Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl-KCl Molten Salt at High Temperatures
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials Reparation
2.2. Corrosion Experiment
2.3. Characterization
3. Result and Discussion
3.1. Corrosion Properties
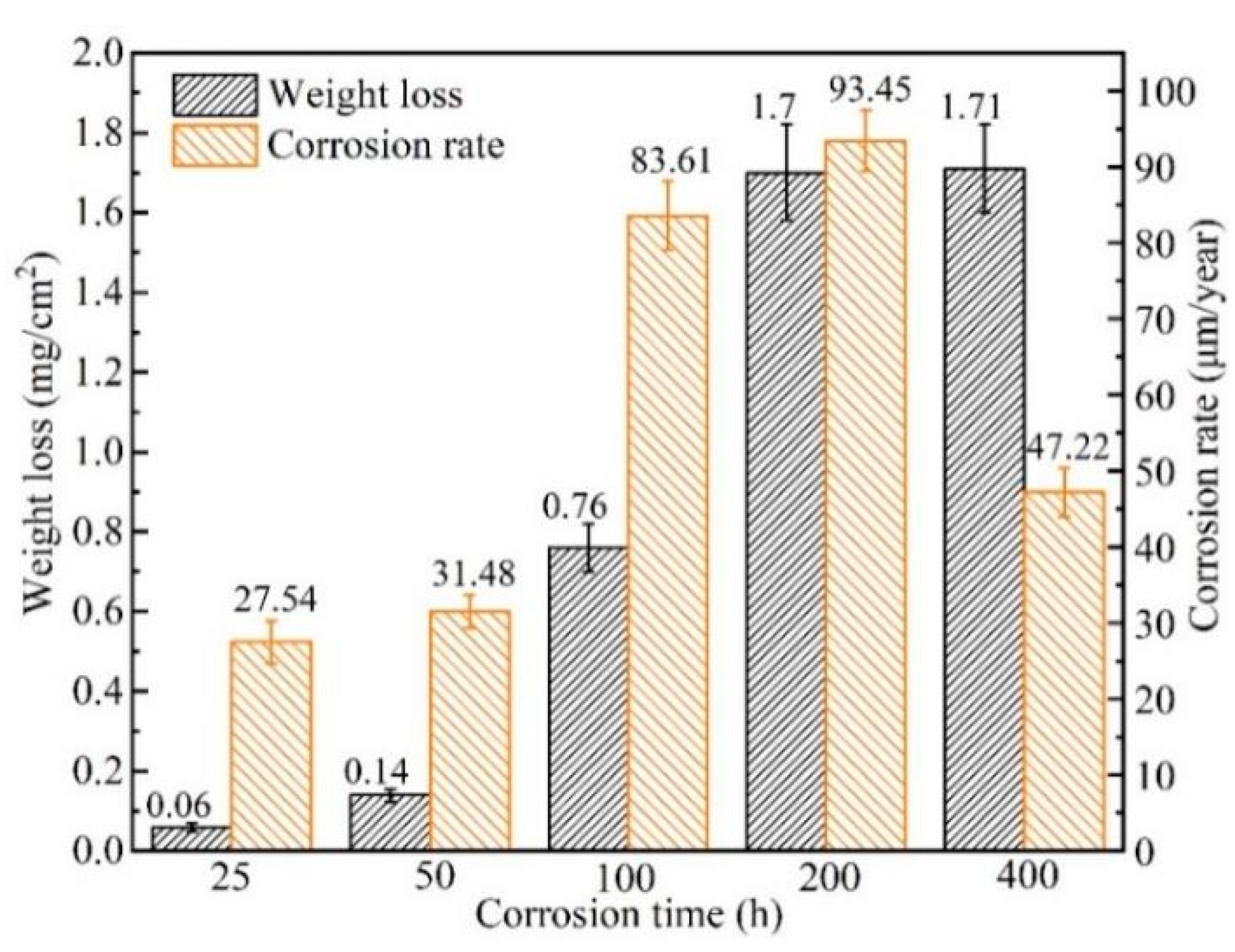
3.1.1. Weight Change
3.1.2. Elemental Distribution and Surface Morphology
3.1.3. Crystalline Structure
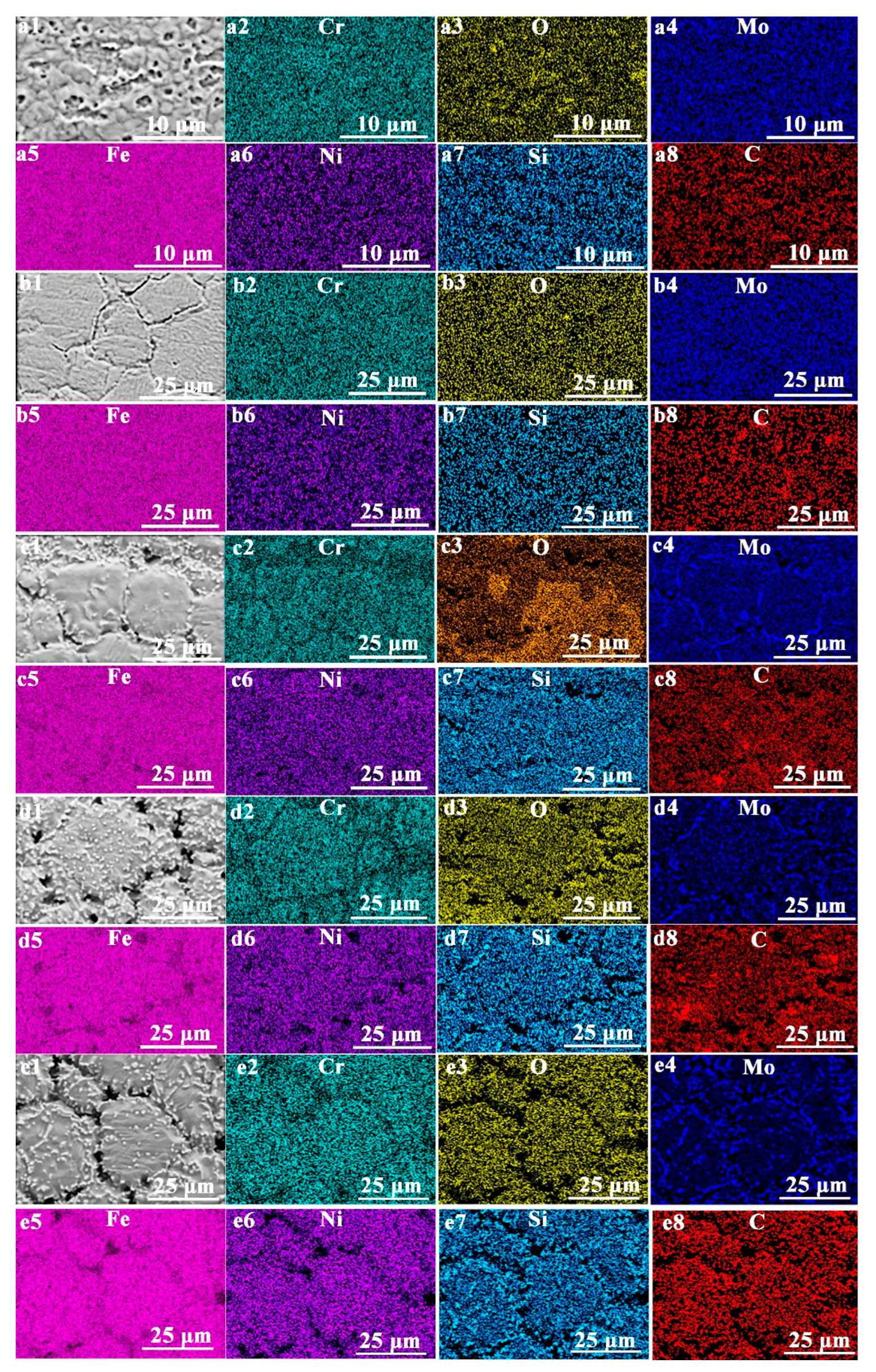
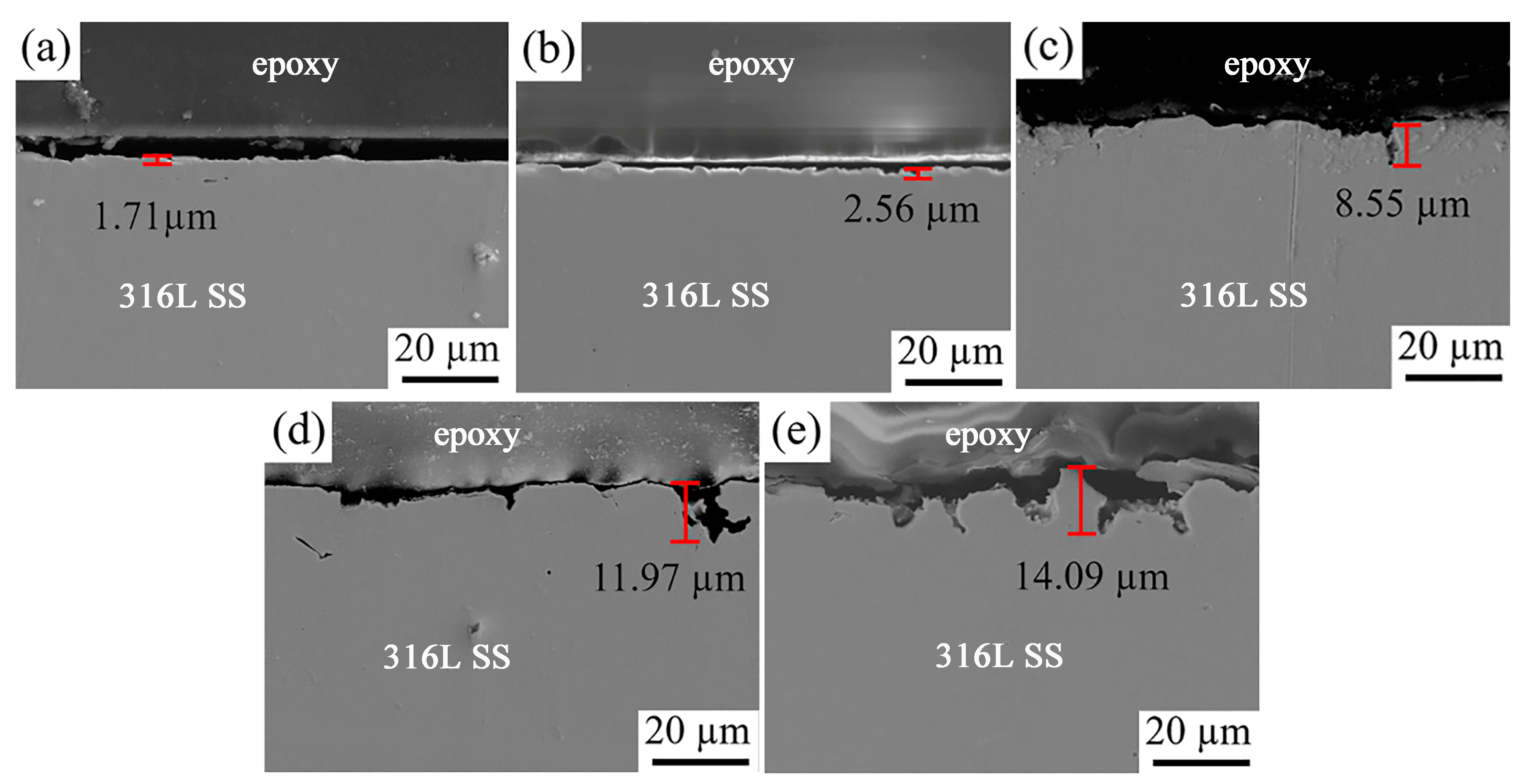
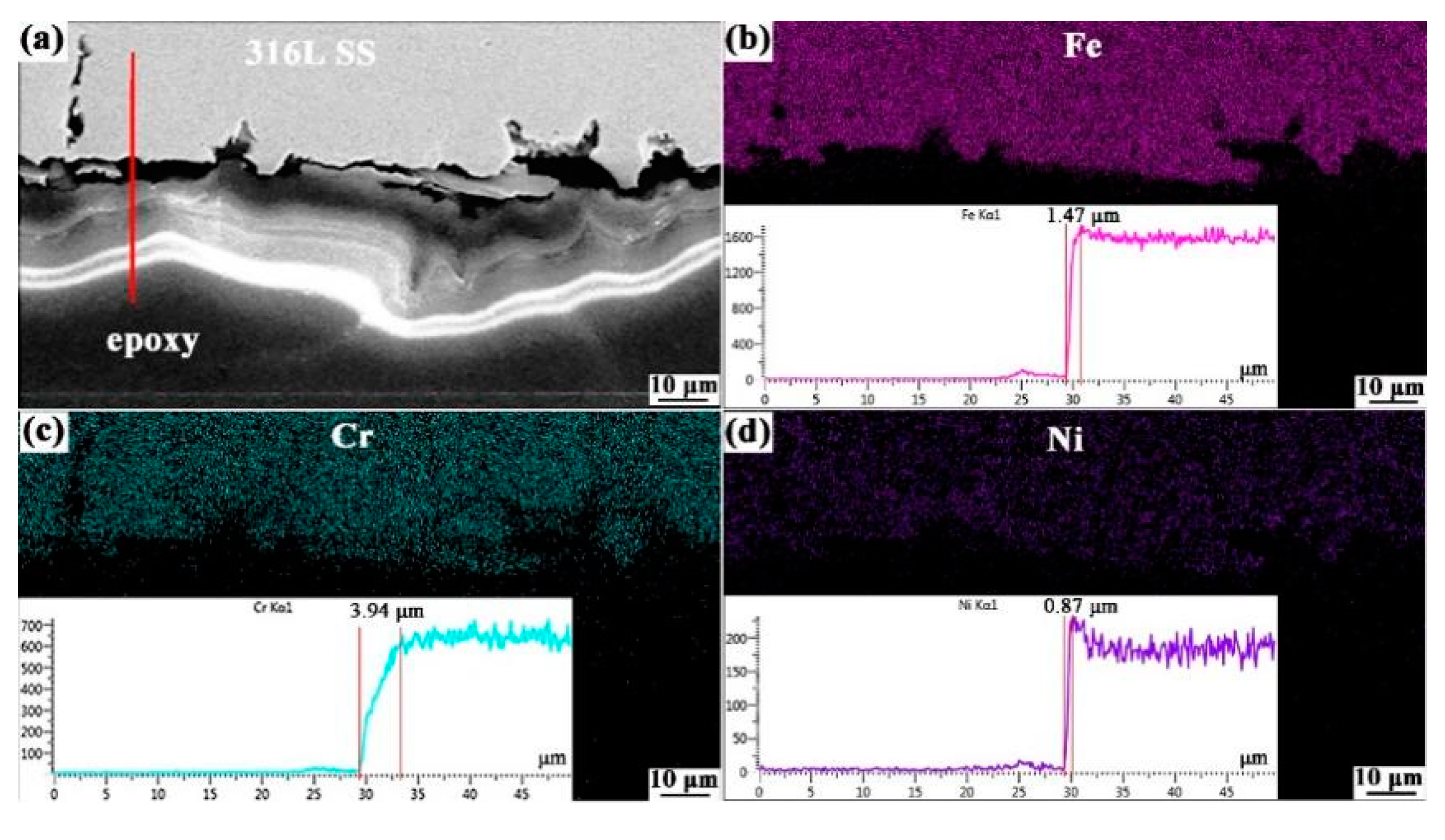
3.1.4. Elemental Distribution and Cross-Sectional Morphology
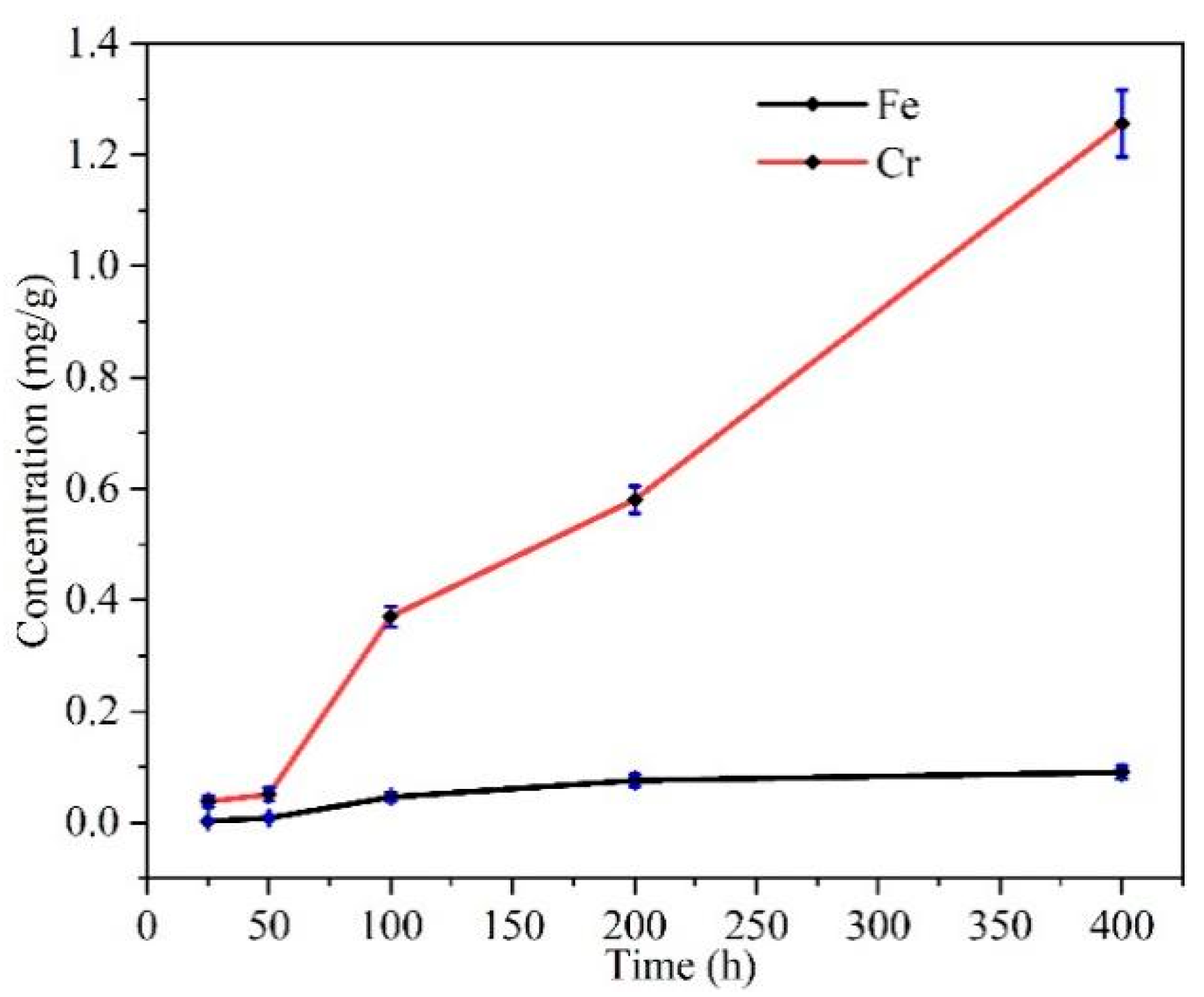
3.1.5. Cr and Fe Concentration in the Molten Salt After Corrosion
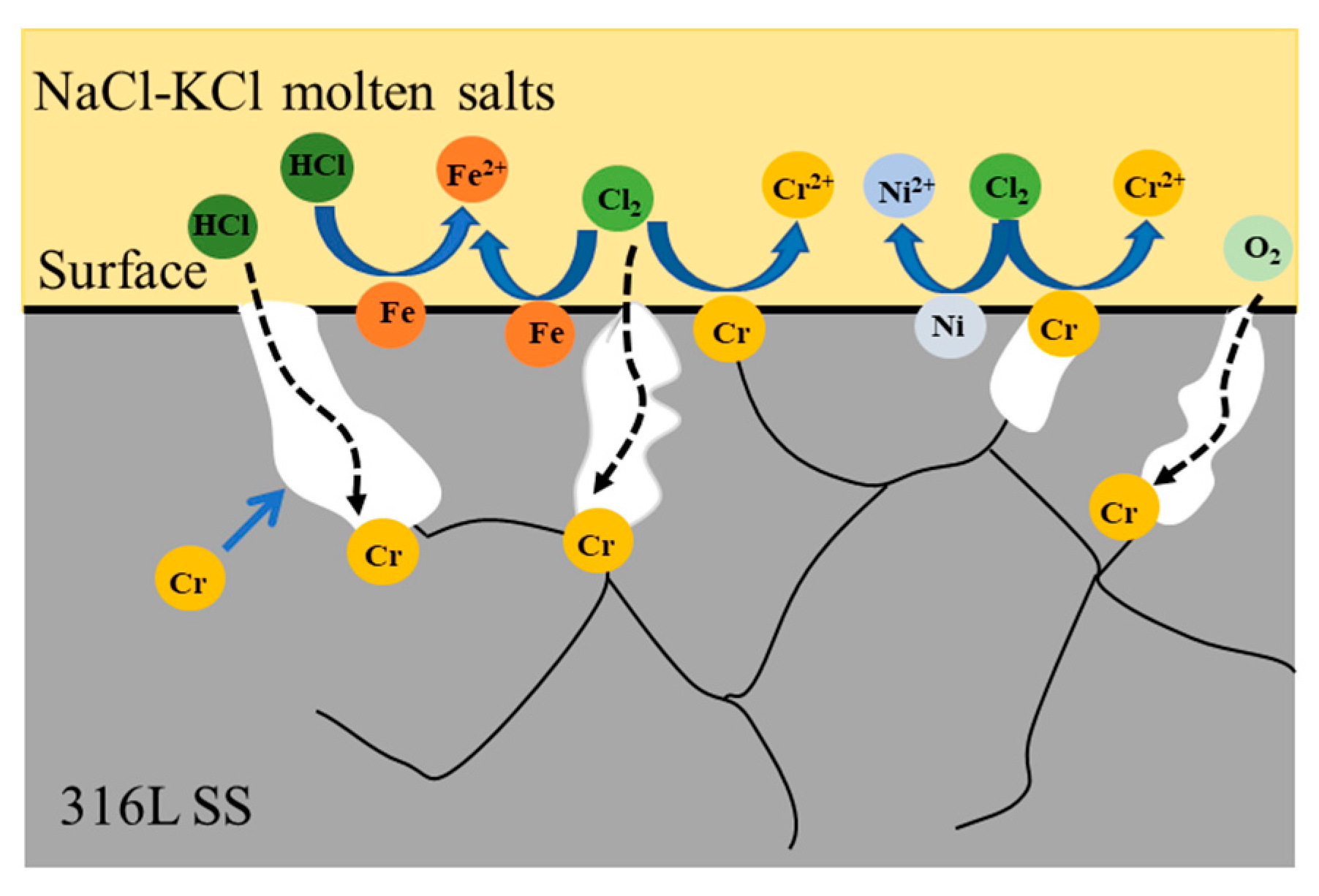
3.2. Corrosion Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Guillot, S.; Faik, A.; Rakhmatullin, A.; Lambert, J.; Veron, E.; Echegut, P.; Bessada, C.; Calvet, N.; Py, X. Corrosion effects between molten salts and thermal storage material for concentrated solar power plants. Appl. Energy 2012, 94, 174–181. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.H.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Li, X.L.; Chang, L.T.; Liu, C.P.; Leng, B.; Ye, X.X.; Han, F.F.; Yang, X.M. Effect of thermal aging on corrosion behavior of type 316H stainless steel in molten chloride salt. Corros. Sci. 2021, 191, 109784. [Google Scholar] [CrossRef]
