Macroscopic Dehydration Control of ZnSO4·7H2O: Infrared and Raman Spectra of ZnSO4 Hydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Microscopic DSC-TGA and Macroscopic TGA of ZnSO4·7H2O
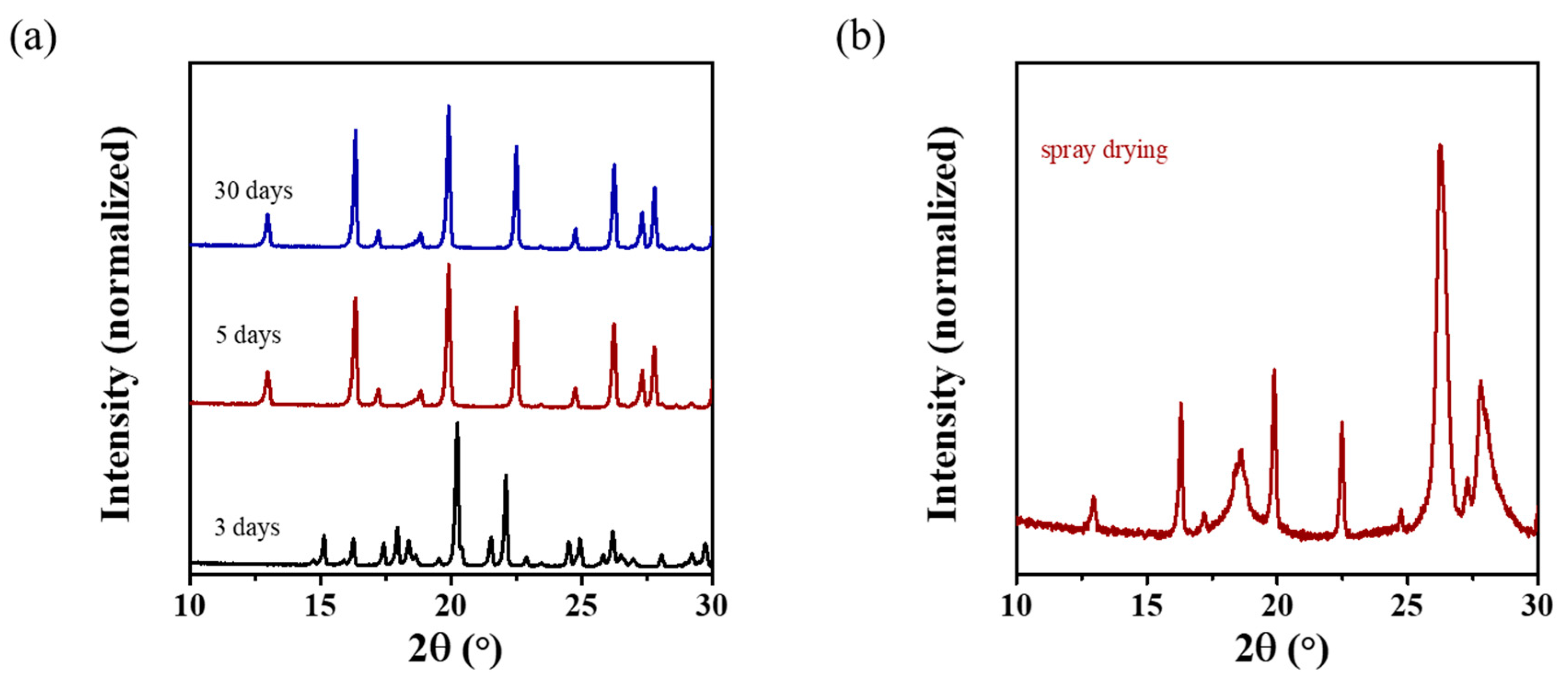
3.2. Macroscopic Dehydration Control of ZnSO4·7H2O
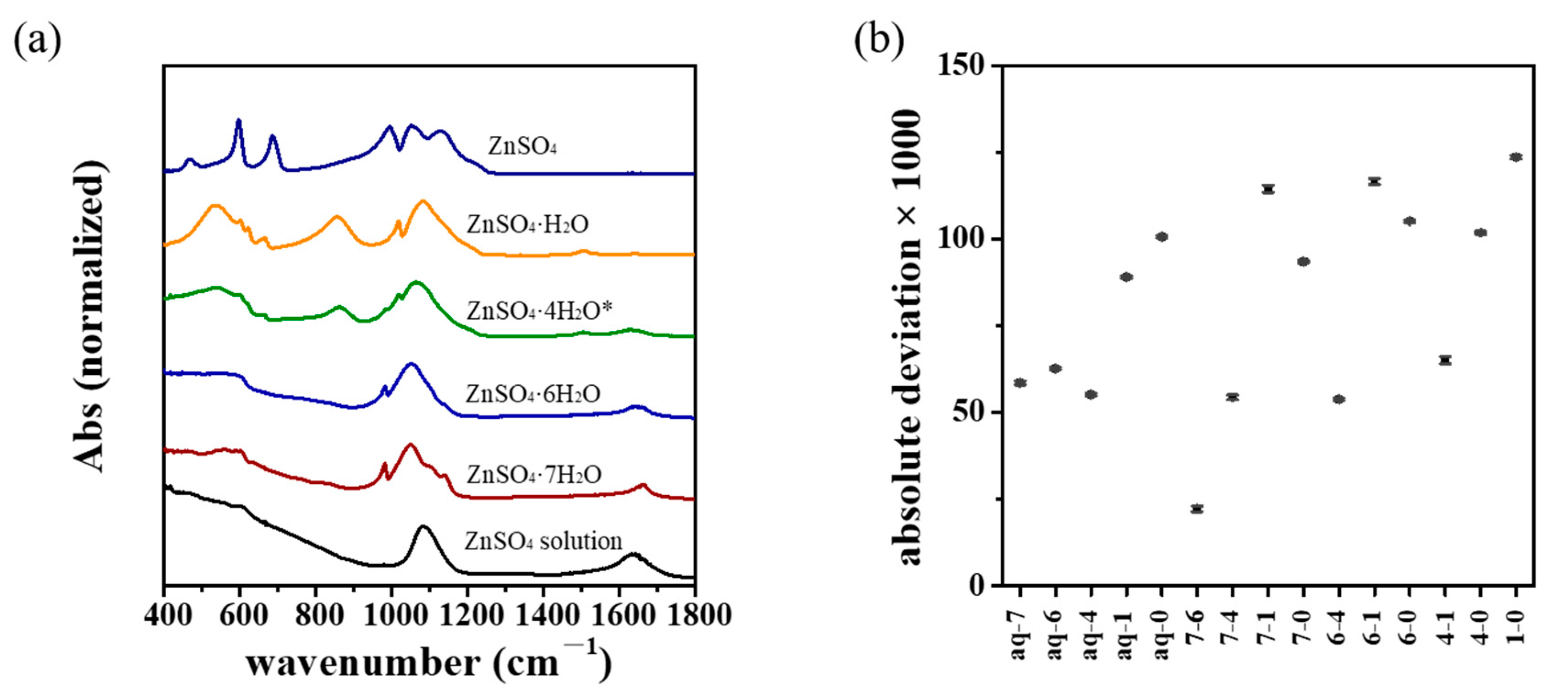
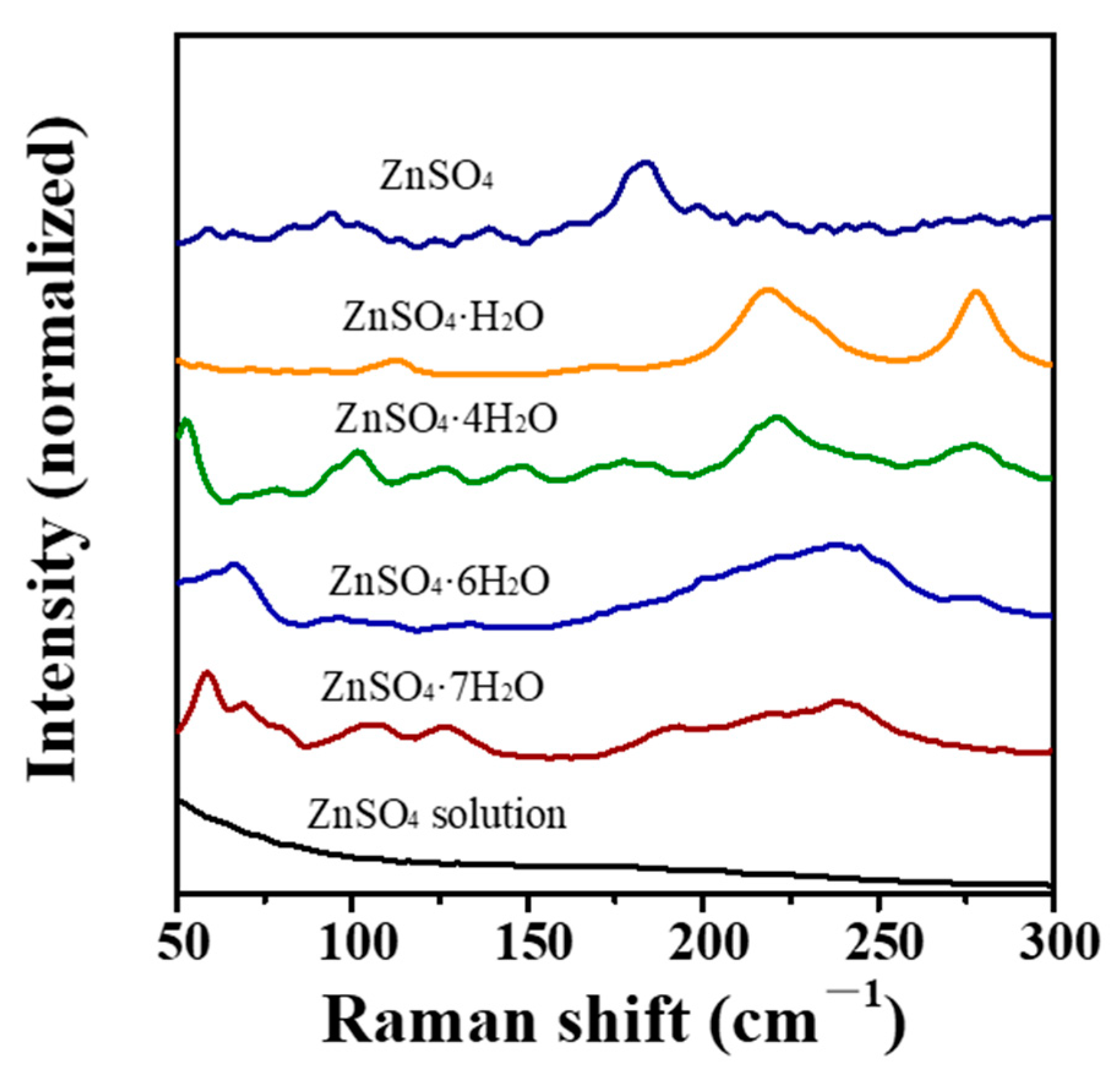
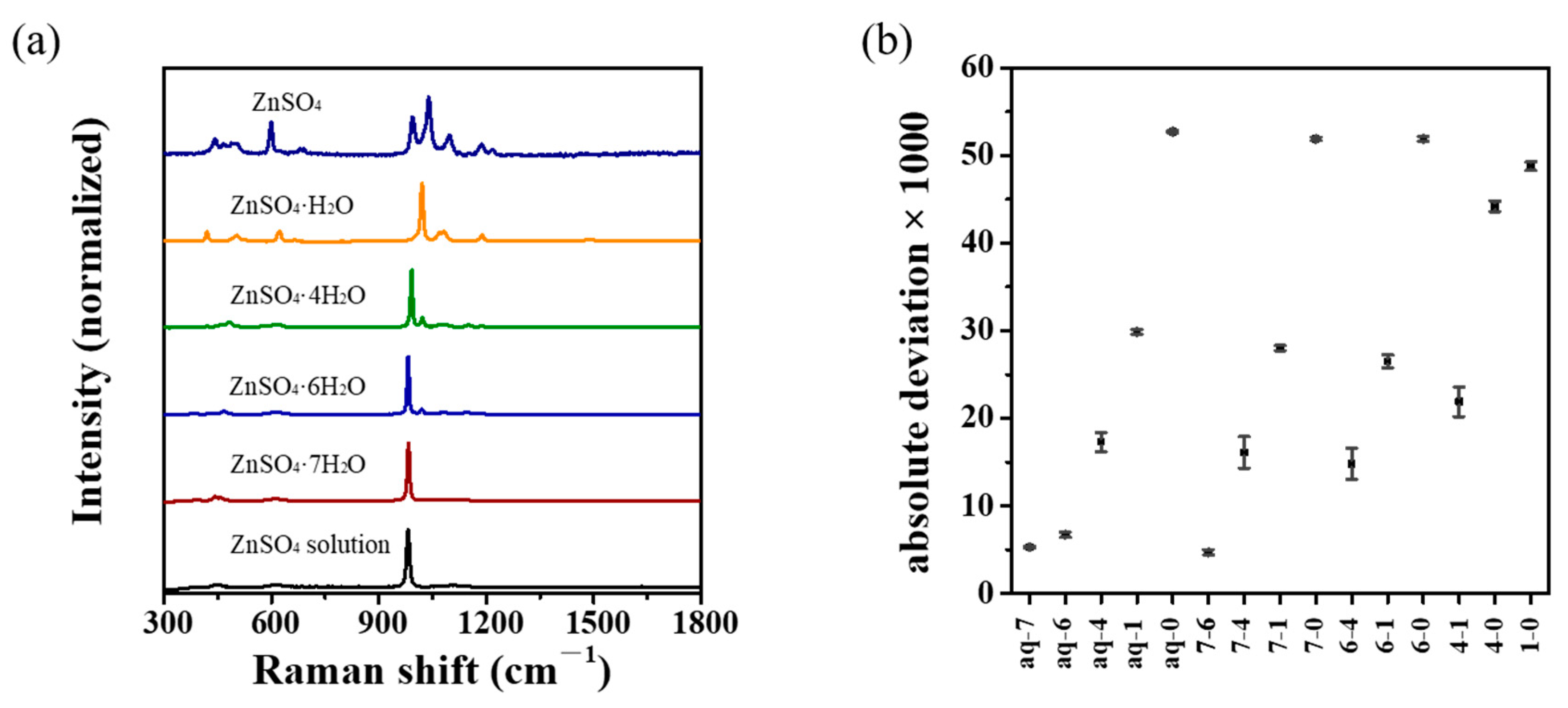
3.3. Vibrational Spectroscopic Analysis
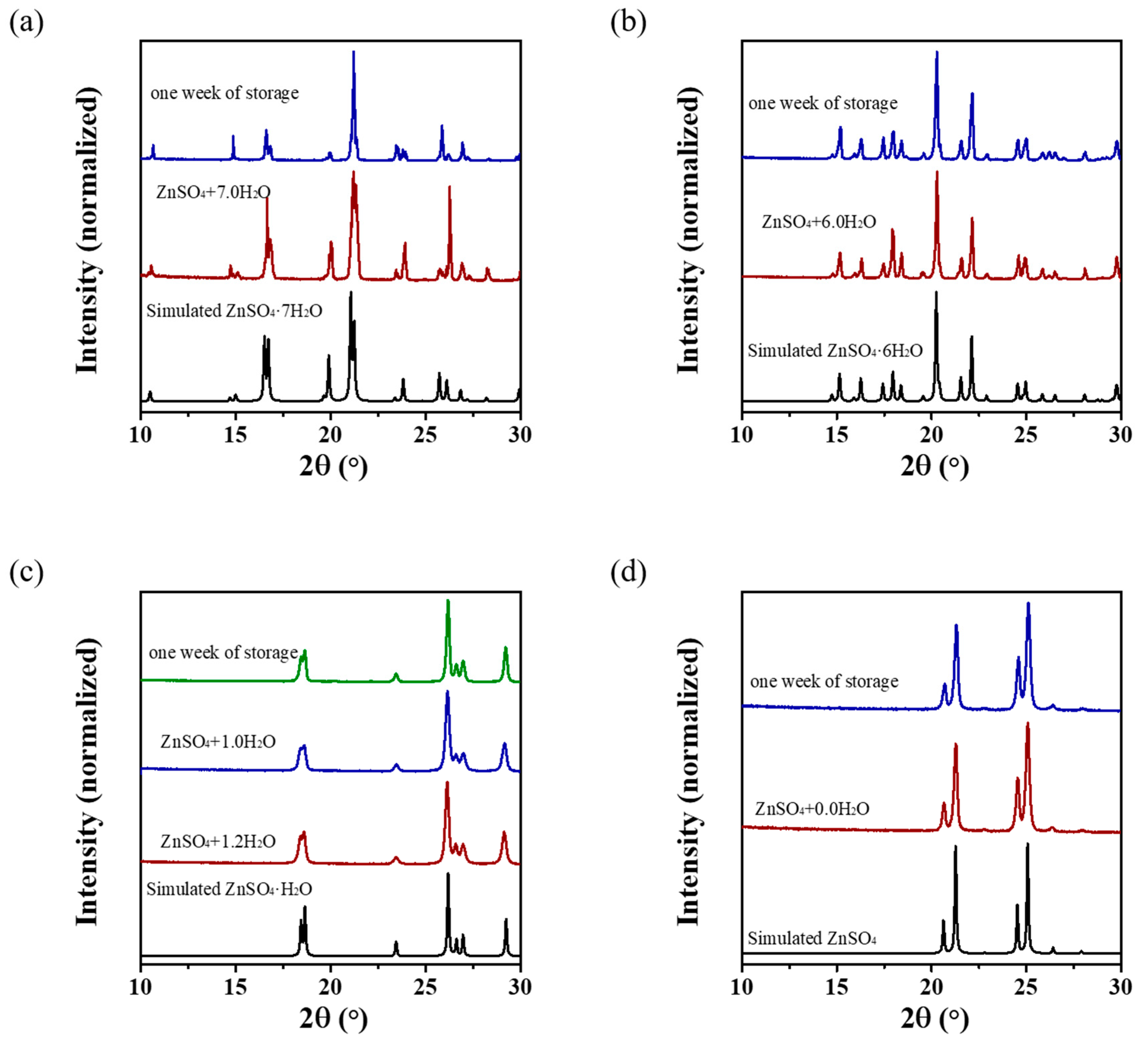
3.4. Crystallographic Analysis
3.5. Similarity Analysis
4. Discussion
4.1. Macroscopic TGA Combined with PXRD
4.2. Preparations of ZnSO4·4H2O
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makatun, V.N.; Shchegrov, L.N. State of water in inorganic crystal hydrates and the characteristic features of their dehydration. Russ. Chem. Rev. 1972, 41, 905. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Lin, N.; Xie, B.; Zhang, D.; Chen, J. Review on tailored phase change behavior of hydrated salt as phase change materials for energy storage. Mater. Today Energy 2021, 22, 100866. [Google Scholar] [CrossRef]
- Hua, W.; Yan, H.; Zhang, X.; Xu, X.; Zhang, L.; Shi, Y. Review of salt hydrates-based thermochemical adsorption thermal storage technologies. J. Energy Storage 2022, 56, 106158. [Google Scholar] [CrossRef]
- Yan, T.S.; Li, T.X.; Xu, J.X.; Chao, J.W. Understanding the transition process of phase change and dehydration reaction of salt hydrate for thermal energy storage. Appl. Therm. Eng. 2020, 166, 114655. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Yan, T.; Chao, J.; Wang, R. Dehydration kinetics and thermodynamics of magnesium chloride hexahydrate for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110819. [Google Scholar] [CrossRef]
