Using a Combined FE-CA Approach to Investigate Abnormally Large Grains Formed by the Limited Recrystallization Mechanism in a Powder Metallurgy Nickel-Based Superalloy
Abstract
:1. Introduction
2. Materials and Methods
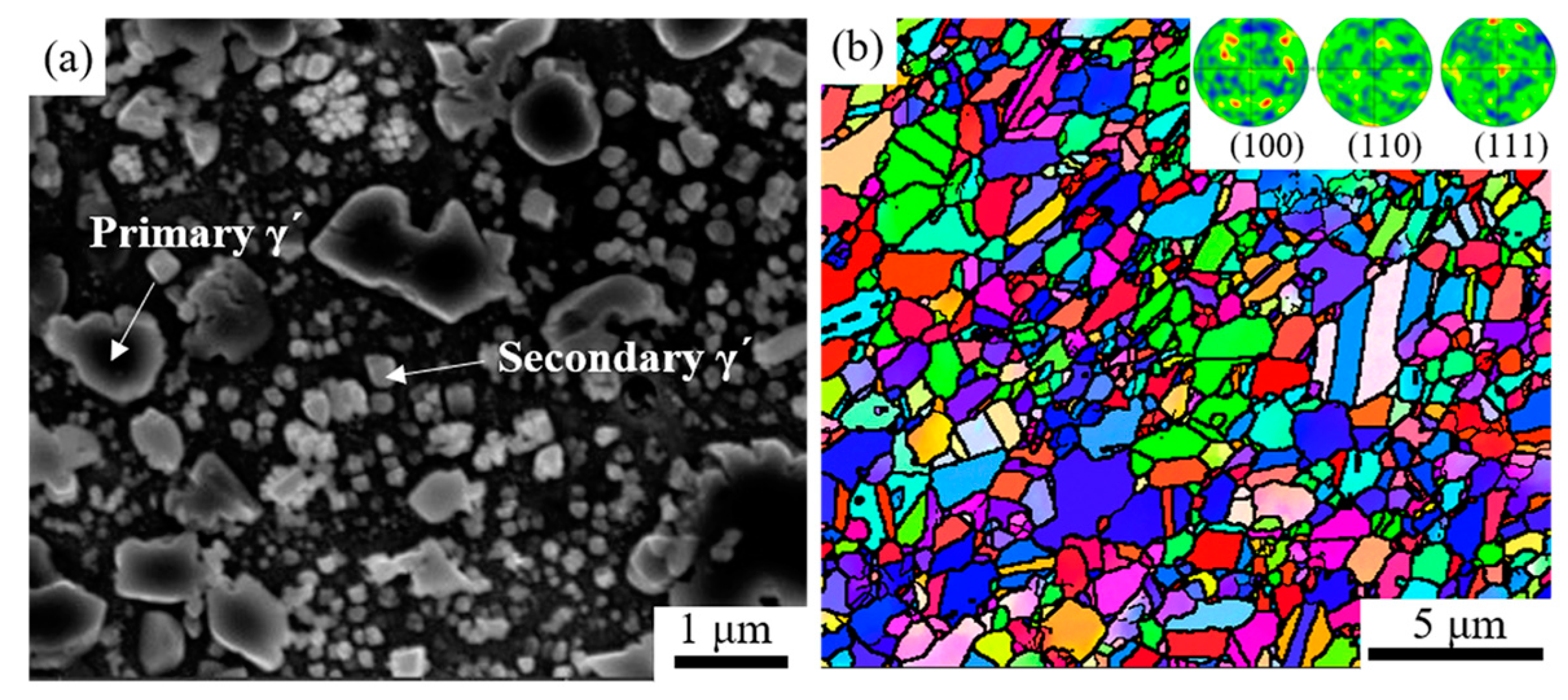
2.1. Materials
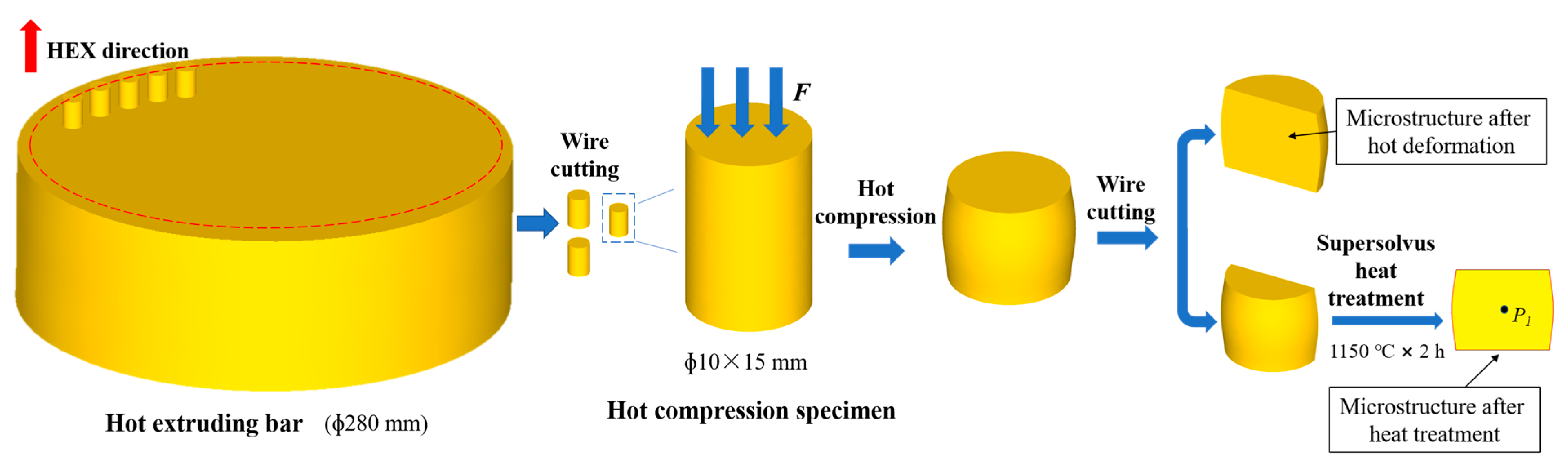
2.2. Isothermal Hot Compression Experiment
2.3. Supersolvus Heat Treatment
2.4. Microstructure Characterization
2.5. Finite Element Simulation
2.6. Cellular Automata Model
- (1)
- Color variable: Different colors were used to distinguish different grains.
