Effect of Nickel Content and Cooling Rate on the Microstructure of as Cast 316 Stainless Steels
Abstract
1. Introduction
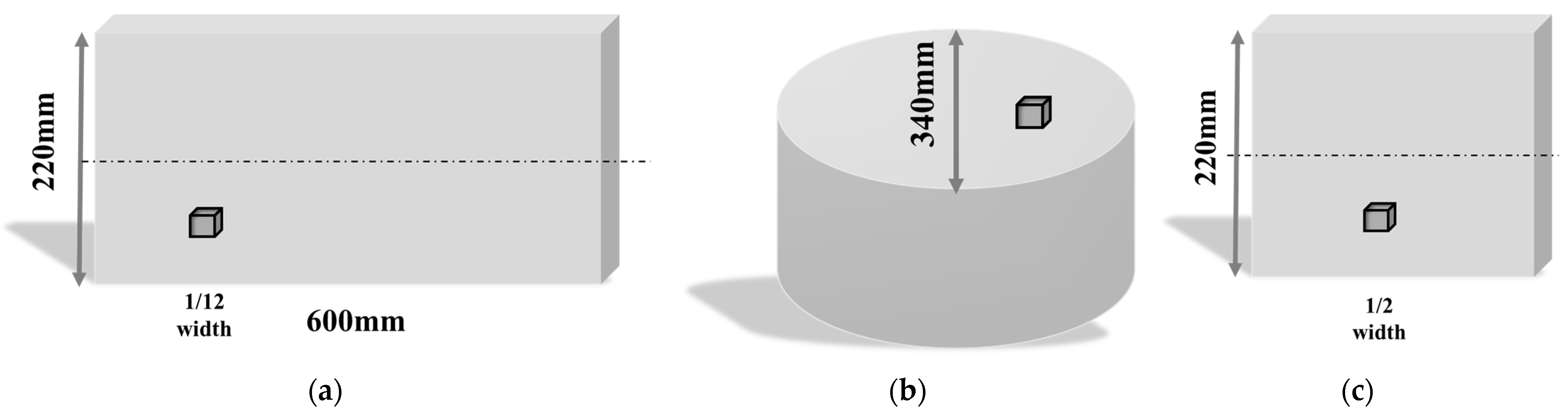
2. Materials and Methods
3. Results
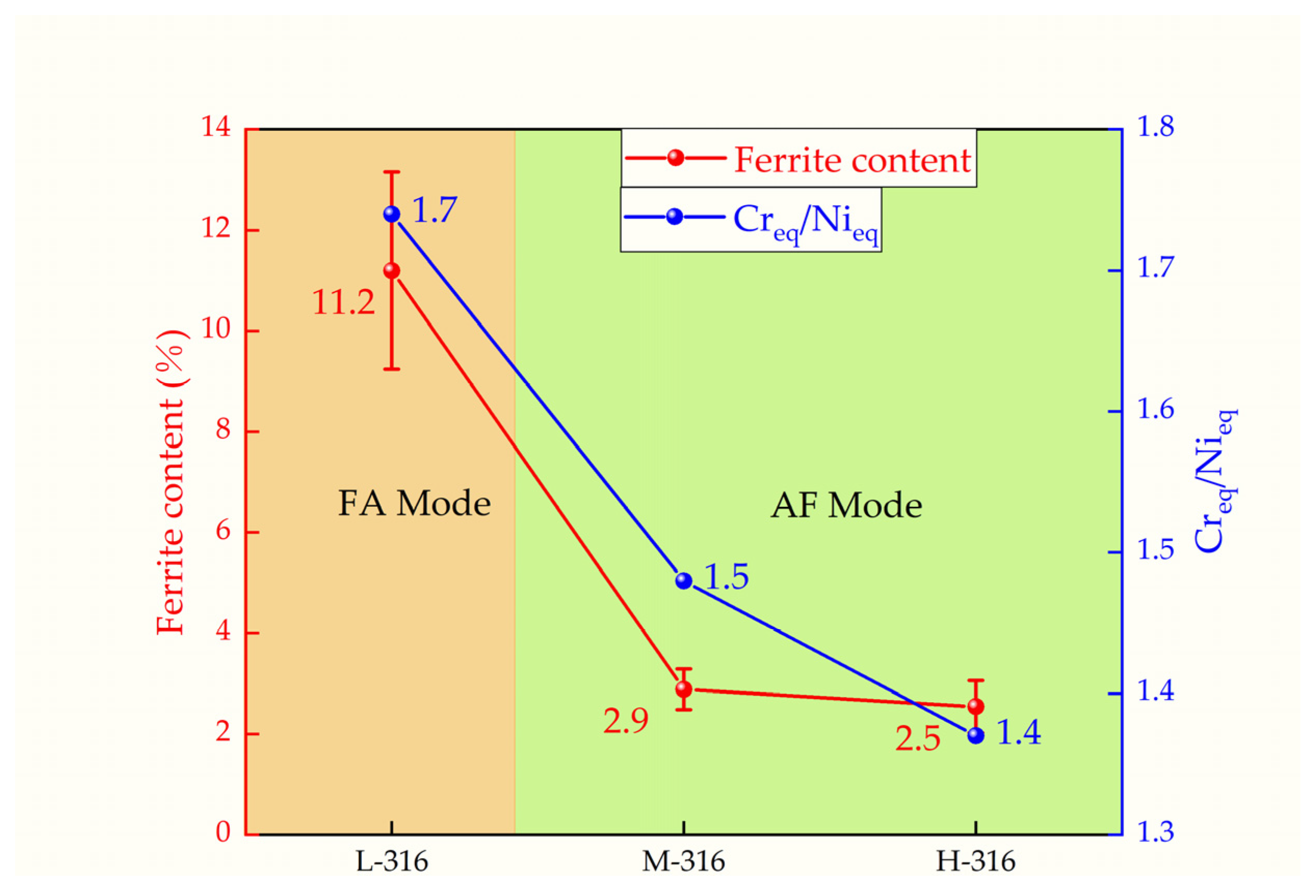
3.1. Effect of Composition on the Microstructure and Ferrite Content
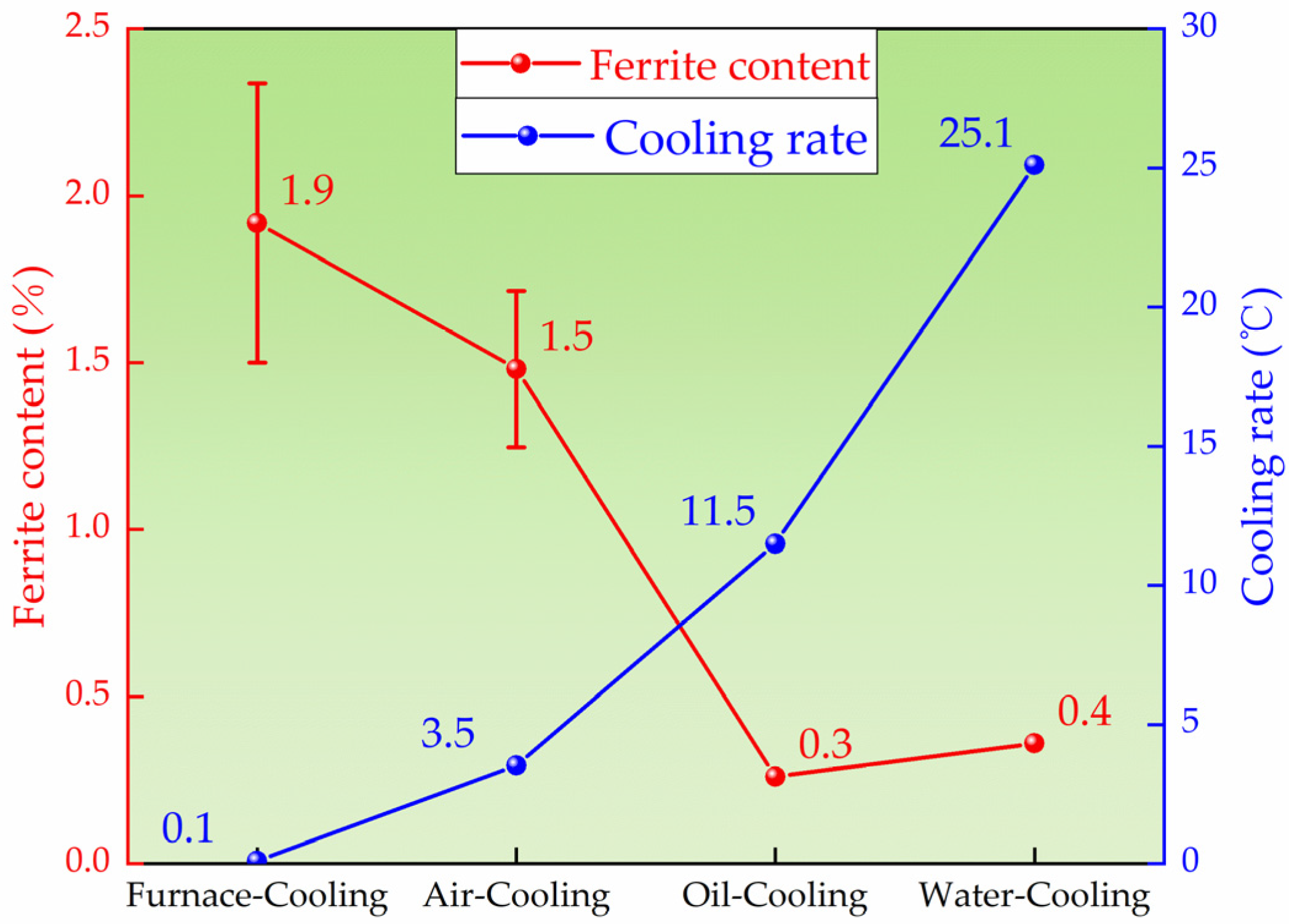
3.2. Effect of Cooling Rate on the Microstructure of High-Ni-Content 316 Stainless Steel
4. Discussion
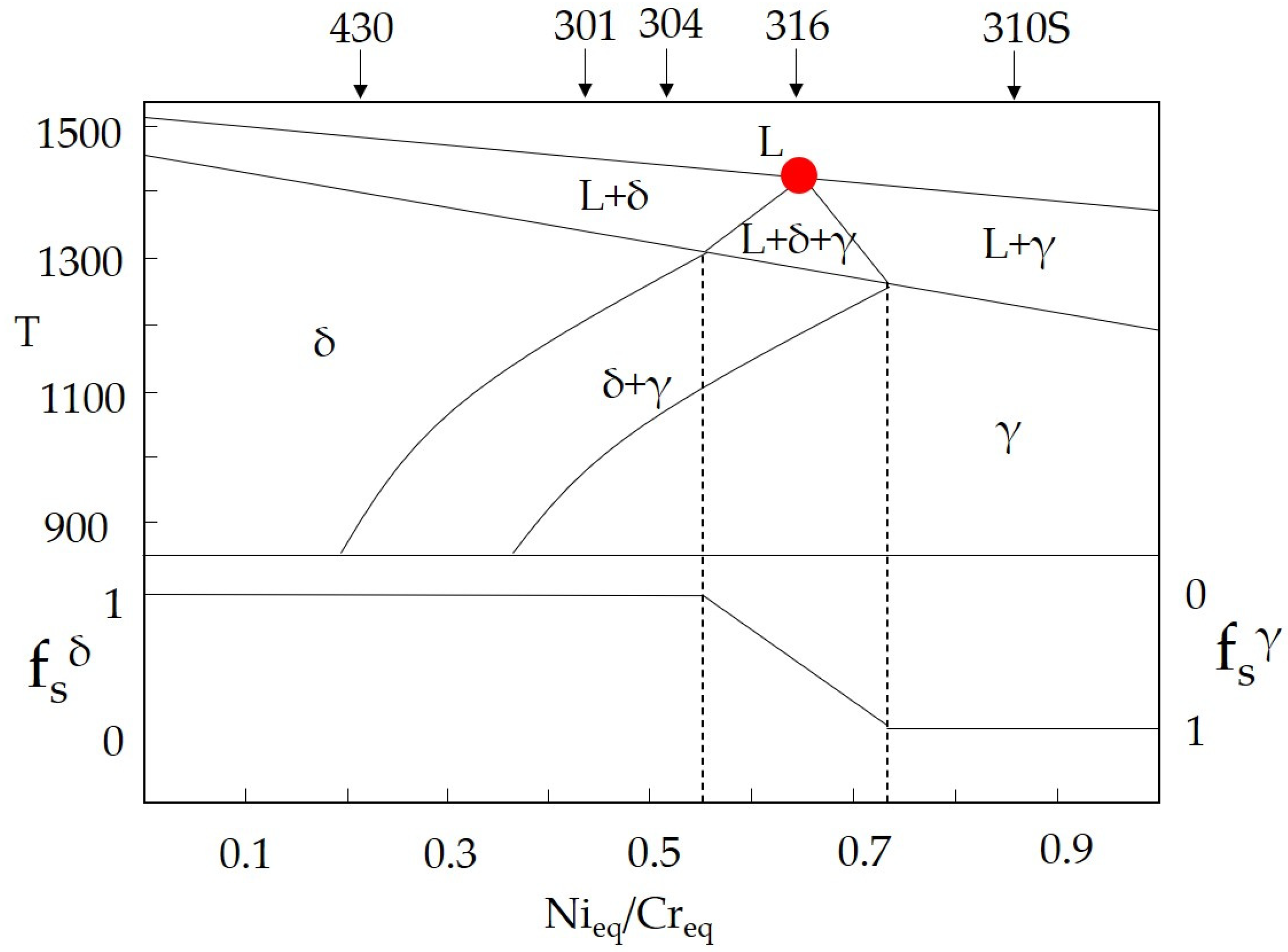
4.1. Solidification Mode Predicted by Empirical Models