- Turchi, S.; Vidal, J.; Bauer, M. Molten salt power towers operating at 600–650 °C: Salt selection and cost benefits. Sol. Energy 2018, 164, 38–46. [Google Scholar] [CrossRef]
- Castro-Quijada, M.; Faundez, D.; Rojas, R.; Videla, A. Improving the working fluid based on a NaNO3-KNO3-NaCl-KCl molten salt mixture for concentrating solar power energy storage. Sol. Energy 2022, 231, 464–472. [Google Scholar] [CrossRef]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Gore, P.; Singh, M.P.; Suryateja, D.; Basu, B.; Chattopadhyay, K. Early-stage corrosion of IN 740H alloy in eutectic NaCl-KCl molten salt at high temperatures. Sol. Energy 2023, 252, 330–341. [Google Scholar] [CrossRef]
- Grégoire, B.; Oskay, C.; Meißner, T.; Galetz, M.J. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part I: NaCl–KCl binary system. Sol. Energy Mater. Sol. Cells 2020, 215, 110659. [Google Scholar] [CrossRef]
- Salinas-Solano, G.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Salinas-Bravo, V.M.; Ascencio-Gutierrez, J.A.; Martinez-Gomez, L. High Temperature Corrosion of Inconel 600 in NaCl–KCl Molten Salts. Adv. Mater. Sci. Eng. 2014, 2014, 696081. [Google Scholar] [CrossRef]
- Guo, L.L.; Liu, Q.; Yin, H.Q.; Pan, T.J.; Tang, Z.F. Excellent corrosion resistance of 316 stainless steel in purified NaCl–MgCl eutectic salt at high temperature. Corros. Sci. 2020, 166, 108473. [Google Scholar] [CrossRef]
