Kinetic Phase Behavior of Binary Mixtures of Tri-Saturated Triacylglycerols Containing Lauric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC)
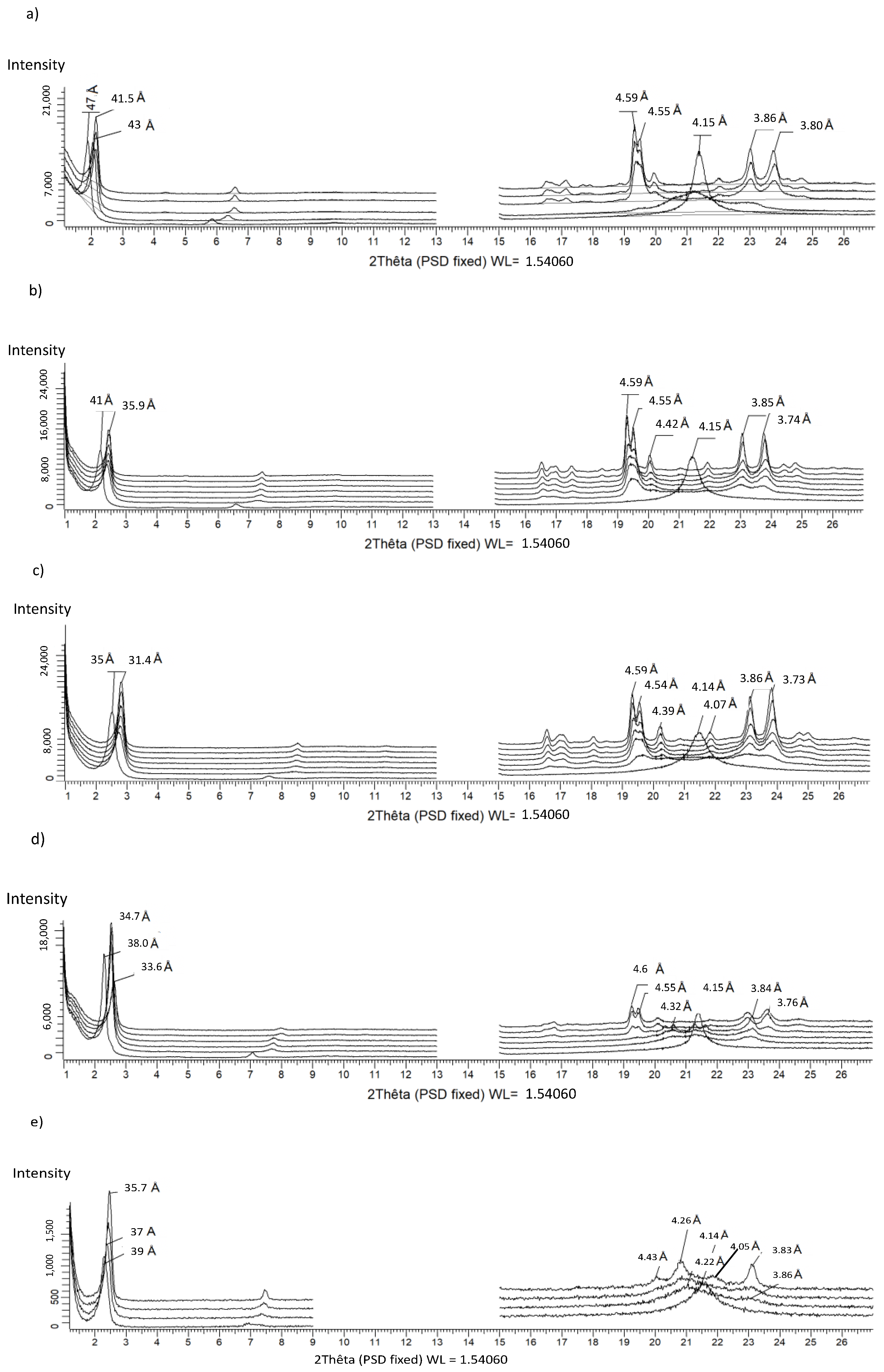
2.2.2. Powder X-ray Diffractometry (PXRD)
3. Results
