First Examples of Metal-Organic Frameworks with Pore-Encapsulated [Co(CO)4]− Anions: Facile Synthesis, Crystal Structures and Stability Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis of MOFs 1–3
2.3. X-ray Crystallographic Analysis
3. Results
3.1. Synthesis and Stability Studies
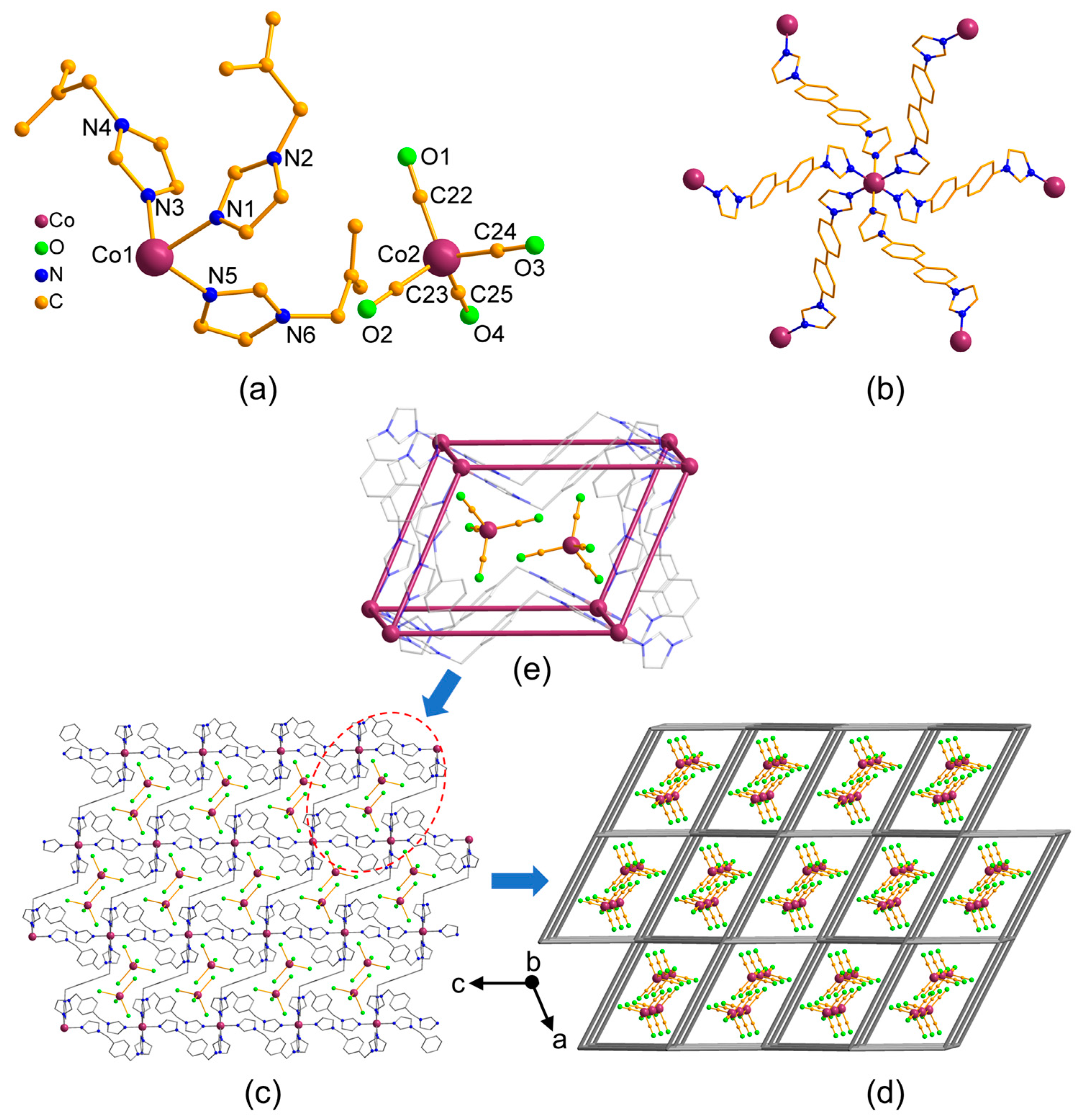
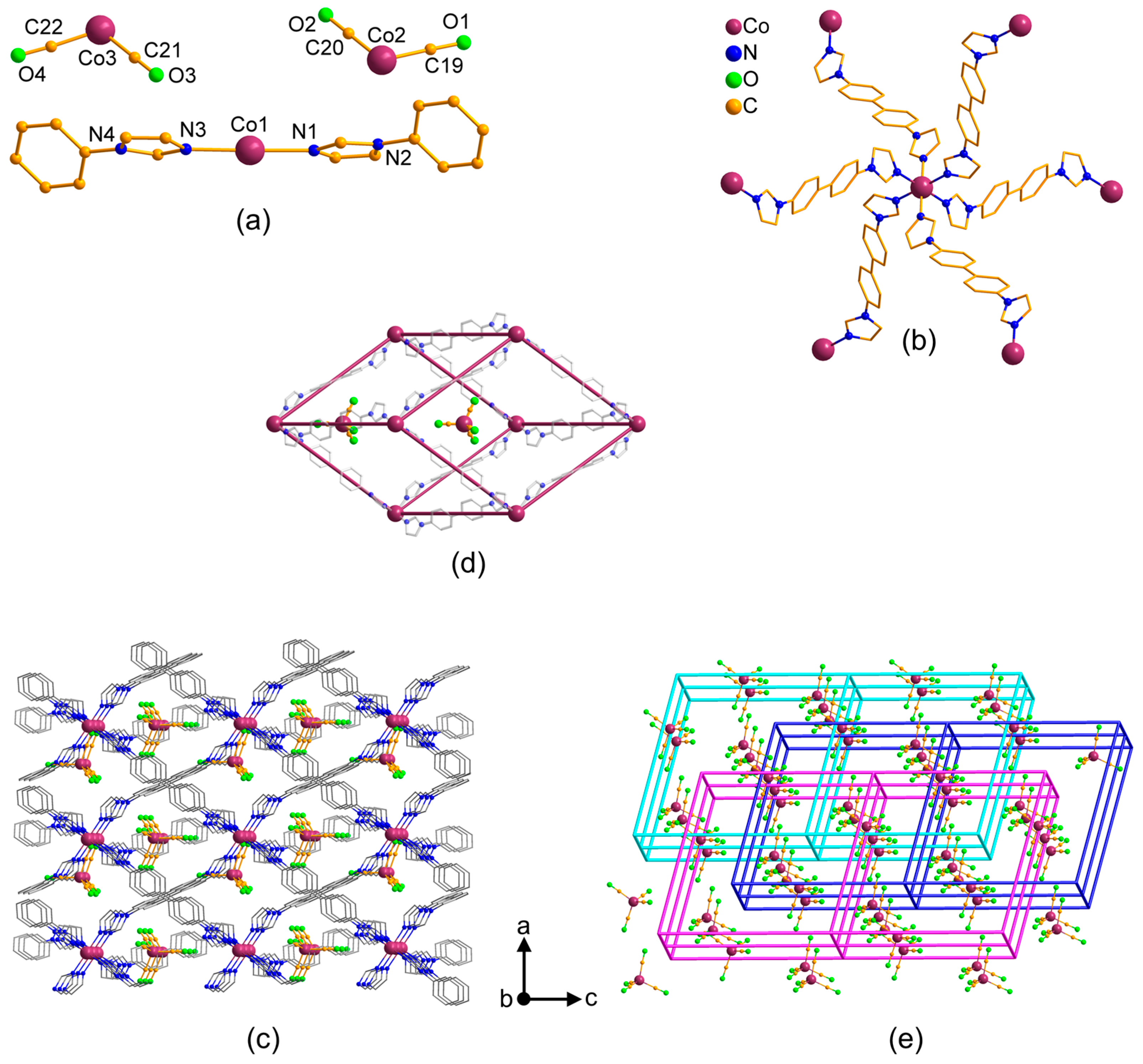
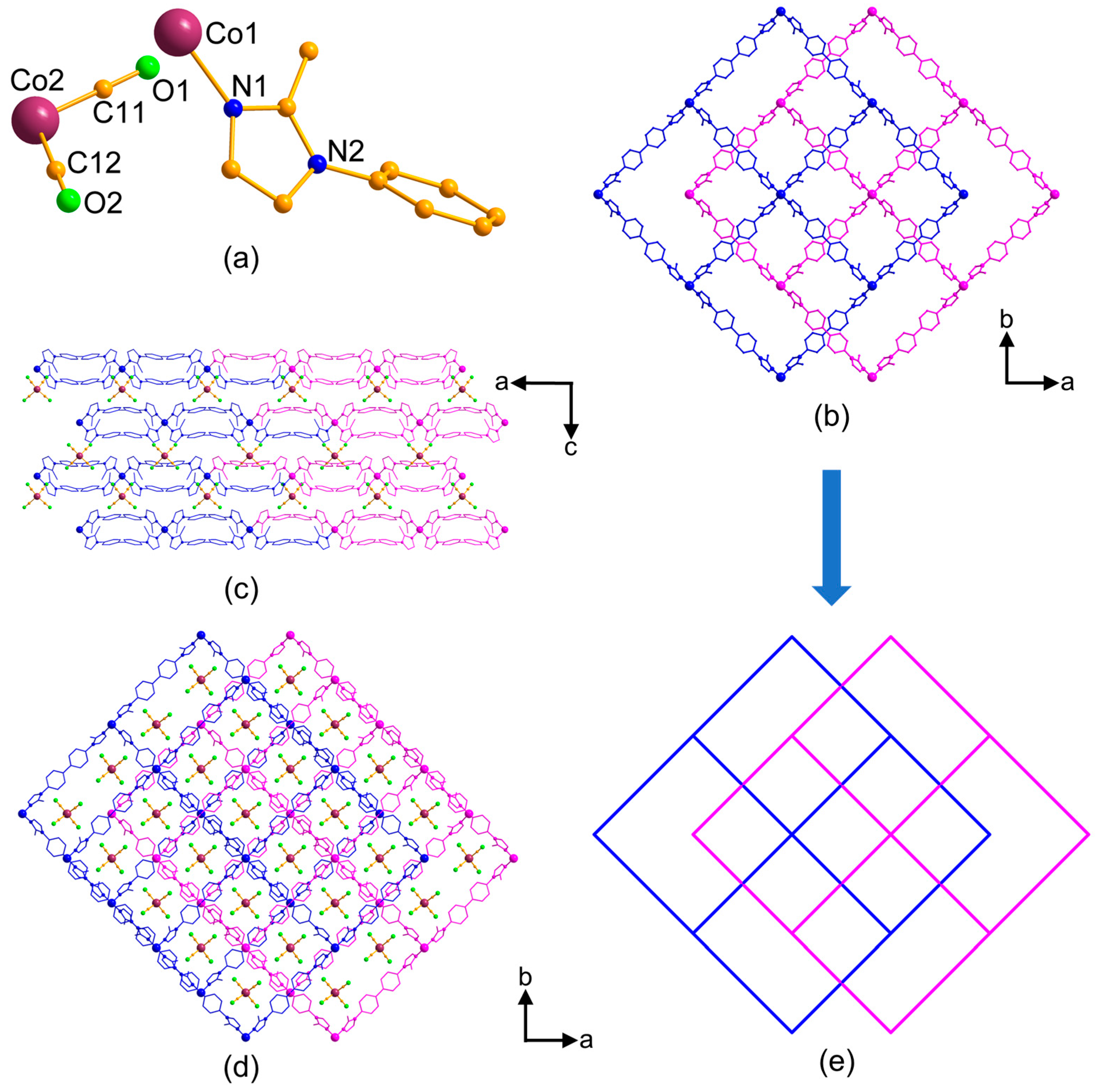
3.2. Structural Description of MOFs 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakano, K.; Nozaki, K. Carbonylation of Epoxides. In Catalytic Carbonylation Reactions. Topics in Organometallic Chemistry; Beller, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 18, pp. 223–238. [Google Scholar]
- Budiman, A.W.; Nam, J.S.; Park, J.H.; Mukti, R.I.; Chang, T.S.; Bae, J.W.; Choi, M.J. Review of Acetic Acid Synthesis from Various Feedstocks through Different Catalytic Processes. Catal. Surv. Asia 2016, 20, 173–193. [Google Scholar] [CrossRef]
- Peng, J.-B.; Wu, F.-P.; Wu, X.-F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bhanage, B.M. Double Carbonylation Reactions: Overview and Recent Advances. Adv. Synth. Catal. 2020, 362, 3022–3058. [Google Scholar] [CrossRef]
- Lee, J.T.; Thomas, P.J.; Alper, H. Synthesis of β-Lactones by the Regioselective, Cobalt and Lewis Acid Catalyzed Carbonylation of Simple and Functionalized Epoxides. J. Org. Chem. 2001, 66, 5424–5426. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.W.; Lobkovsky, E.B.; Coates, G.W. Practical β-Lactone Synthesis: Epoxide Carbonylation at 1 atm. Org. Lett. 2006, 8, 3709–3712. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.M.; Lobkovsky, E.B.; Coates, G.W. Catalytic Double Carbonylation of Epoxides to Succinic Anhydrides: Catalyst Discovery, Reaction Scope, and Mechanism. J. Am. Chem. Soc. 2007, 129, 4948–4960. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.W.; Lamb, J.R.; LaPointe, A.M.; Coates, G.W. Carbonylation of Ethylene Oxide to β-Propiolactone: A Facile Route to Poly(3-hydroxypropionate) and Acrylic Acid. ACS Catal. 2016, 6, 8219–8223. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Yang, L.; Xie, R.; Yang, G.-W.; Wu, G.-P. Perfectly Alternating Copolymerization of CO and Epoxides to Aliphatic Polyester Oligomers via Cooperative Organoboron–Cobalt Complexes. Macromolecules 2021, 54, 9427–9436. [Google Scholar] [CrossRef]
- Denmark, S.E.; Ahmad, M. Carbonylative Ring Opening of Terminal Epoxides at Atmospheric Pressure. J. Org. Chem. 2007, 72, 9630–9634. [Google Scholar] [CrossRef]
- Allmendinger, M.; Eberhardt, R.; Luinstra, G.; Rieger, B. The Cobalt-Catalyzed Alternating Copolymerization of Epoxides and Carbon Monoxide: A Novel Approach to Polyesters. J. Am. Chem. Soc. 2002, 124, 5646–5647. [Google Scholar] [CrossRef]
- Getzler, Y.D.Y.L.; Mahadevan, V.; Lobkovsky, E.B.; Coates, G.W. Synthesis of β-Lactones: A Highly Active and Selective Catalyst for Epoxide Carbonylation. J. Am. Chem. Soc. 2002, 124, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.R.; Mahadevan, V.; Getzler, Y.D.Y.L.; Coates, G.W. A Readily Synthesized and Highly Active Epoxide Carbonylation Catalyst Based on a Chromium Porphyrin Framework: Expanding the Range of Available β-Lactones. Org. Lett. 2004, 6, 373–376. [Google Scholar] [CrossRef]
- Church, T.L.; Getzler, Y.D.Y.L.; Coates, G.W. The Mechanism of Epoxide Carbonylation by [Lewis Acid]+[Co(CO)4]− Catalysts. J. Am. Chem. Soc. 2006, 128, 10125–10133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.R.; Lobkovsky, E.B.; Coates, G.W. Chromium(III) Octaethylporphyrinato Tetracarbonylcobaltate: A Highly Active, Selective, and Versatile Catalyst for Epoxide Carbonylation. J. Am. Chem. Soc. 2005, 127, 11426–11435. [Google Scholar] [CrossRef] [PubMed]