4.2. Equilibrium Solidification by Thermodynamic Calculations
4.3. Effect of Composition on the Microstructure
4.4. Effect of Cooling Rate on the Microstructure
5. Conclusions
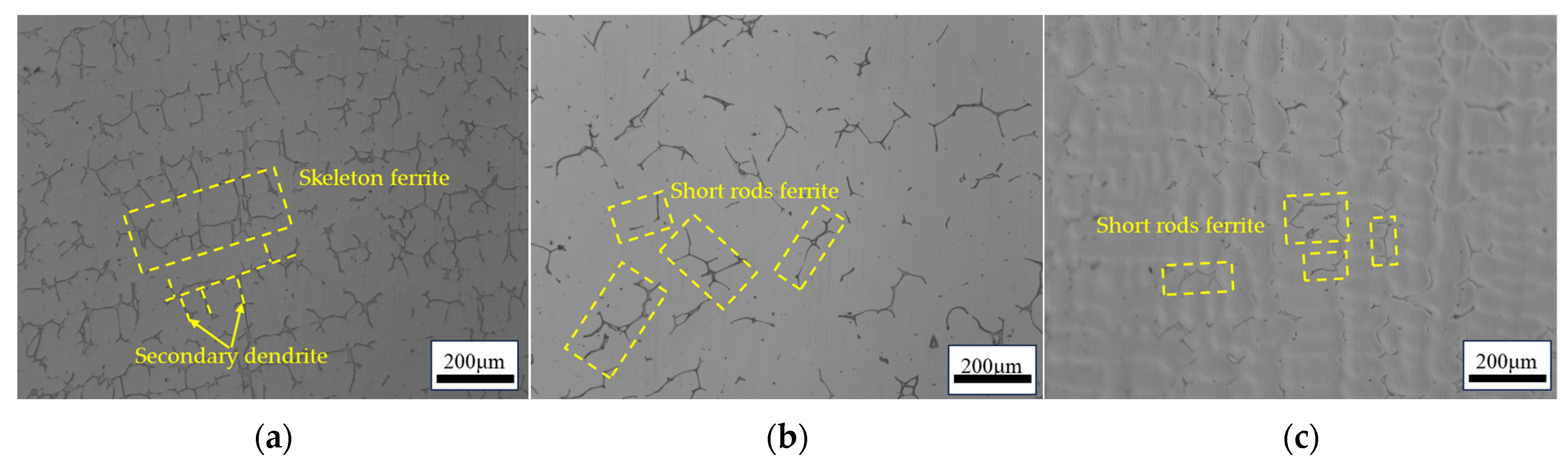
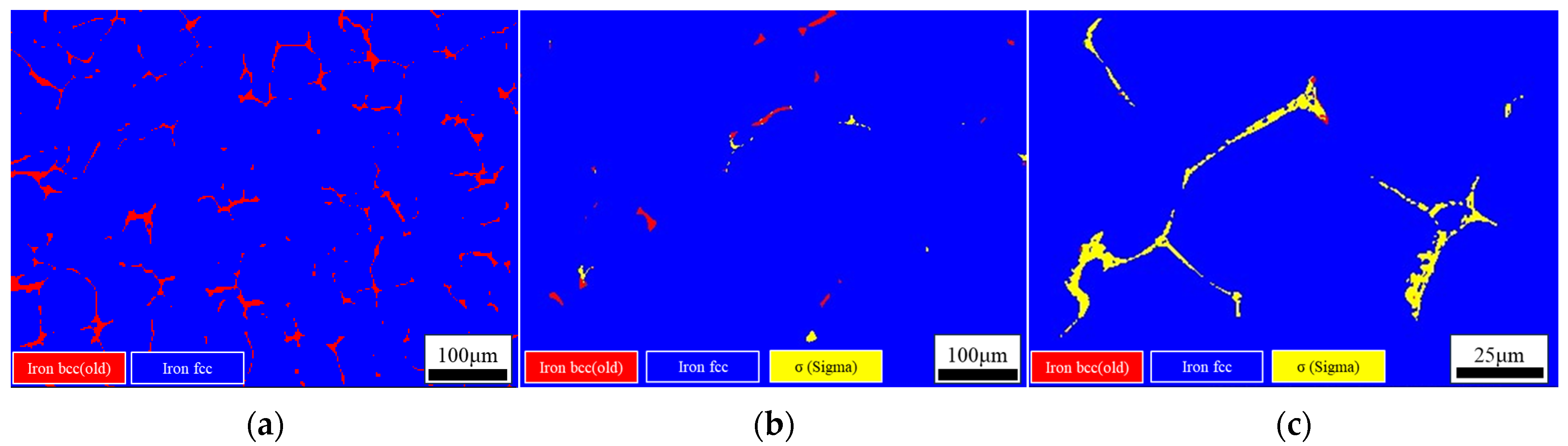
- Three different compositions of 316 stainless steel continuous casting samples are studied: L-316, M-316, and H-316. The primary difference among the three castings is the Ni content, which is 10%, 12%, and 14%, respectively. The ferrite content of the L-316, M-316, and H-316 castings is found to be 11.20%, 2.88%, and 2.45%, respectively. The microstructure of the L-316 casting is mainly composed of the austenitic phase and ferritic phase. The microstructure of the M-316 casting is composed of austenite, ferrite, and a small amount of the sigma phase, with a small amount of ferrite transformed into the sigma phase. The microstructure of the H-316 casting is basically composed of austenite and the sigma phase, and the ferrite is completely transformed into the sigma phase.
