkh, M.; Bati, A.S.; Yang, D.; Jiang, Y.; Priya, S. Multifunctional nanostructured materials for next generation photovoltaics. Nano Energy 2020, 70, 104480. [Google Scholar] [CrossRef]
- Ghos, B.C.; Farhad, S.F.U.; Patwary, M.A.M.; Majumder, S.; Hossain, A.; Tanvir, N.I.; Rahman, M.; Tanaka, T.; Guo, Q. Influence of the Substrate, Process Conditions, and Postannealing Temperature on the Properties of ZnO Thin Films Grown by the Successive Ionic Layer Adsorption and Reaction Method. ACS Omega 2021, 6, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, I.; Viespe, C. ZnO metal oxide semiconductor in surface acoustic wave sensors: A review. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef] [PubMed]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Rahman, F. Zinc oxide light-emitting diodes: A review. Opt. Eng. 2019, 58, 010901. [Google Scholar] [CrossRef]
- Hong, T.; Liu, Z.; Liu, H.; Liu, J.; Zhang, X.; Han, J.; Wang, B. Preparation and enhanced photoelectrochemical performance of selenite-sensitized zinc oxide core/shell composite structure. J. Mater. Chem. A 2015, 3, 4239–4247. [Google Scholar] [CrossRef]
- Maswanganye, M.W.; Kabongo, G.L.; Mathevula, L.E.; Mothudi, B.M.; Dhlamini, M.S. Unveiling the effect of strain engineering on the electrochemical properties of hydrothermally grown nanostructured indium doped ZnSeO3 for photoanode applications. Sci. Rep. 2023, 13, 20131. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Akylbekova, A.; Sarsekhan, G.; Usseinov, A.; Baimukhanov, Z.; Kozlovskiy, A.; Vlasukova, L.A.; Komarov, F.F.; Popov, A.I.; Akilbekov, A.T. Ion-Track Template Synthesis and Characterization of ZnSeO3 Nanocrystals. Crystals 2022, 12, 817. [Google Scholar] [CrossRef]
- Polivtseva, S.; Spalatu, N.; Abdalla, A.; Volobujeva, O.; Hiie, J.; Bereznev, S. Pulsed laser deposition of Zn(O,Se) layers for optoelectronic application. Appl. Energy Mater. 2018, 1, 6505–6512. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, G.H.; Sung, M.C.; Kim, D.W. Orthorhombically distorted perovskite SeZnO3 nanosheets as an electrocatalyst for lithium-oxygen batteries. Chem. Eng. J. 2021, 406, 126896. [Google Scholar] [CrossRef]
- Kaniukov, E.Y.; Ustarroz, J.; Yakimchuk, D.V.; Petrova, M.; Terryn, H.; Sivakov, V.; Petrov, A.V. Tunable nanoporous silicon oxide templates by swift heavy ion tracks technology. Nanotechnology 2016, 27, 115305. [Google Scholar] [CrossRef]