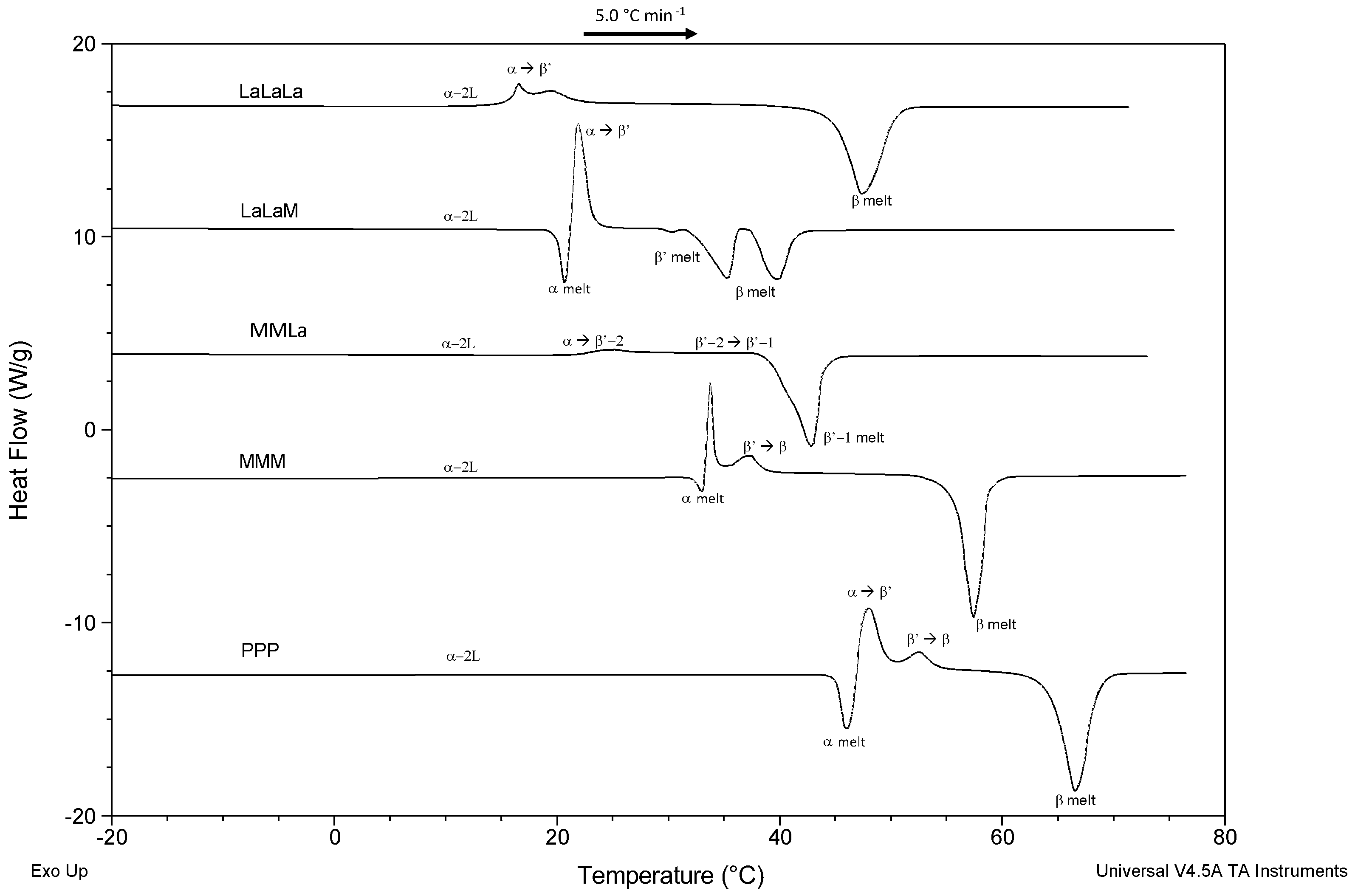
3.1. Polymorphism and Melting Behavior of Pure TAGs (Melting, Quenching, and Reheating at 5 °C min−1)
- (a)
- Monoacid tri-saturated TAGs (PPP, MMM, and LaLaLa).
- (b)
- Mixed acid tri-saturated TAGs (MMLa and LaLaM).
