Abstract
This paper presents a method for obtaining cobalt(II) perrhenate from waste derived from two types of materials, i.e., Li-ion battery scrap, or more precisely, battery mass, and superalloy scrap. Both of the above-mentioned materials are a source of Co. However, a source of rhenium is perrhenic acid produced from ammonium perrhenate (recycled) by the ion exchange method using resins. Co(OH)2 can be precipitated from solutions resulting from the leaching of Li-ion battery mass, sludge from the Zn-Pb industry and superalloy scrap. The compound, after proper purification, can be used in a reaction with perrhenic acid to form Co(ReO4)2. The reaction should be conducted under the following conditions: time 1 h, room temperature, 30% excess of cobalt(II) hydroxide, and rhenium concentration in HReO4 from about 20 g/dm3 to 300 g/dm3. This work shows that with the use of Co(OH)2, obtained from waste, an anhydrous form of cobalt(II) perrhenate can be obtained, containing < 1000 ppm of the cumulative metal impurities.
1. Introduction
Rhenium is a metal that occurs extremely rarely in nature, yet it forms a number of compounds in various oxidation states. The most stable are rhenium compounds in higher oxidation states, i.e., VI and VII. The physicochemical properties of rhenium, including its high melting point (>3180 °C), substantial density (21.0 g/cm3), elevated hardness (7 on the Mohs scale), exceptional strength, remarkable thermal and chemical resistance, as well as its amenability to plastic forming, determine its use in many areas [1,2,3]. Thus, it is used in the following industries: aviation [4,5,6], petrochemical [7,8,9], defense, and catalysis [1,10,11]. It should be emphasized here that 81% of the world production of rhenium is used in the production of alloys and superalloys [12,13,14]. However, only 13% is used in catalysis, mainly in catalytic reforming [15,16,17].
This publication describes methods of obtaining cobalt(II) perrhenate entirely from waste. This compound is used in the production of Co-Re alloy powders [18,19].
There are reports in the literature on the properties, methods of production and use of cobalt(II) perrhenate. Therefore, the first report in the literature regarding the preparation of cobalt(II) perrhenate came from the 1930s, when H. Briscoe, P. Robinson and A. Rudge obtained the above-mentioned compound in the reaction of CoCO3 with HReO4 [20].
In 1949, W. Smith and G. Maxwell obtained Co(ReO4)2∙4H2O in a reaction of HReO4 with CoCO3 or Co(OH)2. Co(ReO4)2∙4H2O was placed in a desiccator with calcium chloride to obtain Co(ReO4)2∙3H2O. Complete dehydration occurred only after drying at 105 °C. This publication also determined the solubility of the produced salts [21].
In 1978, L. Zajtseva and his team determined the composition and physicochemical properties of Co(ReO4)2 and Co(ReO4)2∙4H2O. Cobalt(II) perrhenate tetrahydrate was produced via the reaction between perrhenic acid and basic cobalt(II) carbonate [22].
An article appeared in Zhurnal Neorganicheskoj Khimii in 1990 in which the symmetry and parameters of the crystal lattice of anhydrous cobalt(II) perrhenate were determined. It was found there that this compound is isostructural to Mg(ReO4)2 and crystallizes in a hexagonal Zr(MoO4)2-type crystal system [23].
In 1992, W. Baur, W. Joswig, G. Pieper and D. Kassner determined the crystallographic structure of cobalt(II) perrhenate. They found that Co(ReO4)2 has a rutile-type structure by ordering two cations [24].
The effect of gaseous ammonia solution on crystalline cobalt(II) perrhenate pentahydrate was investigated by L. Maslov and his team in 1994 [25].
In 1995 in Matt. Cryst. Liq. Cryst., an article was published in which insulating layers in ferromagnets were described, consisting of perrhenates of different metals, including Co(ReO4)2 [26].
In the same year, A. Butz and his team obtained and determined the crystal structure of cobalt(II) perrhenate tetrahydrate. Co(ReO4)2∙4H2O was obtained by reacting cobalt(II) carbonate with perrhenic acid, where carbonate was used with a 20% excess. The authors of this work showed that two stages of dehydration occur below 200 °C (first dehydration—120–130 °C, second dehydration—165–175 °C) and in each of these stages, two hydration waters are lost. The publication also specifies the decomposition temperature range of the above-mentioned products: 840–930 °C [27].
In 1997, C. Toradi and his team examined the magnetic properties of anhydrous cobalt(II) perrhenate [28].
The crystal structure of anhydrous cobalt(II) perrhenate was determined in 1998 by A. Butz, G. Miehe, H. Paulus, P. Strauss and H. Fuess. Co(ReO4)2 was obtained by dehydration of the hydrated form. Thermal analysis revealed that within a narrow temperature range, between the Co(ReO4)2∙4H2O and Co(ReO4)2∙2H2O structures, the trihydrate form occurs. The research suggested that Co(ReO4)2∙3H2O molecule should be made from Co(ReO4)2∙6H2O molecules [29].
In 1998, C. Mujica examined and consequently determined the crystallographic structure of Co(ReO4)2∙4H2O. The analysis results indicated that the cobalt ion is coordinated in an octahedral arrangement, with two perrhenate anions and four water molecules surrounding the central cobalt atom [30].
In 1999, M. Varfolomeev determined the crystallographic structure of cobalt(II) perrhenate tetrahydrate. He showed that single crystals are triclinic and isostructural to Cu(ReO4)2∙4H2O [31].
In 2010, L. Liu and his team examined the antifriction properties of cobalt(II) perrhenate tetrahydrate in temperatures spanning from 25 to 750 °C. Co(ReO4)2∙4H2O was obtained in a reaction of CoO with Re2O7 and water [32].
In 2018, J. Wang and his team produced a composite in the form of Co(ReO4)2/MoS2 as an additive that could improve oil lubricity and reduce friction. The tests were carried out at temperatures up to 600 °C. It has been shown that at temperatures above 450 °C, cobalt(II) perrhenate plays the main role as a friction reducing agent [33].
2. Materials and Methods
Perrhenic acid was obtained by leaching the superalloy scrap, with a granulation of less than 1 mm. This process involved using a solution of sulfuric acid with oxidizing agents (hydrogen peroxide and/or nitric acid) as a leaching agent. Table 1 presents the varied compositions of the superalloy scrap. Besides rhenium, these materials also contained significant amounts of nickel (43–53%), cobalt (7–22%), chromium (4–10%), aluminum (13–15%) and iron (up to 1.3%). After leaching, a solution containing 1.5 g/dm3 of rhenium, nickel, cobalt, iron, chromium and aluminum was produced.