- Lu, P.F.; Guo, L.L.; Liu, Q.; Liu, L.L.; Pan, T.J.; Tang, Z.F. Excellent high temperature corrosion resistance of 304 stainless steel immersed in purified NaCl–MgCl2 eutectic salts. Mater. Chem. Phys. 2023, 296, 127216. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Zhao, Z.X.; Song, J.L.; Tang, Z.F.; Tao, Z.C.; Liu, Z.J.; Yin, N.; Shi, Q. Chloride salts/graphite foam composites prepared by vacuum impregnation with high thermal conductivity for medium temperature thermal energy storage. Therm. Sci. Eng. Prog. 2023, 44, 102026. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, W.; Bonk, A.; Bauer, T.; Shi, H.; Weisenburger, A.; Yu, R.; Wang, D. Molten Chloride Salt Technology for Next-Generation CSP Plants: Compatibility of cost-effective Fe-based steels with Mg-purified molten MgCl2-KCl-NaCl at 700 °C. Appl. Energy 2022, 324, 119708. [Google Scholar] [CrossRef]
- Gong, Q.; Hanke, A.; Kessel, F.; Bonk, A.; Bauer, T.; Ding, W. Molten chloride salt technology for next-generation CSP plants: Selection of cold tank structural material utilizing corrosion control at 500 °C. J. Mater. Res. Technol. 2023, 253, 112233. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, R.D.; Wang, L.; Ge, F.F.; Gao, M.Y.; Si, Y.; Li, B.S. Corrosion behavior of iron-based and Ni-based alloys melted in NaCl–MgCl2–KCl mixed molten salt under vacuum atmosphere. J. Mater. Res. Technol.-JmrT 2024, 28, 1915–1923. [Google Scholar] [CrossRef]