- Based on Creq/Nieq and ferrite morphology, the solidification mode of the 316 stainless steel castings with different composition is determined. It is found that the solidification mode is FA in the L-316 casting and AF in the M-316 and H-316 castings. However, the thermodynamic calculation results indicate that the solidification modes of the three castings are FA, FA, and AF, respectively. It is found that the influence of composition on the ferrite content manifests mainly through the altering of the solidification mode. In the 316 stainless steel solidifying in the FA mode, the ferrite content is much higher than that in the 316 stainless steel solidifying in the AF mode. Moreover, for the 316 stainless steel solidifying in the AF mode, changes in Creq/Nieq have little impact on the ferrite content.
- The high-Ni-content 316 stainless steel remelting ingots obtained by different cooling methods are studied. It is found that the ferrite content of the ingots obtained under furnace cooling (0.1 °C/s) and air cooling (3.5 °C/s) is 1.94% and 1.49%, respectively. The content of ferrite is less than 1% in both oil-cooled and air-cooled ingots. In the furnace-cooled ingot, most of the ferrite is transformed into the sigma phase. In the other three cooling methods, only a very small amount of the ferrite is transformed into the sigma phase. It is found that the ferrite content of the 316 stainless steel solidified in AF mode decreases with the increase in the cooling rate. In the studied H-316 steel, the ferrite content can be controlled below 1% when the cooling rate is above 11.5 °C/s.
- Through the above remelting experiments, it is found that the cooling rate has a great influence on the ferrite content and the formation of the sigma phase. With the increase in the cooling rate, the ferrite content in the H-316 ingot decreases. The solidification mode of the H-316 casting is AF and the increase in the cooling rate reduces the element diffusion rate during solidification, reduces the element segregation behavior at the grain boundary, and makes the formation of ferrite at the grain boundary more difficult. At the same time, the increase in the cooling rate also inhibits the transformation of ferrite into the sigma phase by decreasing the diffusion time of the element.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yang, S.; Ma, J.Y.; Chen, C.; Zhang, C.L.; Ren, J.Y.; Jiang, Z.H.; Fan, G.W.; Han, P.D. Effects of B and Ce Grain Boundary Segregation on Precipitates in Super Austenitic Stainless Steel. Metals 2023, 13, 326. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wang, J.; Chen, C.; Tian, H.Y.; Han, P.D. Effect of boron on the solidification characteristics and constitutive equation of S31254 superaustenitic stainless steel. Steel Res. Int. 2024, 95, 2400050. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.F.; Zhang, Z. EBSD parameter assessment and constitutive models of crept HR3C austenitic steel. J. Iron Steel Res. Int. 2023, 30, 772–781. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, C.J.; Liu, C. Effect of heat treatment on microstructure and mechanical properties of selective laser-melted PH13-8Mo stainless steel. J. Iron Steel Res. Int. 2024, 31, 945–955. [Google Scholar] [CrossRef]
- Hou, Y.H.; Xu, Z.K.; Zhou, S.L.; Li, G.Q. Atomic-scale mechanism of effect of co-separation of elements at interface of ferrite and austenite on Cr-depleted zone of duplex stainless steel. J. Iron Steel Res. Int. 2024, 31, 710–718. [Google Scholar] [CrossRef]
- Xu, S.G.; He, J.S.; Zhang, R.Z.; Zhang, F.C.; Wang, X.T. Static recrystallization behaviors and mechanisms of 7Mo super-austenitic stainless steel with undissolved sigma precipitates during double-stage hot deformation. J. Iron Steel Res. Int. 2024, 31, 475–487. [Google Scholar] [CrossRef]
- Toppo, A.; Pujar, M.G.; Sreevidya, N.; Philip, J. Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments. Def. Technol. 2018, 14, 226–237. [Google Scholar] [CrossRef]
- Li, J.S.; Mao, Q.Z.; Chen, M.; Qin, W.B.; Lu, X.K.; Liu, T.; She, D.S.; Kang, J.J.; Wang, G.; Zhu, X.B.; et al. Enhanced pitting resistance through designing a high-strength 316L stainless steel with heterostructure. J. Mater. Res. Technol. 2021, 10, 132–137. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zheng, Z.B.; Long, J.; Xu, Z.B.; Jiao, S.H.; Qiao, Y.X.; Zheng, K.H.; Yin, F.X. Corrosion behaviour of hot-rolled 316L stainless steel-A6 carbon steel composite steel plate for marine environment. J. Mater. Res. Technol. 2023, 26, 556–570. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, F.K.; Zhang, H.; Li, J.; Wang, Y.; Lu, Y.Y.; Xiong, L.; Ni, H.W. Mechanisms of interfacial reactions between 316L stainless steel and MnO–SiO2 oxide during isothermal heating. J. Iron Steel Res. Int. 2023, 30, 1511–1523. [Google Scholar] [CrossRef]
- Han, S.B.; Song, H.; Park, S.H. Improvement of tensile properties through Nb addition and heat treatment in additively manufactured 316L stainless steel using directed energy deposition. J. Mater. Res. Technol. 2024, 29, 4806–4821. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Chen, S.H.; Xie, A.; Lv, X.L.; Chen, S.H.; Yan, C.G.; Jiang, H.C.; Rong, L.J. Tailoring Microstructure of Austenitic Stainless Steel with Improved Performance for Generation-IV Fast Reactor Application: A Review. Crystals 2023, 13, 268. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, Q.Y.; Jiang, H.C.; Rong, L.J. Effect of δ-ferrite on Hot Deformation and Recrystallization of 316KD Austenitic Stainless Steel for Sodium-Cooled Fast Reactor Application. Acta Metall. Sin. 2024, 60, 367–376. Available online: https://www.ams.org.cn/CN/10.11900/0412.1961.2022.00039 (accessed on 5 February 2025).
