Review of Molten Salt Corrosion in Stainless Steels and Superalloys
Abstract
1. Introduction
2. Research Status of Key CSP Technologies
2.1. Investigation of Corrosion-Resistant Materials for High-Temperature Environments
2.1.1. Stainless Steel
2.1.2. High-Temperature Alloys and Superalloys
2.2. Molten Salt for High-Temperature Heat Storage and Heat Transfer
2.2.1. Nitrate-Based Molten Salts
2.2.2. Carbonates
2.2.3. Sulfates
2.2.4. Chloride Salts
2.2.5. Fluorine Salts
2.3. Phase-Change Materials
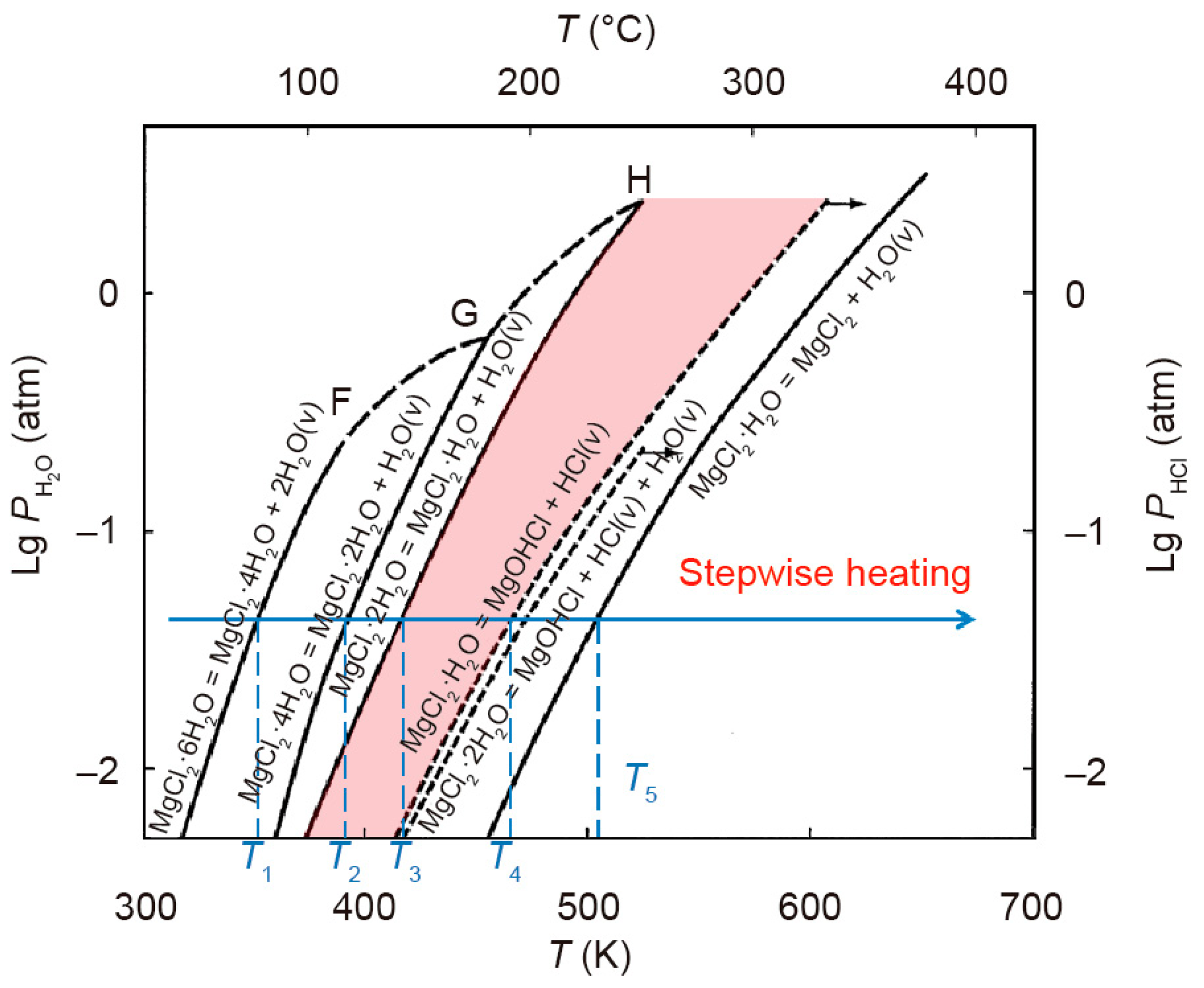
2.4. Impurities in Molten Salt
3. Corrosion Types and Mechanisms of Alloy Materials in Molten Salt Environments
3.1. Corrosion Types
- (1)
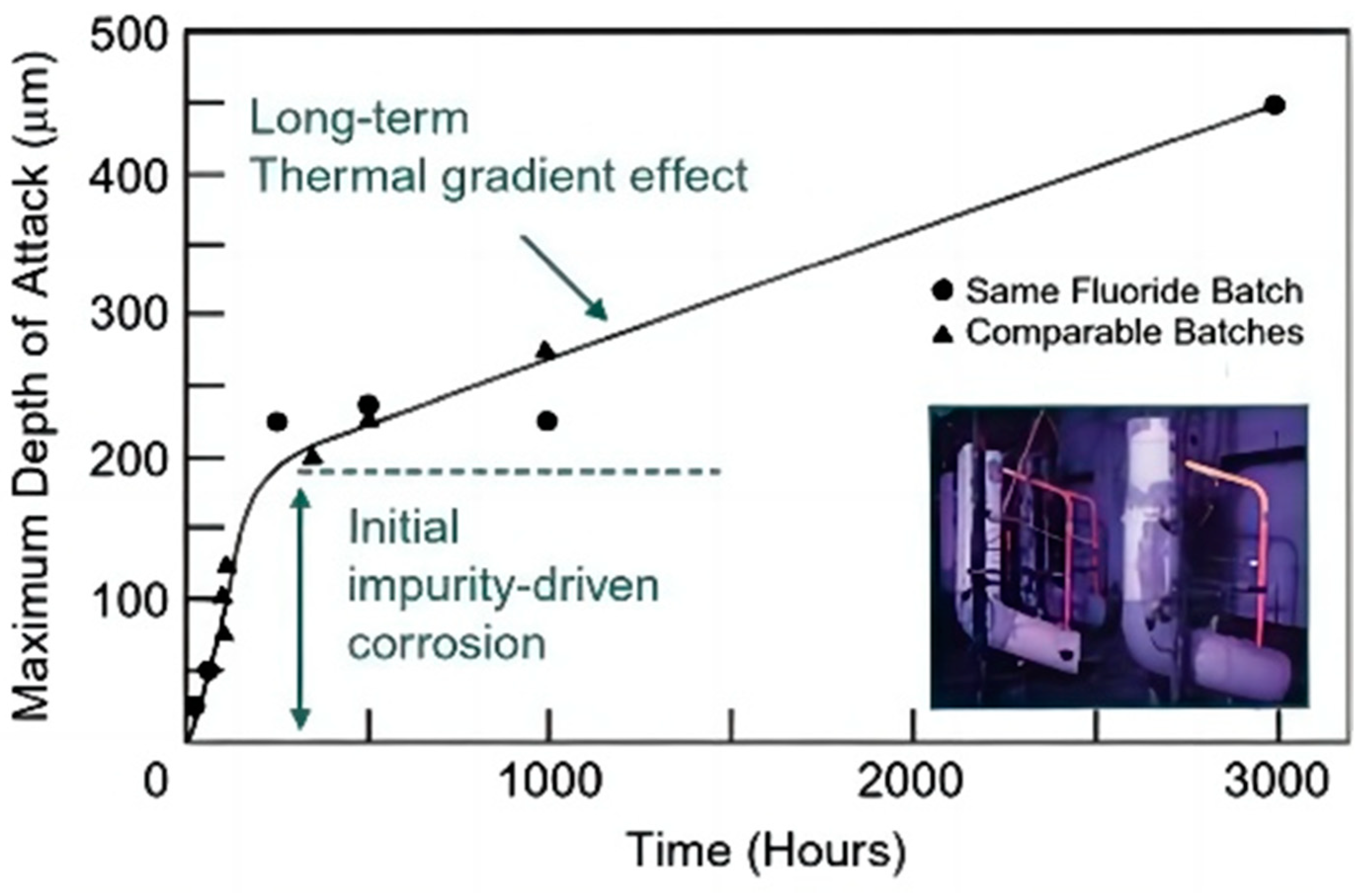
- At a given temperature, regardless of the salt type, both the corrosion rate and the thickness of the corrosion layer increase with the number of thermal cycles.