- (2)
- Orientation variable: To distinguish different grain orientations, we used integers between 1 and 180.
- (3)
- Dislocation density variable: We used the dislocation density variable to represent the storage energy size of grains.
- (4)
- Recrystallization variables: Cells that underwent recrystallization are represented by 1, while cells that did not undergo recrystallization are represented by 0.
- (5)
- Grain boundary variables: Adjacent grains were distinguished by grain boundary variables, where 1 represents the grain boundary and 0 represents the interior of the grain.
- (6)
- Distance variable: We used distance variables to represent the distance at which grain boundaries migrated during the growth process.
3. Results
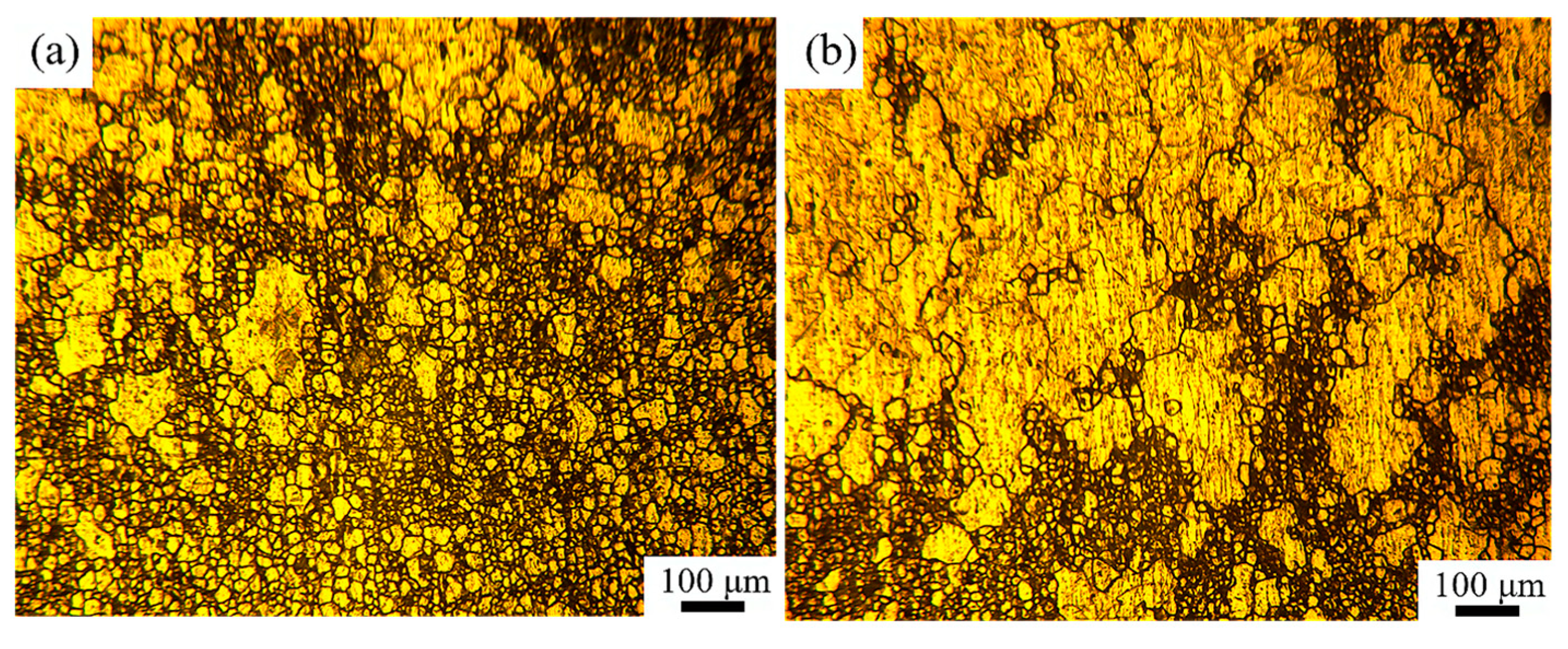
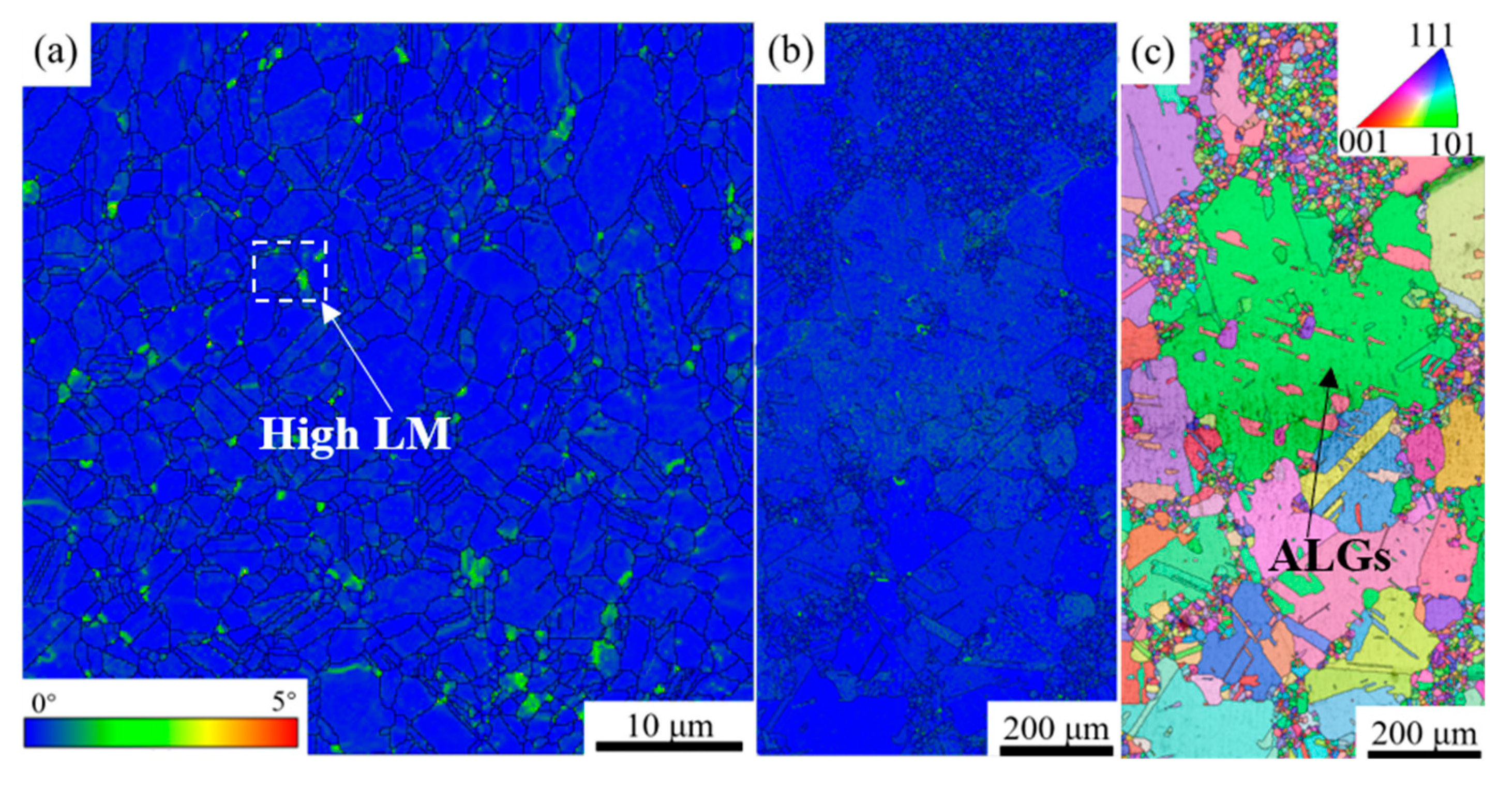
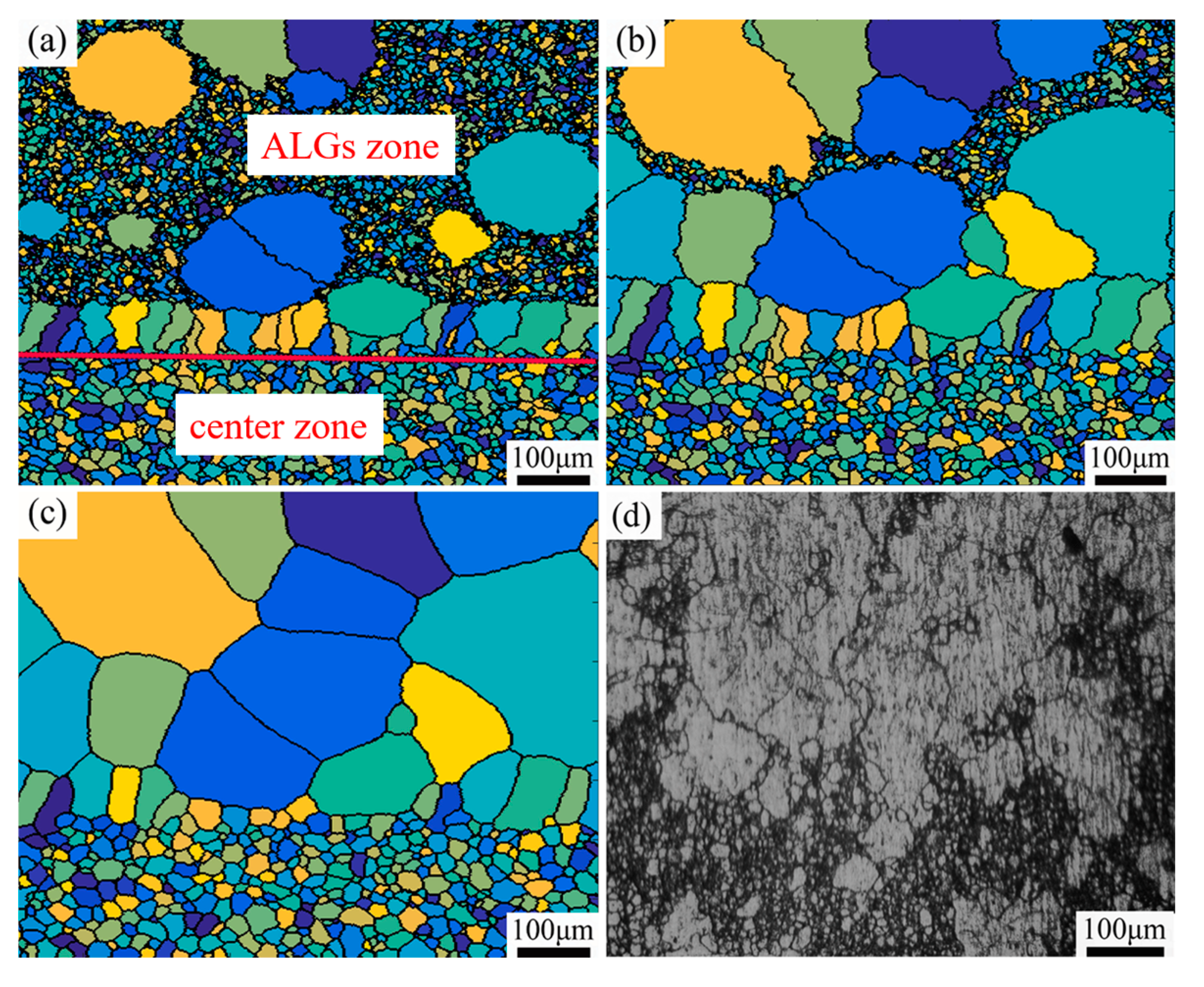
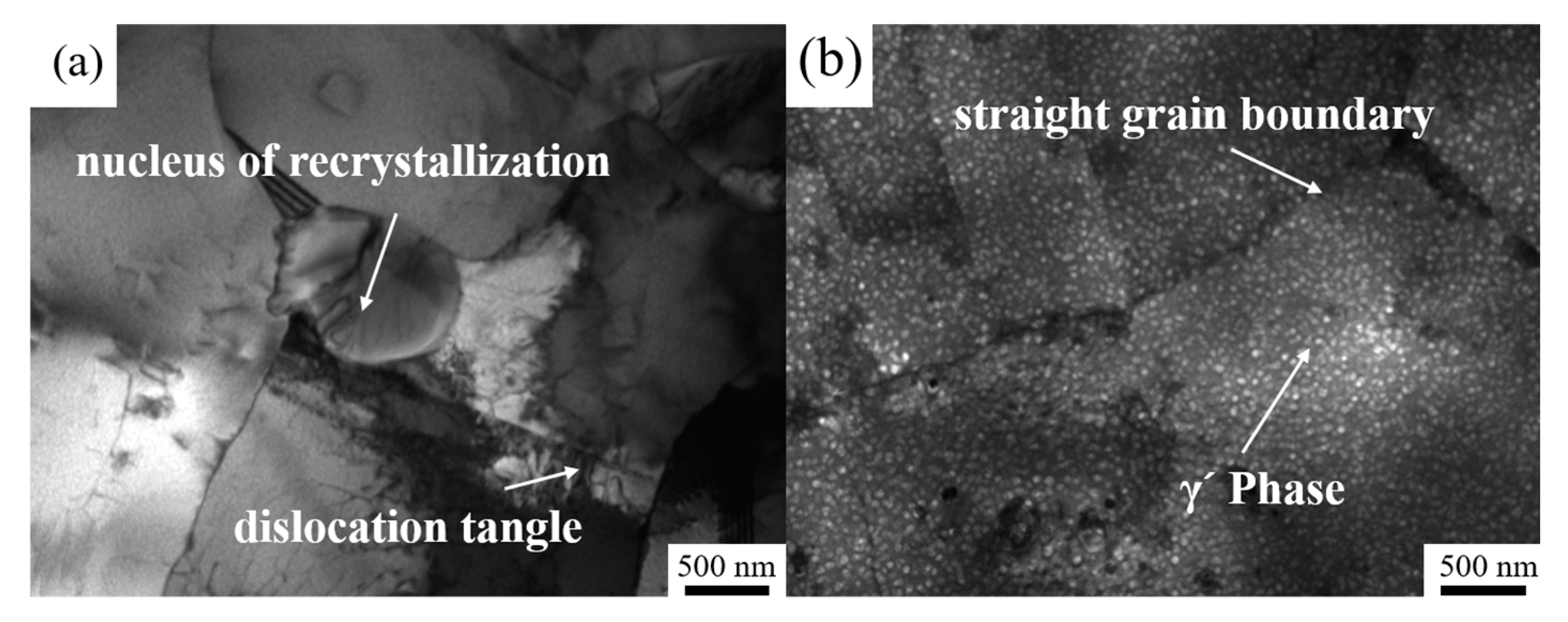
3.1. Microstructure of Over-Solution Treatment
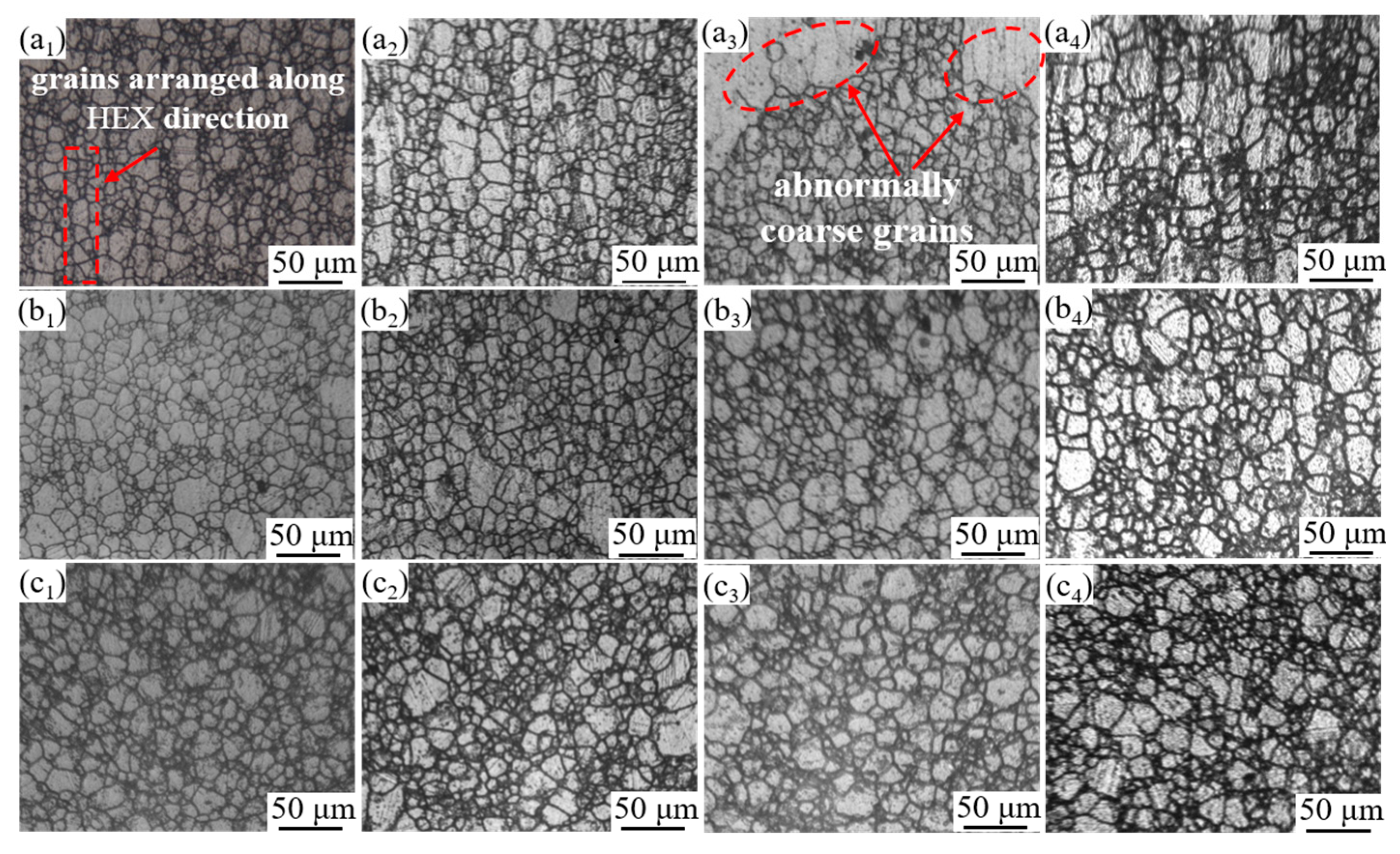
3.2. Thermal Deformation Conditions for Producing Abnormal Large Grains