- Okhrimenko, L.; Dussouillez, J.; Johannes, K.; Kuznik, F. Thermodynamic equilibrium and kinetic study of lanthanum chloride heptahydrate dehydration for thermal energy storage. J. Energy Storage 2022, 48, 103562. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic salt hydrate for thermal energy storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Man, X.; Lu, H.; Xu, Q.; Wang, C.; Ling, Z. Review on the thermal property enhancement of inorganic salt hydrate phase change materials. J. Energy Storage 2023, 72, 108699. [Google Scholar] [CrossRef]
- Raijada, D.; Arnfast, L.; Bond, A.D.; Aho, J.; Bøtker, J.; Sandler, N.; Rantanen, J. Dehydration of nitrofurantoin monohydrate during melt extrusion. Cryst. Growth Des. 2017, 17, 3707–3715. [Google Scholar] [CrossRef]
- Preturlan, J.G.D.; Vieille, L.; Quiligotti, S.; Favergeon, L. Kinetics and mechanism of the dehydration of calcium sulfate dihydrate: A comprehensive approach for studying the dehydration of ionic hydrates under controlled temperature and water vapor pressure. J. Phys. Chem. C 2020, 124, 26352–26367. [Google Scholar] [CrossRef]
- Eaby, A.C.; Myburgh, D.C.; Kosimov, A.; Kwit, M.; Esterhuysen, C.; Janiak, A.M.; Barbour, L.J. Dehydration of a crystal hydrate at subglacial temperatures. Nature 2023, 616, 288–292. [Google Scholar] [CrossRef]
- Koplík, J.; Švec, J.; Másilko, J.; Sedlačík, M.; Bartoníčková, E. Synthesis of calcium aluminate hydrates, their characterization and dehydration. Ceram. Int. 2025, 51, 5536–5543. [Google Scholar] [CrossRef]
- de Castelnuovo, S.; Harness, J.B.; McColm, I.J. The role of liquid water in crystalline hydrate dehydration: Copper sulphate pentahydrate. J. Therm. Anal. Calorim. 2001, 63, 233–247. [Google Scholar] [CrossRef]