- (2)
- During thermal cycling, thermal stress induces defects in the corrosion products, weakening the bond between the corrosion layer and the base metal and thereby compromising its integrity and enhancing corrosivity relative to static corrosion.
- (3)
- The structure of the corrosion layer formed during thermal cycling differs from that observed under static conditions. In particular, thermal cycling in eutectic chloride molten salts can cause the corrosion layer to fracture and spall off the sample surface.
- (4)
- Under cyclic conditions in a mixture of eutectic carbonates and magnesium oxide, typical iron and chromium oxides can form in the corrosion products, a phenomenon not observed under isothermal conditions.
3.2. Corrosion Mechanism
4. Corrosion Resistance Technology
4.1. Protective Passivation Films
4.2. Surface-Treatment Preoxidation
4.3. Nanoparticles
4.4. Surface Coating
5. Conclusions
6. Challenges and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSP | Concentrated solar power |
| HTF | Heat transfer fluid |
| IEA | International Energy Agency |
| TES | Thermal energy storage |
| PCM | Phase change material |
| AFA | Alumina-forming austenitic |
| CV | Cyclic voltammetry |
| PDP | Polarization dissolution potential |
| MSRE | Molten Salt Reactor Experiment |
| SWCNT | Single-walled carbon nanotubes |
| ORNL | Oak Ridge National Laboratory |
| HTHC | High-temperature hot corrosion |
| LTHC | Low-temperature hot corrosion |
| SCC | Stress corrosion cracking |
| SFGE | Steel in fuel-grade ethanol |
| TWHs | Terawatt-hours |
References
- IEA. World Energy Outlook 2024; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 28 January 2025).
- Shahabuddin, M.; Alim, M.A.; Alam, T.; Mofijur, M.; Ahmed, S.F.; Perkins, G. A critical review on the development and challenges of concentrated solar power technologies. Sustain. Energy Technol. Assess. 2021, 47, 101434. [Google Scholar] [CrossRef]
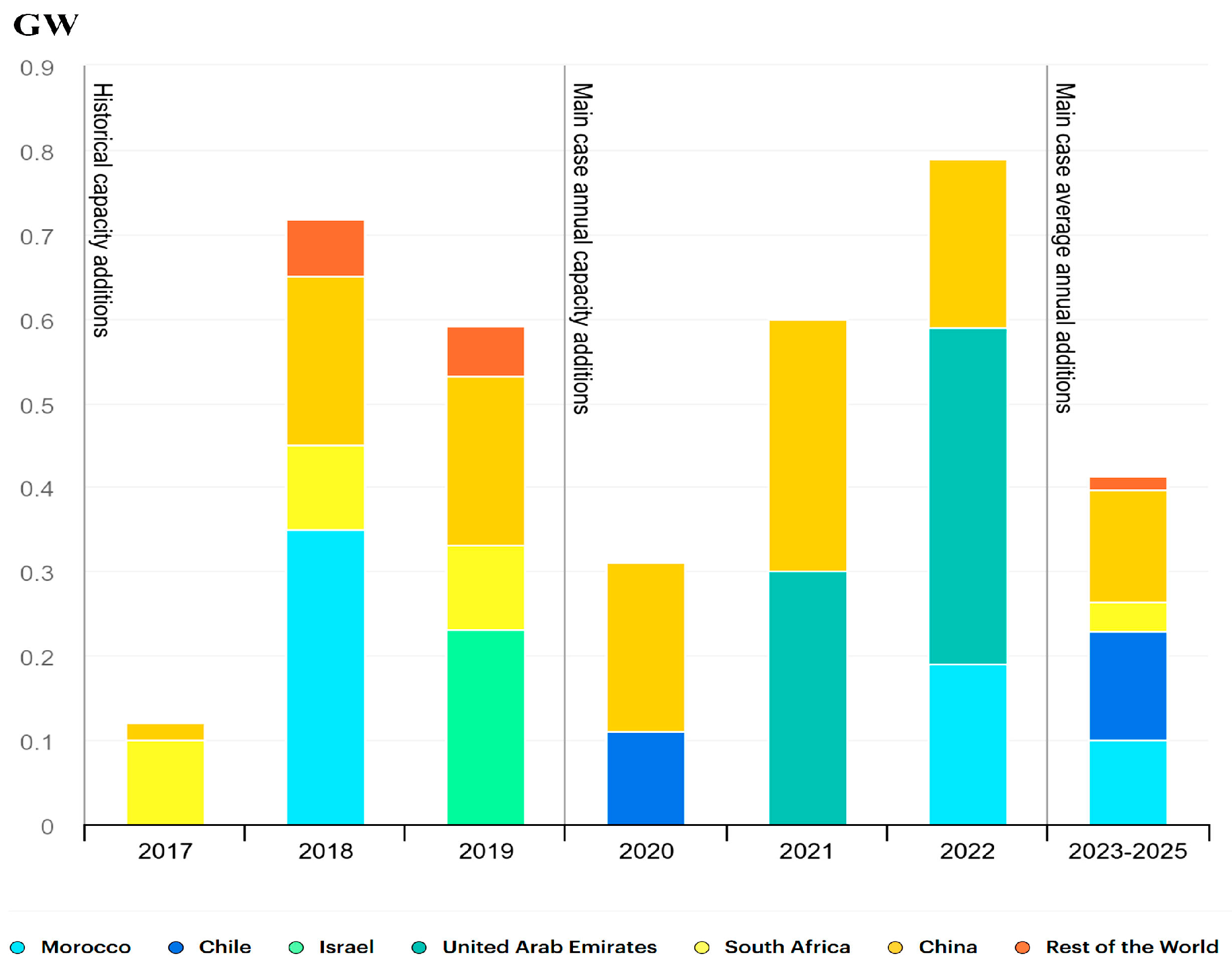
- IEA. CSP Capacity Additions in Selected Countries, 2017–2025; IEA: Paris, France, 2020; Available online: https://www.iea.org/data-and-statistics/charts/csp-capacity-additions-in-selected-countries-2017-2025 (accessed on 28 January 2025).
- U.S. Department of Energy. Generation 3 Concentrating Solar Power Systems (Gen3 CSP). 2021. Available online: https://www.energy.gov/eere/solar/generation-3-concentrating-solar-power-systems-gen3-csp (accessed on 28 January 2025).