- Yakimchuk, D.; Bundyukova, V.; Smirnov, A.; Kaniukov, E. Express method of estimation of etched ion track parameters in silicon dioxide template. Phys. Status Solidi B 2019, 256, 1800316. [Google Scholar] [CrossRef]
- Benvagoub, A.; Toulemonde, M. Ion tracks in amorphous silica. J. Mater. Res. 2015, 30, 1529–1543. [Google Scholar] [CrossRef]
- Demyanov, S.E.; Kaniukov, E.Y.; Petrov, A.V.; Belonogov, E.K.; Streltsov, E.A.; Ivanov, D.K.; Sivakov, V. On the morphology of Si/SiO2/Ni nanostructures with swift heavy ion tracks in silicon oxide. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2014, 8, 805–813. [Google Scholar] [CrossRef]
- Xie, Q.; Zhan, P.; Wang, W.; Li, Z.; Zhang, Z. Enhanced ultraviolet and visible photoluminescence of ZnO/Zn2SiO4/SiO2/Si multilayer structure. J. Alloys Compd. 2015, 642, 131–135. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Chen, Y.W.; Chang, S.J.; Wang, S.F.; Hsu, C.L.; Lin, Y.R.; Chen, I.C. ZnO nanowire-based CO sensors prepared on patterned ZnO: Ga/SiO2/Si templates. Sens. Actuators B Chem. 2007, 125, 498–503. [Google Scholar] [CrossRef]
- Charykova, M.V.; Fokina, E.L.; Klimova, E.V.; Krivovichev, V.G.; Semenova, V.V. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. IX. Physicochemical Formation Conditions and Thermal Stability of Zinc Selenites. Geol. Ore Depos. 2014, 56, 546–552. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Shlimas, D.I.; Borgekov, D.B.; Zdorovets, M.V.; Shakirziyanov, R.I.; Garanin, Y.A.; Volodina, N.O.; Popov, A.I. Study of the Effect of Nanostructured Grains on the Radiation Resistance of Zirconium Dioxide Ceramics During Gas Swelling under High-dose Irradiation with Helium Ions. ES Mater. Manuf. 2024, 24, 1165. [Google Scholar] [CrossRef]
- Inerbaev, T.; Akilbekov, A.; Kenbayev, D.; Dauletbekova, A.; Shalaev, A.; Polisadova, E.; Konuhova, M.; Piskunov, S.; Popov, A.I. Color Centers in BaFBr Crystals: Experimental Study and Theoretical Modeling. Materials 2024, 17, 3340. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Kozlovskiy, A.A.; Konuhova, M.; Popov, A.I.; Shlimas, D.D.; Borgekov, D.B. Determination of gamma radiation shielding efficiency by radiation-resistant composite ZrO2–Al2O3–TiO2–WO3–Nb2O5 ceramics. Opt. Mater. 2024, 154, 115752. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Shlimas, D.I.; Zdorovets, M.V.; Elsts, E.; Konuhova, M.; Popov, A.I. Investigation of the Effect of PbO Doping on Telluride Glass Ceramics as a Potential Material for Gamma Radiation Shielding. Materials 2023, 16, 2366. [Google Scholar] [CrossRef]