- Lv, X.L.; Chen, S.H.; Rong, L.J. Evolution of precipitate and its effect on the degradation of impact toughness in a carbon and nitrogen-controlled 316 stainless steel during thermal aging at 650 °C. J. Mater. Res. Technol. 2024, 33, 3728–3742. [Google Scholar] [CrossRef]
- Li, X.; Gao, F.; Jiao, J.H.; Cao, G.M.; Wang, Y.; Liu, Z.Y. Influences of cooling rates on delta ferrite of nuclear power 316H austenitic stainless steel. Mater. Charact. 2021, 174, 111029. [Google Scholar] [CrossRef]
- Wang, Z.G.; Gao, F.; Cao, G.M.; Tang, S.; Liu, Z.Y. Elemental inter-diffusions for the transformation of δ/M23C6/γ phases during heat treatment processes in heavy gage 316H-type austenitic stainless steel. Metall. Mater. Trans. A 2022, 53, 2652–2664. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Ren, R.J.; Xue, Z.X.; Wang, H.Z.; Zhang, Y.Z.; Wang, J.X.; Wang, J.; Chen, L.; Mu, W.Z. Ferrite formation and decomposition in 316H austenitic stainless steel electro slag remelting ingot for nuclear power applications. Mater. Charact. 2024, 218, 114581. [Google Scholar] [CrossRef]
- Lv, X.L.; Chen, S.H.; Rong, L.J. Absence of dynamic strain aging induced by precipitation in 316 austenitic stainless steel during thermal aging at 750 °C. Mater. Charact. 2024, 207, 113580. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Chen, S.H.; Rong, L.J. δ-ferrite formation and its effect on the mechanical properties of heavy-section AISI 316 stainless steel casting. Metall. Mater. Trans. A 2020, 51, 2998–3008. [Google Scholar] [CrossRef]
- Lv, X.; Chen, S.; Wang, Q.; Jiang, H.; Rong, L. Temperature Dependence of Fracture Behavior and Mechanical Properties of AISI 316 Austenitic Stainless Steel. Metals 2022, 12, 1421. [Google Scholar] [CrossRef]
- Xie, A.; Chen, S.H.; Chen, S.H.; Jiang, H.C.; Rong, L.J. Austenite decomposition behavior adjacent to δ-ferrite in a Si-modified Fe-Cr-Ni austenitic stainless steel during thermal aging at 550 °C. Acta Mater. 2024, 272, 119948. [Google Scholar] [CrossRef]
- Sasikala, G.; Mathew, M.D.; Rao, K.B.S.; Mannan, S.L. Creep deformation and fracture behaviour of a nitrogen-bearing type 316 stainless steel weld metal. J. Nucl. Mater. 1999, 273, 257–264. [Google Scholar] [CrossRef]
- Warren, A.D.; Griffiths, I.J.; Harniman, R.L.; Flewitt, P.E.J.; Scott, T.B. The role of ferrite in Type 316H austenitic stainless steels on the susceptibility to creep cavitation. Mater. Sci. Eng. A 2015, 635, 59–69. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Chen, S.H.; Lv, X.; Jiang, H.C.; Rong, L.J. Role of δ-ferrite in fatigue crack growth of AISI 316 austenitic stainless steel. J. Mater. Sci. Technol. 2022, 114, 7–15. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, Q.Y.; Xie, A.; Rong, L.J. Corrosion behavior of δ-ferrite in AISI 316 austenitic stainless steel exposed to oxygen-saturated lead-bismuth eutectic at 550 °C. Mater. Mater. Charact. 2024, 211, 113930. [Google Scholar] [CrossRef]
- Ciuffini, A.F.; Di Giovanni, F.; Mombelli, D. Utilizzo di un sistema di ispezione ottica automatica atto al rilevamento dei difetti di colata continua basato su algoritmi di Machine Learning per l’analisi dell’incidenza delle marche di oscillazione su bramme di acciaio inossidabile austenitico AISI 316L e 316LI. La Metall. Ital. 2022, 59, 59–68. Available online: https://www.aimnet.it/eng/prodotto.php?id=730&idc=11 (accessed on 5 February 2025). (In Italian).