- Klasing, F.; Schmitz, M.; Gerdes, C.; Odenthal, C.; Bauer, T. Critical diameter for a single-tank molten salt storage–Parametric study on structural tank design. J. Energy Storage 2024, 101, 113870. [Google Scholar] [CrossRef]
- Klasing, F.; Prenzel, M.; Bauer, T. Repurposing of supercritical coal plants into highly flexible grid storage with adapted 620 °C nitrate salt technology. Appl. Energy 2025, 377, 124524. [Google Scholar] [CrossRef]
- Sarvghad, M.; Steinberg, T.A.; Will, G. Corrosion of stainless steel 316 in eutectic molten salts for thermal energy storage. Sol. Energy 2018, 172 Pt 2, 198–203. [Google Scholar]
- Fernández, A.G.; Gomez-Vidal, J.; Oró, E.; Kruizenga, A.; Solé, A.; Cabeza, L.F. Mainstreaming commercial CSP systems: A technology review. Renew. Energy 2019, 140, 152–176. [Google Scholar]
- Sarvghad, M.; Maher, S.D.; Collard, D.; Tassan, M.; Will, G. Materials compatibility for the next generation of Concentrated Solar Power plants. Energy Storage Mater. 2018, 14, 179–198. [Google Scholar]
- Gomez-Vidal, J.C.; Tirawat, R. Corrosion of alloys in a chloride molten salt (NaCl-LiCl) for solar thermal technologies. Sol. Energy Mater. Sol. Cells 2016, 157, 234–244. [Google Scholar]
- Sah, S.P.; Tada, E.; Nishikata, A. Corrosion behaviour of austenitic stainless steels in carbonate melt at 923 K under controlled CO2-O2 environment. Corros. Sci. 2018, 133, 310–317. [Google Scholar]
- Wang, W.; Guan, B.; Li, X.; Lu, J.; Ding, J. Corrosion behavior and mechanism of austenitic stainless steels in a new quaternary molten salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2019, 194, 36–46. [Google Scholar]
- Pooja, M.; Ravishankar, K.S.; Madav, V. High temperature corrosion behaviour of stainless steels and Inconel 625 in hydroxide salt. Mater. Today Proc. 2021, 46, 2612–2615. [Google Scholar]
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot corrosion behavior of commercial alloys in thermal energy storage material of molten MgCl2/KCl/NaCl under inert atmosphere. Sol. Energy Mater. Sol. Cells 2018, 184, 22–30. [Google Scholar]
- Sun, H.; Wang, J.; Li, Z.; Zhang, P.; Su, X. Corrosion behavior of 316SS and Ni-based alloys in a ternary NaCl-KCl-MgCl2 molten salt. Sol. Energy 2018, 171, 320–329. [Google Scholar]
- Luo, J.; Deng, C.K.; Li, N.; Han, R.; Liu, H.; Wang, J.; Cui, X.; Xiong, T. Corrosion behavior of SS316L in ternary Li2CO3–Na2CO3–K2CO3 eutectic mixture salt for concentrated solar power plants. Sol. Energy Mater. Sol. Cells 2020, 217, 110679. [Google Scholar]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. I: Pre-oxidation treatment and isothermal corrosion tests. Sol. Energy Mater. Sol. Cells 2017, 166, 222–233. [Google Scholar]
- Wang, J.W.; Zhang, C.Z.; Li, Z.H.; Zhou, H.X.; He, J.X.; Yu, J.C. Corrosion behavior of nickel-based superalloys in thermal storage medium of molten eutectic NaCl-MgCl2 in atmosphere. Sol. Energy Mater. Sol. Cells 2017, 164, 146–155. [Google Scholar]
- Gage, S.H.; Kesseli, D.; Dupree, J.; Kimbal, C.; Rigby, J.; Yates, J.; Morrison, B.; Bigham, G.; Turchi, C.S. Technical and economic feasibility of molten chloride salt thermal energy storage systems. Sol. Energy Mater. Sol. Cells 2021, 226, 111099. [Google Scholar] [CrossRef]
- Du, B.C.; He, Y.L.; Qiu, Y.; Liang, Q.; Zhou, Y. Investigation on heat transfer characteristics of molten salt in a shell-and-tube heat exchanger. Int. Commun. Heat Mass Transf. 2018, 96, 61–68. [Google Scholar]
- Liu, S.; Liu, Z.; Wang, Y.; Tang, J. A comparative study on the high temperature corrosion of TP347H stainless steel, C22 alloy and laser-cladding C22 coating in molten chloride salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar]
- Guo, L.; Liu, Q.; Yin, H.; Pan, T.J.; Tang, Z. Excellent corrosion resistance of 316 stainless steel in purified NaCl-MgCl2 eutectic salt at high temperature. Corros. Sci. 2020, 166, 108473. [Google Scholar]
- Fernández, A.G.; Cabeza, L.F. Corrosion evaluation of eutectic chloride molten salt for new generation of CSP plants. Part 2: Materials screening performance. J. Energy Storage 2020, 29, 101381. [Google Scholar]
- Fernández, A.G.; Muñoz-Sánchez, B.; Nieto-Maestre, J.; García-Romero, A. High temperature corrosion behavior on molten nitrate salt-based nanofluids for CSP plants. Renew. Energy 2019, 130, 902–909. [Google Scholar]
- Grosu, Y.; Bondarchuk, O.; Faik, A. The effect of humidity, impurities and initial state on the corrosion of carbon and stainless steels in molten HitecXL salt for CSP application. Sol. Energy Mater. Sol. Cells 2018, 174, 34–41. [Google Scholar]