- Cheng, L.; Li, W.; Li, Y.; Yang, Y.; Li, Y.; Cheng, Y.; Song, D. Thermal analysis and decomposition kinetics of the dehydration of copper sulfate pentahydrate. J. Therm. Anal. Calorim. 2019, 135, 2697–2703. [Google Scholar] [CrossRef]
- Gamo, I. Infrared absorption spectra of water of crystallization in copper sulfate penta- and monohydrate crystals. Bull. Chem. Soc. Jpn. 1961, 34, 764–766. [Google Scholar] [CrossRef]
- White, R.L. Variable temperature infrared study of copper sulfate pentahydrate dehydration. Thermochim. Acta 2012, 528, 58–62. [Google Scholar] [CrossRef]
- Widjaja, E.; Chong, H.H.; Tjahjono, M. Use of thermo-Raman spectroscopy and chemometric analysis to identify dehydration steps of hydrated inorganic samples—Application to copper sulfate pentahydrate. J. Raman Spectrosc. 2010, 41, 181–186. [Google Scholar] [CrossRef]
- Rabbering, G.; Wanrooy, J.; Schuijff, A. The dehydration of ZnSO4·7H2O and NiSO4·6H2O. Thermochim. Acta 1975, 12, 57–63. [Google Scholar] [CrossRef]
- Anderson, J.L.; Peterson, R.C.; Swainson, I.P. Combined neutron powder and X-ray single-crystal diffraction refinement of the atomic structure and hydrogen bonding of goslarite (ZnSO4·7H2O). Mineral. Mag. 2005, 69, 259–271. [Google Scholar] [CrossRef]
- Anderson, J.L.; Peterson, R.C.; Swainson, I.P. The atomic structure of deuterated boyleite ZnSO4·4D2O, ilesite MnSO4·4D2O, and bianchite ZnSO4·6D2O. Am. Mineral. 2012, 97, 1905–1914. [Google Scholar] [CrossRef]
- Chihara, H.; Seki, S. Studies of crystalline hydrates. II. Thermal transition and dehydration of Ni-, Fe-, Co-, Zn-, Mn-, and Mg-sulfate hydrates. Bull. Chem. Soc. Jpn. 1953, 26, 88–92. [Google Scholar] [CrossRef]