- Pietraru, M.H.; Ponsard, L.; Lentz, N.; Thuéry, P.; Nicolas, E.; Cantat, T. Fluorophosphoniums as Lewis acids in organometallic catalysis: Application to the carbonylation of β-lactones. Chem. Commun. 2024, 60, 1043–1046. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Dyson, P.J.; Ellis, D.J.; Welton, T. 1-Butyl-3-methylimidazolium cobalt tetracarbonyl [bmim][Co(CO)4]: A catalytically active organometallic ionic liquid. Chem. Commun. 2001, 1862–1863. [Google Scholar] [CrossRef]
- Deng, F.-G.; Hu, B.; Sun, W.; Chen, J.; Xia, C.-G. Novel pyridinium based cobalt carbonyl ionic liquids: Synthesis, full characterization, crystal structure and application in catalysis. Dalton Trans. 2007, 4262–4267. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Jiang, Y.; Zhou, C.; Guo, Z.; Ma, X.; Chen, Q.; Nie, T.; Song, H. Synthesis and evaluation of stable, efficient, and recyclable carbonylation catalysts: Polyether-substituted imidazolium carbonyl cobalt ionic liquids. J. Mol. Catal. A Chem. 2016, 415, 89–95. [Google Scholar] [CrossRef]
- Rajendiran, S.; Park, G.; Yoon, S. Direct Conversion of Propylene Oxide to 3-Hydroxy Butyric Acid Using a Cobalt Carbonyl Ionic Liquid Catalyst. Catalysts 2017, 7, 228. [Google Scholar] [CrossRef]
- Zhang, W.; Han, F.; Tong, J.; Xia, C.; Liu, J. Cobalt carbonyl ionic liquids based on the 1,1,3,3-tetra-alkylguanidine cation: Novel, highly efficient, and reusable catalysts for the carbonylation of epoxides. Chin. J. Catal. 2017, 38, 805–812. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Wang, Y.-N.; Lu, H.-M.; Liang, S.; Xu, B.-L.; Fan, Y.-N. Immobilization of Carbonylcobalt Catalyst by Poly (4-vinylpyridine) (P4VP) through N→Co Coordination Bonds: The Promotional Effect of Pyridine and the Reusability of Polymer Catalyst. Chem. Asian J. 2016, 11, 3159–3164. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, P.; Yang, W.; Niu, H.; Li, S.; Liang, C. Chemical kinetics and promoted Co-immobilization for efficient catalytic carbonylation of ethylene oxide into methyl 3-hydroxypropionate. Front. Chem. 2022, 10, 945028. [Google Scholar] [CrossRef]
- Park, H.D.; Dincă, M.; Román-Leshkov, Y. Heterogeneous Epoxide Carbonylation by Cooperative Ion-Pair Catalysis in Co(CO)4−-Incorporated Cr-MIL-101. ACS Cent. Sci. 2017, 3, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Yoon, S. Cr-Phthalocyanine Porous Organic Polymer as an Efficient and Selective Catalyst for Mono Carbonylation of Epoxides to Lactones. Catalysts 2020, 10, 905. [Google Scholar] [CrossRef]
- Rajendiran, S.; Natarajan, P.; Yoon, S. A covalent triazine framework-based heterogenized Al–Co bimetallic catalyst for the ring-expansion carbonylation of epoxide to β-lactone. RSC Adv. 2017, 7, 4635–4638. [Google Scholar] [CrossRef]
- Rajendiran, S.; Gunasekar, G.H.; Yoon, S. A heterogenized cobaltate catalyst on a bis-imidazolium-based covalent triazine framework for hydroesterification of epoxides. New J. Chem. 2018, 42, 12256–12262. [Google Scholar] [CrossRef]
- Rajendiran, S.; Ganesan, V.; Yoon, S. Balancing between Heterogeneity and Reactivity in Porphyrin Chromium-Cobaltate Catalyzed Ring Expansion Carbonylation of Epoxide into β-Lactone. Inorg. Chem. 2019, 58, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Chen, B. Reducing CO2 with Stable Covalent Organic Frameworks. Joule 2018, 2, 1030–1032. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS 97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sumner, G.G.; Klug, H.P.; Alexander, L.E. The crystal structure of dicobalt octacarbonyl. Acta Cryst. 1964, 17, 732–742. [Google Scholar] [CrossRef]