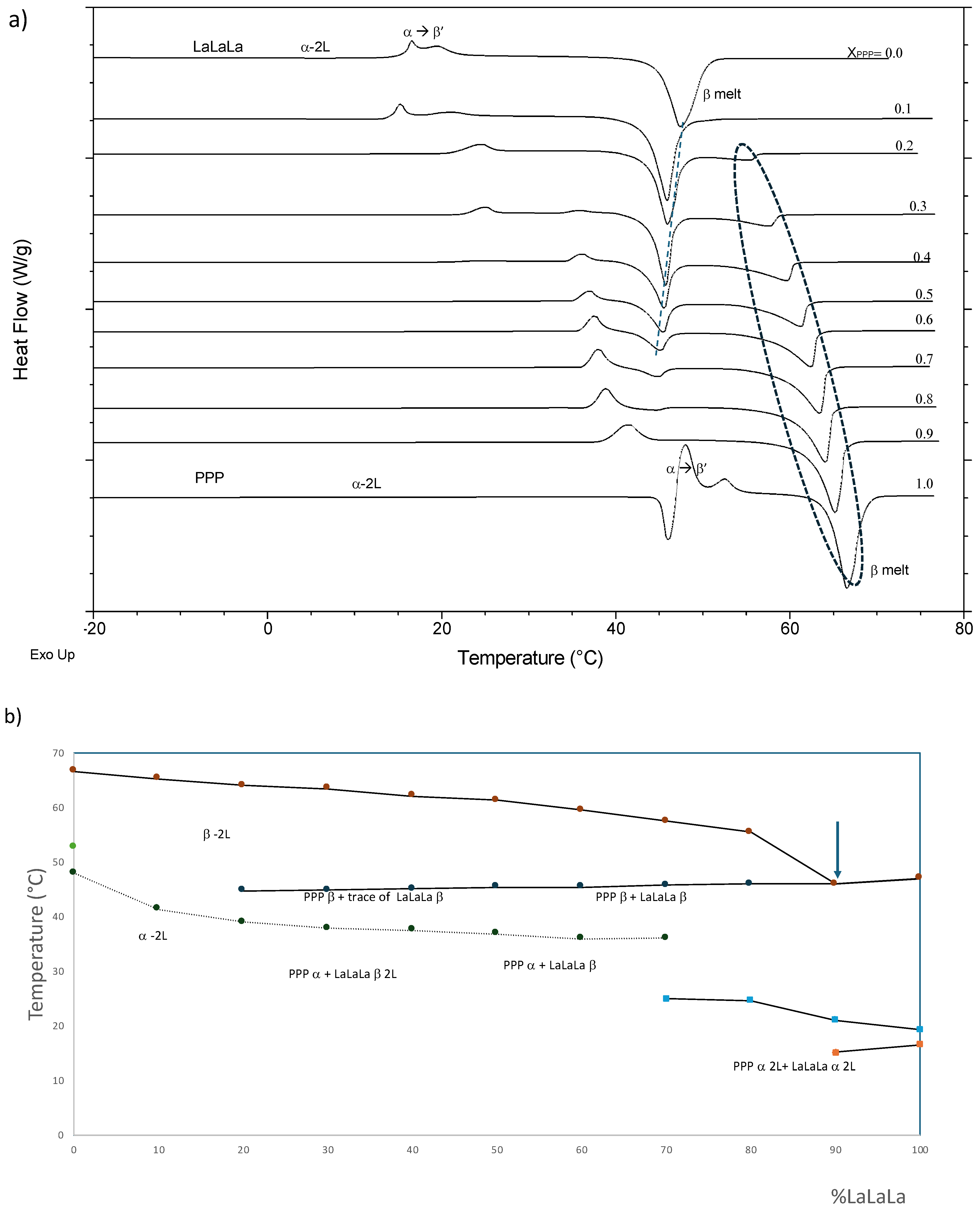
3.2. Binary Phase Diagrams
3.2.1. LaLaLa/MMM (Figure 3)

3.2.2. LaLaLa/MMLa (Figure 4)
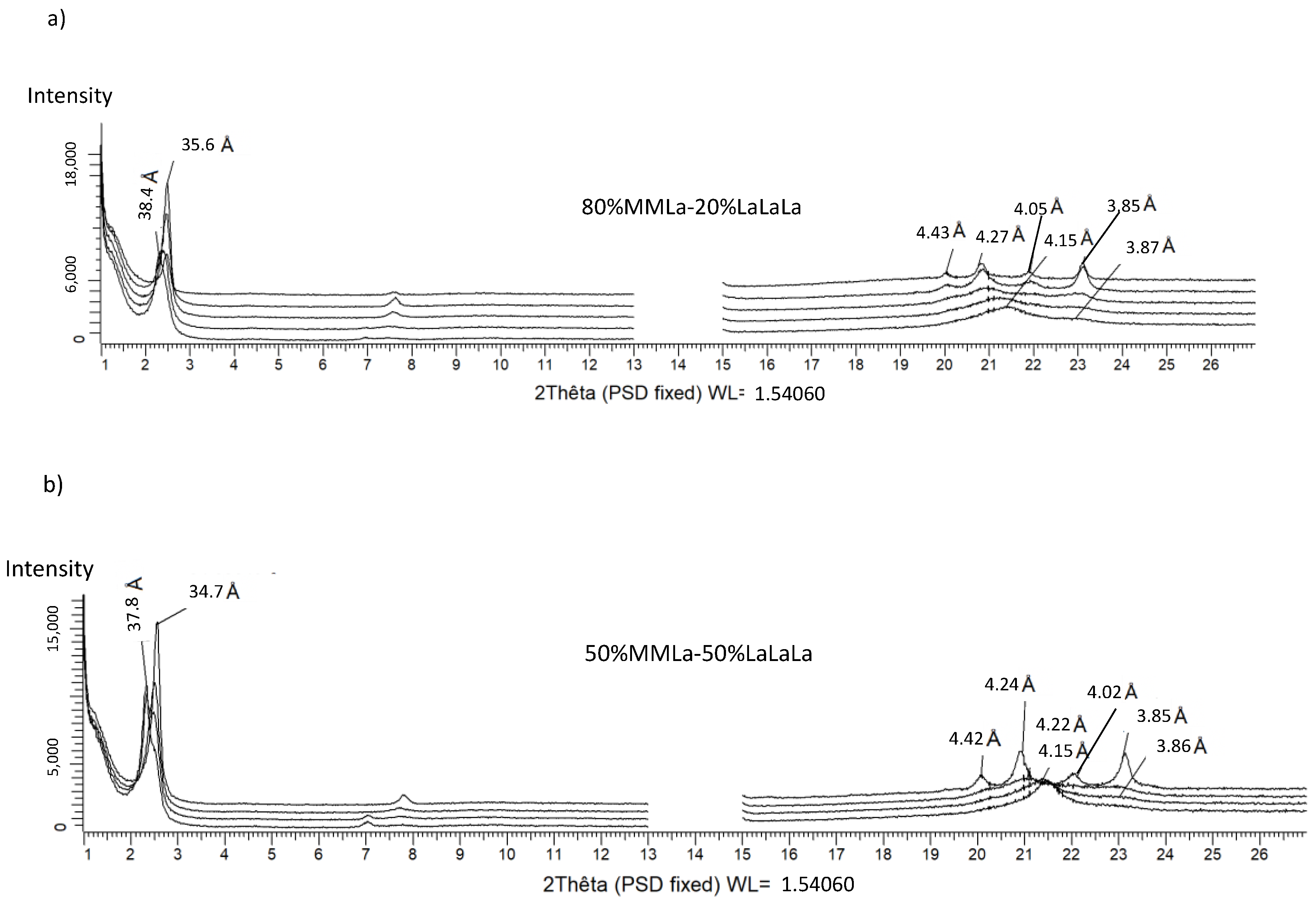
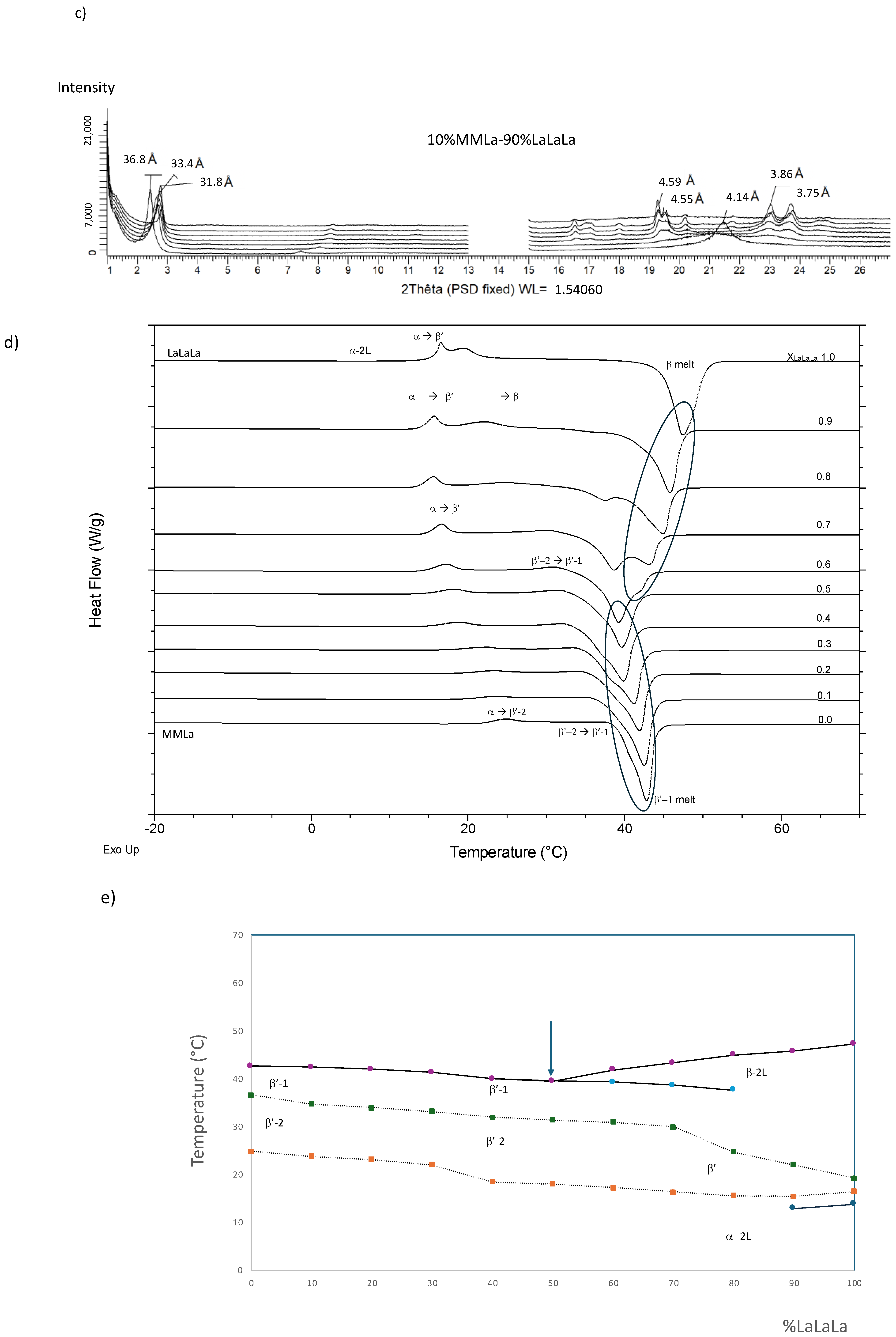
3.2.3. LaLaLa/LaLaM (Figure 5)

3.2.4. MMLa/MMM (Figure 6)
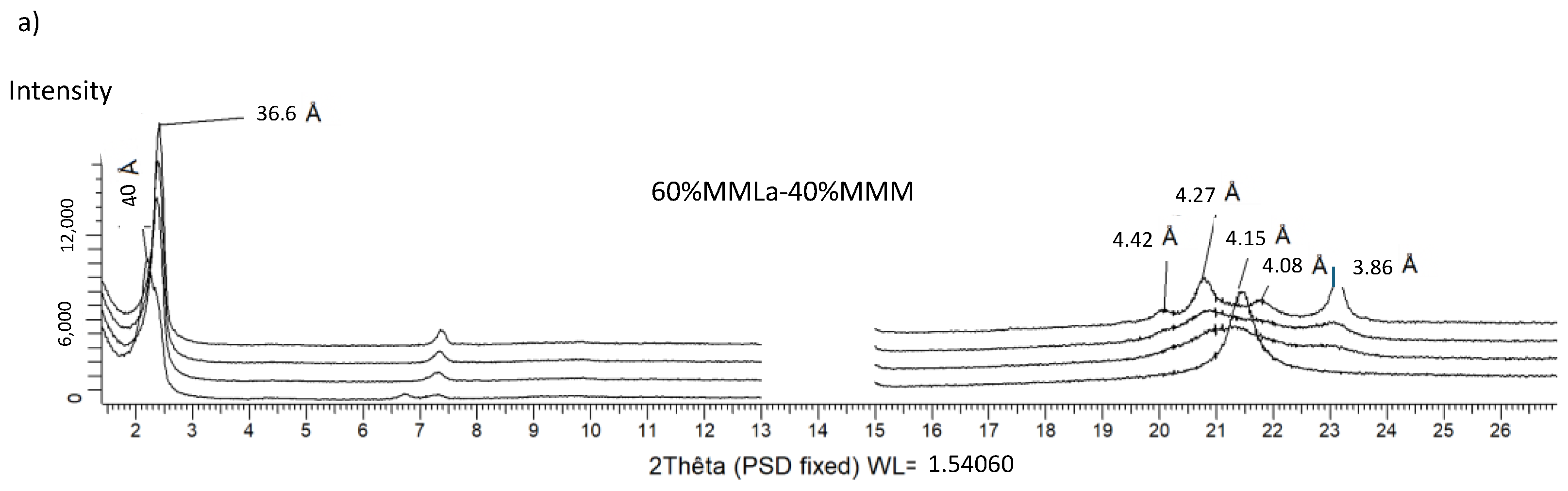
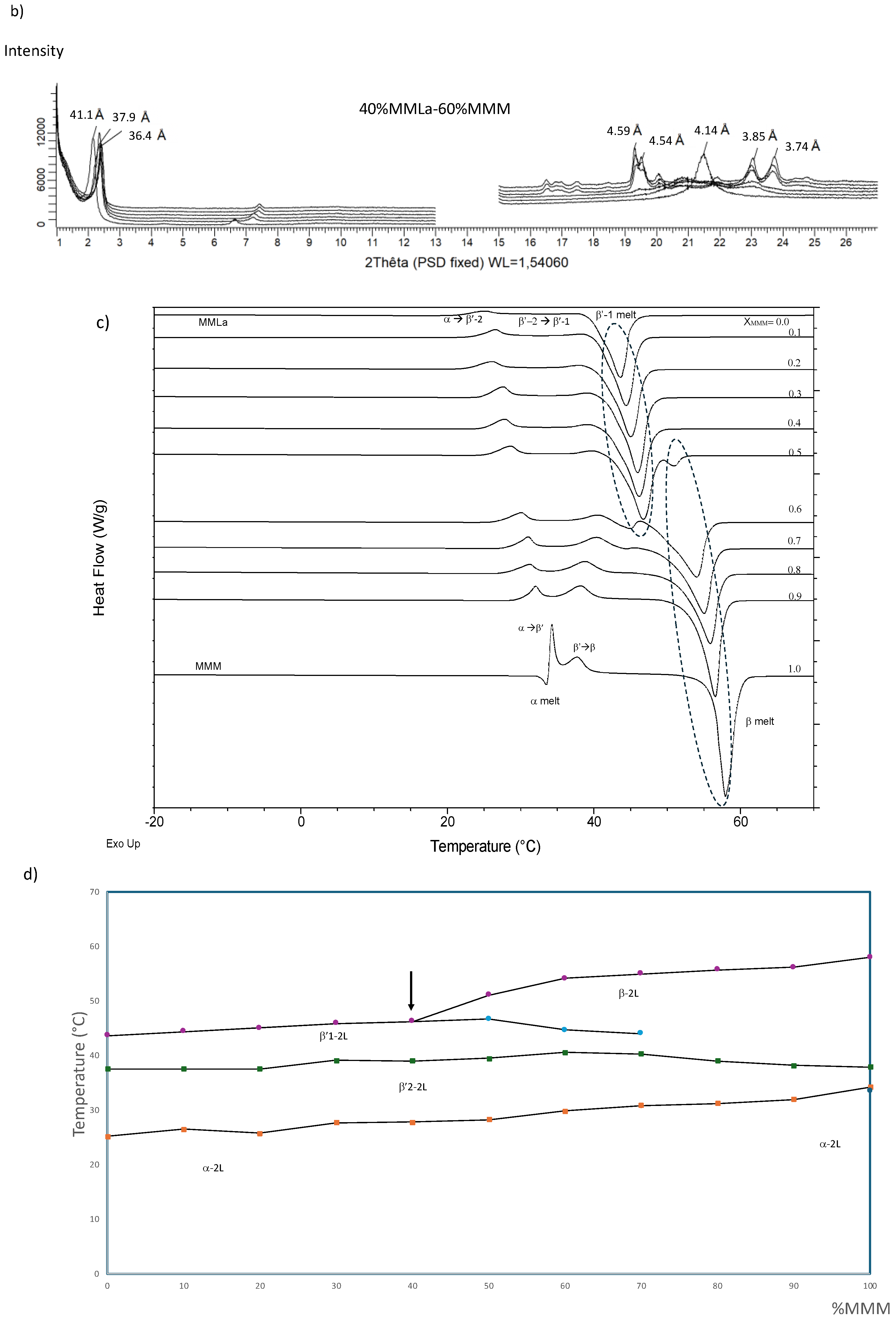
3.2.5. LaLaLa–PPP (Figure 7)
3.2.6. MMLa/LaLaM (Figure 8)

4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamoneka, J.; Malumba, P.; Blecker, C.; Gindo, M.; Richard, G.; Fauconnier, M.L.; Lognay, G.; Danthine, S. Physicochemical properties and thermal behaviour of African wild mango (Irvingia gabonensis) seed fat. LWT 2015, 64, 989–996. [Google Scholar] [CrossRef]
- Anihouvi, A.; Blecker, C.; Dombrée, A.; Danthine, S. Comparative Study of Thermal and Structural Behavior of Four Industrial Lauric Fats. Food Bioprocess. Technol. 2013, 6, 3381–3391. [Google Scholar] [CrossRef]
- Gibon, V. Chapter 12: Palm Oil and Palm Kernel Oil Refining and Fractionation Technology. In Palm Oil; AOCS Press: Champaign, IL, USA, 2012; pp. 329–375. [Google Scholar]
