Surface Modification of Chromium–Nickel Steel by Electrolytic Plasma Nitriding Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- -
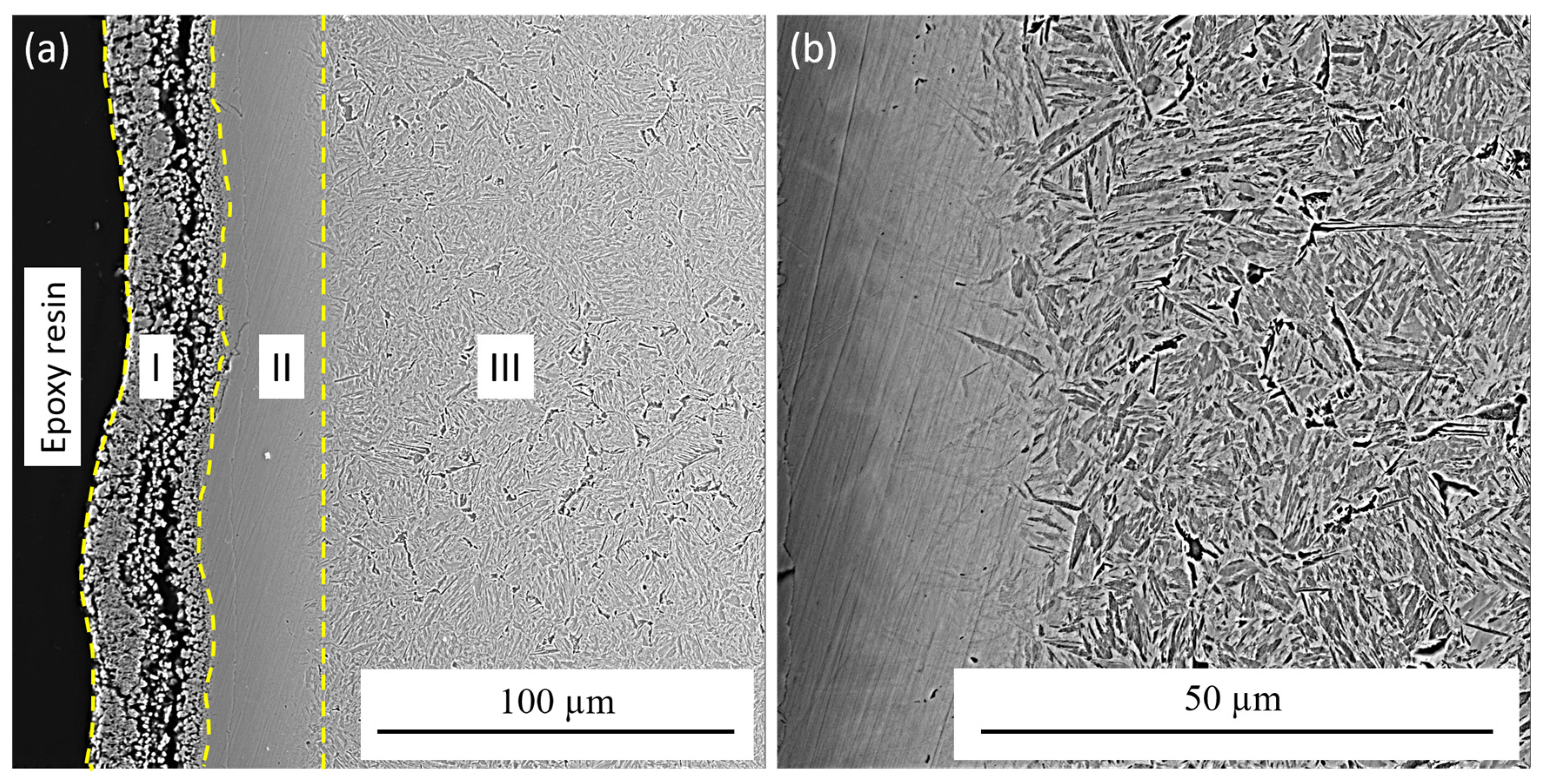
- On the basis of metallographic and X-ray diffraction analyses, it was determined that after electrolyte–plasma nitriding, multilayer diffusion layers are formed. In the surface layer, the formed oxide–nitride zone 25–30 μm thick, containing iron nitride Fe2N and iron oxide Fe3O4. Under this zone is located a nitride–martensitic sublayer. This is followed by the third zone of internal nitriding.