- Tong, M.-L.; Ru, J.; Wu, Y.-M.; Chen, X.-M.; Chang, H.-C.; Mochizuki, K.; Kitagawa, S. Cation-templated construction of three-dimensional α-Po cubic-type [M(dca)3]− networks. Syntheses, structures and magnetic properties of A[M(dca)3] (dca = dicyanamide; for A = benzyltributylammonium, M = Mn2+, Co2+; for A = benzyltriethylammonium, M = Mn2+, Fe2+). New J. Chem. 2003, 27, 779–782. [Google Scholar]
- Adarsh, N.N.; Novio, F.; Ruiz-Molin, D. Coordination polymers built from 1,4-bis(imidazol-1-ylmethyl)benzene: From crystalline to amorphous. Dalton Trans. 2016, 45, 11233–11255. [Google Scholar] [CrossRef]
- Wang, F.; Ke, X.; Zhao, J.; Deng, K.; Leng, X.; Tian, Z.; Wen, L.; Li, D. Six new metal–organic frameworks with multi-carboxylic acids and imidazole-based spacers: Syntheses, structures and properties. Dalton Trans. 2011, 40, 11856–11865. [Google Scholar] [CrossRef]
- Fan, L.; Fan, W.; Li, B.; Liu, X.; Zhao, X.; Zhang, X. Syntheses, structures, topologies, and luminescence properties of four coordination polymers based on bifunctional 6-(4-pyridyl)-terephthalic acid and bis(imidazole) bridging linkers. RSC Adv. 2015, 5, 14897–14905. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, D.-C.; Yao, X.-Q.; Yang, Y.-X.; Liu, J.-C. Four cobalt(II) complexes based on a new tricarboxylate with naphthalene ring and different N-containing ligands: Synthesis, crystal structures and magnetic properties. New J. Chem. 2016, 40, 5010–5018. [Google Scholar] [CrossRef]
- Yan, M.-X.; Chen, L.-J.; Ma, R.; Cai, J.-N.; Shi, Y.-D.; Lin, S. Three 3D Co(II) cluster-based MOFs constructed from polycarboxylate acids and bis(imidazole) ligands and their derivatives: Magnetic properties and catalytic performance for the ORR. Dalton Trans. 2019, 48, 13369–13377. [Google Scholar]