3.3. Finite Element Simulation Results
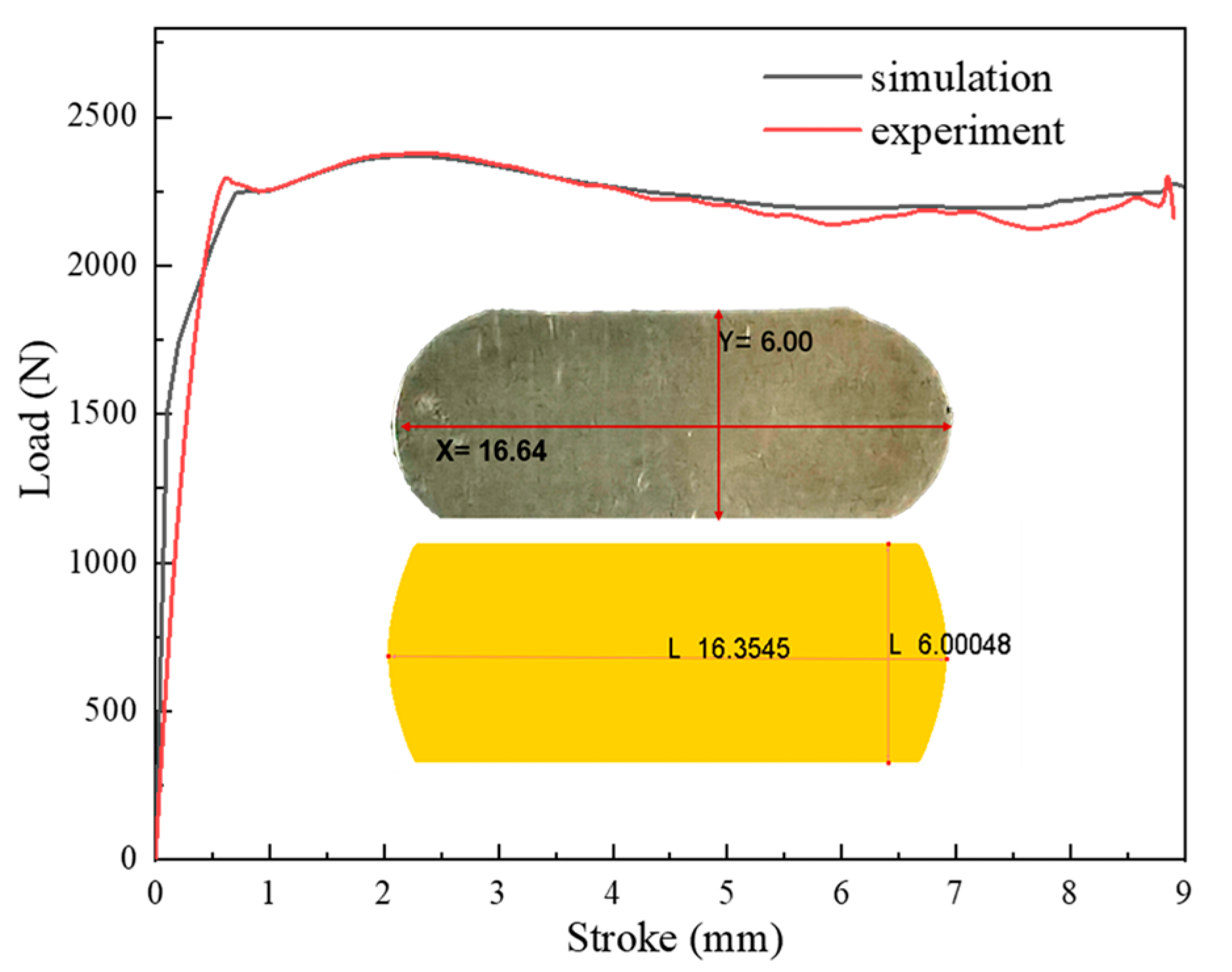
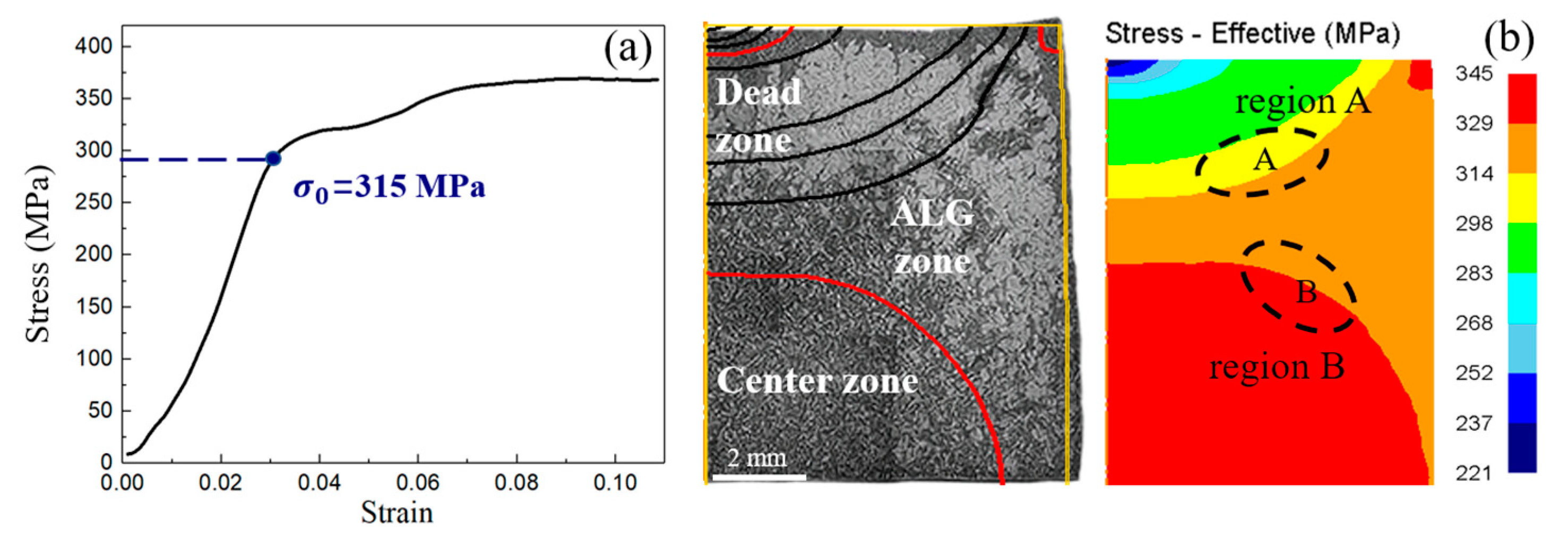
3.3.1. Finite Element Model Validation
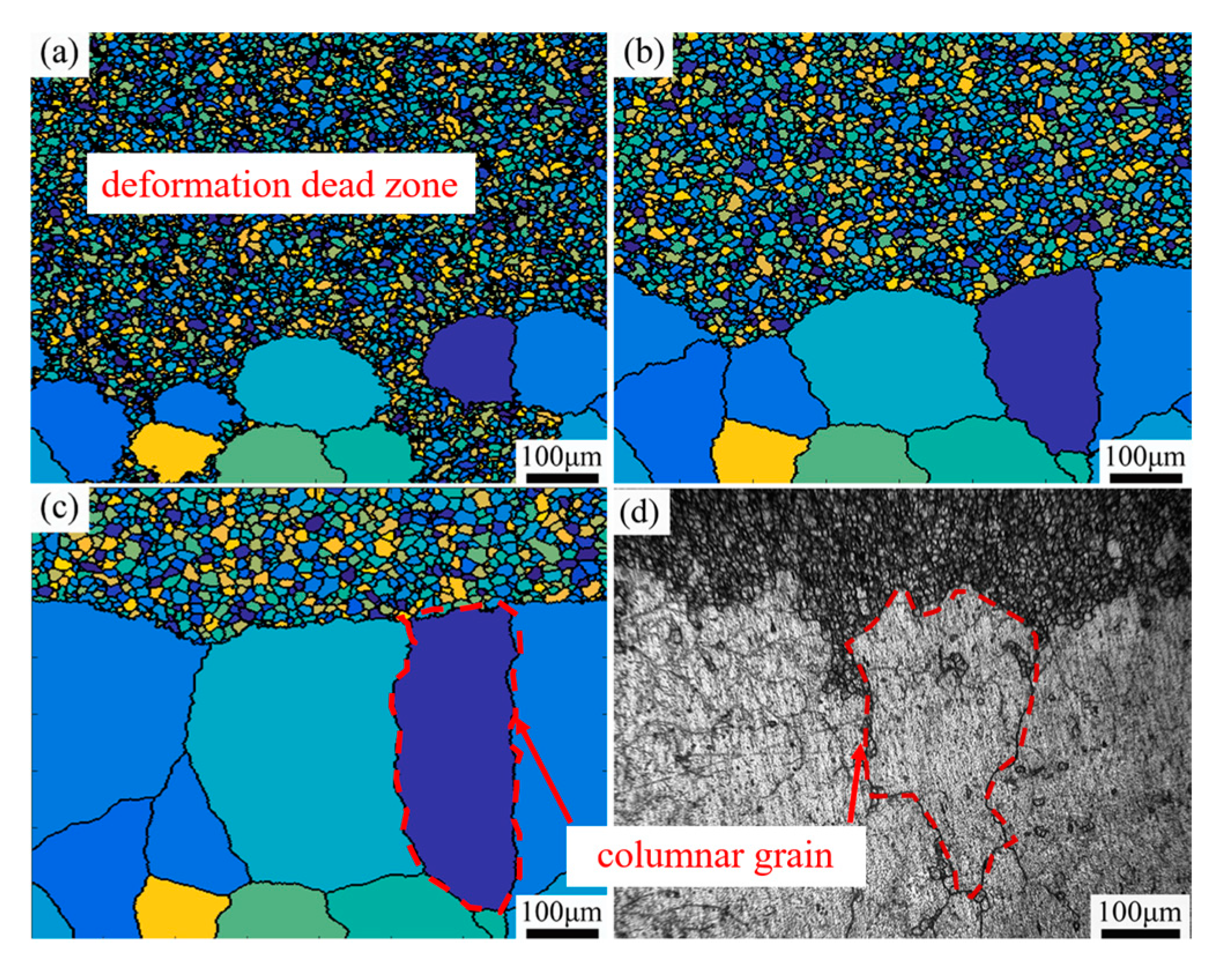
3.3.2. The Distribution of ALGs Under Different Thermal Deformation Conditions
4. Discussion
4.1. Formation Mechanism of ALGs with Different Distribution Characteristics
4.2. CA Simulation of Finite Recrystallization Formation Mechanism of ALGs
5. Conclusions
- (1)
- The hot deformation conditions, including the hot deformation temperature, reduction, and reduction rate, significantly influence the formation of ALGs in powder nickel-based superalloys after solution treatment. Combined with the finite element simulation and experimental results, the hot deformation conditions required for the alloy to form ALGs are obtained. ALGs only appeared at deformation temperatures of 1020 °C and 1070 °C, and no ALGs were found when the deformation exceeded 60%.
- (2)
- In this study, the grain coarsening caused by the dissolution of the local γ′ phase during solid solution treatment did not produce ALGs. The formation mechanism of ALGs after over-solution is influenced by the distribution of equivalent stress. After thermal deformation, if the partial equivalent stress of the ALG formation region is higher than the yield strength under corresponding conditions, the main formation mechanism of ALGs is limited recrystallization. Otherwise, the main formation mechanism of ALGs is the uneven distribution of storage energy; that is, the grains with low storage energy after thermal deformation swallow the grains with high storage energy.