Table 1.
Superalloy scrap composition.
This solution was used in the recovery of rhenium in the form of NH4ReO4 (catalytic purity, Innovator, Gliwice, Poland), using ion exchange technology developed at the Łukasiewicz Research Network—Institute of Non-Ferrous Metals (Łukasiewicz-IMN). Then, using this technology, perrhenic acid was obtained, containing 15–900 g/dm3 of Re and <0.0001% of each of the following impurities: Ca, K, Mg, Cu, Na, Mo, Pb, Fe, Ni, NH4+, Bi, Zn, W, As and Al (Innovator; Gliwice; Polska) [19,34,35].
Three types of waste were tested as a source of cobalt: two from recycling, i.e., nickel-based superalloy scrap (Table 1, Figure 1) and battery mass (black mass) made from Li-ion battery waste (Table 2), as well as sludge from a national zinc producer using the electrolytic method (Table 3) [36]. Figure 2 shows a scheme for obtaining a multi-component solution, containing cobalt, resulting from the leaching of superalloy scrap. Figure 3 shows a scheme for the further processing of the solution to obtain materials suitable for cobalt recovery and/or production of cobalt(II) perrhenate. Figure 4 shows XRD analyzes of battery masses. Figure 5 shows a scheme for obtaining a solution for cobalt recovery and/or production of cobalt(II) perrhenate from battery mass, and Figure 6 shows a scheme for obtaining a solution for cobalt recovery and/or production of cobalt(II) perrhenate from sludge from the Zn-Pb industry.

Figure 1.
Superalloy scrap pictures. (A) superalloy waste with granulation <10 mm. (B) scrap blade made of superalloys.

Table 2.
Compositions of battery masses used in the research.

Table 3.
Composition of the selected sludges.

Figure 2.
Scheme of superalloy scrap processing, highlighting the selected solution for the recovery of cobalt.

Figure 3.
Scheme for obtaining a solution for cobalt recovery.