- Palacios, A.; Navarro, M.E.; Jiang, Z.; Avila, A.; Qiao, G.; Mura, E.; Ding, Y. High-temperature corrosion behaviour of metal alloys in commercial molten salts. Sol. Energy 2020, 201, 437–452. [Google Scholar]
- Kondo, M.; Nagasaka, T.; Sagara, A.; Noda, N.; Muroga, T.; Xu, Q.; Nagura, M.; Suzuki, A.; Terai, T. Metallurgical study on corrosion of austenitic steels in molten salt LiF–BeF2 (Flibe). J. Nucl. Mater. 2009, 386, 685–688. [Google Scholar]
- Atmani, H.; Rameau, J.J. Stress corrosion cracking of 304L stainless steel in molten salts media. Corros. Sci. 1984, 24, 279–285. [Google Scholar]
- García-Martín, G.; Lasanta, M.I.; Encinas-Sánchez, V.; Migue, M.T.; Pérez, F.J. Evaluation of corrosion resistance of A516 Steel in a molten nitrate salt mixture using a pilot plant facility for application in CSP plants. Sol. Energy Mater. Sol. Cells 2017, 161, 226–231. [Google Scholar]
- Ruiz-Cabañas, F.J.; Prieto, C.; Madina, V.; Fernández, A.; Cabeza, L. Materials selection for thermal energy storage systems in parabolic trough collector solar facilities using high chloride content nitrate salts. Sol. Energy Mater. Sol. Cells 2017, 163, 134–147. [Google Scholar]
- Sumit, K.; Hanke, A.; Bonk, A.; Bauer, T. Influence of atmosphere and austenitic stainless steel on the solar salt corrosivity. Heliyon 2024, 10, e25966. [Google Scholar]
- Ding, W.; Bonk, A.; Gussone, J.; Bauer, T. Electrochemical measurement of corrosive impurities in molten chlorides for thermal energy storage. J. Energy Storage 2018, 15, 408–414. [Google Scholar]
- Swaminathan, S.; Kumar, S.; Kranzmann, A.; Hesse, R.; Goldbeck, H.; Fantin, A. Corrosion characteristics of 316L stainless steel in oxide-rich molten solar salt at 600 °C. Sol. Energy Mater. Sol. Cells 2024, 278, 113176. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Wen, B.; Ye, Q.; Zhang, N. Corrosion behaviours of iron-chromium-aluminium steel near the melting point of various eutectic salts. Sol. Energy Mater. Sol. Cells 2020, 210, 110510. [Google Scholar] [CrossRef]
- Bhuyan, P.; Pradhan, S.K.; Mitra, R.; Mandal, S. Evaluating the efficiency of grain boundary serrations in attenuating high-temperature hot corrosion degradation in Alloy 617. Corros. Sci. 2019, 149, 164–177. [Google Scholar] [CrossRef]
- Pillai, R.; Raiman, S.S.; Pint, B.A. First steps toward predicting corrosion behavior of structural materials in molten salts. J. Nucl. Mater. 2021, 546, 152755. [Google Scholar] [CrossRef]
- Sun, H.; Ding, X.; Ai, H.; Lei, G.; Yang, X.; Wang, J. Interaction mechanisms of a Hastelloy N-316L stainless steel couple in molten LiF-NaF-KF salt. Corros. Sci. 2020, 164, 108317. [Google Scholar] [CrossRef]
- Cho, H.S.; Van Zee, J.W.; Shimpalee, S.; Tavakoli, B.A.; Weidner, J.W.; Garcia-Diaz, B.L.; Martinez-Rodriguez, M.J.; Olson, L.; Gray, J. Dimensionless analysis for predicting Fe-Ni-Cr alloy corrosion in molten salt systems for concentrated solar power systems. Corrosion 2016, 72, 742–760. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, J.; Zheng, Y.; Yu, H.; Yang, P.; La, P. Corrosion Behavior and Mechanism of High-Aluminum Inconel 625 in Chlorinated Salts. Crystals 2025, 15, 144. [Google Scholar] [CrossRef]
- Nishikata, A.; Numata, H.; Tsuru, T. Electrochemistry of molten salt corrosion. Mater. Sci. Eng. A 1991, 146, 15–31. [Google Scholar] [CrossRef]
- Bell, S.; Steinberg, T.; Will, G. Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renew. Sustain. Energy Rev. 2019, 114, 109328–109336. [Google Scholar] [CrossRef]
- Raiman, S.S.; Lee, S. Aggregation and data analysis of corrosion studies in molten chloride and fluoride salts. J. Nucl. Mater. 2018, 511, 523–535. [Google Scholar] [CrossRef]
- Mohan, G.; Venkataraman, M.; Gomez-Vidal, J.; Coventry, J. Assessment of a novel ternary eutectic chloride salt for next generation high-temperature sensible heat storage. Energy Convers. Manag. 2018, 167, 156–164. [Google Scholar] [CrossRef]
- Chen, H.Z.; Li, B.R.; Wen, B.; Ye, Q.; Zhang, N. Corrosion resistance of iron-chromium-aluminium steel in eutectic molten salts under thermal cycling conditions. Corros. Sci. 2020, 173, 108798. [Google Scholar]
- Yu, Y.; Zhao, C.; Tao, Y.; Chen, X.; He, Y. Superior thermal energy storage performance of NaCl-SWCNT composite phase change materials: A molecular dynamics approach. Appl. Energy 2021, 290, 116799–116805. [Google Scholar]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar]