- Sonwai, S.; Podchong, P.; Rousseau, D. Crystallization Kinetics of Coconut Oil in the Presence of Sorbitan Esters with Different Fatty Acid Moieties. J. Am. Oil Chem. Soc. 2016, 93, 849–858. [Google Scholar] [CrossRef]
- Salas, J.; Bootello, M.; Martinez-Force, E.; Garces, R. Tropical vegetable fats and butters: Properties and new alternatives. Ol. Corps Gras Lipides 2009, 16, 254–258. [Google Scholar] [CrossRef]
- Srisuksai, K.; Limudomporn, P.; Kovitvadhi, U.; Thongsuwan, K.; Imaram, W.; Lertchaiyongphanit, R.; Sareepoch, T.; Kovitvadhi, A.; Fungfuang, W. Physicochemical properties and fatty acid profile of oil extracted from black soldier fly larvae (Hermetia illucens). Vet. World 2024, 7, 518–526. [Google Scholar] [CrossRef]
- Tsuiji, H.; Takeuchi, H.; Nakamura, M.; Okazaki, M.; Kondo, K. Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J. Nutr. 2001, 131, 2853–2859. [Google Scholar] [CrossRef]
- Sonwai, S.; Rungprasertphol, P.; Nantipipat, N.; Tungvongcharoan, S.; Laiyangkoon, N. Characterization of Coconut Oil Fractions Obtained from Solvent Fractionation Using Acetone. J. Oleo Sci. 2016, 66, 951–961. [Google Scholar] [CrossRef]