- Wheeler, R.C.; Frost, G.B. A comparative study of the dehydration kinetics of several hydrated salts. Can. J. Chem. 1955, 33, 546–561. [Google Scholar] [CrossRef]
- Razouk, R.I.; Mikhail, R.S.; Ghorab, H.Y. Dehydration and rehydration behaviour of zinc and magnesium sulphate heptahydrates. J. Appl. Chem. 1969, 19, 329–335. [Google Scholar] [CrossRef]
- Grønvold, F.; Meisingset, K.K. Thermodynamic properties and phase transitions of salt hydrates between 270 and 400 K I. NH4Al(SO4)2 · 12H2O, KAl(SO4)2 · 12H2O, Al2(SO4)3 · 17H2O, ZnSO4 · 7H2O, Na2SO4 · 10H2O, and Na2S2O3 · 5H2O. J. Chem. Thermodyn. 1982, 14, 1083–1098. [Google Scholar] [CrossRef]
- Straszko, J.; Olszak-Humienik, M.; Możejko, J. Kinetics of thermal decomposition of ZnSO4·7H2O. Thermochim. Acta 1997, 292, 145–150. [Google Scholar] [CrossRef]
- Lin, F.; Yang, S. The study of the dehydrating mechanism of zinc sulfate heptahydrate. J. Chongqing Norm. Uni. 2006, 23, 55–57. [Google Scholar]
- Saha, J.; Podder, J. Crystallization of zinc sulphate single crystals and its structural, thermal and optical characterization. J. Bangladesh Acad. Sci. 2011, 35, 203–210. [Google Scholar] [CrossRef]
- Attia, S.; Hevroni, L.; Danon, A.; Meyerstein, D.; Koresh, J.E.; Finkelstein, Y. The role of the cation in the oxygen isotopic exchange in crystalline sulfate salt hydrates. Adsorption 2013, 19, 821–833. [Google Scholar] [CrossRef]
- Carsky, M.; Veselý, V.; Hartman, M.; Haluza, J. Dehydration of zinc sulphate in a fluidised bed drier. S. Afr. J. Chem. Eng. 2014, 19, 1–8. [Google Scholar]