- Li, Q.; Li, C.; Du, Z.; Jiang, F.; Ding, Y. A review of performance investigation and enhancement of shell and tube thermal energy storage device containing molten salt based phase change materials for medium and high temperature applications. Appl. Energy 2019, 255, 113806–113811. [Google Scholar]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar]
- Ding, W.; Bauer, T. Progress in Research and Development of Molten Chloride Salt Technology for Next Generation Concentrated Solar Power Plants. Engineering 2021, 7, 334–347. [Google Scholar]
- Grégoire, B.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part I: NaCl–KCl binary system. Sol. Energy Mater. Sol. Cells 2020, 215, 110659. [Google Scholar] [CrossRef]
- Ong, T.C.; Sarvghad, M.; Lippiatt, K.; Griggs, L.; Ryan, H.; Will, G.; Steinberg, T.A. Review of the solubility, monitoring, and purification of impurities in molten salts for energy storage in concentrated solar power plants. Renew. Sustain. Energy Rev. 2020, 131, 110006–110012. [Google Scholar] [CrossRef]
- Ding, W.; Yang, F.; Bonk, A.; Bauer, T. Molten chloride salts for high-temperature thermal energy storage: Continuous electrolytic salt purification with two Mg-electrodes and alternating voltage for corrosion control. Sol. Energy Mater. Sol. Cells 2021, 223, 110979. [Google Scholar]
- Fernandez, A.G.; Cabeza, L.F. Corrosion evaluation of eutectic chloride molten salt for new generation of CSP plants. Part 1: Thermal treatment assessment. J. Energy Storage 2020, 27, 101125.1–101125.7. [Google Scholar]
- Mohan, G.; Venkataraman, M.; Gomez-Vidal, J.; Coventry, J. Thermo-economic analysis of high-temperature sensible thermal storage with different ternary eutectic alkali and alkaline earth metal chlorides. Sol. Energy 2018, 176, 350–357. [Google Scholar]
- Tian, H.; Wang, W.; Ding, J.; Wei, X.L. Thermal performance and economic evaluation of NaCl–CaCl2 eutectic salt for high-temperature thermal energy storage. Energy 2021, 227, 120412–120420. [Google Scholar]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar]
- Grosu, Y.; Udayashankar, N.; Bondarchuk, O.; González-Fernández, L.; Faik, A. Unexpected effect of nanoparticles doping on the corrosivity of molten nitrate salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 91–97. [Google Scholar]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar]
- Hu, Q.; Qiu, Y.B.; Guo, X.P.; Huang, J.Y. Crevice corrosion of Q235 carbon steels in a solution of NaHCO3 and NaCl. Corros. Sci. 2010, 52, 1205–1212. [Google Scholar]
- Cao, L.; Frankel, G.S. Effect of chloride on stress corrosion cracking susceptibility of carbon steel in simulated fuel grade ethanol. Electrochim. Acta 2013, 104, 255–266. [Google Scholar]
- Yang, M.Z.; Wilmott, M.; Luo, J.L. Crevice corrosion behavior of A516-70 carbon steel in solutions containing inhibitors and chloride ions. Thin Solid Film. 1998, 326, 180–188. [Google Scholar]
- Seifert, H.P.; Ritter, S. The influence of ppb levels of chloride impurities on the stress corrosion crack growth behaviour of low-alloy steels under simulated boiling water reactor conditions. Corros. Sci. 2016, 108, 134–147. [Google Scholar]
- Ning, F.; Tan, J.; Zhang, Z.; Wang, X.; Wu, X.; Han, E.; Ke, W. Nodular corrosion inside the crevice of Alloy 690 in deaerated high-temperature chloride solution. Corros. Sci. 2021, 185, 109442. [Google Scholar]
- Ma, H. Corrosion of Metallic Materials in High-Temperature Chloride Salt Environment. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2003. [Google Scholar]
- Haanappel, V.A.C.; Fransen, Τ.; Gellings, P.J. Chlorine-Induced High Temperature Corrosion: II. The Tedmon Equation as a Theoretical Approach of the Kinetics. High Temp. Mater. Process. 1992, 10, 2. [Google Scholar] [CrossRef]
- Kochmańska, A.E.; Kochmański, P. Failure analysis of grate in a municipal solid waste incineration plant. Eng. Fail. Anal. 2024, 165, 108823. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, G.; Liu, Z.; She, W.; Zhang, Y. Corrosion Behavior and Mechanism of Alloy Steel Submitted to Chloride and Sulfate. Mater. Rep. 2025, 39, 24030019. [Google Scholar]
- Yang, S.; Xia, X.; Zhang, L.; Bai, X.; Yao, H. Dissolved species predominance diagram and Pourbaix diagram of Fe-H2O system at different temperatures. Min. Metall. 2023, 32, 64–73. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina forming alloys against molten chlorides for energy production. II: Electrochemical impedance spectroscopy under thermal cycling conditions. Sol. Energy Mater. Sol. Cells 2017, 166, 234–245. [Google Scholar] [CrossRef]