- (3)
- The FE-CA approach can accurately simulate the formation process of ALGs generated by the limited recrystallization mechanism. Moreover, for ALGs formed by the limited nucleation recrystallization mechanism, the grain size of ALGs is related to the equivalent stress value in this region, and the higher the stress value, the smaller the grain size of the ALGs finally formed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.S.S.; Raghu, T.; Bhattacharjee, P.P.; Appa Rao, G.; Borah, U. Evolution of microstructure and microtexture during hot deformation in an advanced P/M nickel base superalloy. Mater. Charact. 2018, 146, 217–236. [Google Scholar] [CrossRef]
- Gessinger, G.H.; Bomford, M.J. Powder metallurgy of superalloys. Int. Metall. Rev. 1974, 19, 51–76. [Google Scholar] [CrossRef]
- Li, Z.; Qin, H.; Xu, K.; Xie, Z.; Ji, P.; Jia, M. Fatigue Behavior of the FGH96 Superalloy under High-Temperature Cyclic Loading. Materials 2023, 16, 5883. [Google Scholar] [CrossRef]
- Sreenu, B.; Sarkar, R.; Kumar, S.S.S.; Chatterjee, S.; Rao, G.A. Microstructure and mechanical behaviour of an advanced powder metallurgy nickel base superalloy processed through hot isostatic pressing route for aerospace applications. Mater. Sci. Eng. A 2020, 797, 140254. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ando, S.; Futami, S.; Kitamura, T.; Koiwa, M. Superplastic deformation and microstructure evolution in PM IN-100 superalloy. J. Mater. Sci. 1990, 25, 4712–4716. [Google Scholar] [CrossRef]
- Gong, X.; Duan, Z.; Pei, W.; Chen, H. Superplastic Deformation Mechanisms of Superfine/Nanocrystalline Duplex PM-TiAl-Based Alloy. Materials 2017, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, H.; Wang, Y.; Yang, L.; Qiao, S.; Huang, Z.; Liu, M. Hot working characteristics of HEXed PM nickel-based superalloy during hot compression. Trans. Nonferrous Met. Soc. China 2020, 30, 2709–2723. [Google Scholar] [CrossRef]
- Song, K.; Aindow, M. Grain growth and particle pinning in a model Ni-based superalloy. Mater. Sci. Eng. A 2008, 479, 365–372. [Google Scholar] [CrossRef]
- Gabb, T.P.; Kantzos, P.T.; Telesman, J.; Gayda, J.; Sudbrack, C.K.; Palsa, B. Fatigue resistance of the grain size transition zone in a dual microstructure superalloy disk. Int. J. Fatigue 2011, 33, 414–426. [Google Scholar] [CrossRef]
- Kirchmayer, A.; Pröbstle, M.; Huenert, D.; Neumeier, S.; Göken, M. Influence of Grain Size and Volume Fraction of η/δ Precipitates on the Dwell Fatigue Crack Propagation Rate and Creep Resistance of the Nickel-Base Superalloy ATI 718Plus. Metall. Mater. Trans. A 2023, 54, 2219–2226. [Google Scholar] [CrossRef]
- Benz, M.G.; Blankenship, C.P.; Henry, M.F.; Huron, E.S.; Kissinger, R.D.; Raymond, E.L. Control of Grain Size of Nickel-Base Superalloy. U.S. Patent US5759305A, 4 February 1997. [Google Scholar]
- Carter, J.L.W.; Kuper, M.W.; Uchic, M.D.; Mills, M.J. Characterization of localized deformation near grain boundaries of superalloy René-104 at elevated temperature. Mater. Sci. Eng. A 2014, 605, 127–136. [Google Scholar] [CrossRef]
- Charpagne, M.; Franchet, J.; Bozzolo, N. Overgrown grains appearing during sub-solvus heat treatment in a polycrystalline γ-γ’ Nickel-based superalloy. Mater. Des. 2018, 144, 353–360. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, Y.; Lv, L.; Huang, Z.; Tan, G. Normal and abnormal grain growth in a FGH96 superalloy during thermomechanical treatment. J. Mater. Res. Technol. 2021, 15, 7033–7049. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Cai, B.; Zhou, N.; Magdysyuk, O.; Gao, Y.; Srivatsa, S.; Tan, L.; Jiang, L. Formation mechanism of abnormally large grains in a polycrystalline nickel-based superalloy during heat treatment processing. Acta Mater. 2019, 168, 287–298. [Google Scholar] [CrossRef]
- Hardy, M.; Huron, E.; Glatzel, U.; Griffin, B.; Lewis, B.; Rae, C.; Seetharaman, V.; Tin, S. Inhomogeneous Grain Coarsening Behavior in Supersolvus Heat Treated Nickel-Based Superalloy RR1000. In Proceedings of the Superalloys 2016: Proceedings of the 13th Intenational Symposium of Superalloys, Seven Spring, PA, USA, 11 September 2016. [Google Scholar]
- Miao, J.; Pollock, T.M.; Wayne Jones, J. Crystallographic fatigue crack initiation in nickel-based superalloy René 88DT at elevated temperature. Acta Mater. 2009, 57, 5964–5974. [Google Scholar] [CrossRef]
- Huron, E.; Srivatsa, S.; Raymond, E. Control of Grain Size Via Forging Strain Rate Limits for R88DT. Superalloys 2000, 2000, 49–58. [Google Scholar]
- Rios, P.R.; Zöllner, D. Critical assessment 30: Grain growth—Unresolved issues. Mater. Sci. Technol. 2018, 34, 629–638. [Google Scholar] [CrossRef]
- Kern, M.; Bernhard, M.; Presoly, P.; Brandl, D.; Bernhard, C. Critical Examination of the Representativeness of Austenite Grain Growth Studies Performed In Situ Using HT-LSCM and Application to Determine Growth-inhibiting Mechanisms. Bhm Berg-Und Hüttenmännische Monatshefte 2024, 169, 161–166. [Google Scholar] [CrossRef]
- DeMania, D.A. Recovery and Recrystallization in Nickel-Based Superalloy René 88 DT; University of Virginia: Charlottesville, VA, USA, 2002; p. 114. [Google Scholar]
- Rios, P.R. Abnormal growth in the presence of coarsening particles. Acta Metall. Et Mater. 1992, 40, 649–651. [Google Scholar] [CrossRef]
- Rios, P.R. Abnormal grain growth in materials containing particles. Acta Metall. Et Mater. 1994, 42, 839–843. [Google Scholar] [CrossRef]
- Kronberg, M.L.; Wilson, U.F. Secondary recrystallization in copper. JOM 1949, 1, 501–514. [Google Scholar] [CrossRef]
- Jung, S.; Yoon, D.Y.; Kang, S.L. Mechanism of abnormal grain growth in ultrafine-grained nickel. Acta Mater. 2013, 61, 5685–5693. [Google Scholar] [CrossRef]
- Schirra, J.J. Recovery And Recrystallization After Critical Strain In The Nickel-Based. Structure 2004, 34, 38. [Google Scholar]
- Blankenship, C.P.; Henry, M.F.; Hyzak, J.M.; Rohling, R.B.; Hall, E.L. Hot-Die Forging of P/M Ni-Base Superalloys. Superalloys 1966, 1996, 653–662. [Google Scholar]
- He, G.; Tan, L.; Liu, F.; Huang, L.; Huang, Z.; Jiang, L. Unraveling the formation mechanism of abnormally large grains in an advanced polycrystalline nickel base superalloy. J. Alloys Compd. 2017, 718, 405–413. [Google Scholar] [CrossRef]
- Zener, C.; Hollomon, J.H. Effect of Strain Rate Upon Plastic Flow of Steel. J. Appl. Phys. 1944, 15, 22–32. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Chen, X.; Wang, X.; Sun, J. Modeling and simulation of grain growth for FGH96 superalloy using a developed cellular automaton model. Model. Simul. Mater. Sci. Eng. 2024, 32, 55011. [Google Scholar] [CrossRef]
- GB/T 6394-2017[S]; Determination of Estimating the Average Grain Size of Metal. General Administration of Quality Supervision, Inspection and Quarantine of P.R.C: Beijing, China, 2017.