- Yang, W.; Jun, Y. Gibbs free energy minimization and TG-DTA analysis for thermal decomposition of zinc sulfate heptahydrate. Inorg. Chem. Ind. 2014, 46, 31–33. [Google Scholar]
- Höffler, F.; Müller, I.; Steiger, M. Thermodynamic properties of ZnSO4 (aq) and phase equilibria in the ZnSO4 –H2O system from 268 K to 373 K. J. Chem. Thermodyn. 2018, 116, 279–288. [Google Scholar] [CrossRef]
- Rehman, A.U.; Maosheng, Z.; Hayat, A. Hydration performance and cycling stability of threeTCM:MgSO4, ZnSO4 and FeSO4. Int. J. Energy Res. 2020, 44, 6981–6990. [Google Scholar] [CrossRef]
- Liu, H.; Chen, G.-M.; Ma, J.; Fan, Y.-X.; Tao, Z.-Y. Zinc sulfate hydrates characterization by terahertz time domain spectroscopy. Optik 2022, 253, 168594. [Google Scholar] [CrossRef]
- Rego, A.S.C.; Navarro, R.C.S.; Brocchi, E.A.; Souza, R.F.M. Kinetic modeling of the thermal decomposition of zinc sulfate through a global optimization method. Metall. Mater. Trans. B 2022, 53, 4105–4113. [Google Scholar] [CrossRef]
- Bertouche, S.; Tomao, V.; Ruiz, K.; Hellal, A.; Boutekedjiret, C.; Chemat, F. First approach on moisture determination in food products using alpha-pinene as an alternative solvent for Dean–Stark distillation. Food Chem. 2012, 134, 602–605. [Google Scholar] [CrossRef]
- Moya, M.; Sánchez, D.; Romero, J.Á.; Villar-García, J.R. Influence of Moisture Content on Some Mechanical Properties of Wheat. Agronomy 2024, 14, 347. [Google Scholar] [CrossRef]
- Sivakumar, A.; Sahaya Jude Dhas, S.; Elberin Mary Theras, J.; Jose, M.; Sivaprakash, P.; Arumugam, S.; Martin Britto Dhas, S.A. Spectroscopic and diffraction studies of polycrystalline copper sulfate pentahydrate at shocked conditions. Solid State Sci. 2021, 121, 106751. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Fan, Y.; Xue, R.; Chen, F. Explanation and prediction for the product of trehalose dihydrate selective dehydration process using mid-frequency Raman difference spectra. Vib. Spectrosc. 2024, 130, 103626. [Google Scholar] [CrossRef]