- Maruyama, J.M.; Soares, F.; D’Agostinho, N.R.; Goncalves, M.; Gioielli, L.; da Silva, R.C. Effects of emulsifier addition on the crystallization and melting behavior of palm olein and coconut oil. J. Agric. Food Chem. 2014, 62, 2253–2263. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Shamsi, K.; Yusoff, M.S.A.; Jinap, S. A study on the crystal structure of palm oil-based whipping cream. J. Am. Oil Chem. Soc. 2003, 80, 409–415. [Google Scholar] [CrossRef]
- Md Ali, A.R.; Dimick, P.S. Melting and solidification characteristics of confectionery fats: Anhydrous milk fat, cocoa butter and palm kernel stearin blends. J. Am. Oil Chem. Soc. 1994, 71, 803–806. [Google Scholar] [CrossRef]
- Pantzaris, T.P.; Basiron, Y. The lauric (coconut and palm kernel) oils. In Vegetable Oils in Food Technology: Composition, Properties and Uses; Gunstone, F.D., Ed.; Blackwell: Oxford, UK, 2002; pp. 157–202. [Google Scholar]
- Tan, C.P.; Che Man, Y.B. Differential scanning calorimetric analysis of edible oils: Comparison of thermal properties and chemical composition. J. Am. Oil Chem. Soc. 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Noordin, M.I.; Chung, L.Y. Thermostability and polymorphism of theobroma oil and palm kernel oil as suppository bases. J. Therm. Anal. Calorim. 2009, 95, 891–894. [Google Scholar] [CrossRef]