- Rios, P.R. Some Theoretical Considerations on Abnormal Grain Growth. Mater. Sci. Forum 1996, 204–206, 247–256. [Google Scholar] [CrossRef]
- Rios, P.R.; Glicksman, M.E. Topological theory of abnormal grain growth. Acta Mater. 2006, 54, 5313–5321. [Google Scholar] [CrossRef]
- Novikov, V.Y. Microstructure evolution during grain growth in materials with disperse particles. Mater. Lett. 2012, 68, 413–415. [Google Scholar] [CrossRef]
- Semiatin, S.L.; McClary, K.E.; Rollett, A.D.; Roberts, C.G.; Payton, E.J.; Zhang, F.; Gabb, T.P. Plastic Flow and Microstructure Evolution during Thermomechanical Processing of a PM Nickel-Base Superalloy. Metall. Mater. Trans. A 2013, 44, 2778–2798. [Google Scholar] [CrossRef]
- Srolovitz, D.J.; Grest, G.S.; Anderson, M.P. Computer simulation of grain growth-V. Abnormal grain growth. Acta Metall. 1985, 33, 2233–2247. [Google Scholar] [CrossRef]
- Lee, S.B.; Yoon, D.Y.; Henry, M.F. Abnormal grain growth and grain boundary faceting in a model Ni-base superalloy. Acta Mater. 2000, 48, 3071–3080. [Google Scholar] [CrossRef]
- Zheng, C.; Xiao, N.; Li, D.; Li, Y. Microstructure prediction of the austenite recrystallization during multi-pass steel strip hot rolling: A cellular automaton modeling. Comput. Mater. Sci. 2008, 44, 507–514. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, Y.; Chen, M.; Huang, M.; Ma, X.; Long, Z. Study of static recrystallization behavior in hot deformed Ni-based superalloy using cellular automaton model. Mater. Des. 2016, 99, 107–114. [Google Scholar] [CrossRef]






| Parameters | Value |
|---|---|
| Temperature (°C) | 1020, 1050, 1070, 1090 |
| Strain rate (s−1) | 0.001, 0.003, 0.005, 0.01, 0.03, 0.1, 0.3, 0.5, 1 |
| Reduction amount (%) | 10, 30, 60 |
| Thermal Deformation Conditions | Temperature (°C) | Reduction Rate (s−1) | Reduction | Strain | Strain Rate (s−1) | Stress (MPa) |
|---|---|---|---|---|---|---|
| #1020-0.05-10 | 1020 | 0.05 | 10% | 0.099~0.110 | 0.049~0.056 | 176~188 |
| #1020-0.05-30 | 0.05 | 30% | 0.133~0.265 | 0.027~0.052 | 141~164 | |
| #1020-0.1-10 | 0.1 | 10% | 0.106~0.137 | 0.106~0.142 | 249~268 | |
| #1020-0.3-10 | 0.3 | 10% | 0.108~0.123 | 0.087~0.103 | 242~267 | |
| #1020-0.3-30 | 0.3 | 30% | 0.040~0.268 | 0.040~0.278 | 187~244 | |
| #1020-0.5-30 | 0.5 | 30% | 0.039~0.211 | 0.0676~0.468 | 194~274 | |
| #1020-1-10 | 1 | 10% | 0.056~0.107 | 0.341~1.16 | 276~329 | |
| #1070-0.1-30 | 1070 | 0.1 | 30% | 0.268~0.374 | 0.151~0.224 | 119~145 |
| #1070-0.3-30 | 0.3 | 30% | 0.125~0.351 | 0.028~0.315 | 132~185 | |
| #1070-0.3-30 | 0.5 | 30% | 0.143~0.285 | 0.245~0.452 | 149~199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, B.; Chen, X.; Wang, X.; Feng, Y.; Su, L.; Liang, Z.; Liu, Y. Using a Combined FE-CA Approach to Investigate Abnormally Large Grains Formed by the Limited Recrystallization Mechanism in a Powder Metallurgy Nickel-Based Superalloy. Crystals 2025, 15, 91. https://doi.org/10.3390/cryst15010091
Yang Y, Zhang B, Chen X, Wang X, Feng Y, Su L, Liang Z, Liu Y. Using a Combined FE-CA Approach to Investigate Abnormally Large Grains Formed by the Limited Recrystallization Mechanism in a Powder Metallurgy Nickel-Based Superalloy. Crystals. 2025; 15(1):91. https://doi.org/10.3390/cryst15010091
Chicago/Turabian StyleYang, Yanhui, Boyan Zhang, Xiuquan Chen, Xiaoxuan Wang, Yankai Feng, Lidong Su, Zhengfei Liang, and Yifan Liu. 2025. "Using a Combined FE-CA Approach to Investigate Abnormally Large Grains Formed by the Limited Recrystallization Mechanism in a Powder Metallurgy Nickel-Based Superalloy" Crystals 15, no. 1: 91. https://doi.org/10.3390/cryst15010091
APA StyleYang, Y., Zhang, B., Chen, X., Wang, X., Feng, Y., Su, L., Liang, Z., & Liu, Y. (2025). Using a Combined FE-CA Approach to Investigate Abnormally Large Grains Formed by the Limited Recrystallization Mechanism in a Powder Metallurgy Nickel-Based Superalloy. Crystals, 15(1), 91. https://doi.org/10.3390/cryst15010091