- Ou, K.; Wang, S.; Huang, M.; Zhang, Y.; Wang, Y.; Duan, X.; Yi, L. Influence of thickness and annealing on photoluminescence of nanostructured ZnSe/ZnS multilayer thin films prepared by electron beam evaporation. J. Lumin. 2019, 199, 34–38. [Google Scholar] [CrossRef]
- Ramya, E.; Rao, M.V.; Jyothi, L.; Rao, D.N. Photoluminescence and nonlinear optical properties of transition metal (Ag, Ni, Mn) doped ZnO nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 7072–7077. [Google Scholar] [CrossRef]
- Kazmersky, L.L. Polycrystalline and Amorphous Thin Films and Devices, 1st ed.; Academic Press: New York, NY, USA, 1980; pp. 135–152. [Google Scholar]
- Gao, F.; Naik, S.P.; Sasaki, Y.; Okubo, T. Preparation and opt ical property of nanosized ZnO electrochemically deposited in mesoporous silica films. Thin Solid Films 2006, 495, 68–72. [Google Scholar] [CrossRef]
- Ravindra, C.A. X-ray Diffraction and Photoluminescence Studies on ZnO Nanoparticles Synthesized by Sol Gel Auto Combustion Method Glycine Assisted Chelating Agent. J. Emerg. Technol. Innov. Res. 2021, 8, 143–147. [Google Scholar]
- Zuala, L.; Agarwal, P. Growth and characterization of ZnSe nanocrystals synthesized using solvothermal process. J. Mater. Sci. Mater. Electron. 2020, 31, 14756–14766. [Google Scholar] [CrossRef]
- Sukharev, V.Y. Percolation model of adsorption-induced response of the electrical characteristics of polycrystalline semiconductor adsorbents. J. Chem. Soc. Faraday Trans. 1993, 89, 559–572. [Google Scholar] [CrossRef]
- Glot, A.B. A model of non-Ohmic conduction in ZnO varistors. J. Mater. Sci. Mater. Electron. 2006, 17, 755–765. [Google Scholar] [CrossRef]
- Belyaev, A.P.; Rubets, V.P.; Antipov, V.V.; Grishin, V.V. Electrical and galvanomagnetic properties of cadmium telluride films synthesized under highly nonequilibrium conditions. Semiconductors 2008, 42, 1282–1285. [Google Scholar] [CrossRef]
- Shklovskii, B.I.; Efros, A.L. Electronic Properties of Doped Semiconductors; Springer: Berlin/Heidelberg, Germany, 1984; p. 388. [Google Scholar] [CrossRef]
- Belyaev, A.P.; Rubets, V.P.; Nuzhdin, M.Y. Electrical properties of cadmium telluride films synthesized in a thermal field with a temperature gradient. Semiconductors 2003, 37, 646–648. [Google Scholar] [CrossRef]


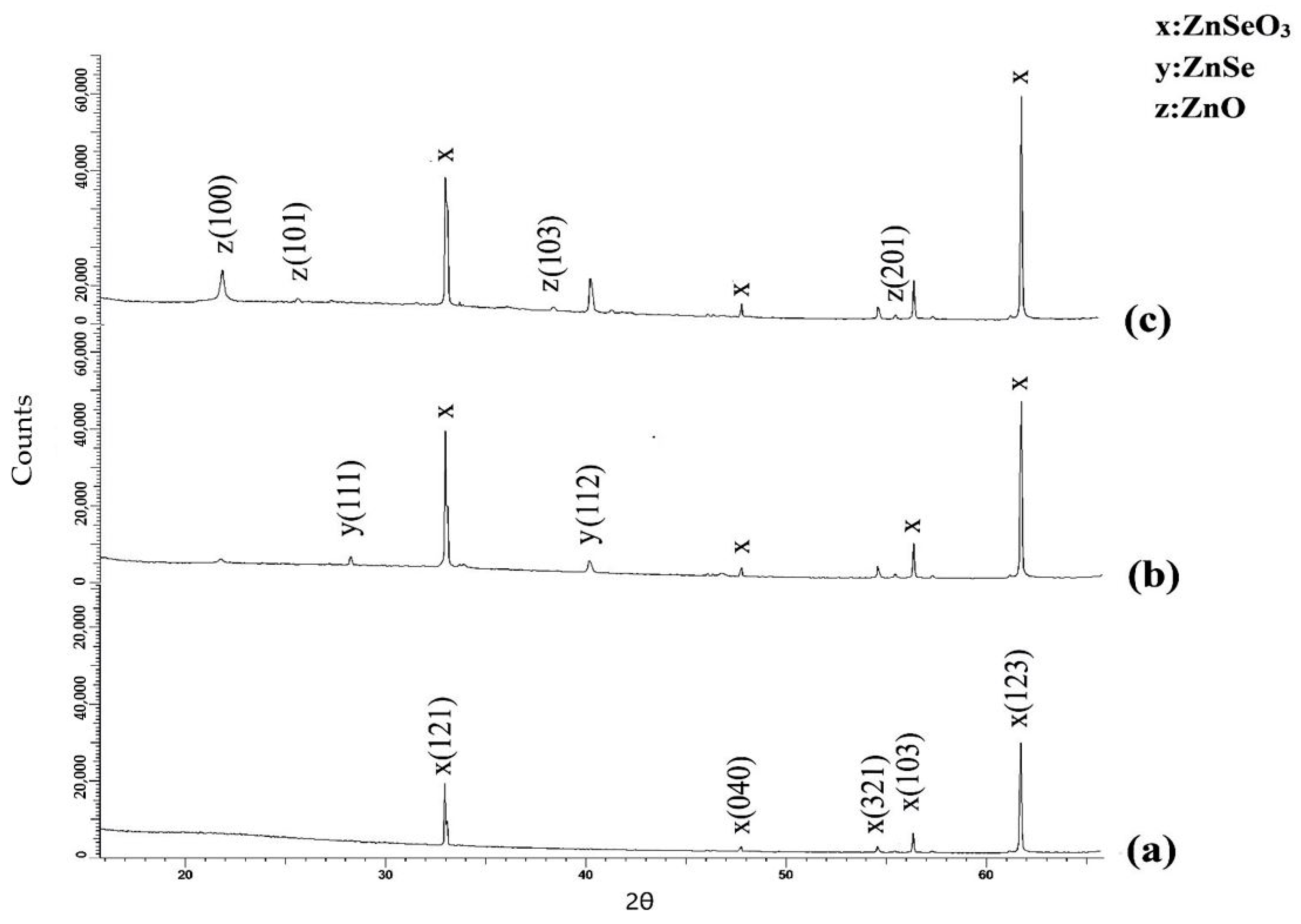

| Phase | Structure Type | Space Group | hkl | 2θ° | d, Å | Crystalline Size, nm | Cell Parameters, Å | Phase Content, % | Volume, Å3 |
