A New Type of Acidic OH-Groups in the LTL Zeolite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterisation
3. Results and Discussion
3.1. SEM-EDX
3.2. N2 Physisorption
3.3. XRD Analysis
3.4. FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCusker, L.B.; Baerlocher, C. Zeolite structures. In Introduction to Zeolite Science and Practice, 3rd ed.; Studies in Surface Science and Catalysis; Cejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 13–37. [Google Scholar]
- McCusker, L.B.; Olson, D.H.; Baerlocher, C. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Csicsery, S.M. Shape-selective catalysis in zeolites. Zeolites 1984, 4, 202–213. [Google Scholar]
- Khouw, C.B.; Davis, M.E. Shape-Selective Catalysis with Zeolites and Molecular Sieves; ACS Publications: Washington, DC, USA, 1993. [Google Scholar]
- Uguina, M.; Serrano, D.; Van Grieken, R.; Venes, S. Adsorption, acid and catalytic changes induced in ZSM-5 by coking with different hydrocarbons. Appl. Catal. A-Gen. 1993, 99, 97–113. [Google Scholar]
- Kareem, A.; Chand, S.; Mishra, I. Disproportionation of Toluene to Produce Benzene and p-Xylene–A Review; NISCAIR-CSIR: New Delhi, India, 2001. [Google Scholar]
- Tan, K.H.; Cham, H.Y.; Awala, H.; Ling, T.C.; Mukti, R.R.; Wong, K.L.; Mintova, S.; Ng, E.P. Effect of Extra Framework Cations of LTL Nanozeolites to Inhibit Oil Oxidation. Nanoscale Res. Lett. 2015, 10, 253. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; Wiley-Interscience: Hoboken, NJ, USA, 2003. [Google Scholar]
- Jasra, R.V.; Choudary, N.V.; Bhat, S.G.T. Correlation of sorption behavior of nitrogen, oxygen, and argon with cation locations in zeolite X. Ind. Eng. Chem. Res. 1996, 35, 4221–4229. [Google Scholar]
- Barrer, R.M.; Galabova, I.M. Ion-exchanged forms of zeolite L, erionite, and offretite and sorption of inert-gases. Adv. Chem. 1973, 121, 356–373. [Google Scholar]