- Abu-warda, N.; García-Rodríguez, S.; Torres, B.; Utrilla, M.V.; Rams, J. Impact of the additive manufacturing process on the high-temperature corrosion of 316L steel in the presence of NaCl–KCl–ZnCl molten solar salt. J. Mater. Res. Technol. 2022, 20, 3949–3961. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, L.L.; Lu, P.F.; Liu, W.H.; Zhang, F.; Tang, Z.F. Corrosion behavior and mechanism of 316 stainless steel in NaCl-KCl-ZnCl molten salts at high temperature. Mater. Today Commun. 2022, 31, 103297. [Google Scholar] [CrossRef]
- Chen, S.J.; Liu, Q.; Yang, Z.G.; Guo, F.Y.; Chen, L.P.; Zhang, Z.G.; Tang, Z.F. Corrosion behaviours of Ni-Mo-Cr alloy and 316 stainless steel in molten NaCl-KCl-AlCl salts at high temperature. Corros. Eng. Sci. Technol. 2023, 58, 103–107. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, H.; Guo, F.; Chen, L.; Lai, X.; Tang, Z. Corrosion behavior and mechanism of 316 stainless steel in NaCl–KCl–FeCl3 molten salt and vapor at 300 °C. Mater. Corros. 2024, 75, 266–274. [Google Scholar] [CrossRef]
- Wang, L.L.; Wallace, T.C. Vacuum evaporation of KCl-NaCl salts. Part 1: Thermodynamic modeling of vapor pressures of solid and liquid solutions. Metall. Trans. 1996, 27, 915086. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl system. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, Z.; Zhang, P.; Su, X. Corrosion behavior of 316SS and Ni-based alloys in a ternary NaCl–KCl–MgCl2 molten salt. Sol. Energy 2018, 171, 320–329. [Google Scholar] [CrossRef]
- Ernst, F.; Cao, Y.; Michal, G.; Heuer, A. Carbide precipitation in austenitic stainless steel carburized at low temperature. Acta Mater. 2007, 55, 1895–1906. [Google Scholar] [CrossRef]
- Lai, X.; Yin, H.; Li, P.; Liu, B.; Gao, L.; Tang, Z. Design optimization and thermal storage characteristics of NaNO3-NaCl-NaF molten salts with high latent heat and low cost for the thermal energy storage. Mater. Corros. 2022, 52, 104805. [Google Scholar] [CrossRef]
- Liang, J.; Wang, W.; Cao, Z.; Guo, J.; Sun, Z.; Hai, Y. Corrosion resistance and mechanism of high-entropy alloys: A review. Mater. Corros. 2024, 75, 424–432. [Google Scholar] [CrossRef]
- Shankar, A.R.; Mudali, U.K. Corrosion of type 316L stainless steel in molten LiCl-KCl salt. Mater. Corros.-Werkst. Und Korros. 2008, 59, 878–882. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Q.; Bao, H.; Pan, T.; Tang, Z. Effect of FeCl3 in NaCl-MgCl2 molten salts on the corrosion behavior of 316 stainless steel at 600 °C. Corros. Sci. 2023, 212, 110961. [Google Scholar] [CrossRef]