Figure 4.
XRD patterns of the battery masses used in the research. (A) Battery masses from cars. (B) Battery masses from tablets. (C) Battery masses from mobile phones.
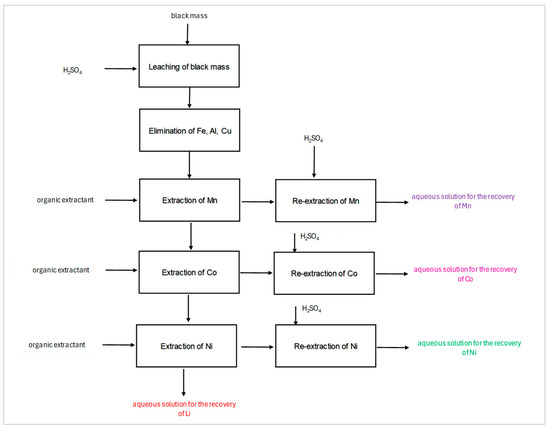
Figure 5.
Scheme of processing the battery masses, highlighting the identified solutions for recovering cobalt and other metals.
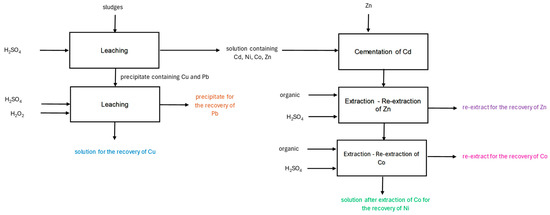
Figure 6.
Scheme of sludge processing with the identified solutions for testing the production of cobalt(II) perrhenate.
In Poland, rhenium is recovered from nickel-based superalloys, Ni-Co-Cr-Al concentrate, and refractory metal concentrate recovery. The potential of combining the processing of these two materials (black masses and waste of superalloys) is an exciting solution that needs to be developed. However, the material streams are complicated to clean and process, and the investor involved must be wealthy. The need for investment in this crucial area is pressing, but there is no such possibility at the moment [36].
3. Methods
3.1. Methodology for Producing Co(OH)2 from the Solutions Obtained from the Processing of Superalloy Scrap
A method was developed to recover Co in the form of cobalt(II) hydroxide from the superalloy scrap. The scheme of the technology is presented in Figure 3. Thus, the solutions intended for testing, which were obtained after the elimination of Fe, Cr and Al, were characterized by pH in the range of 5.5–5.8. The manuscript did not include tests for removing Fe, Cr and Al. This was because these elements were not the primary focus of the research study. The solutions were directed to the extraction of Co, using an extractant known from the literature, i.e., Cyanex 272 dissolved in Exxsol 80 (room temperature, contact time = 1 h, volume ratio of the aqueous phase to the organic phase = 5:1). Cobalt re-extraction was conducted using sulfuric acid solution, with a concentration ranging from 10 to 30% (room temperature, contact time = 1 h, volume ratio of the aqueous phase to the organic phase = 1:3). The organic phase formed after the re-extraction was recirculated to the next extraction.
3.2. Methodology for Producing Co(OH)2 from the Solutions Obtained during the Processing of Battery Mass
A method was developed to recover Co in the form of cobalt(II) hydroxide from the battery mass. The scheme of the technology is presented in Figure 5. In the research, solutions after the removal of Fe, Cu, Mn and Al were used. They were characterized by pH in the range of 5.0–5.8. The manuscript did not include tests for removing Fe, Cu, Mn and Al. This was because these elements were not the primary focus of the study. The solutions were sent for Co extraction using an extractant known from the literature, i.e., Cyanex 272 dissolved in Exxsol 80 (room temperature, contact time = 1 h, volume ratio of the aqueous phase to the organic phase = 2:1). Sulfuric acid solution with a concentration ranging from 5 to 20% was used in cobalt re-extraction (room temperature, contact time = 1 h, volume ratio of the aqueous phase to the organic phase = 1:2). The organic phase obtained after the re-extraction was returned to the next extraction.
3.3. Methodology for Producing Co(OH)2 from the Solutions Obtained from the Processing of Waste from the Zn-Pb Industry
A method was developed to recover Co in the form of cobalt(II) hydroxide from the waste of the Zn-Pb industry. The scheme of the technology is presented in Figure 6. The manuscript did not include tests for removing Pb, Cu, Cd, Zn and Ni. This was because these elements were not the primary focus of the study. The re-extract resulting from the re-extraction of Co was sent to the cobalt(II) hydroxide precipitation stage using an aqueous NaOH solution. At pH >8.0, cobalt(II) hydroxide was precipitated, filtered and sent to the purification stage, resulting in a solution with a Co concentration of <0.01 g/dm3.
3.4. Methodology for Obtaining Various Forms of Cobalt(II) Perrhenate
An aqueous solution of 100–400 g/dm3 perrhenic acid was added to the precipitated Co(OH)2 remaining after the last filtration, so that the final pH of the solution was 6.0–8.0. This mixture was stirred intensively at a temperature not exceeding 75 °C, for 60 min, while constantly controlling the pH. Then, the remaining precipitate of unreacted cobalt(II) hydroxide was filtered from the solution, and the solution with a pH of 6.5–8.2 was evaporated to dryness. The solution underwent evaporation at a controlled temperature maintained below 55 °C. The resulting precipitate, consisting of hydrated cobalt(II) perrhenate, was then subjected to a drying process. This drying phase took place at an elevated temperature of 180 °C and continued until all moisture was removed, yielding a dry mass. The final product of this process was a precipitate of anhydrous cobalt(II) perrhenate. The compounds were analyzed and stored in air-free conditions.
4. Analytical Methods
The analytical procedures were carried out at two specialized Centres—Analytical Chemistry Centre and the Functional Materials Centre (Łukasiewicz Research network—Institute of Non-Ferrous Metals).
To determine the rhenium concentration in Co(ReO4)2 and HReO4, an X-ray fluorescence spectrometer (ZSX Primus, Rigaku, Tokyo, Japan) was used. For the detection of ammonium ions in the aqueous perrhenic acid solution, a two-step process was utilized. First, the sample underwent distillation, followed by titration using the Nessler method.
The following instrumental techniques were utilized to analyze the concentrations of Zn, Pb, Bi, As, Al, Mn, Cu, Ca, K, Mg, Mo, W, Cr, Na, Fe, Ni and Co:
- Graphite furnace atomic absorption spectroscopy (Z-2000, HITACHI, Tokyo, Japan);
- Inductively coupled plasma–optical emission spectroscopy (ULTIMA 2, HORIBA Jobin-Ivon, Kyoto, Japan and Optima 5300V, PerkinElmer, Waltham, MA, USA);
- Inductively coupled plasma–mass spectroscopy (Nexion, PerkinElmer, Waltham, MA, USA);
- Flame atomic absorption spectrometry (THERMO SOLAAR S4, Thermo Fisher Scientific, Waltham, MA, USA, supplied with a flame module and deuterium background correction).
The Rigaku MiniFlex 600 XRD diffractometer, supplied with an X-ray tube with a wavelength of 1.5406 Å and a stripe detector, silicon D/TeX and a high-resolution 2.5” Soller slit on the primary and scattered beam, was used to perform XRD analyses.
The microstructural and elemental analyses were conducted using a high-resolution scanning electron microscope (SEM, Zeiss Gemini 1525, Carl Zeiss NTS GmbH, Oberkochen, Germany). This instrument was equipped with a Quantax xFlash®6 Bruker Nano X-ray, enabling energy-dispersive X-ray spectroscopy (EDS) for compositional analysis.
5. Results and Discussion
5.1. Results of the Tests of Producing Co(OH)2 from the Solutions Obtained from the Processing of Superalloy Scrap
Using a solution obtained after the elimination of Fe, Al and Cr, tests were conducted on the extraction and re-extraction of Co. The research was conducted in a wide range. Figure 7 shows the influence of the solution’s pH on the Co extraction efficiency. The influence of acid concentration on the efficiency of Co re-extraction is presented in Figure 8.