- Breck, D.W.; Flanigen, E.M. Molecular Sieves; Society of Chemical Industry: London, UK, 1968; p. 47. [Google Scholar]
- Breck, D.W.; Acara, N.A. Crystalline zeolite L. US Patent 3216789, 9 November 1965. [Google Scholar]
- Pichat, P.; Franco-Parra, C.; Barthomeuf, D. Infrared structural study of various type L zeolites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 991–996. [Google Scholar]
- Barrer, R.M.; Villiger, H. The crystal structure of the synthetic zeolite L. Z. Krist.-New Cryst. Struct. 1969, 128, 352–370. [Google Scholar]
- Ohsuna, T.; Horikawa, Y.; Hiraga, K.; Terasaki, O. Surface Structure of Zeolite L Studied by High-Resolution Electron Microscopy. Chem. Mater. 1998, 10, 688–691. [Google Scholar]
- Hassani, S.S.; Salehirad, F.; Aghabozorg, H.R.; Sobat, Z. Synthesis and morphology of nanosized zeolite L. Cryst. Res. Technol. 2010, 45, 183–187. [Google Scholar]
- Newsam, J.M.; Melchior, M.T.; Malone, H. Full profile analysis of the 29Si NMR spectra of LTL-framework zeolites. Solid State Ion. 1988, 26, 125–131. [Google Scholar]
- Li, P.; Wang, Y.; Li, H.; Calzaferri, G. Luminescence Enhancement after Adding Stoppers to Europium(III) Nanozeolite L. Angew. Chem. Int. Ed. 2014, 53, 2904–2909. [Google Scholar]
- Wang, Y.; Li, H.; Gu, L.; Gan, Q.; Li, Y.; Calzaferri, G. Thermally stable luminescent lanthanide complexes in zeolite L. Microporous Mesoporous Mater. 2009, 121, 1–6. [Google Scholar]
- Bhat, S.D.; Niphadkar, P.S.; Gaydhankar, T.R.; Awate, S.V.; Belhekar, A.A.; Joshi, P.N. High temperature hydrothermal crystallization, morphology and yield control of zeolite type K-LTL. Microporous Mesoporous Mater. 2004, 76, 81–89. [Google Scholar]
- Garces, L.J.; Makwana, V.D.; Hincapie, B.; Sacco, A.; Suib, S.L. Selective N,N-methylation of aniline over cocrystallized zeolites RHO and zeolite X (FAU) and over Linde type L (Sr,K-LTL). J. Catal. 2003, 217, 107–116. [Google Scholar]