- Sim, J.-H.; Kim, Y.-S.; Cho, I.-J. Corrosion behavior induced by LiCl-KCl in type 304 and 316 stainless steel and copper at low temperature. Nucl. Eng. Technol. 2017, 49, 769–775. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Zhang, X.; Wang, Q.; Wu, J.; Liu, Z.; Zeng, C.; Zhu, S.J. Effect of enamel coating on the hot corrosion of 304 stainless steel beneath KCl–ZnCl2 deposits at 450 °C. J. Mater. Res. Technol. 2023, 23, 245–257. [Google Scholar] [CrossRef]
- Polovov, I.B.; Abramov, A.V.; Rebrin, O.I.; Volkovich, V.A.; Denisov, E.; Griffiths, T.R.; May, I.; Kinoshita, H. Corrosion of Stainless Steel in NaCl-KCl Based Melts. ECS Trans. 2010, 33, 321. [Google Scholar] [CrossRef]
- Lai, X.; Yin, H.; Yang, Z.; Tang, Z. Synergistic effect of Cl− and F− on the corrosion behavior and mechanism of 316 stainless steel in NaNO3-based molten salts and vapor. J. Energy Storage 2023, 65, 107243. [Google Scholar] [CrossRef]
- Brito-Hernandez, D.; Lopez-Sesenes, R.; Haro, S.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G. Corrosion behavior of UCX, KHR35, and KHR45 alloys in molten nitrates. Mater. Corros. 2020, 71, 1783–1793. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Zuo, Y.; Song, Y.-L.; Tang, R.; Qian, Y. A novel purification method for fluoride or chloride molten salts based on the redox of hydrogen on a nickel electrode. RSC Adv. 2021, 11, 35069–35076. [Google Scholar] [CrossRef]