- Awad, T.; Sato, K. Acceleration of crystallisation of palm kernel oil in oil-in-water emulsion by hydrophobic emulsifier additives. Colloids Surf. B Biointerfaces 2002, 25, 45–53. [Google Scholar] [CrossRef]
- Cornacchia, L.; Roos, Y.H. Solid–liquid transition and stability of HPKO-in-water systems emulsified by dairy proteins. Food Biophys. 2011, 6, 288–294. [Google Scholar] [CrossRef]
- Cornacchia, L.; Roos, Y.H. Lipid and water crystallization in protein-stabilised oil-in-water emulsions. Food Hydrocoll. 2011, 25, 1726–1736. [Google Scholar] [CrossRef]
- Relkin, P.; Ait-Taleb, A.; Sourdet, S.; Fosseux, P.-Y. Thermal behavior of fat droplet as related to adsorbed milk proteins in complex food emulsions. A DSC study. J. Am. Oil Chem. Soc. 2003, 80, 741–746. [Google Scholar] [CrossRef]
- Relkin, P.; Sourdet, S.; Fosseux, P.-Y. Fat crystallization in complex food emulsions. Effects of adsorbed milk proteins and of a whipping process. J. Therm. Anal. Calorim. 2003, 71, 187–195. [Google Scholar] [CrossRef]