- Song, J.; Ma, H.; Tian, Z.; Yan, L.; Xu, Z.; Liu, Q.; Qu, W. The effect of Fe on Pt particle states in Pt/KL catalysts. Appl. Catal. Gen. 2015, 492, 31–37. [Google Scholar]
- Derouane, E.G.; Vanderveken, D.J. Structural recognition and preorganization in zeolite catalysis: Direct aromatization of n-hexane on zeolite L-based catalysts. Appl. Catal. 1988, 45, 15–22. [Google Scholar]
- Larsen, G.; Haller, G.L. Metal-support effects in Pt/L-zeolite catalysts. Catal. Lett. 1989, 3, 103–110. [Google Scholar]
- Bernard, J.R.; Ward, D.W. Hydrocarbons Aromatization on Platinum Alkaline Zeolites. In Proceedings of the 5th Interantional Conference on Zeolites, Naples, Italy, 2–6 June 1980; Philadelphia Heyden & Son Ltd.: London, UK, 1980; pp. 686–695. [Google Scholar]
- Besoukhanova, C.; Guidot, J.; Barthomeuf, D.; Breysse, M.; Bernard, J.R. Platinum-zeolite interactions in alkaline L zeolites. Correlations between catalytic activity and platinum state. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 1595–1604. [Google Scholar]
- Cho, S.J.; Ryoo, R. Characterization of PtSn narioparticles in KL zeolite and n-hexane aromatization activity. Catal. Lett. 2004, 97, 71–75. [Google Scholar]
- Tarach, K.; Tekla, J.; Filek, U.; Szymocha, A.; Tarach, I.; Góra-Marek, K. Alkaline-acid treated zeolite L as catalyst in ethanol dehydration process. Microporous Mesoporous Mater. 2017, 241, 132–144. [Google Scholar]
- Al-Ani, A.; Freitas, C.; Zholobenko, V. Nanostructured large-pore zeolite: The enhanced accessibility of active sites and its effect on the catalytic performance. Microporous Mesoporous Mater. 2020, 293, 109805. [Google Scholar]
- McCusker, L.B.; Baerlocher, C.; Wilson, S.T.; Broach, R.W. Synthesis and Structural Characterization of the Aluminosilicate LZ-135, a Zeolite Related to ZSM-10. J. Phys. Chem. 2009, 113, 9838–9844. [Google Scholar]