- Wang, J.-w.; Zhang, C.-z.; Li, Z.-h.; Zhou, H.-x.; He, J.-x.; Yu, J.-c. Corrosion behavior of nickel-based superalloys in thermal storage medium of molten eutectic NaCl-MgCl2 in atmosphere. Sol. Energy Mater. Sol. Cells 2017, 164, 146–155. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Tang, Z.J. Corrosion behavior and mechanism of GH3539 alloy in molten NaCl-KCl-CaCl2 salts at 800 °C. J. Energy Storage 2024, 98, 112942. [Google Scholar] [CrossRef]
- Ren, S.; Chen, Y.; Ye, X.-X.; Jiang, L.; Yan, S.; Liang, J.; Yang, X.; Leng, B.; Li, Z.; Chen, Z. Corrosion behavior of carburized 316 stainless steel in molten chloride salts. Sol. Energy 2021, 223, 1–10. [Google Scholar] [CrossRef]
- Singh, H.; Pandey, O. Novel process for synthesis of nanocrystalline WC from wolframite ore. Ceram. Int. 2015, 41, 10481–10487. [Google Scholar] [CrossRef]
- Epifano, E.; Monceau, D. Ellingham diagram: A new look at an old tool. Corros. Sci. 2023, 217, 111113. [Google Scholar] [CrossRef]
- Wang, Y.; Sridharan, K.; Couet, A.J. Method for identification of redox control parameters for corrosion mitigation in molten fluoride salts. J. Nucl. Mater. 2021, 543, 152624. [Google Scholar] [CrossRef]
- Luo, A.; Jacobson, D.; Ponnappan, R.J. Compatibility of inconel 617R alloy with LIF-MGF2-kf thermal energy storage salts and vacuum at high temperature. J. Mater. Eng. Perform. 1992, 1, 755–761. [Google Scholar] [CrossRef]