- Relkin, P.; Sourdet, S. Factors affecting fat droplet aggregation in whipped frozen protein-stabilized emulsions. Food Hydrocoll. 2005, 19, 503–511. [Google Scholar] [CrossRef]
- Gibon, V.; Danthine, S. Systematic Investigation of Co-Crystallization Properties in Binary and Ternary Mixtures of Triacylglycerols Containing Palmitic and Oleic Acids in Relation with Palm Oil Dry Fractionation. Foods 2020, 9, 1891. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ueno, S.; Sato, K. Synchrotron Radiation SAXS/WAXS Study of Polymorph-Dependent Phase Behavior of Binary Mixtures of Saturated Monoacid Triacylglycerols. Cryst. Growth Des. 2003, 3, 369–374. [Google Scholar] [CrossRef]
- Macridachis-Gonzales, J.; Bayes-Garcia, L.; Calvet, T. An Insight into the Solid-State Miscibility of Triacylglycerol Crystals. Molecules 2020, 25, 4562. [Google Scholar] [CrossRef] [PubMed]
- Bhaggan, K.; Smith, K.; Blecker, C.; Danthine, S. Binary Mixtures of Tripalmitoylglycerol (PPP) and 1,3-Dipalmitoyl-2-stearoyl-sn-glycerol (PSP): Polymorphism and Kinetic Phase Behavior. Eur. J. Lipid Sci. Technol. 2018, 120, 1700306. [Google Scholar] [CrossRef]