- Liu, Z. Study on the Influence of Aluminum Element on the Microstructure and Properties of HP40 Heat-Resistant Casting Alloy. Ph.D. Thesis, Lanzhou University of Technology, Lanzhou, China, 2008. [Google Scholar]
- La, P.; Liu, H.; Meng, Q.; Wei, Y. Microstructure and Mechanical Properties of 310S Heat-resistant Steel with Different Al Contents after Hot Rolling. J. Lanzhou Univ. Technol. 2012, 38, 15–20. [Google Scholar]
- La, P.; Sa, X.; Liu, H.; Meng, Q.; Wei, Y.; Lu, X. Welding Performance of High-aluminum 310S Heat-resistant Steel Plate. J. Iron Steel Res. 2014, 26, 34–40. [Google Scholar]
- Liu, H. Microstructure and Properties of Hot-Rolled High-Aluminum 310S Heat-Resistant Steel and the Action Mechanism of Aluminum Element. Ph.D. Thesis, Lanzhou University of Technology, Lanzhou, China, 2012. [Google Scholar]
- Xue, P.; La, P.; Liu, H.; Meng, Q.; Wei, Y. Influence of Al on High-temperature Tensile Properties of 310S Heat-resistant Steel. J. Iron Steel Res. 2015, 27, 46–51. [Google Scholar]
- La, P.; Wang, H.; Liu, S.; Shen, D.; Liu Xi Ju, Q. Influence of Al Element on High-temperature Compressive Properties of 310S Heat-resistant Steel. J. Lanzhou Univ. Technol. 2010, 36, 9–12. [Google Scholar]
- Meng, Q. Microstructure Evolution of Cast and Hot-Rolled High-Aluminum 304, 316L and 310S Stainless Steels and the Action Mechanism of Aluminum Element. Ph.D. Thesis, Lanzhou University of Technology, Lanzhou, China, 2016. [Google Scholar]
- Wei, Y.; Zhan, F.; Li, Z.; Shi, Y.; Zhu, M.; Zheng, Y.; Sheng, J.; La, P. Superior strength and ductility of 316L stainless steel induced by micro/nano/ultrafine-grains multiphase complex structures. Mater. Sci. Eng. A 2022, 859, 144194. [Google Scholar] [CrossRef]
- Edeleanu, C.; Littlewood, R. Thermodynamics of corrosion in fused chlorides. Electrochim. Acta 1960, 3, 195–207. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, M.; Pei, Y.; Li, S.; Gong, S. Study on abnormal hot corrosion behavior of nickel-based single-crystal superalloy at 900 °C after drilling. npj Mater. Degrad. 2021, 5, 21. [Google Scholar]
- Wei, Y.; La, P.; Jin, J.; Du, M.; Zheng, Y.; Zhan, F.; Sheng, J.; Yu, H.; Zhu, M. Corrosion Behavior of Aluminum-Forming Alloy 310S for Application in Molten Chloride Salt CSP Thermal Storage Tank. Front. Mater. 2022, 9, 886285. [Google Scholar]
- Wei, Y.; Cao, J.; Yu, H.; Sheng, J.; La, P. Effect of Mg Addition on Molten Chloride Salt Corrosion Resistance of 310S Stainless Steel with Aluminum. Metals 2024, 14, 1109. [Google Scholar] [CrossRef]
- Mahobia, G.S.; Paulose, N.; Mannan, S.L.; Sudhakar, R.G.; Chattopadhyay, K.; Santhi Srinivas, N.C.; Singh, V. Effect of hot corrosion on low cycle fatigue behavior of superalloy IN718. Int. J. Fatigue 2014, 59, 272–281. [Google Scholar]
- Nithiyanantham, U.; Grosu, Y.; Gonzalez-Fernandez, L.; Zaki, A.; Mirena Igartua, J.; Faik, A. Corrosion aspects of molten nitrate salt-based nanofluids for thermal energy storage applications. Sol. Energy 2019, 189, 219–227. [Google Scholar]
- Ma, B.; Shin, D.; Banerjee, D. One-step synthesis of molten salt nanofluid for thermal energy storage application–a comprehensive analysis on thermophysical property, corrosion behavior, and economic benefit. J. Energy Storage 2021, 35, 102278. [Google Scholar] [CrossRef]
- Hu, S.; Finklea, H.; Liu, X. A review on molten sulfate salts induced hot corrosion. J. Mater. Sci. Technol. 2021, 90, 243–254. [Google Scholar]
- Xiang, Z.D.; Rose, S.R.; Datta, P.K. Pack codeposition of Al and Cr to form diffusion coatings resistant to high temperature oxidation and corrosion for γ-TiAl. Mater. Sci. Technol. 2002, 18, 1479–1484. [Google Scholar]

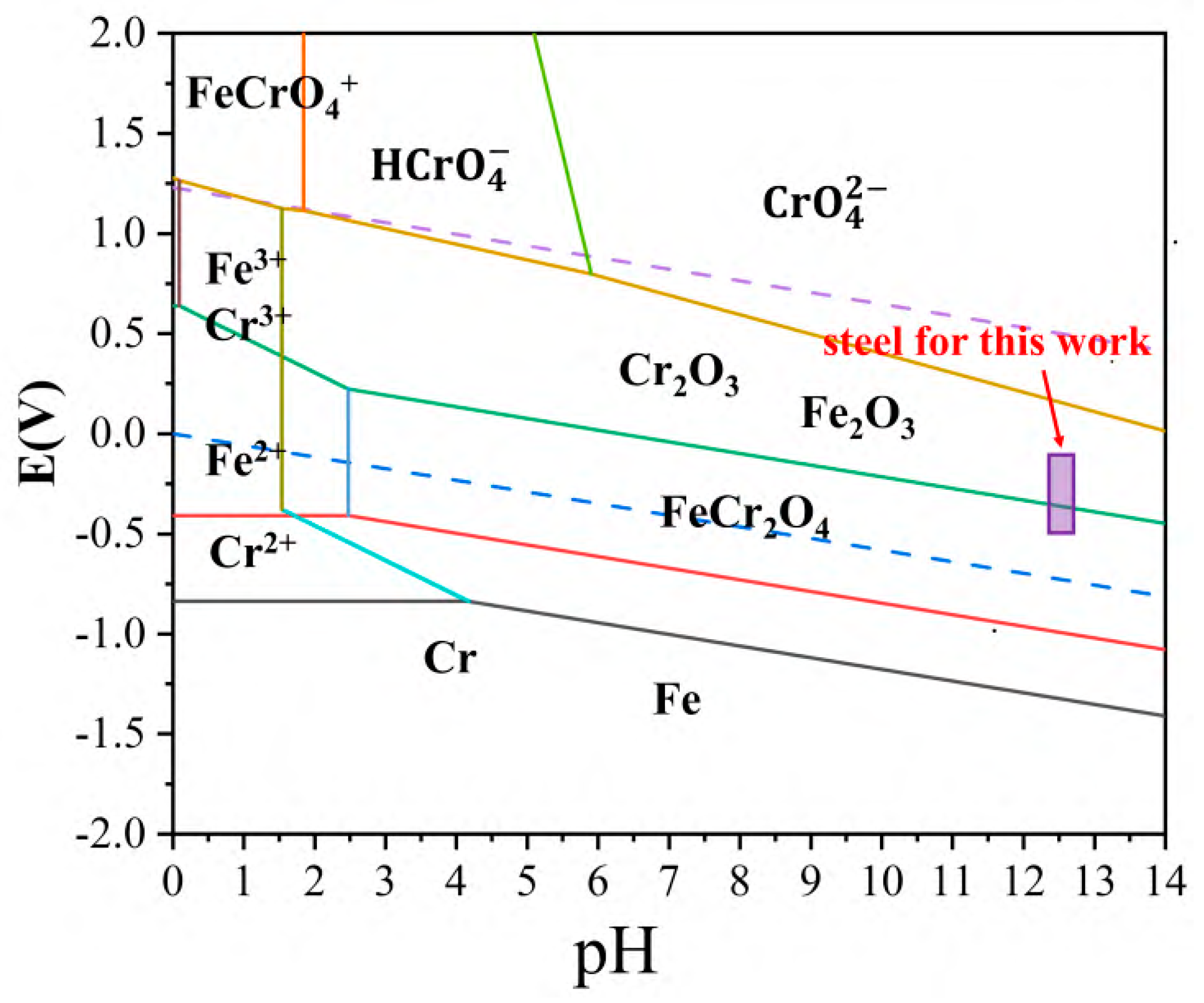
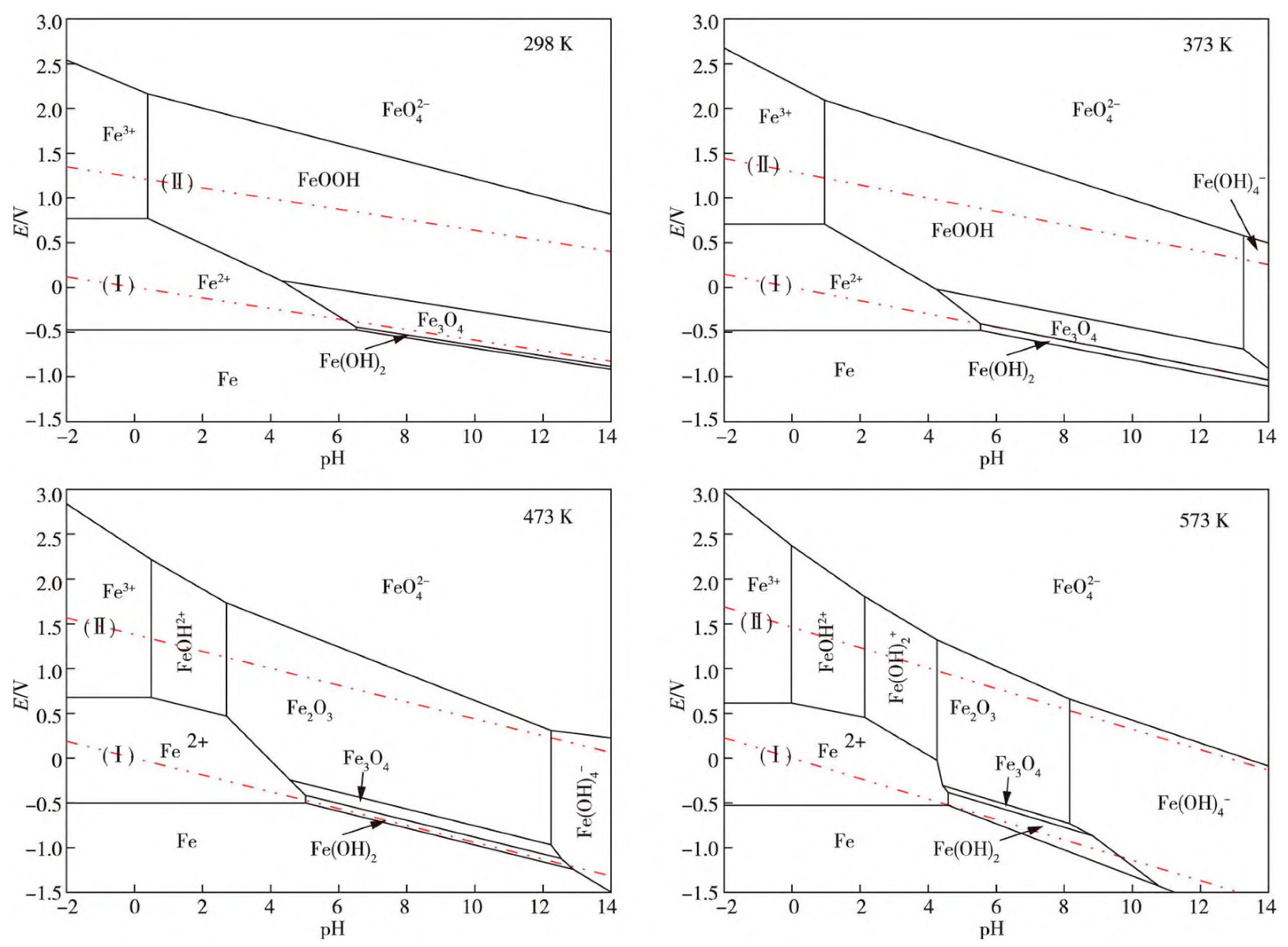
| No. | Alloy | Molten Salt | Corrosion Condition | Corrosion Rate (μm) | Corrosion Products |
|---|---|---|---|---|---|
| [12] | 310S 316L 321 | KNO3/NaNO2/NaNO3/KCl | Atmosphere 500 °C 1008 h | 2.22 µm/year 2.96 µm/year 3.63 µm/year | Fe3O4 > NiCr2O4 > (Fe, Ni) Fe2O4 |
| [21] | TP347H | KCl/NaCl (98.6–1.4 wt.%) | 750 °C 96 h | 6400 g/m2 | Cr2O3, NiO; Fe2O3, Fe3O4, NiFe2O4/Ni1.43Fe1.7O4 |
| [32] | 310S 316L 304 | Li2CO3/Na2CO3/ K2CO3 | 700 °C Electrochemical corrosion | 0.5 ± 0.1 mm/year 2.9 ± 0.4 mm/year 3.5 ± 0.1 mm/year | Cr-rich oxide, LiCrO2, LiFeO2, Fe3O4, Li0.3Ni0.7O |
| [13] | 316 310 | NaOH | 700 °C 48 h | 11.1 mm/year 9.1 mm/year | NiO, Cr2O3 |
| [7] | SS304 316L | LiF-BeF2 | 600 °C 1000 h | 10.6 µm/year 5.4 µm/year | |
| [2] | SS316 | Solar salt | 600 °C Atmosphere | 15.9 µm/year | |