Figure 7.
The influence of the solution’s pH on the Co extraction efficiency.

Figure 8.
The influence of acid concentration on the Co re-extraction efficiency.
The application of the process conditions allowed for the Co extraction efficiency to reach the range of 89–94%. A Co extraction efficiency of 94% was obtained for the solution with pH 5.6. In the case of Co re-extraction, the efficiencies were in the range of 81–92%. A Co extraction efficiency of 92% was obtained for the sulfuric acid concentration of >15%. The re-extract obtained after the re-extraction of Co was directed to the stage of cobalt(II) hydroxide precipitation using an aqueous NaOH solution. At pH > 8.0, cobalt(II) hydroxide was precipitated, filtered and sent to the purification stage, and the resulting solution with a cobalt concentration of <0.01 g/dm3 was recirculated. Purification was carried out cyclically with water (2–6 cycles), using 0.03 dm3 of water in one cycle for every 10 g of precipitate, in such a way that Co(OH)2 precipitate was mixed with water for 30 min at a temperature of up to 40 °C. Table 4 shows the effect of cyclic purification on the purity of the target product.

Table 4.
Results of Co(OH)2 cyclic purification.
As can be seen in Table 4, the use of three purification cycles allowed us to obtain a high-purity product, i.e., cobalt(II) hydroxide, containing up to 80 ppm of metallic impurities in the form of Ni, Cr, Al and Fe. Figure 9 shows the developed scheme for obtaining Co(OH)2. The efficiency of the entire process was approximately 83%.
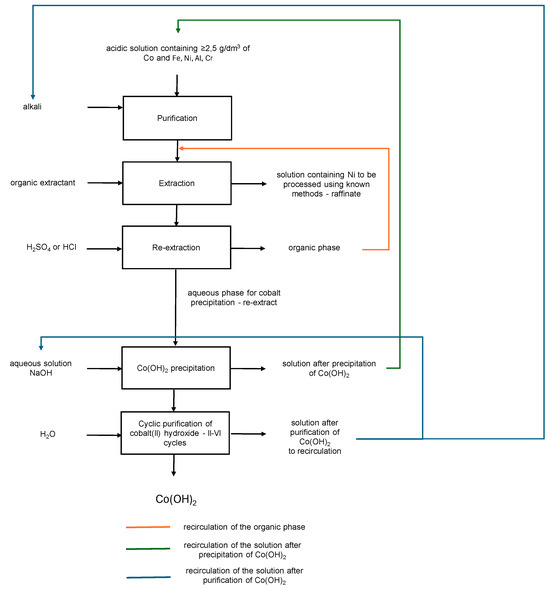
Figure 9.
Scheme of obtaining Co(OH)2.
5.2. Results of the Tests of Producing Co(OH)2 from the Solutions Obtained during the Processing of Battery Mass
Using the solution obtained after the removal of Fe, Cu, Mn and Al (20.5 g/dm3 of Co, 0.6 g/dm3 of Ni, 0.1/dm3 of Li, 0.2 g/dm3 of Fe, 0.2 g/dm3 of Cu, 0.5 g/dm3 of Mn), tests were conducted on the extraction and re-extraction of Co. Figure 10 shows the influence of the solution’s pH on the Co extraction efficiency. The effect of the acid concentration on the Co re-extraction efficiency is shown in Figure 11.