- -
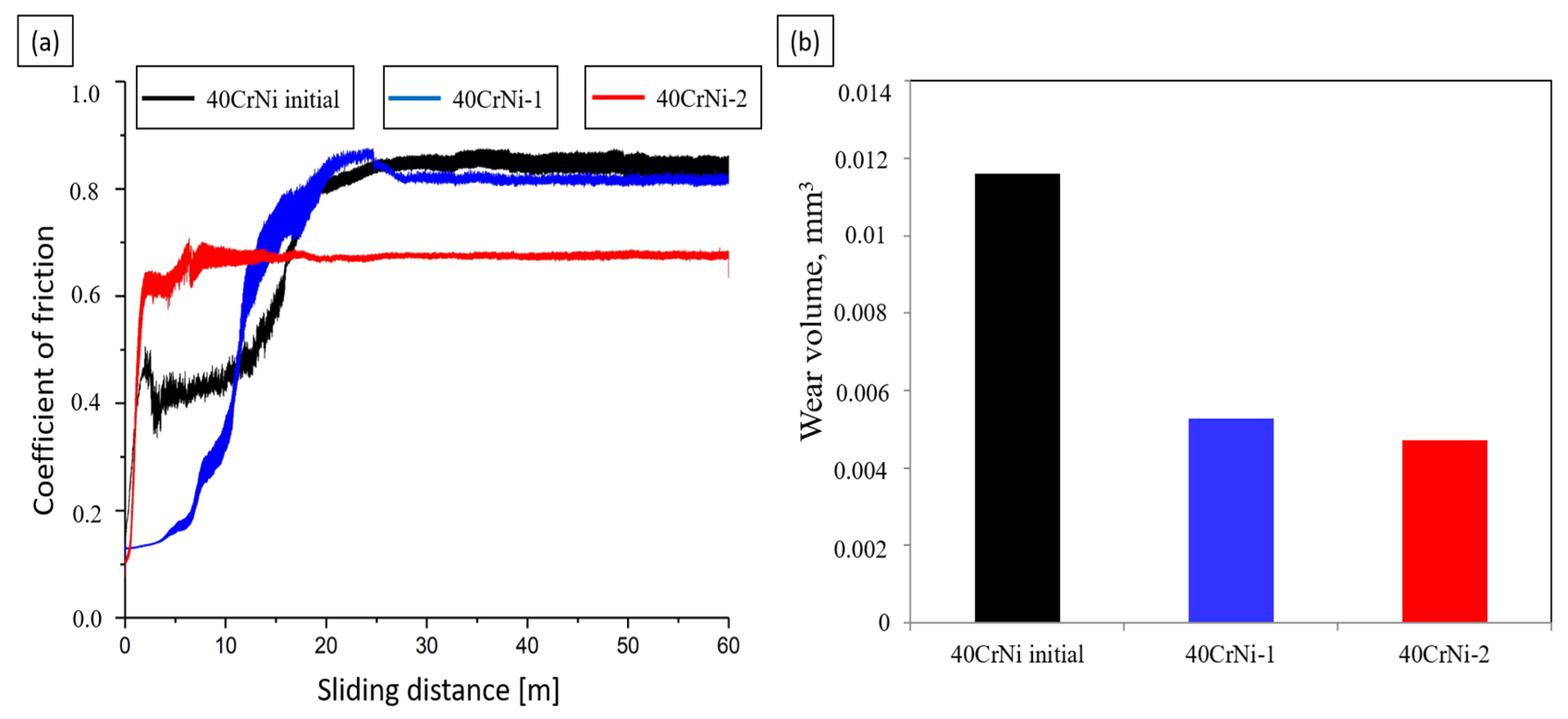
- Tribological tests showed that the hardened samples have high wear resistance compared to the original samples. After nitriding steel in solutions of carbamide, the coefficient of dry friction decreases from 0.82 in the untreated sample to 0.63 in the sample after nitriding within 7 min, and the wear resistance increases by 1.5 times.