|---|---|---|---|---|---|---|---|---|---|
| x: ZnSeO3 | Orthorhombic | Pnma (62) | 121 | 33.00 | 2.71 | 21.17 | a = 5.94 b = 7.61 c = 5.10 | 100 | 231.41 |
| 040 | 47.76 | 1.90 | |||||||
| 321 | 54.53 | 1.68 | |||||||
| 103 | 56.41 | 1.63 | |||||||
| 123 | 61.66 | 1.50 |
| Sample | Phase | Space Group | 2θ° | Lattice Parameter, Å | Crystalline Size, nm | Phase Content, % | Volume, Å3 |
|---|---|---|---|---|---|---|---|
| After annealing at 800 °C | y: ZnSe (PDF-01-077-8903), Tetragonal | P4/nmm (129) | 28.0 40.2 | a = 3.97 c = 5.23 | 25.7 | 68.5 | 82.57 |
| x: ZnSeO3 (JCPDS-78-0446) Orthorhombic | Pnma (62) | 33.002 47.76 54.53 56.41 61.656 | a = 5.94 b = 7.61 c = 5.10 | 26.52 | 31.5 | 231.41 | |
| After annealing at 1000 °C | z: ZnO (PDF-01-070-8070), Hexagonal | P63mc (186) | 21.9 25.8 38.3 55.5 | a = 3.27 c = 5.23 | 37.5 | 77.3 | 48.47 |
| x: ZnSeO3 (JCPDS-78-0446) Orthorhombic | Pnma (62) | 33.002 47.76 54.53 56.41 61.656 | a = 5.94 b = 7.61 c = 5.10 | 36.17 | 22.7 | 231.41 |
| Value | As-Deposited Samples | After Annealing at 800 °C | After Annealing at 1000 °C |
|---|---|---|---|
| Specific conductivity of the structure containing the nanocrystal arrays, σ, Ohm−1·cm−1 | 1.0 × 10−7 | 3.2 × 10−7 | 5.9 × 10−8 |
| Specific conductivity of a conical wire consisting of nanocrystals σW, Ohm−1·cm−1 | 2.7 × 10−6 | 8.5 × 10−6 | 1.5 × 10−6 |
| Random potential Δ, eV | 0.012 | 0.012 | 0.012 |
| Ohmic conductivity for low-field σ0, Ohm−1·cm−1 | 2.0 × 10−7 | 6.0 × 10−7 | 1.2 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aralbayeva, G.; Sarsekhan, G.; Akylbekova, A.; Vlasukova, L.A.; Baimukhanov, Z.; Yuvchenko, V.; Bazarbek, A.-D.; Dauletbekova, A.; Kabdrakhimova, G.; Akilbekov, A.T. The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates. Crystals 2024, 14, 730. https://doi.org/10.3390/cryst14080730
Aralbayeva G, Sarsekhan G, Akylbekova A, Vlasukova LA, Baimukhanov Z, Yuvchenko V, Bazarbek A-D, Dauletbekova A, Kabdrakhimova G, Akilbekov AT. The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates. Crystals. 2024; 14(8):730. https://doi.org/10.3390/cryst14080730
Chicago/Turabian StyleAralbayeva, Gulnara, Gulnaz Sarsekhan, Aiman Akylbekova, Liudmila A. Vlasukova, Zein Baimukhanov, Vera Yuvchenko, Assyl-Dastan Bazarbek, Alma Dauletbekova, Gaukhar Kabdrakhimova, and Abdirash T. Akilbekov. 2024. "The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates" Crystals 14, no. 8: 730. https://doi.org/10.3390/cryst14080730