- Flanigen, E.M.; Kellberg, E.R. Synthetic Crystalline Zeolite and Process for Preparing Same. US Patent No. 4241036, 23 December 1980. [Google Scholar]
- Galli, E. Mazzite, a Zeolite. Cryst. Struct. Commun. 1974, 3, 339–344. [Google Scholar]
- Galli, E.; Passaglia, E.; Pongiluppi, D.; Rinaldi, R. Mazzite, a new mineral, the natural counterpart of the synthetic zeolite Ω. Contrib. Mineral. Petrol. 1974, 45, 99–105. [Google Scholar]
- Rinaldi, R.; Pluth, J.J.; Smith, J.V. Crystal structure of mazzite dehydrated at 600 °C. Acta Crystallogr. Sect. B Struct. Sci. 1975, 31, 1603–1608. [Google Scholar]
- McQueen, D.; Chiche, B.; Fajula, F.; Auroux, A.; Guimon, C.; Fitoussi, F.; Schulz, P. A Multitechnique Characterization of the Acidity of Dealuminated Mazzite. J. Catal. 1996, 161, 587–596. [Google Scholar]
- Shigeishi, R.; Chiche, B.; Fajula, F. CO adsorption on superacid sites on dealuminated mazzite. Microporous Mesoporous Mater. 2001, 43, 211–226. [Google Scholar]
- Goossens, A.M.; Feijen, E.P.; Verhoeven, G.; Wouters, B.H.; Grobet, P.J.; Jacobs, P.A.; Martens, J.A. Crystallization of MAZ-type zeolites using tetramethylammonium, sodium and n-hexane derivatives as structure- and composition-directing agents. Microporous Mesoporous Mater. 2000, 35–36, 555–572. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar]
- Lutterotti, L. Maud: A Rietveld Analysis Program Designed for the Internet and Experiment Integration. Acta Crystallogr. Sect. A Found. Crystallogr 2000, 56, s54. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD (Material Analysis Using Diffraction): A user friendly java program for Rietveld texture analysis and more. In Proceeding of the Twelfth International Conference on Textures of Materials (ICOTOM-12), Montreal, QC, Canada, 9–13 August 1999; Volume 1, p. 1599. [Google Scholar]