- -
- It is determined that after electrolyte–plasma nitriding, the surface hardness increases significantly. Nitriding regimes and the amount of alloying elements significantly affect the hardness of the diffusion zone.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belkin, P.N.; Kusmanov, S.A. Plasma electrolytic hardening of steels: Review. Surf. Eng. Appl. Electrochem. 2016, 52, 531–546. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and corrosion resistance of laser surface hardened structural steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Laser surface modification of carburized and borocarburized 15CrNi6 steel. Mater. Charact. 2007, 58, 461–470. [Google Scholar] [CrossRef]
- Hurtado-Delgado, E.; Huerta-Larumbe, L.; Miranda-Pérez, A.; Aguirre-Sánchez, Á. Microcracks reduction in laser hardened layers of ductile iron. Coatings 2021, 11, 3. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Miniyazov, A.Z.; Skakov, M.K.; Sagdoldina, Z.B.; Tulenbergenov, T.R.; Sapataev, E.E. Structural modification and erosion of plasma-irradiated tungsten and molybdenum surfaces. Technol. Phys. 2020, 65, 382–391. [Google Scholar] [CrossRef]
- Zhu, X. Effect of fast cooling on mechanical properties of continuous annealed cold rolled ultra-high strength steel. Cailiao Rechuli Xuebao/Trans. Mater. Heat Treat. 2006, 27, 49–52. [Google Scholar]
- Tyurin, Y.N.; Pogrebnjak, A.D. Electric heating using a liquid electrode. Surf. Coat. Technol. 2001, 142–144, 293–299. [Google Scholar] [CrossRef]
- Ayday, A.; Durman, M. Effect of different surface-heat-treatment methods on the surface properties of AISI 4140 steel. Mater. Tehnol. 2014, 48, 787–790. [Google Scholar]
- Ayday, A.; Kırsever, D.; Demirkıran, A.Ş. The effects of overlapping in electrolytic plasma hardening on wear behavior of carbon steel. Trans. Indian Inst. Met. 2022, 75, 27–33. [Google Scholar] [CrossRef]
- Dyakov, I.G.; Burov, S.V.; Belkin, P.N.; Rozanov, E.V.; Zhukov, S.A. Increasing wear and corrosion resistance of tool steel by anodic plasma electrolytic nitriding. Surf. Coat. Technol. 2019, 362, 124–131. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Silkin, S.A.; Smirnov, A.A.; Belkin, P.N. Possibilities of increasing wear resistance of steel surface by plasma electrolytic treatment. Wear 2017, 386–387, 239–246. [Google Scholar] [CrossRef]
- Kumruoglu, L.C.; Becerik, D.A.; Ozel, A.; Mimaroglu, A. Surface modification of medium carbon steel by using electrolytic plasma thermocyclic treatment. Mater. Manuf. Process. 2009, 24, 781–785. [Google Scholar] [CrossRef]
- Tabiyeva, Y.Y.; Rakhadilov, B.K.; Uazyrkhanova, G.K.; Zhurerova, L.G.; Maulit, A.; Baizhan, D. Surface modification of steel mark 2 electrolytic plasma exposure. Eurasian J. Phys. Funct. Mater. 2019, 3, 355–362. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Parfenov, E.V. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Bayati, M.R.; Molaei, R.; Janghorban, K. Surface alloying of carbon steels from electrolytic plasma. Met. Sci. Heat Treat. 2011, 53, 91–94. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Taheri, P.; Rouhaghdam, A.S.; Dehghanian, C. Systematic study of nanocrystalline plasma electrolytic nitrocarburising of 316 L austenitic stainless steel for corrosion protection. J. Mater. Sci. Technol. 2007, 23, 665–671. [Google Scholar]
- Baizhan, D.; Rakhadilov, B.; Zhurerova, L.; Tyurin, Y.; Sagdoldina, Z.; Adilkanova, M.; Kozhanova, R. Investigation of Changes in the Structural-Phase State and the Efficiency of Hardening of 30CrMnSiA Steel by the Method of Electrolytic Plasma Thermocyclic Surface Treatment. Coatings 2022, 12, 1696. [Google Scholar] [CrossRef]
- Mukhacheva, T.L.; Belkin, P.N.; Dyakov, I.G.; Kusmanov, S.A. Wear mechanism of medium carbon steel after its plasma electrolytic nitrocarburizing. Wear 2020, 462–463, 203516. [Google Scholar] [CrossRef]