- Hu, D.-C.; Wu, Y.-J.; Sun, J.; Shang, K.-X.; Yao, X.-Q.; Yang, Y.-X.; Liu, J.-C. Five coordination polymers based on two asymmetric Semi-rigid carboxylate organic ligands and N-donor 4, 4′-di (1H-imidazol-1-yl)-1, 1′-biphenyl ligand: Solvent-induced synthesis, crystal structures, magnetic and dye absorption properties. Inorg. Chim. Acta 2020, 502, 119356. [Google Scholar] [CrossRef]
- Fan, C.; Wang, L.; Xu, C.; Wang, J.; Zhu, B.; Liu, W.; Zhang, X.; Zong, Z.; Fan, Y. High-Efficiency Organic Contaminants Remover Based on Modulated Self-Assembly of Cobalt Metal−Organic Frameworks. Cryst. Growth Des. 2021, 21, 4305–4317. [Google Scholar] [CrossRef]
- Li, B.-L.; Zhu, X.; Zhou, J.-H.; Zhang, Y. Syntheses and structures of two zinc coordination polymers with a three-dimensional α-polonium cubic network and a two-dimensional (4,4) network. J. Coord. Chem. 2005, 58, 271–278. [Google Scholar] [CrossRef]
- Yu, Y. Nitrate Ions Templated 3D Noninterpenetrating 6-Connected pcu Net: Synthesis, Structure and Properties. Synth. React. Inorg. M 2015, 45, 1710–1712. [Google Scholar] [CrossRef]
- Khalaj, M.; Lalegani, A.; Lyczko, K.; Lipkowski, J. Synthesis and Characterization of Co(II) Coordination Polymer with a Flexible Bidentate Ligand. Crystallogr. Rep. 2019, 64, 1084–1088. [Google Scholar] [CrossRef]
- Duan, Y.; Xia, W.; Tang, P.; Li, D.; Dong, W.; Lu, J.Y. The first 3-fold interpenetrating framework containing both azobenzene-3,3′-dicarboxylicate and 1,2-bis(4-pyridyl)ethylene. Complex Met. 2014, 1, 122–127. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Proserpio, D.M. How 2-periodic coordination networks are interweaved: Entanglement isomerism and polymorphism. CrystEngComm 2017, 19, 1993–2006. [Google Scholar] [CrossRef]


| MOFs | 1 | 2 | 3 |
|---|---|---|---|
| CCDC number | 2,368,487 | 2,368,488 | 2,368,489 |
| Empirical formula | C50H42N12O8Co3 | C62H42N12O8Co3 | C48H36N8O8Co3 |
| Formula weight (g mol−1) | 1115.74 | 1259.85 | 1029.64 |