- Available online: http://www.iza-structure.org/databases/ (accessed on 1 July 2024).
- Available online: https://www.icdd.com (accessed on 1 July 2024).
- Zholobenko, V.; Freitas, C.; Jendrlin, M.; Travert, A.; Bazin, P.; Thibault-Starzyk, F. Probing the acid sites of zeolites with pyridine: Quantitative AGIR measurements of the molar absorption coefficients. J. Catal. 2020, 385, 52–60. [Google Scholar]
- Contini, A.; Jendrlin, M.; Al-Ani, A.; Zholobenko, V. Structural and Acidic Properties of Ion-Exchanged Mazzite. Pet. Chem. 2024, 64, 235–244. [Google Scholar]
- Seoung, D.; Lee, Y.; Kim, S.J.; Lee, H.H.; Ahn, D.; Shin, N.S.; Vogt, T. Pressure-induced hydration and cation migration in a Cs+ exchanged gallosilicate zeolite LTL: Synchrotron X-ray powder diffraction study at ambient and high pressures. Microporous Mesoporous Mater. 2010, 136, 75–82. [Google Scholar]
- Price, L.A.; Jones, Z.; Nearchou, A.; Stenning, G.; Nye, D.; Sartbaeva, A. The Effect of Cation Exchange on the Pore Geometry of Zeolite L. Appl. Chem. 2022, 2, 149–159. [Google Scholar]
- Martucci, A.; Precisvalle, N.; Fois, E.; Ardit, M.; Beltrami, G.; Pasti, L.; Cescon, M.; Suard, E.; Tabacchi, G. Location of Brønsted sites in deuterated L-zeolite: A combined neutron powder diffraction and computer modeling study. Mater. Chem. Phys. 2023, 308, 128250. [Google Scholar]
- Zholobenko, V.L.; Kustov, L.M.; Borovkov, V.Y.; Kazansky, V.B. A new type of acidic hydroxyl groups in ZSM-5 zeolite and in mordenite according to diffuse reflectance IR spectroscopy. Zeolites 1988, 8, 175–178. [Google Scholar]
- Zholobenko, V.L.; Makarova, M.A.; Dwyer, J. Inhomogeneity of Broensted acid sites in mordenite. J. Phys. Chem. 1993, 97, 5962–5964. [Google Scholar]
- Windeck, H.; Berger, F.; Sauer, J. Spectroscopic Signatures of Internal Hydrogen Bonds of Brønsted-Acid Sites in the Zeolite H-MFI. Angew. Chem. Int. Ed. 2023, 62, e202303204. [Google Scholar]