| [2] | SS321 | Hitec | 570 °C Atmosphere | 2 µm/year | |
| [2] | 304 | ZnCl2/NaCl/KCl (68.6–7.5–23.9) | 400 °C Inert gas | 15 µm/year | |
| [10] | 347 | NaCl/LiCl (34.42–65.58) | 650 °C Inert gas Electrochemical corrosion | 7490 µm/year | |
| [10] | 310 | NaCl/LiCl (34.42–65.58) | 650 °C Inert gas/ 700 °C Electrochemical corrosion | 6420 µm/year 12,451 µm/year | |
| [23] | 304 | KCl/MgCl2/NaCl (20.4–55.1–24.5 wt.%) | 720 °C 8 h Electrochemical corrosion | 8.19 mm/year | |
| [14] | SS 310 | MgCl2/KCl/NaCl | 700 °C 500 h | 1581 µm/year | MgO, MgCr2O4, MgSiO3 |
| [29] | A516 carbon steel | NaNO3/KNO3 (60–40 wt.%) | 500 °C 100 h Dynamic test simulation | Dynamic 3.003 mg/cm2 Static 1.935 mg/cm2 | Fe2O3, Fe3O4 |
| [33] | 316L | NaNO3/KNO3 (60/40 wt.%) | 600 °C 168 h | 0.5 mg/cm2 | Cr, Fe3O4, Na, FeO2 |
| No. | Alloy | Molten Salt | Corrosion Condition | Corrosion Rate (μm) | Corrosion Products |
|---|---|---|---|---|---|
| [21] | TP347H Hastelloy C22 | 98.6 wt.% KCl and 1.4 wt.% NaCl | 750 °C 96 h | 6400 g/m2 6391 g/m2 | Cr2O3, NiO; Fe2O3, Fe3O4, NiFe2O4/Ni1.43Fe1.7O4; |
| [13] | Inconel 625 316 310 | NaOH | 700 °C 48 h | 4.93 mm/year 11.1 mm/year 9.1 mm/year | NiO, Cr2O3 |
| [14] | Hastelloy 230 | Solar salt | 600 °C atmosphere | 47 µm/year | |
| [14] | Hastelloy C22 | ZnCl2/NaCl/KCl (68.6–7.5–23.9) | 400 °C/800 °C inert gas | 8 µm/year 12 µm/year | |
| [14] | Hastelloy C276 | ZnCl2/NaCl/KCl (68.6–7.5–23.9) | 500 °C/400 °C/800 °C inert gas | 80 µm/year 3 µm/year 5 µm/year | |
| [14] | Inconel 625 HastelloyX Hastelloy B-3 | MgCl2/NaCl/CaCl2 (14.95–53.43–31.61) | 600 °C atmosphere | 121 µm/year 153 µm/year 145 µm/year | |
| [10] | Incoloy800H | MgCl2/NaCl/CaCl2 (14.95–53.43–31.61) | 650 °C inert gas/ 700 °C electrochemical corrosion | 5940 µm/year 14,311 µm/year | |
| [10] | Inconel 625 | MgCl2/NaCl/CaCl2 (14.95–53.43–31.61) | 650 °C inert gas electrochemical corrosion | 2800 µm/year | |
| [23] | 304 Inconel 702 Haynes 224 | KCl/MgCl2 /NaCl (20.4–55.1–24.5 wt.%) | 720 °C 8 h electrochemical corrosion | 8.19 mm/year 6.34 mm/year 3.12 mm/year | MgCr2O4, Al2O3 |
| [14] | In 800H | KCl/MgCl2/NaCl (20.4–55.1–24.5 wt.%) | 720 °C 8 h electrochemical corrosion | 364 µm/year | MgO, MgCr2O4 |
| [14] | Ha C-276 | KCl/MgCl2/NaCl (20.4–55.1–24.5 wt.%) | 720 °C 8 h electrochemical corrosion | 79 µm/year | MgCr2O4 |
| [15] | 316SS Inconel 617 Haynes 242 Hastelloy C276 Hastelloy C22 Inconel 600 Inconel 625 Haynes 230 | NaCl-KCl-MgCl2 (33–21.6–45.4 mol%) | 700 °C N2 100 h static immersion test | 2.38 ±0.20 mg/cm2 0.85 ± 0.07 mg/cm2 0.62± 0.04 mg/cm2 1.05 ± 0.10 mg/cm2 0.74 ±0.07 mg/cm2 2.16 ±0.02 mg/cm2 0.67 ± 0.09 mg/cm2 0.82 ±0.05 mg/cm2 | |
| [18] | Ni GH4033 GH4169 | NaCl-MgCl2 | 500 °C 160 h | 57.0 ± 9.0 µm/year 141.9 ± 11.2 µm/year 246.4 ± 13.4 µm/year | Ni: MgO, carbides GH4033: Ni, MgO, MgCr2O4, NiCr2O4 GH4169: (Ni,Fe), Ni3Fe, MgO, MgFe2O4, NiFe2O4 |
| Heat Transfer and Heat Storage Technology | Advantages | Challenges | Demonstration Project |
|---|---|---|---|
| Chloride salt | It has similar thermophysical properties to nitrate. High thermal stability and maximum operating temperature of 800 °C; chloride salt: abundant and inexpensive. | Corrosive to materials; receivers, heat storage systems, valves and pumps, steam generators, and other components to adapt to higher operating temperatures, and the temperature is not too high or too low. | FASTR, USA Avanza, Spain |
| Solid particles | The maximum working temperature is up to 1000 °C; simple processing can be done at different temperatures in the atmosphere. | The price is low. Low thermal conductivity; to adapt to new working components such as a receiver, heat storage, particle transport, steam generator, etc., there is a particle loss. | G3P3, USA CentRec, Germany |
| Salt-phase change materials (PCMs) | High energy density, the maximum working temperature is 600–1000 °C, and materials are abundant and inexpensive. Corrosive to materials. | Enhanced cost-effectiveness to overcome low thermal conductivity; improve the stability of the material cycle; system integration with PCMs. | |
| Gas | Low-cost, mature technology; compatible with many heat storage technologies. | The system is complex and requires increased costs; fluid circulation brings high energy losses. | VHTR, USA |
| Liquid metal | High thermal conductivity and high thermal stability; toxic substances with experience in the field of nuclear energy. | Corrosion control, high administrative costs, and low heat storage material costs. | Vast Solar, Australia |