- Wang, Y.; Chen, L.; Mu, W.Z.; Zhang, Z.R.; Wang, J.; Chen, C. Solidification mode and residual ferrite characteristics of high nickel 316L stainless steel continuous casting billet. Iron Steel 2024, 59, 80–91. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G.G. Delta-ferrite distribution in a continuous casting slab of Fe-Cr-Mn austenitic stainless steel. Metall. Mater. Trans. B 2017, 48, 2324–2333. [Google Scholar] [CrossRef]
- Hou, Z.B.; Cheng, G.G.; Wu, C.C.; Chen, C. Time-Series Analysis Technologies Applied to the Study of Carbon Element Distribution along Casting Direction in Continuous-Casting Billet. Metall. Mater. Trans. B 2012, 43, 1517–1529. [Google Scholar] [CrossRef]
- Pang, J.C.; Qian, G.Y.; Pang, S.; Ma, W.H.; Cheng, G.G. Design of a submerged entry nozzle for optimizing continuous casting of stainless steel slab. J. Iron Steel Res. Int. 2023, 30, 2229–2241. [Google Scholar] [CrossRef]
- Kim, S.K.; Shin, Y.K.; Kim, N.J. Distribution of δ ferrite content in continuously cast type 304 stainless steel slabs. Ironmak. Steelmak. 1995, 22, 316–325. [Google Scholar]
- Spaccarotella, A.; Ridolf, M.R.; Picht, G.; Scheller, P.R. Control of δ-γ Transformation during Solidification of Stainless Steel Slabs in the Mould. Steel Res. Int. 2003, 74, 693–699. [Google Scholar] [CrossRef]
- Niessen, F.; Tiedje, N.S.; Hald, J. Kinetics modeling of delta-ferrite formation and retainment during casting of supermartensitic stainless steel. Mater. Des. 2017, 118, 138–145. [Google Scholar] [CrossRef]
- Zargar, T.; Sadeghi, F.; Kim, J.W.; Lee, J.S.; Heo, Y.U.; Yim, C.H. Kinetic model to investigate the effect of cooling rate on δ-Ferrite behavior and its application in continuous casting of AISI 304 stainless steel. Metals Mater. Int. 2022, 28, 2263–2276. [Google Scholar] [CrossRef]
- dos Santos, F.A.M.; Martorano, M.A.; Padilha, A.F. Delta ferrite formation and evolution during slab processing from an 80-ton industrial heat of AISI 304 austenitic stainless steel. REM Int. Eng. J. 2023, 76, 47–54. [Google Scholar] [CrossRef]
- Suutala, N.; Takalo, T.; Moisio, T. The relationship between solidification and microstructure in austenitic and austenitic-ferritic stainless steel welds. Metall. Mater. Trans. A 1979, 10, 512–514. [Google Scholar] [CrossRef]
- Wang, Y.; Sukenaga, S.; Shibata, H.; Wang, Q.; Mu, W. Combination of In Situ Confocal Microscopy and Calorimetry to Investigate Solidification of Super-and Hyper-Duplex Stainless Steels. Steel Res. Int. 2023, 94, 2200960. [Google Scholar] [CrossRef]
- Li, Y.N.; Zou, D.N.; Li, M.M.; Tong, L.B.; Zhang, Y.B.; Zhang, W. Effect of Cooling Rate on δ-Ferrite Formation and Sigma Precipitation Behavior of 254SMO Super-Austenitic Stainless Steel During Solidification. Metall. Mater. Trans. B 2023, 54, 3497–3507. [Google Scholar] [CrossRef]
- Hao, Y.S.; Cao, G.M.; Li, C.G.; Liu, W.C.; Li, J.; Liu, Z.Y.; Gao, F. Solidification structures of Fe–Cr–Ni–Mo–N super austenitic stainless steel processed by twin-roll strip casting and ingot casting and their segregation evolution behaviors. ISIJ Int. 2018, 58, 1801–1810. [Google Scholar] [CrossRef]