Figure 10.
The influence of the solution’s pH on the Co extraction efficiency.
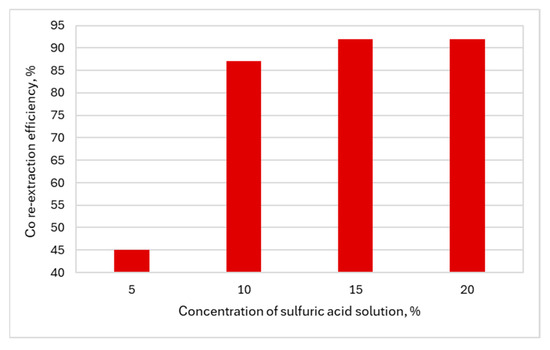
Figure 11.
The influence of acid concentration on the Co re-extraction efficiency.
The application of the researched conditions allowed the Co extraction efficiency to reach the range of 76–94%. A Co extraction efficiency of 94% was obtained for the solution with pH 5.6. In the case of Co re-extraction, the efficiencies were in the range of 45–92%. A Co extraction efficiency of 92% was obtained for the sulfuric acid concentration of >15%. The re-extract obtained after the re-extraction of Co was directed to the stage of cobalt(II) hydroxide precipitation using an aqueous NaOH solution. At pH > 8.0, cobalt(II) hydroxide was precipitated, filtered and sent to the purification stage, and the resulting solution with a cobalt concentration of <0.01 g/dm3 was recirculated. Purification was carried out cyclically with water (2–6 cycles), using 0.03 dm3 of water in one cycle for every 10 g of precipitate, in such a way that Co(OH)2 precipitate was mixed with water for 30 min at a temperature of up to 40 °C. Table 5 shows the effect of cyclic purification on the purity of the target product.

Table 5.
Results of Co(OH)2 cyclic purification.
As can be seen in Table 5, the use of three purification cycles allowed us to obtain a high-purity product, i.e., cobalt(II) hydroxide, containing up to 140 ppm of metallic impurities in the form of Ni, Cr, Al and Fe. Figure 12 shows the developed scheme for obtaining Co(OH)2. The efficiency of the entire process was approximately 82%.

Figure 12.
Scheme of obtaining Co(OH)2 from battery masses.
5.3. Results of the Tests of Producing Co(OH)2 from the Solutions Obtained from the Processing of Was from the Zn-Pb Industry
The re-extract resulting from the re-extraction of Co was sent to the cobalt(II) hydroxide precipitation stage using an aqueous NaOH solution. At pH > 8.0, cobalt(II) hydroxide was precipitated, filtered and sent to the purification stage, resulting in a solution with a concentration of <0.01 g/dm3 of Co. The same purification procedure was used for processing Co(OH)2 obtained from superalloy scrap. The use of three purification cycles allowed us to obtain a high-purity product, i.e., cobalt(II) hydroxide, containing no more than 40 ppm of metallic impurities in the form of Ni (<5 ppm), Zn (<10 ppm), Cd (<10 ppm), Cu (<10 ppm) and Pb (<1 ppm). The efficiency of the entire process was approximately 86%.
5.4. Results of the Tests of Producing Co(ReO4)2
Despite obtaining high-purity solutions in all cases (metal contamination at the level <0.001 g/dm3) containing significant amounts of Co (>2.5 g/dm3), it was not possible to obtain an appropriately qualified rhenium(VII) salt from them, i.e., cobalt(II) perrhenate. This was not possible due to the significant content of sulfates >2% in the precipitated compounds. For this reason, Co(OH)2—obtained from all three types of wastes—was selected for further research. The data showcasing the impact of rhenium concentration in perrhenic acid on the precipitation efficiency of Co(ReO4)2 are displayed in Table 6 and Figure 13.

Table 6.
Research results of the influence of Re concentration in HReO4 on the efficiency of Co(ReO4)2 precipitation.

Figure 13.
The impact of rhenium concentration in HReO4 on cobalt(II) perrhenate precipitation efficiency.
The rhenium concentration in perrhenic acid demonstrated a notable effect within the examined range. A positive correlation was observed between the increasing concentration and the precipitation efficiency of cobalt(II) perrhenate. Across the selected concentration range, the precipitation efficiencies of the target compound varied from 60% to 92%. This indicates that cobalt(II) perrhenate can be successfully precipitated from perrhenic acid solutions with a broad range of rhenium concentrations. However, when considering the development of technologies aligned with sustainable development principles, it is crucial to minimize the production of waste solutions. This objective can be effectively achieved by utilizing more concentrated perrhenic acid solutions. Such an approach not only enhances the precipitation efficiency but also aligns with environmentally conscious practices by reducing the volume of waste generated during the process.
The next phase of the research focused on examining how pH affects the precipitation efficiency of cobalt(II) perrhenate. Experiments were conducted under the following conditions: pH range: 7.7 to 9.4, 1 h, temperature 75 °C, Re concentrations in perrhenic acid: 100 g/dm3 and 350 g/dm3. The results illustrating the relationship between pH and cobalt(II) perrhenate precipitation efficiency are presented in Figure 14 and Figure 15.
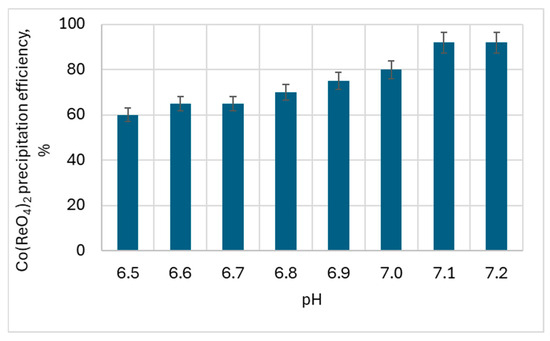
Figure 14.
The influence of the pH on cobalt(II) perrhenate precipitation efficiency—100 g/dm3 of Re in HReO4.
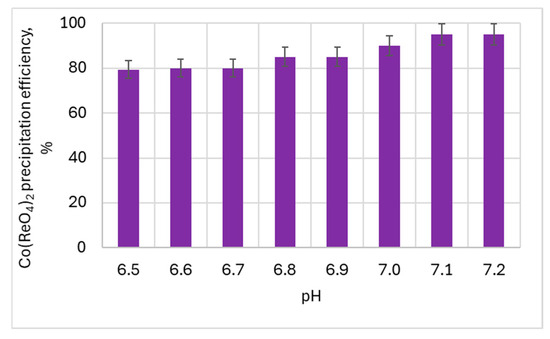
Figure 15.
The influence of the pH on cobalt(II) perrhenate precipitation efficiency—350 g/dm3 of Re in HReO4.
An important influence of pH on the cobalt(II) perrhenate precipitation efficiency was found. The best results for both acid concentrations were obtained at pH >7.1.
So it can be said with certainty that in order to precipitate cobalt(II) perrhenate with an efficiency of over 92%, the process must be carried out at pH 7.1. It was possible to obtain Co(ReO4)2 even at a pH over 6.5, but then the efficiency was in the range of 60–79%.
For the selected cobalt(II) perrhenate, its composition was analyzed. It had the following composition: 66.6% of Re and 10.5% of Co, and <10 ppm of Na, <10 ppm of Zn, <10 ppm of Al, <10 ppm of Ca, <10 ppm of K, <10 ppm of Fe, <5 ppm of Ni, <5 ppm of Cu, <5 ppm of Co, <5 ppm of Mg. The impurities allow for the use of the compound in the defense and aviation industries.
Additionally, XRD analysis was performed for cobalt(II) perrhenate precipitated at pH 7.1—Figure 16.