| Molten Salts (wt.%) | Melting Point (°C) | Stability Limit (°C) | Density (g/cm3) | Heat Capacity (kJ/kg·K) | Material Cost (USD/kg) |
|---|---|---|---|---|---|
| Solar Salt: KNO3/NaNO3 (40/60) | 240 | 530–565 | 1.8 (400 °C) | 1.5(400 °C) | 0.50–0.80 |
| HITEC: KNO3/NaNO3/NaNO2 (53/7/40) | 142 | 450–540 | 1.8 (400 °C) | 1.5 (400 °C) | 0.90 |
| K2CO3/Li2CO3/Na2CO3 (32/35/33) | 397 | >650 | 2.0 (700 °C) | 1.9 (700 °C) | 1.30–2.50 |
| KF/LiF/NaF (59/29/12) | 454 | >700 | 2.0 (700 °C) | 1.9 (700 °C) | >2.00 |
| KCl/NaCl/ZnCl2 (23.9/7.5/68.6) | 204 | 850 | 2.0 (600 °C) | 0.8 (300–600 °C) | <1.00 |
| KaCl/MgCl2/NaCl (17.8/68.2/14.0) | 380 | >800 | 1.7 (600 °C) | 1.0 (500–800 °C) | <0.35 |
| Molten Salt Formula | Author | Time | Country |
|---|---|---|---|
| NaCl/LiCl (34.42/65.58 wt.%) | Gomez-Vidal J C [10] | 2016 | US |
| MgCl2/KCl (35.59 /64.41 wt.%) | Gomez-Vidal J C [10] | 2016 | US |
| MgCl2/KCl/NaCl (60/20/20 mol%) | Ding W [32] | 2018 | Germany |
| NaCl/KCl/MgCl2 (24.5/20.5/55 wt.%) | Mohan G [43,54] | 2018 | Australia |
| NaCl/KCl/MgCl2 (33/21.6/45.4 mol%) | Sun H [37] | 2018 | China |
| NaCl-KCl-ZnC2 NaCl-CaCl2-MgCl | Grégoire B [50] | 2020 | Germany |
| NaCl-KCl-MgCl2 KCl/MgCl2/NaCl (20.4/55.1/24.5 wt.%) | Fernández A G [23] | 2020 | Spain |
| MgCl2/KCl/NaCl | Samuel H. Gage [19] | 2021 | US |
| NaCl/CaCl2 (52/48 mol%) | Heqing Tian [55] | 2021 | China |
| Molten Salt Composition (wt.%) | Melting Point (°C) | Molten Salt Composition (KJ/Kg) | Density (Kg/m3) | Specific Heat (KJ/Kg·K) Solid/Liquid | |
|---|---|---|---|---|---|
| LiOH/KOH (40/60) | 314 | 341 | |||
| KNO3/KCl (95.5/45) | 320 | 74 | 2100 | 1.21 | |
| KNO3/KCl (96/4) | 320 | 150 | |||
| KNO3/KBr/KCl (80/10/10) | 342 | 140 | |||
| NaCl/KCl/LiCl (33/24/43) | 346 | 281 | |||
| NaOH/NaCl (80/20) | 370 | 370 | |||
| MgCl2/KCl/NaCl (60/20.4/19.6) | 380 | 400 | 1800 | 0.96 | |
| Li2CO3/K2CO3/NaCO3 (32.1/34.5/33.4) | 397 | 276 | |||
| MgCl2/KCl (39/61) | 435 | 351 | 2110 | 0.80 | 0.96 |
| MgCl2/NaCl (52/48) | 450 | 430 | 2230 | 092 | 1.00 |
| MgCl2/KCl (64/36) | 470 | 388 | 2190 | 0.84 | 0.96 |
| MgCl2/KCl/CaCl2 (48/25/27) | 487 | 342 | 2530 | 0.80 | 0.92 |
| CaCl2/NaCl (67/33) | 500 | 281 | 2160 | 0.84 | 1.00 |
| NaCl/KCl/CaCl2 (29/5/66) | 504 | 279 | 2150 | 1.17 | 1.00 |
| BaCl2/KCl/NaCl (53/28/19) | 542 | 221 | 3020 | 0.63 | 0.80 |
| BaCl2/KCl/CaCl2 (47/24/29) | 551 | 219 | 2930 | 0.67 | 0.84 |
| LiF/MgF2/KF (64/30/6 mol%) | 710 | 782 | |||
| LiF/CaF2(80.5/19.5 mol%) | 767 | 790 | |||
| Molten Salt | Corrosion Mechanism |
|---|---|
| Nitrate | 1. Oxidation reactions: NO3− + 2e = NO2 +O2−; M + O2− = MO + 2e−; 3MO + O2− = M3O4 + 2e− 2M + 3O2− = M2O3 + 6e−; M: metals 2. Effect of impurities H2O: H2O + NO3− + 2e = NO2− + 2OH− 3. Nitrate pyrolysis, NO2 reacts with H2O to form HNO3: 2M(NO3)2 = 2MO + 4NO2 + O2 3NO2 + H2O = 2HNO3 + NO |
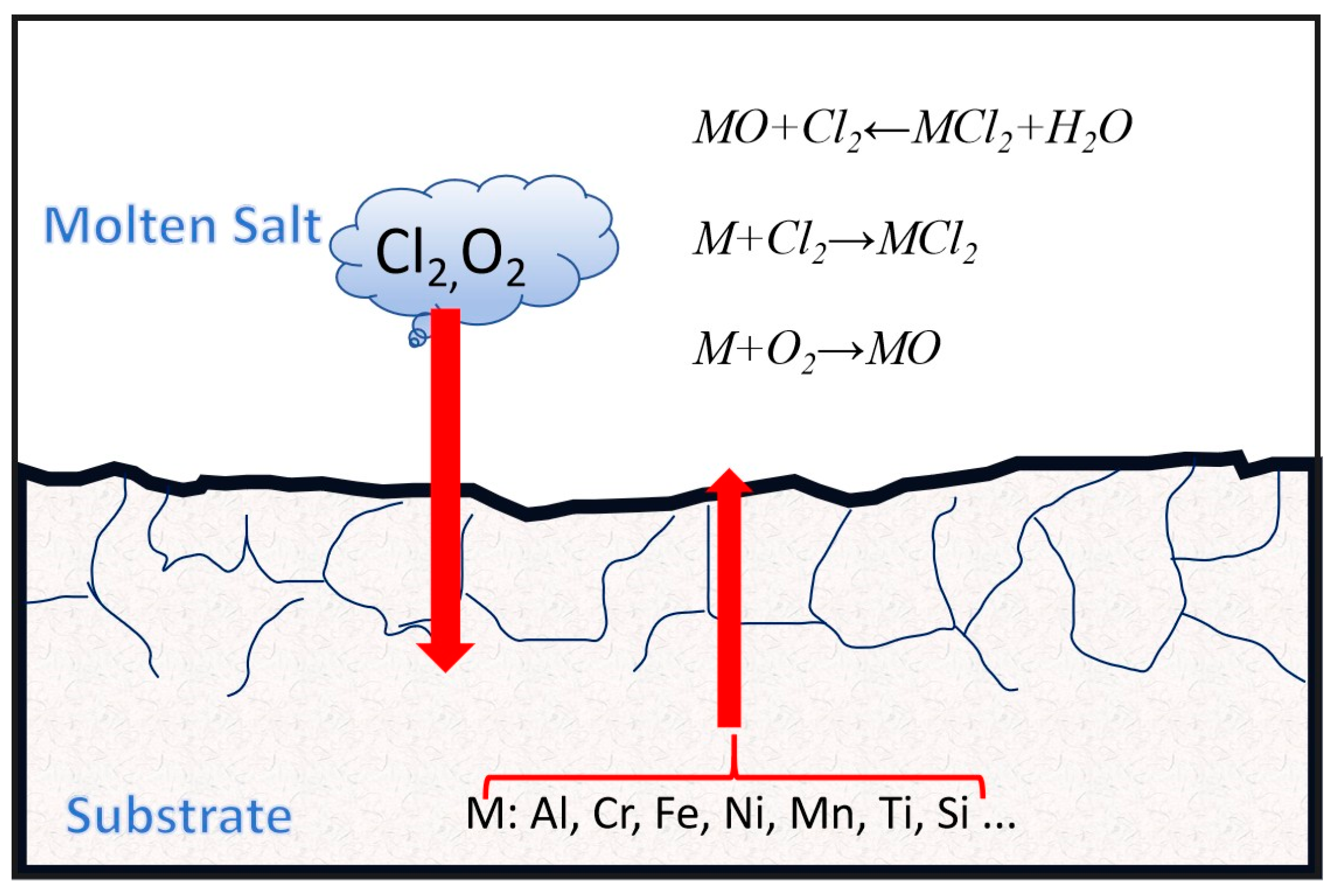
| Chloride | 1. The impurity H2O reacts with alkaline earth metal chloride salts to form HCl: MeCl(l) + H2O(g) = Me(OH)(l) + HCl(g) 2MeCl(l) + H2O(g) = Me2O(l) + 2HCl(g) 2. HCl reacts with O2 to form Cl2, and both can react chemically with metal M: 4HCl(l) + O2(g) = 2H2O(g) + 2Cl2(g) Xm + y/2O2(g) = MxOy M + Z/2Cl2(g) = MClZ 3. Either HCl or H2O can react chemically with metal M to release H2, and alkaline earth metal oxide Me2O will also react with HCl: xM(s) + yH2O(g) = MxOy(s) + yH2(g) xM(l) + yHCl(g) = MxCly(l) + y/2H2(g) Me2O(l) + 2HCl(g) = 2MeCl(l) + H2O(g) 4. Metal and metal impurity elements in molten salt undergo displacement reactions with metal oxides: NiCl2 + Cr = CrCl2 + H2 Mg2+ + Si4+ + 3O2− = MgSiO3(S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; La, P.; Zheng, Y.; Zhan, F.; Yu, H.; Yang, P.; Zhu, M.; Bai, Z.; Gao, Y. Review of Molten Salt Corrosion in Stainless Steels and Superalloys. Crystals 2025, 15, 237. https://doi.org/10.3390/cryst15030237
Wei Y, La P, Zheng Y, Zhan F, Yu H, Yang P, Zhu M, Bai Z, Gao Y. Review of Molten Salt Corrosion in Stainless Steels and Superalloys. Crystals. 2025; 15(3):237. https://doi.org/10.3390/cryst15030237
Chicago/Turabian StyleWei, Ying, Peiqing La, Yuehong Zheng, Faqi Zhan, Haicun Yu, Penghui Yang, Min Zhu, Zemin Bai, and Yunteng Gao. 2025. "Review of Molten Salt Corrosion in Stainless Steels and Superalloys" Crystals 15, no. 3: 237. https://doi.org/10.3390/cryst15030237
APA StyleWei, Y., La, P., Zheng, Y., Zhan, F., Yu, H., Yang, P., Zhu, M., Bai, Z., & Gao, Y. (2025). Review of Molten Salt Corrosion in Stainless Steels and Superalloys. Crystals, 15(3), 237. https://doi.org/10.3390/cryst15030237