- Chu, H.Y.; Shiue, R.K.; Cheng, S.Y. The Effect of Homogenization Heat Treatment on 316L Stainless Steel Cast Billet. Materials 2024, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.D.; DuPont, J.N.; Perricone, M.J.; Marder, A.R. Phase Transformations and Microstructural Evolution of Mo-Bearing Stainless Steels. Metall. Mater. Trans. A 2007, 38, 86–99. [Google Scholar] [CrossRef]
- Zhai, R.Z.; Zhang, H.L.; Xu, B.; Liu, S.; Xie, B.J.; Sun, M.Y. Elimination of δ-ferrite in N50 steel and its effect on cryogenic mechanical properties. Cryogenics 2022, 126, 03522. [Google Scholar] [CrossRef]
- Kumar, A.; Ganesh, P.; Sharma, V.K.; Manekar, M.; Gupta, R.K.; Singh, R.; Singh, M.K.; Mundar, G.; Kaul, R. Development of Low-Magnetic-Permeability Welds of 316L Stainless Steel. Weld. J. 2021, 100, 323. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Harendranath, C.S.; Raman, R.; Kulkarni, S.D. Microstructural evolution during solidification of austenitic stainless steel weld metals: A color metallographic and electron microprobe analysis study. Mater. Charact. 1997, 38, 53–65. [Google Scholar] [CrossRef]
- Mazumdar, S.; Ray, S.K. Solidification control in continuous casting of steel. Sādhanā 2001, 26, 179–198. [Google Scholar] [CrossRef]
- Wu, C.F.; Li, S.L.; Zhan, C.H.; Wang, X.T. Microstructural evolution in 316LN austenitic stainless steel during solidification process under different cooling rates. J. Mater. Sci. 2016, 51, 2529–2539. [Google Scholar] [CrossRef]
- Chun, E.J.; Baba, H.; Nishimoto, K.; Said, K. Precipitation of sigma and chi phases in δ-ferrite of Type 316FR weld metals. Mater. Charact. 2013, 86, 152–166. [Google Scholar] [CrossRef]
- Padilha, A.F.; Tavares, C.F.; Martorano, M.A. Delta Ferrite Formation in Austenitic Stainless Steel Castings. Mater. Sci. Forum 2012, 730, 733–738. [Google Scholar] [CrossRef]
- Hull, F.C. Delta ferrite and martnesite formation in stainless steels. Weld. J. 1973, 52, 193. [Google Scholar]
- Wang, Y.; Chen, C.; Yang, X.Y.; Zhang, Z.R.; Wang, J.; Li, Z.; Chen, L.; Mu, W.Z. Solidification modes and delta-ferrite of two types 316L stainless steels: A combination of as-cast microstructure and HT-CLSM research. J. Iron Steel Res. Int. 2025, 1–11. [Google Scholar] [CrossRef]
- Siegel, U.; Spies, H.-J.; Eckstein, H.-J. Effect of solidification conditions on the solidification sequence of austenitic chromium-nickel stainless steels. Steel Res. 1986, 57, 25–32. [Google Scholar] [CrossRef]
- Scheller, P.R.; Flesch, R.; Bleck, W. Solidification Morphology and Microstructure Properties at Increased Cooling Rates for 18-8 Cr-Ni Stainless Steel. Adv. Eng. Mater. 1999, 1, 209–214. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, W. Effect of Cooling Rate on Crystallization Behavior during Solidification of Hyper Duplex Stainless Steel S33207: An In Situ Confocal Microscopy Study. Crystals 2023, 13, 1114. [Google Scholar] [CrossRef]
- Chu, H.Y.; Wu, M.C.; Shiue, R.K.; Cheng, S.Y. Investigating the sigma formation in homogenization treatment of the 309L stainless cast billet. J. Mater. Res. Technol. 2025, 35, 2354–2368. [Google Scholar] [CrossRef]
- Niagaj, J.; Mazur, Ł. Review of methods for measurement of ferrite content in high alloyed steels and their welded joints. Weld. Int. 2014, 28, 345–353. [Google Scholar] [CrossRef]