Figure 16.
XRD patterns of cobalt(II) perrhenate produced entirely from waste.
6. Conclusions
This study demonstrated the feasibility of synthesizing anhydrous cobalt(II) perrhenate with a high purity, containing no more than 100 ppm of total metallic impurities. The process utilizes cobalt(II) hydroxide, derived from three distinct waste sources, in combination with perrhenic acid. To achieve optimal results, the reaction should be conducted under the following specific conditions: time 1 h, room temperature pH = 7.1, rhenium concentration in HReO4 350 g/dm3.
Drawing from the research findings presented, a novel hydrometallurgical process has been created for the production of cobalt(II) perrhenate. This innovative technology is notable for its complete reliance on solutions derived from waste leaching processes. The method is comprehensive and consists of a sequence of ten distinct stages, which are purification, extraction, re-extraction, precipitation of cobalt(II) hydroxide, cyclic purification, preparation of cobalt–rhenium mixture, filtration of the unreacted Co(OH)2, evaporation and drying. Furthermore, the process requires filtration steps between certain operations.
In Figure 17, a scheme of the developed technology is presented in its entirety, with all the recirculation.

Figure 17.
Scheme of obtaining cobalt(II) perrhenate from waste.
7. Patents
A portion of the research findings from this study has been submitted to the Patent Office of the Republic of Poland for consideration and potential patent protection, on June 14, 2024, entitled: Sposób otrzymywania renianu(VII) kobaltu(II) z wykorzystaniem materiałów odpadowych (English title: Method of obtaining cobalt(II) perrhenate using waste materials).
Author Contributions
Conceptualization, K.L.-S., K.P., D.K. and G.B.; methodology, K.L.-S., G.B. and M.C.; validation, K.P., K.L.-S. and D.K.; investigation, K.P. and J.M.; resources, K.L.-S., G.B. and D.K.; data curation, K.L.-S., A.G., G.B. and D.K.; writing—original draft preparation, K.L.-S., A.T. and A.G.; writing—review and editing, K.L.-S., K.G., A.T. and A.G.; visualization, K.L.-S., J.M., K.G., P.K., S.O. and A.T.; supervision, G.B., D.K., M.C. and K.L.-S.; project administration, K.L.-S.; funding acquisition, K.L.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was co-funded by the Norwegian Financial Mechanism 2014–2021—Small Grant 2020 NOR/SGS//RenMet/0049/2020-00 (11/PE/0146/21), entitled: Innovative hydrometallurgical technologies for the production of rhenium compounds from recycled waste materials for catalysis, electromobility, aviation and defense industry.
Data Availability Statement
Data available on request due to restrictions of privacy. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patent application and project contract.
Acknowledgments
The authors would like to express their thanks for the paid quantitative chemical analyses carried out in the Łukasiewicz Research Network-Institute of Non-Ferrous Metals, Centre of Analytical Chemistry, and for the XRD qualitative analyses conducted in the Łukasiewicz Research Network-Institute of Non-Ferrous Metals, Centre of Functional Materials (especially dr. Łukasz Hawełek and Tymon Warski) and Centre of Advanced Materials Technologies (Grzegorz Muzia).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Roskill. Rhenium: Outlook to 2029, 11th ed.; Roskill Information Services Ltd.: London, UK, 2019; ISBN 978-1-910-92279-8. [Google Scholar]
- Huang, M.; Zhu, J. An Overview of Rhenium Effect in Single-Crystal Superalloys. Rare Met. 2016, 35, 127–139. [Google Scholar] [CrossRef]