- Koskela, J.; Sutton, J.J.; Lipiäinen, T.; Gordon, K.C.; Strachan, C.J.; Fraser-Miller, S.J. Low- versus mid-frequency Raman spectroscopy for in situ analysis of crystallization in slurries. Mol. Pharm. 2022, 19, 2316–2326. [Google Scholar] [CrossRef]
- Robert, C.; Fraser-Miller, S.J.; Berzins, K.; Okeyo, P.O.; Rantanen, J.; Rades, T.; Gordon, K.C. Monitoring the isothermal dehydration of crystalline hydrates using low-frequency Raman spectroscopy. Mol. Pharm. 2021, 18, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liang, C.; Li, Y.; Xiao, W.; Niu, Y.; Jin, H.; Xue, R.; Chen, F. Explanation and prediction of the selective crystallization of boscalid by mid-frequency Raman difference spectroscopy analysis. CrystEngComm 2023, 25, 3548–3555. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chan, C.K. Understanding the hygroscopic properties of supersaturated droplets of metal and ammonium sulfate solutions using Raman spectroscopy. J. Phys. Chem. A 2002, 106, 285–292. [Google Scholar] [CrossRef]
- Hayes, A.C.; Kruus, P.; Adams, W.A. Raman spectroscopic study of aqueous (NH4)2SO4 and ZnSO4 solutions. J. Sol. Chem. 1984, 13, 61–75. [Google Scholar] [CrossRef]
- Kurban, G.V.T.; Rego, A.S.C.; Mello, N.M.; Brocchi, E.A.; Navarro, R.C.S.; Souza, R.F.M. Thermodynamics and kinetic modeling of the ZnSO4·H2O thermal decomposition in the presence of a Pd/Al2O3 catalyst. Energies 2022, 15, 548. [Google Scholar] [CrossRef]