| Temperature (K) | 293 | 293 | 293 |
| Crystal system | monoclinic | trigonal | tetragonal |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Space group | C2/c | P3 | P−421c |
| a (Å) | 23.741(12) | 11.6083(6) | 12.2559(6) |
| b (Å) | 12.626(6) | 11.6083(6) | 12.2559(6) |
| c (Å) | 19.300(10) | 13.5342(14) | 15.4132(16) |
| β (°) | 108.642(9) | 120 | 90 |
| V (Å3) | 5482(5) | 1579.4(2) | 2315.2(3) |
| Z | 8 | 3 | 8 |
| Dc (g cm−3) | 1.352 | 1.325 | 1.477 |
| μ (mm−1) | 0.958 | 0.840 | 1.125 |
| θmin-max (°) | 5.328–55.08 | 5.048–54.968 | 4.246–55.038 |
| Index ranges | −30 ≤ h ≤ 30; −15 ≤ k ≤ 16; −25 ≤ l ≤ 25 | −14 ≤ h ≤ 15; −15 ≤ k ≤ 14; −17 ≤ l ≤ 17 | −15 ≤ h ≤ 15; −15 ≤ k ≤ 15; −20 ≤ l ≤ 19 |
| Measured reflns. | 20,683 | 12,140 | 16,984 |
| Unique reflns./I > 2σ(I) | 6267/4548 | 4795/4497 | 2654/2475 |
| Rint | 0.0194 | 0.0168 | 0.0264 |
| F(000) | 2284 | 643 | 1050 |
| R1 a | 0.0439 | 0.0602 | 0.0431 |
| wR2 b | 0.1226 | 0.1624 | 0.1198 |
| Goodness-of-fit on F2 | 1.046 | 1.081 | 1.002 |
| Largest diff. peak/hole (eÅ−3) | 0.38/−0.36 | 0.74/−1.11 | 0.64/−0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Du, S. First Examples of Metal-Organic Frameworks with Pore-Encapsulated [Co(CO)4]− Anions: Facile Synthesis, Crystal Structures and Stability Studies. Crystals 2024, 14, 731. https://doi.org/10.3390/cryst14080731
Xiao C, Du S. First Examples of Metal-Organic Frameworks with Pore-Encapsulated [Co(CO)4]− Anions: Facile Synthesis, Crystal Structures and Stability Studies. Crystals. 2024; 14(8):731. https://doi.org/10.3390/cryst14080731
Chicago/Turabian StyleXiao, Caihong, and Shaowu Du. 2024. "First Examples of Metal-Organic Frameworks with Pore-Encapsulated [Co(CO)4]− Anions: Facile Synthesis, Crystal Structures and Stability Studies" Crystals 14, no. 8: 731. https://doi.org/10.3390/cryst14080731