- Kemmitt, R.D.W.; Peacock, R.D. The Chemistry of Manganese, Technetium and Rhenium; Pergamon Press: Oxford, UK; New York, NY, USA; Toronto, ON, Canada; Sydney, Australia; Paris, France; Braunschweig, Germany, 1975; ISBN 0 08 018870 2. [Google Scholar]
- Fink, P.J.; Miller, J.L.; Konitzer, D.G. Rhenium Reduction-Alloy Design Using an Economically Strategic Element. Jom 2010, 62, 55–57. [Google Scholar] [CrossRef]
- Dobrzańska-Danikiewicz, A.D.; Wolany, W. A Rhenium Review—From Discovery to Novel Applications. Arch. Mater. Sci. Eng. 2016, 82, 70–78. [Google Scholar] [CrossRef]
- Yagi, R.; Okabe, T.H. Review: Rhenium and Its Smelting and Recycling Technologies. Int. Mater. Rev. 2024, 69, 4–6. [Google Scholar] [CrossRef]
- Kesieme, U.; Chrysanthou, A.; Catulli, M. Assessment of Supply Interruption of Rhenium, Recycling, Processing Sources and Technologies. Int. J. Refract. Met. Hard Mater. 2019, 82, 150–158. [Google Scholar] [CrossRef]
- Deshmukh, S.N.; Varade, A.M. Rhenium in the Earth’s Crust: A Comprehensive Review of Mineralogy, Geochemistry and Economic Significance. Bull. Pure Appl. Sci. 2023, 42, 211–218. [Google Scholar] [CrossRef]
- Kinas, S.; Jermakowicz-Bartkowiak, D.; Pohl, P.; Dzimitrowicz, A.; Cyganowski, P. On the Path of Recovering Platinum-Group Metals and Rhenium: A Review on the Recent Advances in Secondary-Source and Waste Materials Processing. Hydrometallurgy 2024, 223, 106222. [Google Scholar] [CrossRef]
- Leszczynska-Sejda, K.; Benke, G.; Malarz, J.; Ciszewski, M.; Kopyto, D.; Piatek, J.; Drzazga, M.; Kowalik, P.; Zemlak, K.; Kula, B. Rhenium(VII) Compounds as Inorganic Precursors for the Synthesis of Organic Reaction Catalysts. Molecules 2019, 24, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kunkes, E.L.; Simonetti, D.A.; Dumesic, J.A.; Pyrz, W.D.; Murillo, L.E.; Chen, J.G.; Buttrey, D.J. The Role of Rhenium in the Conversion of Glycerol to Synthesis Gas over Carbon Supported Platinum-Rhenium Catalysts. J. Catal. 2008, 260, 164–177. [Google Scholar] [CrossRef]
- Targanov, I.E.; Solodovnikov, M.A.; Troshkina, I.D. Oxidative Leaching of Rhenium from Grinding Waste of Rhenium-Containing Superalloys. Izv. Non-Ferr. Metall. 2023, 29, 25–33. [Google Scholar] [CrossRef]
- Yang, F.M.; Lian, L.X.; Liu, Y.; Gong, X.F. Mechanism of Adding Rhenium to Improve Hot Corrosion Resistance of Nickel-Based Single-Crystal Superalloys. Rare Met. 2021, 40, 2076–2082. [Google Scholar] [CrossRef]
- Wu, W.; Chen, B.; Shen, H.; Ding, Z. Molecular Dynamics Simulation of Rhenium Effects on Creep Behavior of Ni-Based Single Crystal Superalloys. Prog. Nat. Sci. Mater. Int. 2022, 32, 259–266. [Google Scholar] [CrossRef]
- Roslan, N.A.; Mohd Arif, N.N.; Jaspin, J.L.; Mohamed Razali, N.A.; Zainal Abidin, S. Hydrogen Production by Glycerol Dry Reforming over Rhenium Promoted Ni-Based Catalyst Supported on Santa Barbara Amorphous 15 (SBA-15). Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 1495–1507. [Google Scholar] [CrossRef]
- Keshavarz, A.; Salabat, A. An Efficient Strategy in Microemulsion Systems to Prepare Mono- and Bimetallic Platinum-Rhenium Reforming Nanocatalyst with Remarkable Catalytic Performance. ChemistrySelect 2019, 4, 6094–6100. [Google Scholar] [CrossRef]
- Cichy, M.; Pańczyk, M.; Słowik, G.; Zawadzki, W.; Borowiecki, T. Ni–Re Alloy Catalysts on Al2O3 for Methane Dry Reforming. Int. J. Hydrogen Energy 2022, 47, 16528–16543. [Google Scholar] [CrossRef]
- Kopyto, D.; Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Ciszewski, M. Production of Electrolytic Rhenium-Cobalt Alloys. Proc.-Eur. Metall. Conf. EMC 2017 2017, 2. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A. Hydrometallurgical Methods for Production of Nickel(II) and Cobalt(II) Perrhenates—Semi-Products for Manufacture of Re-Ni, Re-Co Alloy Powders. Erzmetall 2013, 66, 267–273. [Google Scholar]