- Mamaeva, A.; Kenzhegulov, A.; Panichkin, A.; Abdulvaliyev, R.; Fischer, D.; Bakhytuly, N.; Toiynbaeva, N. Influence of Current Duty Cycle and Voltage of Micro-Arc Oxidation on the Microstructure and Composition of Calcium Phosphate Coating. Coatings 2024, 14, 766. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Kusmanova, Y.V.; Naumov, A.R.; Belkin, P.N. Features of anode plasma electrolytic nitrocarburising of low carbon steel. Surf. Coat. Technol. 2015, 272, 149–157. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Satbayeva, Z.; Baizhan, D. Effect of electrolytic-plasma surface strengthening on the structure and properties of steel 40 kHN. In Proceedings of the METAL 2019—28th International Conference on Metallurgy and Materials, Conference Proceedings, Brno, Czech Republic, 22–24 May 2019; pp. 950–955. [Google Scholar] [CrossRef]
- Tabieva, E.E.; Zhurerova, L.G.; Baizhan, D. Influence of electrolyte-plasma hardening technological parameters on the structure and properties of banding steel 2. Key Eng. Mater. 2020, 839, 57–62. [Google Scholar] [CrossRef]
- Lu, S.; Sun, X.; Zhang, B.; Wu, J. Review of Cathode Plasma Electrolysis Treatment: Progress, Applications, and Advancements in Metal Coating Preparation. Materials 2024, 17, 3929. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Yu, H.; Cheng, K.; Feng, X.; Liu, Y.; Zhou, J.; Bai, M.; Ni, F.; Wu, J.; et al. Low Current Density Cathode Plasma Electrolytic Deposition of Aluminum Alloy Based on a Bipolar Pulse Power Supply. Coatings 2024, 14, 835. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; He, Y. Solid Lubrication System and Its Plasma Surface Engineering: A Review. Lubricants 2023, 11, 473. [Google Scholar] [CrossRef]
- Kusmanov, S.; Mukhacheva, T.; Tambovskiy, I.; Naumov, A.; Belov, R.; Sokova, E.; Kusmanova, I. Increasing Hardness and Wear Resistance of Austenitic Stainless-Steel Surface by Anodic Plasma Electrolytic Treatment. Metals 2023, 13, 872. [Google Scholar] [CrossRef]
- Pérez, H.; Vargas, G.; Magdaleno, C.; Silva, R. Article: Oxy-Nitriding AISI 304 Stainless Steel by Plasma Electrolytic Surface Saturation to Increase Wear Resistance. Metals 2023, 13, 309. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Choi, J.-Y.; Kim, C.-H.; Lee, I.-S.; Jun, S.; Kim, D.; Cha, B.-C.; Kim, D.-W. N+-Implantation on Nb Coating as Protective Layer for Metal Bipolar Plate in PEMFCs and Their Electrochemical Characteristics. Materials 2022, 15, 8612. [Google Scholar] [CrossRef]
- Bakhtiari-Zamani, H.; Saebnoori, E.; Bakhsheshi-Rad, H.R.; Berto, F. Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding. Materials 2022, 15, 8300. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Kurbanbekov, S.R.; Kilishkhanov, M.K.; Kenesbekov, A.B.; Amanzholov, S. Changing the structure and phasestates and the microhardness of the R6M5 steel surface layer after electrolytic-plasma nitriding. Eurasian J. Phys. Funct. Mater. 2018, 2, 259–266. [Google Scholar] [CrossRef]





| Sample | Electrolyte Composition | Pressures | Time |
|---|---|---|---|
| 40CrNi-1 | 7% ammonium chloride (NH4Cl) 14% urea ((NH2)2CO) 7% sodium carbonate (Na2CO3) 72% distilled water (H2O) | 320B 220B | 7 s 7 min |
| 40CrNi-2 | 10% sodium carbonate (Na2CO3) 20% urea ((NH2)2CO) 70% distilled water (H2O) | 320B 220B | 7 s 7 min |
| Sample | Detected Phases | Phase Content, Mas. % | Lattice Parameters, Å |
|---|---|---|---|
| 40CrNi initial | Fe_229_BCC | 100 | a = 2.8690 |
| 40CrNi-1 | Fe_229_BCC | 91 | a = 2.8688 |
| Fe2N_164_trigonal | 9 | a = 2.6648 c = 4.3371 | |
| 40CrNi-2 | Fe2N_164_trigonal | 30 | a = 2.6650 c = 4.3369 |
| Fe2N_60_orthorombic | 50 | a = 4.3200 b = 5.5793 c = 4.6916 | |
| Fe3O4_166_rhombohedral | 20 | a = 5.8514 c = 14.5110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satbayeva, Z.; Rakhadilov, B.; Turar, Z.; Berdimuratov, N.; Baizhan, D.; Maulit, A. Surface Modification of Chromium–Nickel Steel by Electrolytic Plasma Nitriding Method. Crystals 2024, 14, 759. https://doi.org/10.3390/cryst14090759
Satbayeva Z, Rakhadilov B, Turar Z, Berdimuratov N, Baizhan D, Maulit A. Surface Modification of Chromium–Nickel Steel by Electrolytic Plasma Nitriding Method. Crystals. 2024; 14(9):759. https://doi.org/10.3390/cryst14090759
Chicago/Turabian StyleSatbayeva, Zarina, Bauyrzhan Rakhadilov, Zhangabay Turar, Nurbol Berdimuratov, Daryn Baizhan, and Almasbek Maulit. 2024. "Surface Modification of Chromium–Nickel Steel by Electrolytic Plasma Nitriding Method" Crystals 14, no. 9: 759. https://doi.org/10.3390/cryst14090759