- Freitas, C.; Barrow, N.S.; Zholobenko, V. Accessibility and location of acid sites in zeolites as probed by FTIR and MAS-NMR. Johns. Matthey Technol. Rev. 2018, 62, 279–290. [Google Scholar]
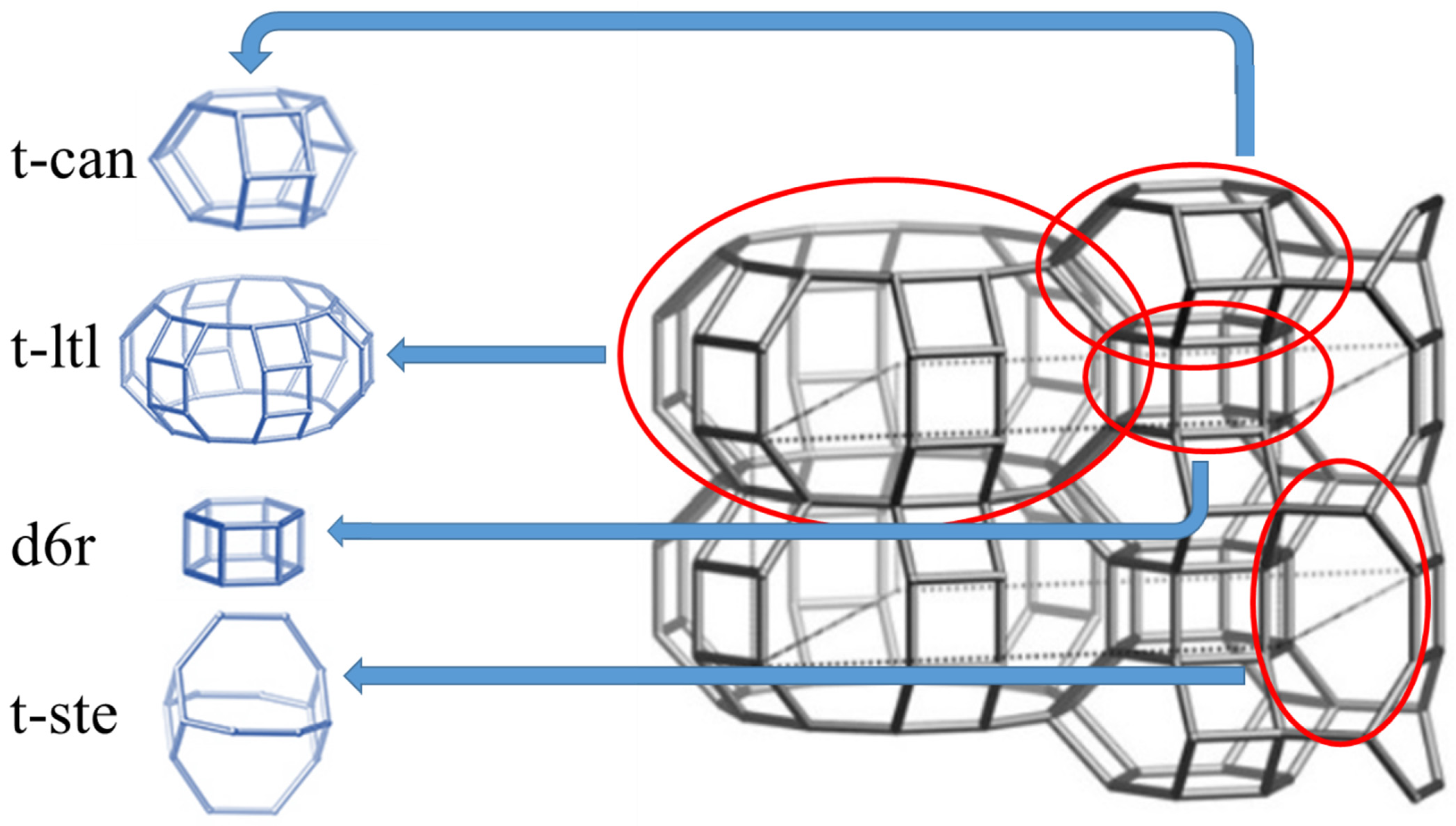

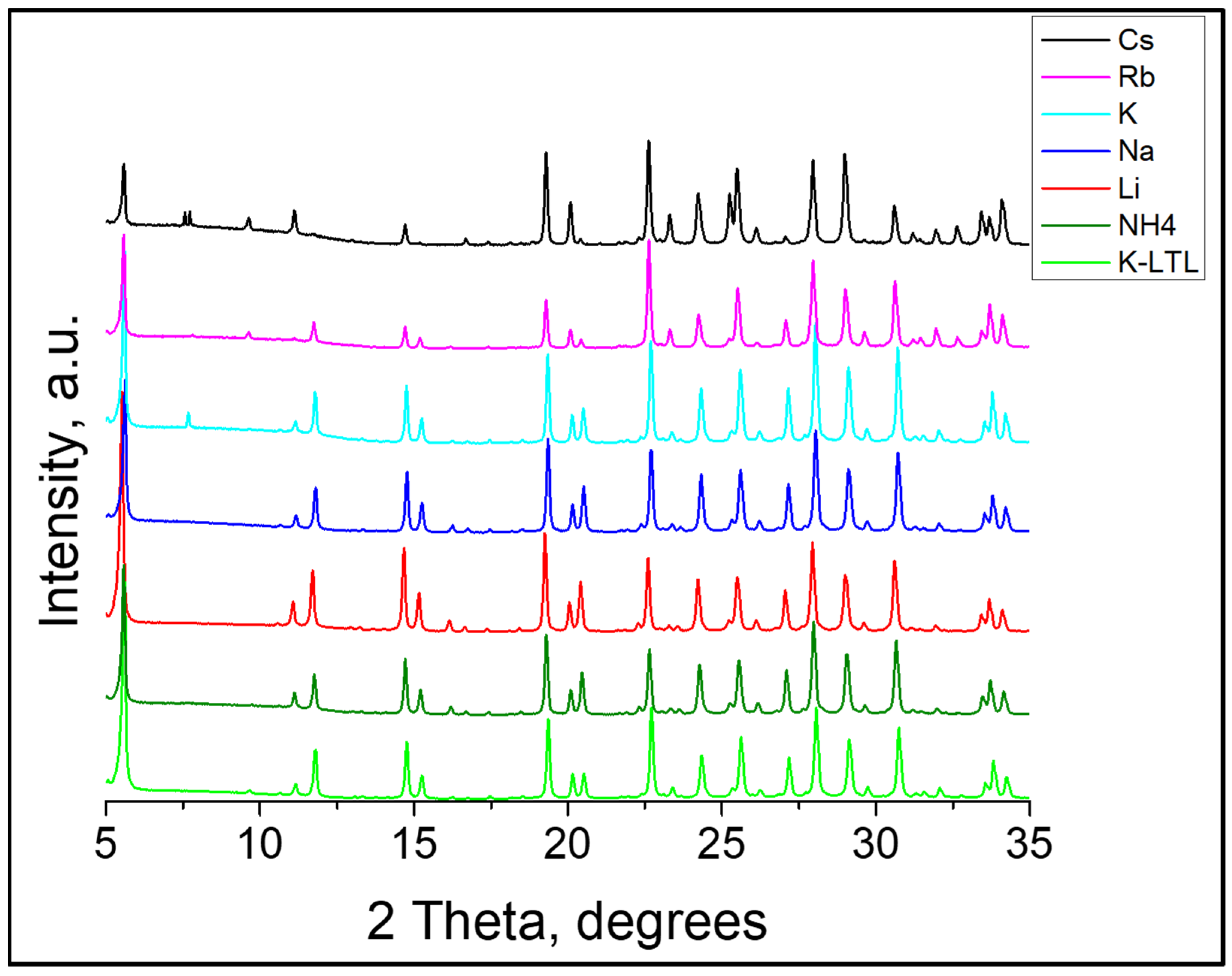
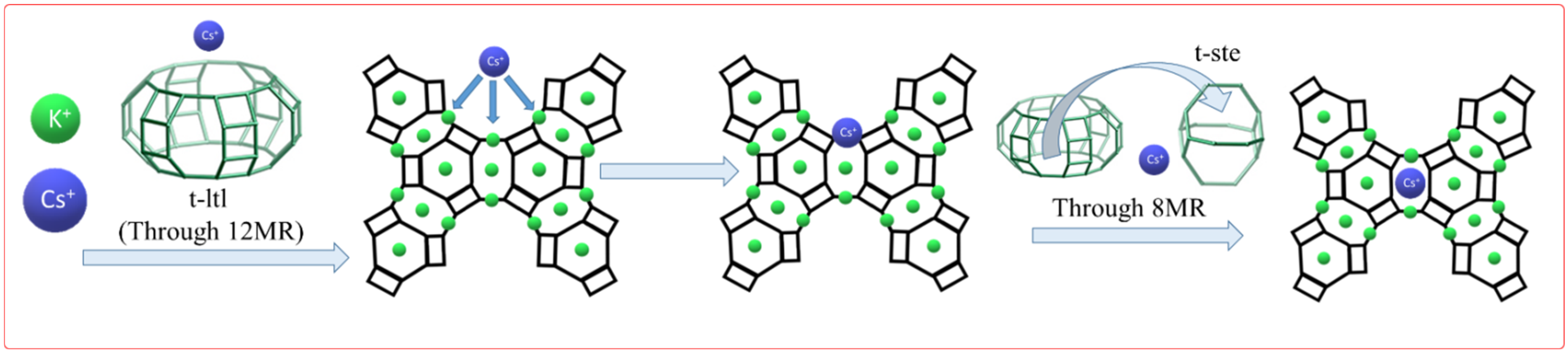



| Sample | Composition a | Crystallinity b, % | S, m2 g−1 | Vmicro c, cm3 g−1 |
|---|---|---|---|---|
| K-LTL | K8.8Al8.6Si27.4O72 | 92 | 346 | 0.12 |
| NH4-K-LTL | (NH4)3.6K5.0Al8.6Si27.4O72 | 89 | 376 | 0.12 |
| Li-NH4-K-LTL | Li0.6(NH4)2.8K5.2Al8.6Si27.4O72 | 90 | 385 | 0.13 |
| Na-NH4-K-LTL | Na1.0(NH4)2.4K5.2Al8.6Si27.4O72 | 88 | 357 | 0.12 |
| K-NH4-K-LTL | (NH4)0.8K7.8Al8.6Si27.4O72 | 87 | 332 | 0.12 |
| Rb-NH4-K-LTL | Rb2.2(NH4)0.8K5.6Al8.5Si27.4O72 | 85 | 295 | 0.10 |
| Cs-NH4-K-LTL | Cs3.1(NH4)0.6K4.8Al8.5Si27.4O72 | 84 | 266 | 0.09 |
| NH4-Na-MAZ | (NH4)4.2Na4.2Al8.4Si27.6O72 | 81 | 105 | 0.02 |
| Sample | BAS, µmol g−1 | LAS, µmol g−1 |
|---|---|---|
| NH4-MAZ | 113 | 89 |
| K-LTL | 0 | 8 |
| NH4-K-LTL | 486 | 204 |
| Li-NH4-K-LTL-4 | 474 | 174 |
| Na-NH4-K-LTL-4 | 469 | 118 |
| K-NH4-K-LTL-4 | 312 | 45 |
| Rb-NH4-K-LTL-4 | 289 | 34 |
| Cs-NH4-K-LTL-4 | 229 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contini, A.; Jendrlin, M.; Zholobenko, V. A New Type of Acidic OH-Groups in the LTL Zeolite. Crystals 2024, 14, 745. https://doi.org/10.3390/cryst14080745
Contini A, Jendrlin M, Zholobenko V. A New Type of Acidic OH-Groups in the LTL Zeolite. Crystals. 2024; 14(8):745. https://doi.org/10.3390/cryst14080745
Chicago/Turabian StyleContini, Alessandro, Martin Jendrlin, and Vladimir Zholobenko. 2024. "A New Type of Acidic OH-Groups in the LTL Zeolite" Crystals 14, no. 8: 745. https://doi.org/10.3390/cryst14080745