- Briscoe, H.V.A.; Robinson, P.L.; Rudge, A.J. The Perrhenates of Copper, Nickel, and Cobalt, and the Ammines of These Compounds. J. Chem. Soc. 1931, 2211–2213. [Google Scholar] [CrossRef]
- Smith, W.T.; Maxwell, G.E. The Salts of Perrhenic Acid. II. The Iron Family and Manganese. J. Am. Chem. Soc. 1949, 71, 578–580. [Google Scholar] [CrossRef]
- Zajtseva, L.; Velichko, A.; Kruglov, A.; Zotov, V. Physicochemical Properties of Cobalt Pertechnetate and Cobalt Perrhenates. Zhurnal Neorg. Khimii 1978, 23, 2396–2401. [Google Scholar]
- Varfolomeev, M.; Shamraj, N.; Lunk, K.; Orlova, T. X-Ray Diffraction Study of Anhydrous Nickel and Cobalt Perrhenates. Zhurnal Neorg. Khimii 1990, 35, 545–546. [Google Scholar]
- Baur, W.H.; Joswig, W.; Pieper, G.; Kassner, D. CoReO4, a New Rutile-Type Derivative with Ordering of Two Cations. J. Solid State Chem. 1992, 99, 207–211. [Google Scholar] [CrossRef]
- Maslov, L.P.; Men`shikov, O.D.; Borisov, V.V.; Sorokin, S.I.; Krutovertsev, S.A.; Kharkevich, S.I.; Ivanova, M. The Effect of Gaseous Ammonia on Cobalt Perrhenate; Dejstvie Gazoobraznogo Ammonia Na Perrenat Kobal ` Ta. Koord. Khimiya 1994, 20, 522–526. [Google Scholar]
- Reiff, W.M.; Dodrill, B.C.; Torardi, C.C. New Insulating Layered Network 3d-Ferromagnets Composed of the Divalent Metal Perrhen Ates: Fe(ReO4)2, Co(ReO4)2 And Ni(ReO4)2. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1995, 274, 137–143. [Google Scholar] [CrossRef]
- Butz, A.; Svoboda, I.; Paulus, H.; Fuess, H. M(ReO4)2·4H2O (M = Co, Zn) Preparation and Crystal Structure Determination. J. Solid State Chem. 1995, 115, 255–259. [Google Scholar] [CrossRef]
- Torardi, C.; Reiff, W.M.; Dodrill, B.C.; Vogt, T. Layered 3-D Ferromagnets AND Antiferromagnets, M2+(ReO4)2 (M=Mn, Fe, Co, Ni, Cu): Importance of Dipolar Interactions. Solid-state Chem. Inorg. Mater. 1997, 453, 399–403. [Google Scholar] [CrossRef]
- Butz, A.; Miehe, G.; Paulus, H.; Strauss, P.; Fuess, H. The Crystal Structures of Mn(ReO4)2·2H2O and of the Anhydrous PerrhenatesM(ReO4)2 of Divalent Manganese, Cobalt, Nickel, and Zinc. J. Solid State Chem. 1998, 138, 232–237. [Google Scholar] [CrossRef]
- Mujica, C.; Peters, K.; Peters, E.; Schnering, H.G. Von Crystal Structure of Tetraaquabis(Perrhenato)Cobalt(II), Co(ReO4)2(H2O)4. 1998, 213, 10. 213.
- Varfolomeev, M.B. Determination of Crystal Structure of Co(ReO4)2∙4H2O and Refinement of Crystal Structure of Mg(ReO4)2∙4H2O. Russ. J. Inorg. Chem. 1999, 44, 1335–1337. [Google Scholar] [CrossRef]
- Lin-lin, L.; Shu, L.; Yang, L. Preliminary Study on the Anti-Friction Behaviors of Perrhenates of Cobalt, Calcium and Copper at High Temperature. Tribology 2010, 30, 554–560. [Google Scholar]
- Wang, J.; Lu, B.; Zhang, L.; Li, T.; Yan, T.; Li, M. An Investigation on the Tribological Properties of Co(ReO4)2/MoS2 Composite as Potential Lubricating Additive at Various Temperatures. Mater. Res. Express 2018, 5, 026522. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Anyszkiewicz, K.; Satora, W.; Kozub, K. Hydrometallurgical Methods for Production of Ni(ReO4)2 and Co(ReO4)2. In Proceedings of the Conference Papers and Proceedings, Congresses European Metallurgical Conference, EMC, Hydrometallurgy, Weimar, Germany, 23–26 June 2013; pp. 885–898. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Krompiec, S.; Michalik, S.; Krompiec, M. Synthesis of Perrhenic Acid Using Ion Exchange Method. Hydrometallurgy 2007, 89, 289–296. [Google Scholar] [CrossRef]
- Śmieszek, Z.; Czernecki, J.; Sak, T.; Madej, P. Metallurgy of Non-Ferrous Metals in Poland. J. Chem. Technol. Metall. 2017, 52, 221–234. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).