- Pandey, R.; Pourhashem, G.; Gladen, A.C. Screening of salt hydrates and cellulose nanocrystal composites for thermochemical energy storage using life cycle assessment. Sustainable Mater. Technol. 2024, 40, e00889. [Google Scholar] [CrossRef]
- Blake, A.J.; Cooke, P.A.; Hubberstey, P.; Sampson, C.L. Zinc(II) sulfate tetrahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, i109–i111. [Google Scholar] [CrossRef]


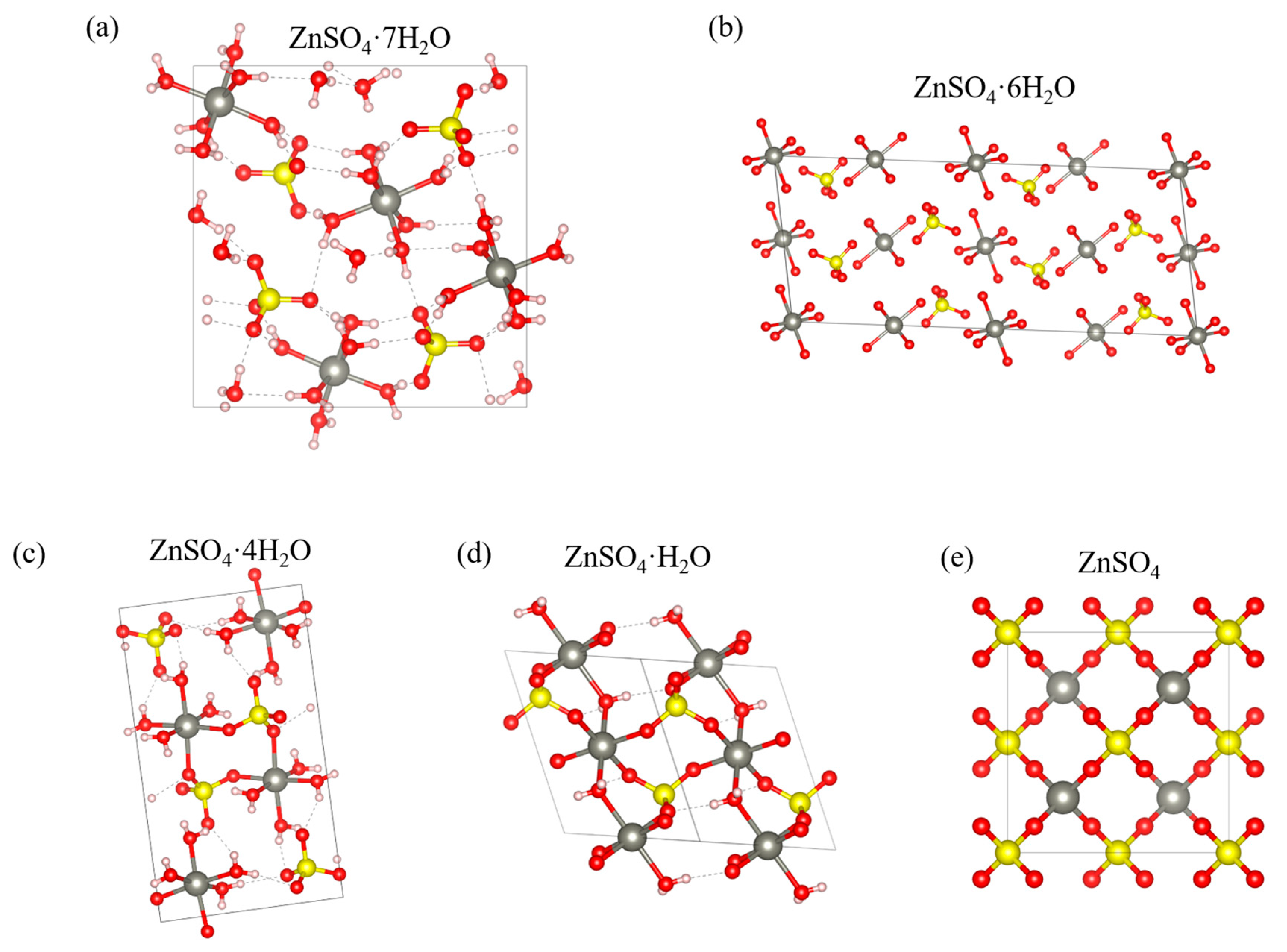

| Year | Process | Method | Refs. |
|---|---|---|---|
| 1953 | 7 → 6 → 1 | DTA | [21] |
| 1955 | 7 → 1 → amorphous | XRD (vacuum) | [22] |
| 1969 | 7 → 6 → 1 | TGA-DTA | [23] |
| 1975 | 7 → 6 → 4 → 1 | DSC, DTA, TGA | [18] |
| 1982 | 7 → 6 → 1 | Calorimetric containers of fused silica | [24] |
| 1997 | 7 → 6 → 1 | DTA, DTG, TG (5 K·min−1, 20–1000 °C) | [25] |
| 2005 | 7 → 6 → 4 → 1 | Neutron PXRD and single-crystal XRD | [19] |
| 2006 | 7 → 6 → 1 → 0 | DTA, TG (5 K·min−1) | [26] |
| 2011 | 7 → 1 | TGA-DTA (30 °C·min−1 in air) | [27] |
| 2012 | 7 → 6 → 4 → 1 | In situ diffraction | [20] |
| 2013 | 7 → 1 → 0 | TPD-MS-SMB | [28] |
| 2014 | 7 → 6 → 1 | TG-DTA, fluidized bed drier | [29] |
| 2014 | 7 → 6 → 1 → X → 0 | TG-DTA (5 K·min−1, 25–1100 °C, air) | [30] |
| 2018 | 7 → 6 → 1 | Water vapor sorption analyzer, microbalance | [31] |
| 2020 | 7 → 5 → 1 | DSC-TG (1 K·min−1, 25–150 °C, N2) | [32] |
| 2022 | 7 → 6 → 1 | All-fiber terahertz time-domain spectroscopy | [33] |
| 2022 | 7 → 6 → 1 | TGA (10 K·min−1, 25–1000 °C, N2) | [34] |
| 7H2O | 6H2O | 4H2O | H2O | Anhydrous | |
|---|---|---|---|---|---|
| Year | 1959 | 1979 | 2001 | 1991 | 1978 |
| ICSD No. | 107287 | 41708 | 280878 | 71348 | 2456 |
| System | Orthorhombic | Monoclinic | Monoclinic | Monoclinic | Cubic |