- Bhaggan, K.; Smith, K.; Blecker, C.; Danthine, S. Polymorphism and Kinetic Behavior of Binary Mixtures of Trisaturated Triacylglycerols Containing Palmitic and Stearic Acid Under Non-Isothermal Conditions. Eur. J. Lipid Sci. Technol. 2018, 120, 1800072. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Takeguchi, S.; Sato, A.; Hondoh, H.; Uehara, H.; Ueno, S. Multiple β Forms of Saturated Monoacid Triacylglycerol Crystals. Molecules 2020, 25, 5086. [Google Scholar] [CrossRef]
- Hagemann, J.W.; Tallent, W.H.; Kolb, K.E. Differential scanning calorimetry of single acid triglycerides: Effect of chain length and unsaturation. J. Am. Oil Chem. Soc. 1972, 49, 118–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danthine, S. Kinetic Phase Behavior of Binary Mixtures of Tri-Saturated Triacylglycerols Containing Lauric Acid. Crystals 2024, 14, 807. https://doi.org/10.3390/cryst14090807
Danthine S. Kinetic Phase Behavior of Binary Mixtures of Tri-Saturated Triacylglycerols Containing Lauric Acid. Crystals. 2024; 14(9):807. https://doi.org/10.3390/cryst14090807
Chicago/Turabian StyleDanthine, Sabine. 2024. "Kinetic Phase Behavior of Binary Mixtures of Tri-Saturated Triacylglycerols Containing Lauric Acid" Crystals 14, no. 9: 807. https://doi.org/10.3390/cryst14090807