- Song, R.B.; Xiang, J.Y.; Hou, D.P. Characteristics of mechanical properties and microstructure for 316L austenitic stainless steel. J. Iron Steel Res. Int. 2011, 18, 53–59. [Google Scholar] [CrossRef]
- Cicutti, C.; Boeri, R. On the relationship between primary and secondary dendrite arm spacing in continuous casting products. Scr. Mater. 2001, 45, 1455–1460. [Google Scholar] [CrossRef]
- Fukumoto, S.; Oikawa, Y.; Tsuge, S. Prediction of σ Phase Formation in Fe–Cr–Ni–Mo–N Alloys. ISIJ Int. 2010, 50, 445–449. [Google Scholar] [CrossRef][Green Version]
- Hammar, O.; Svensson, U. Solidification and Casting of Metals; The Metal Society: London, UK, 1997; p. 401. [Google Scholar]
- Al-Mukhtar, A.M.; Al-Jumaili, S.A.K.; Fahem, A.H. Effect of Heat Treatments on 302 Austenitic Stainless Steel Spot Weld. Adv. Eng. Forum 2018, 29, 19–25. [Google Scholar] [CrossRef]

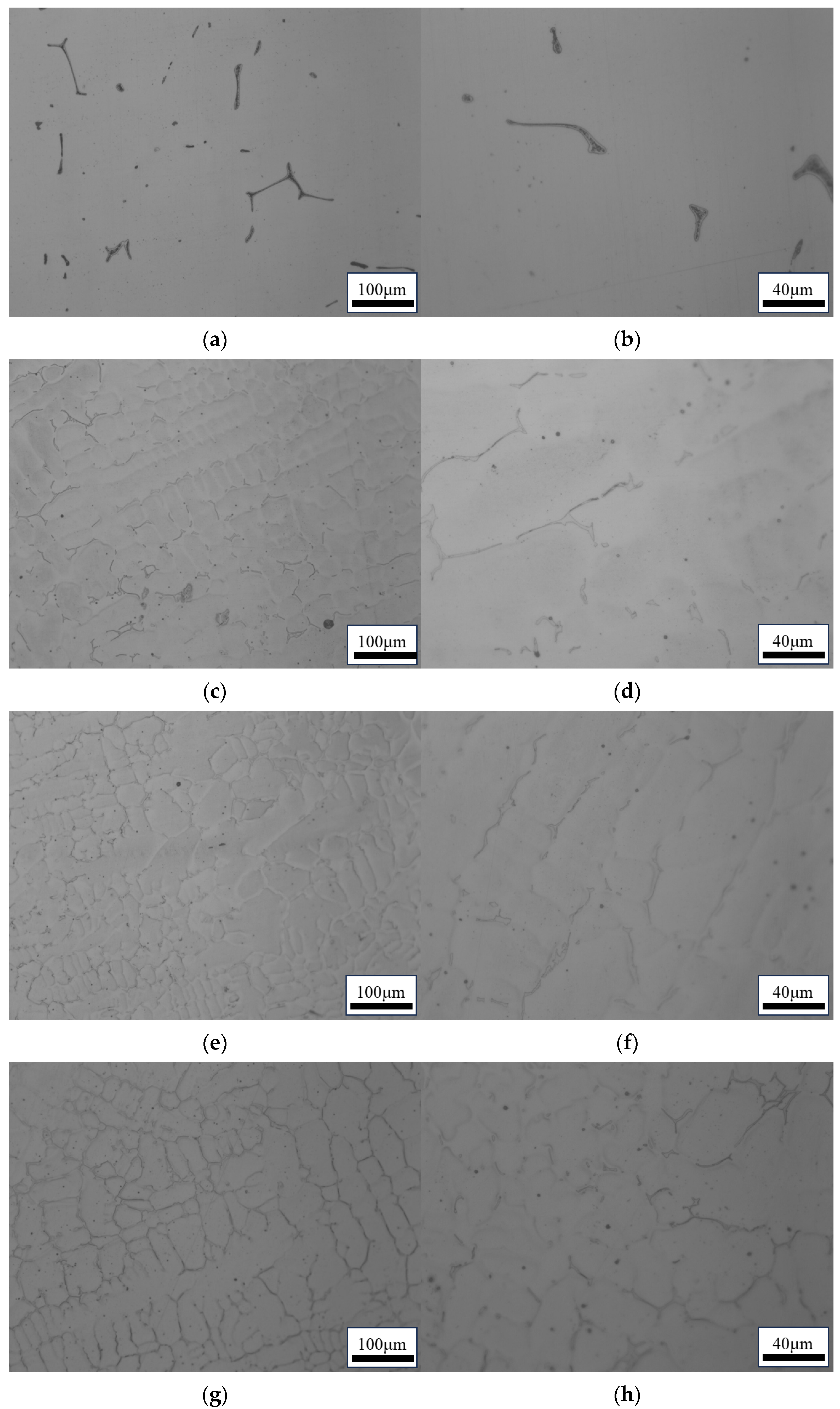
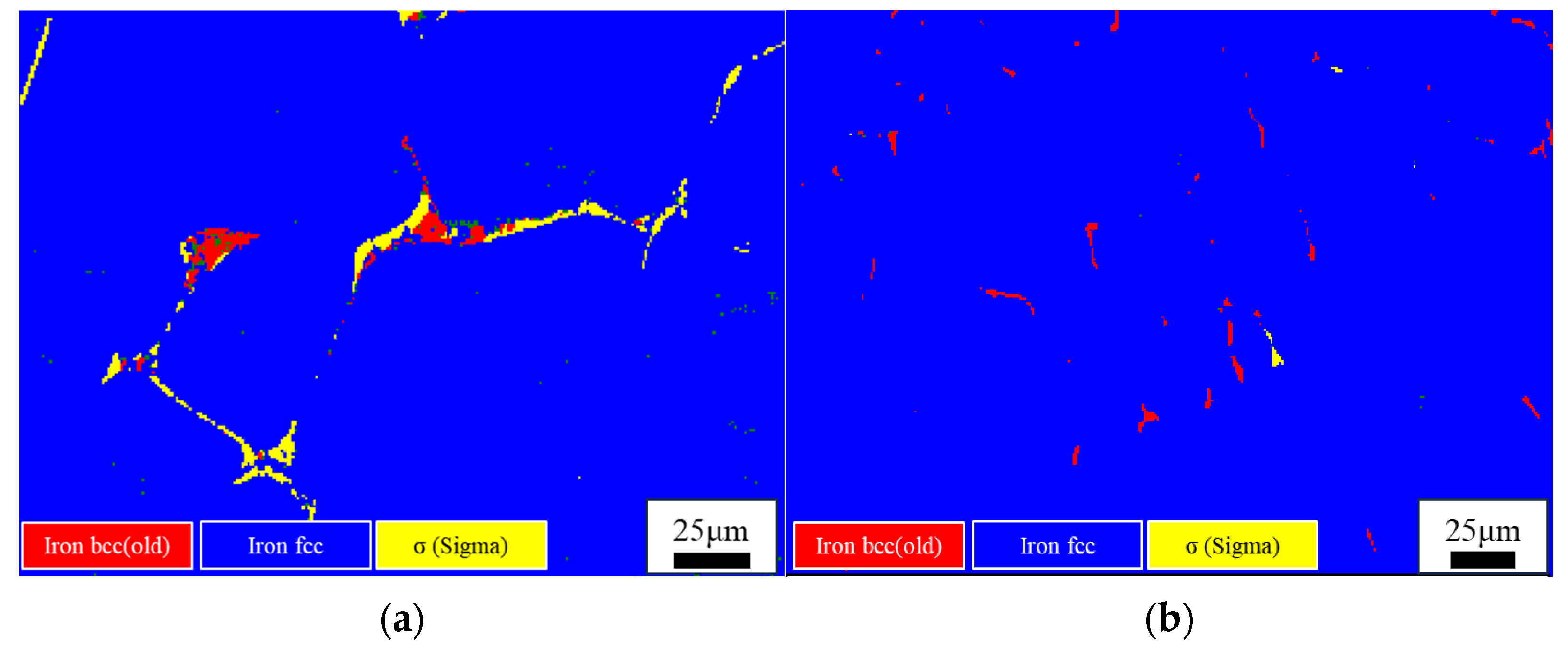
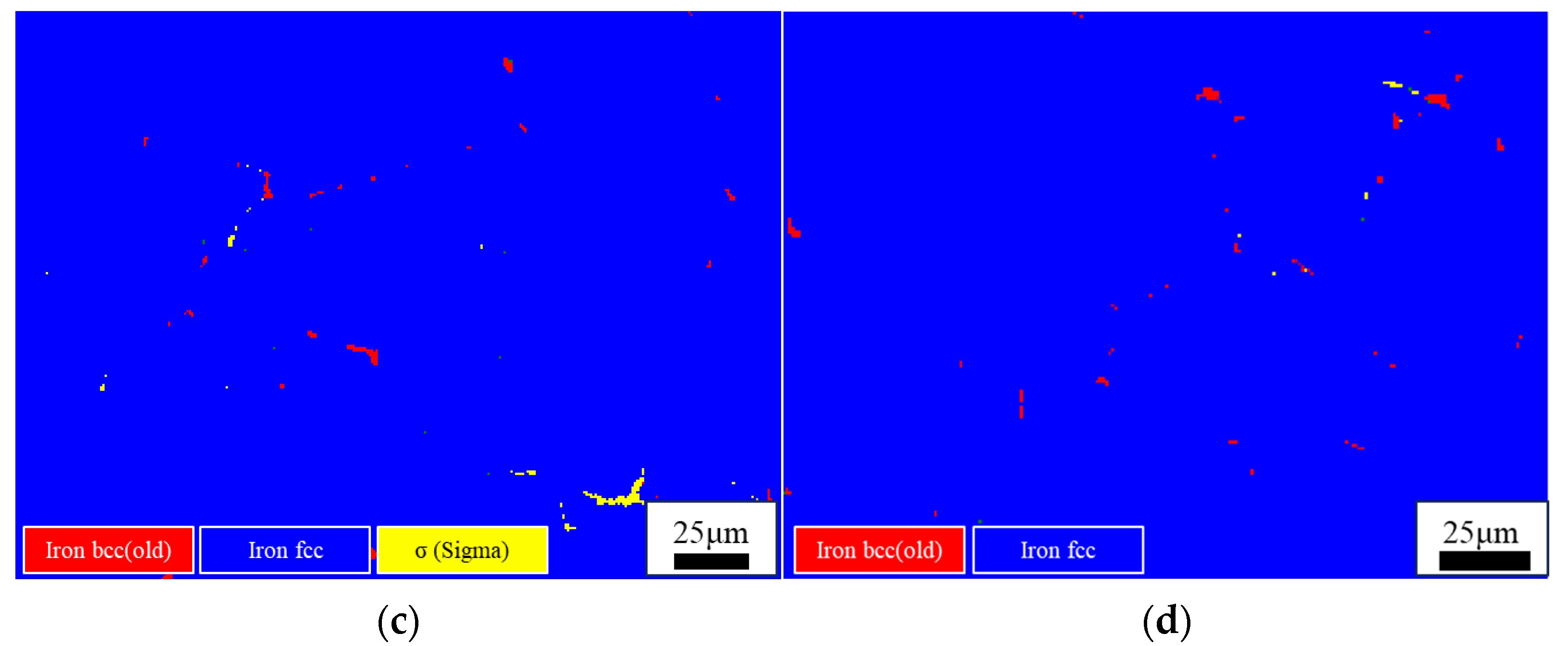


| C | Si | Mn | P | S | Cr | Ni | Mo | N | |
|---|---|---|---|---|---|---|---|---|---|
| AISI 316 | ≤0.080 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 16.00–18.00 | 10.00–14.00 | 2.00–3.00 | ≤0.100 |
| L-316 | 0.022 | 0.52 | 1.14 | 0.035 | 0.001 | 16.58 | 10.00 | 2.01 | 0.043 |
| M-316 | 0.049 | 0.37 | 1.61 | 0.015 | 0.001 | 17.50 | 12.17 | 2.62 | 0.062 |
| H-316 | 0.059 | 0.58 | 1.20 | 0.027 | 0.011 | 17.13 | 14.25 | 2.73 | 0.046 |
| Sample | Cooling Rate (°C/s) | Ferrite Content Average (%) | Ferrite Content Ranges (%) | Phase Constitution | |
|---|---|---|---|---|---|
| Continuous Casting | L-316 | 0.5 | 11.2 | 10.2–13.2 | δ + γ |
| M-316 | 0.4 | 2.9 | 2.6–3.3 | δ + γ + σ (small amount) | |
| H-316 | 0.5 | 2.5 | 2.2–2.7 | δ (small amount) + γ + σ | |
| H-316 Remelting Ingot | Furnace-Cooling | 0.1 | 1.9 | 1.5–2.1 | δ + γ + σ |
| Air-cooling | 3.5 | 1.5 | 1.3–1.8 | δ + γ + σ (small amount) | |
| Oil-cooling | 11.5 | 0.3 | - | δ + γ + σ (small amount) | |
| Water-cooling | 25.1 | 0.4 | - | δ + γ + σ (small amount) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, Y.; Li, Y.; Zhang, Z.; Xue, Z.; Ban, X.; Hu, C.; Li, H.; Tian, J.; Mu, W.; et al. Effect of Nickel Content and Cooling Rate on the Microstructure of as Cast 316 Stainless Steels. Crystals 2025, 15, 168. https://doi.org/10.3390/cryst15020168
Chen L, Wang Y, Li Y, Zhang Z, Xue Z, Ban X, Hu C, Li H, Tian J, Mu W, et al. Effect of Nickel Content and Cooling Rate on the Microstructure of as Cast 316 Stainless Steels. Crystals. 2025; 15(2):168. https://doi.org/10.3390/cryst15020168
Chicago/Turabian StyleChen, Lei, Yang Wang, Yafeng Li, Zhengrui Zhang, Zhixuan Xue, Xinyu Ban, Chaohui Hu, Haixiao Li, Jun Tian, Wangzhong Mu, and et al. 2025. "Effect of Nickel Content and Cooling Rate on the Microstructure of as Cast 316 Stainless Steels" Crystals 15, no. 2: 168. https://doi.org/10.3390/cryst15020168
APA StyleChen, L., Wang, Y., Li, Y., Zhang, Z., Xue, Z., Ban, X., Hu, C., Li, H., Tian, J., Mu, W., Yang, K., & Chen, C. (2025). Effect of Nickel Content and Cooling Rate on the Microstructure of as Cast 316 Stainless Steels. Crystals, 15(2), 168. https://doi.org/10.3390/cryst15020168