| Space Group | P212121 | C2/c | P21/n | C2/c | F-43m |
| a (Å) | 11.779 | 9.981 | 5.904 | 6.925 | 7.176 |
| b (Å) | 12.050 | 7.250 | 13.519 | 7.591 | 7.176 |
| c (Å) | 6.822 | 24.280 | 7.883 | 7.635 | 7.176 |
| α (°) | 90 | 90 | 90 | 90 | 90 |
| β (°) | 90 | 98.45 | 90.26 | 118.19 | 90 |
| γ (°) | 90 | 90 | 90 | 90 | 90 |
| Solution | 7H2O | 6H2O | 4H2O* | 1H2O | Anhydrous | Assignment [38] |
|---|---|---|---|---|---|---|
| 470 | ||||||
| 561 | 557 | 536 | 534 | |||
| 601 | 602 | 597 | 600 | 601 | 599 | δas- |
| 623 | ||||||
| 665 | 665 | 689 | ||||
| 861 | 855 | |||||
| 980 | 982 | 982 | 984 | 992 | ν1- | |
| 1017 | 1017 | |||||
| 1053 | 1053 | 1063 | 1050 | |||
| 1084 | 1082 | ν3- | ||||
| 1142 | 1129 | |||||
| 1504 | 1504 | |||||
| 1643 | 1661 | 1651 | 1632 | δ-OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Zheng, W.; Xue, R.; Chen, F. Macroscopic Dehydration Control of ZnSO4·7H2O: Infrared and Raman Spectra of ZnSO4 Hydrates. Crystals 2025, 15, 257. https://doi.org/10.3390/cryst15030257
Ding C, Zheng W, Xue R, Chen F. Macroscopic Dehydration Control of ZnSO4·7H2O: Infrared and Raman Spectra of ZnSO4 Hydrates. Crystals. 2025; 15(3):257. https://doi.org/10.3390/cryst15030257
Chicago/Turabian StyleDing, Chengang, Wenling Zheng, Rongrong Xue, and Fenghua Chen. 2025. "Macroscopic Dehydration Control of ZnSO4·7H2O: Infrared and Raman Spectra of ZnSO4 Hydrates" Crystals 15, no. 3: 257. https://doi.org/10.3390/cryst15030257
APA StyleDing, C., Zheng, W., Xue, R., & Chen, F. (2025). Macroscopic Dehydration Control of ZnSO4·7H2O: Infrared and Raman Spectra of ZnSO4 Hydrates. Crystals, 15(3), 257. https://doi.org/10.3390/cryst15030257