- Kurley, J.M.; Halstenberg, P.W.; McAlister, A.; Raiman, S.; Dai, S.; Mayes, R.T. Enabling chloride salts for thermal energy storage: Implications of salt purity. RSC Adv. 2019, 9, 25602–25608. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Pavlík, V.; Boča, M. High-temperature corrosion behavior of superalloys in molten salts–a review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 83–97. [Google Scholar] [CrossRef]
- Kawahara, Y. High temperature corrosion mechanisms and effect of alloying elements for materials used in waste incineration environment. Corros. Sci. 2002, 44, 223–245. [Google Scholar] [CrossRef]
- Smith, A. The diffusion of chromium in type 316 stainless steel. Met. Sci. 1975, 9, 375–378. [Google Scholar] [CrossRef]
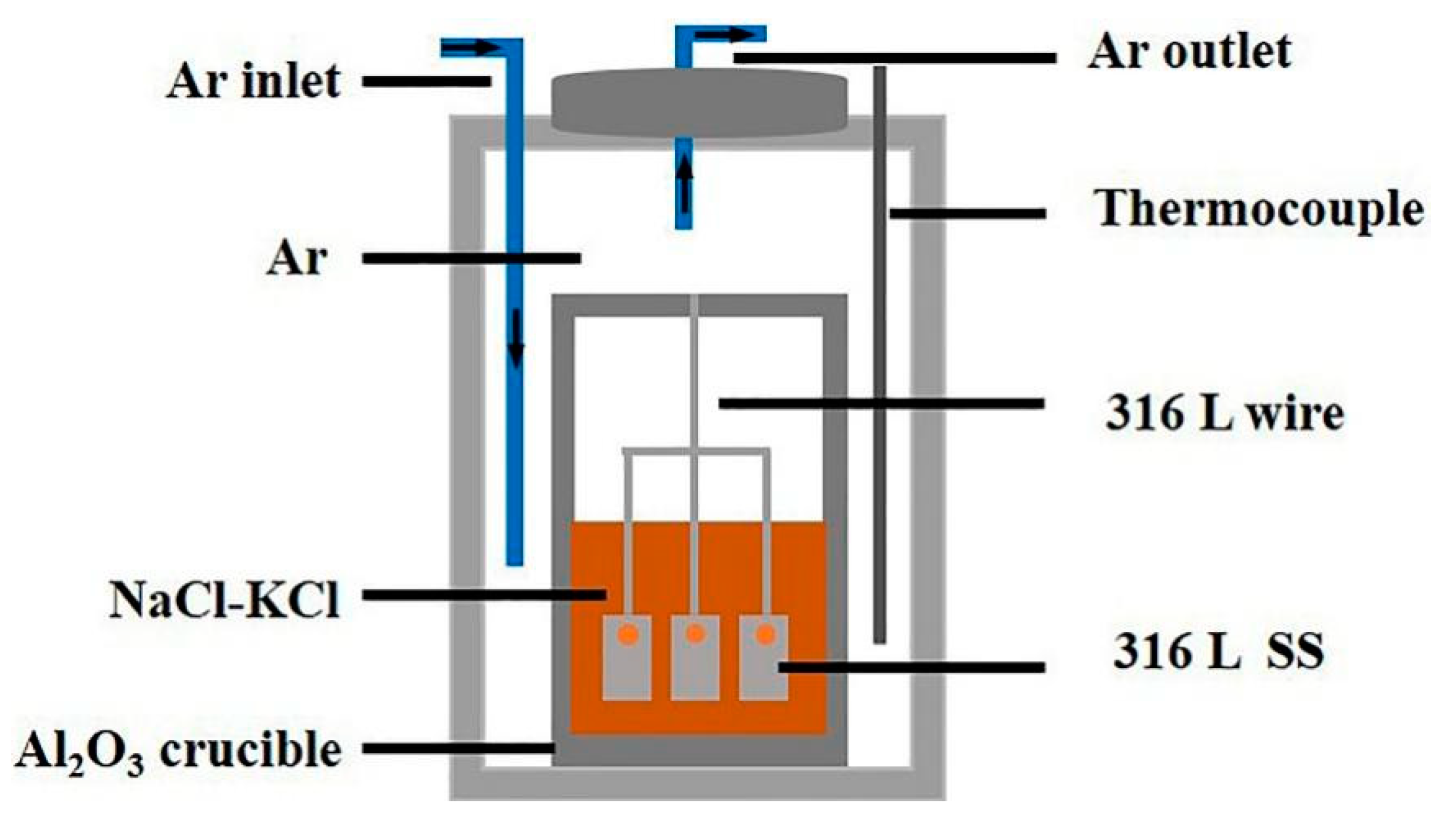

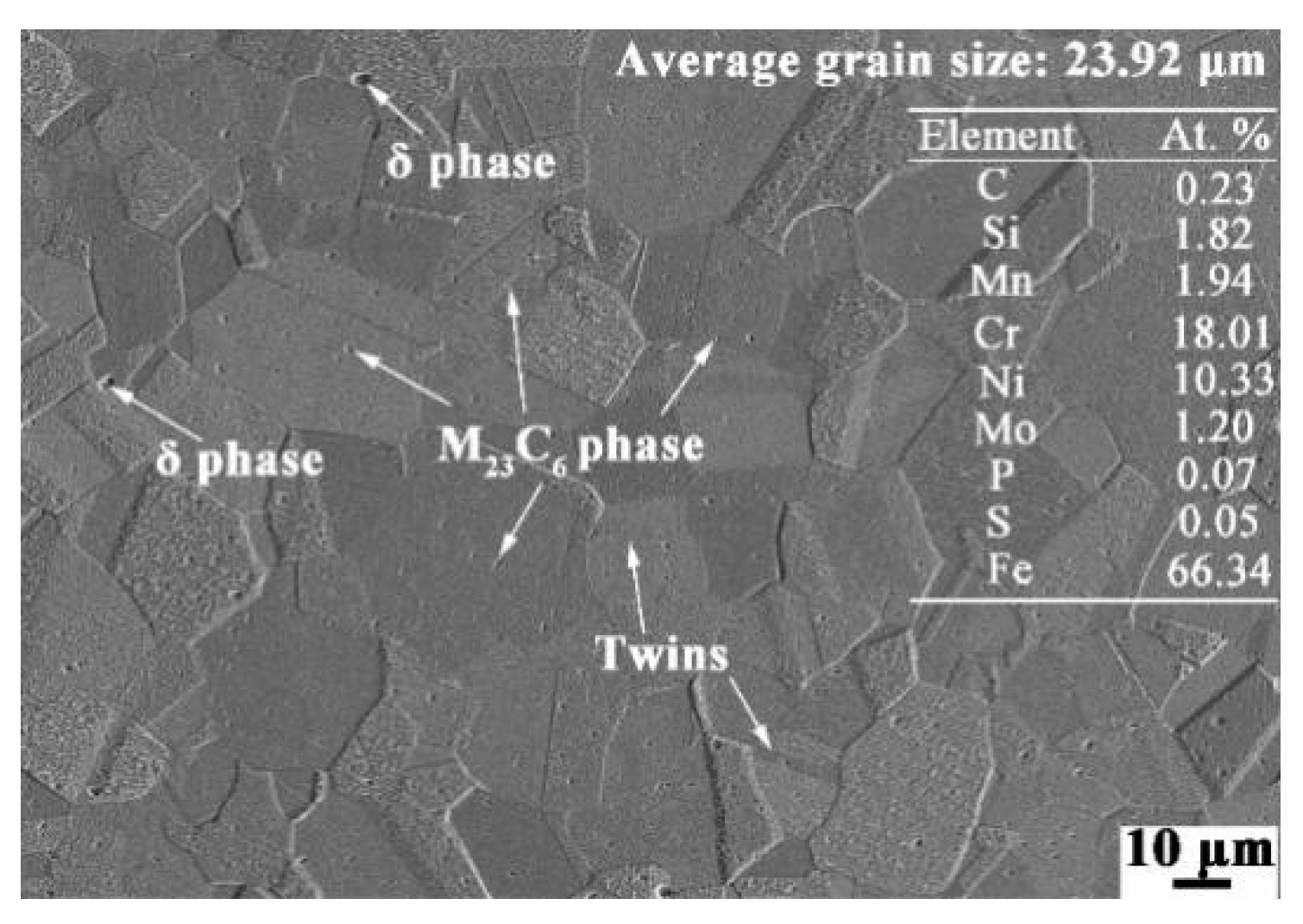



| Element | C | Si | Mn | Cr | Ni | Mo | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.03 | 0.92 | 1.93 | 16.9 | 11 | 2.08 | 0.04 | 0.03 | Bal. |
| Molten Salts System | Temperature (°C) | Time (h) | CR (μm/year) |
|---|---|---|---|
| NaCl-MgCl2 | 700 | 240 | 205.36 [11] |
| NaCl-KCl-MgCl2 | 700 | 100 400 | 261 [23] 51.3 [38] |
| NaCl-KCl-ZnCl2 | 500 | 120 240 350 | 291 [17] 198 [17] 191 [17] |
| NaCl-KCl | 700 | 100 400 | 83.61 (this work) 47.22 (this work) |
| Regions | C | Si | Mn | Cr | Ni | Mo | Fe | O |
|---|---|---|---|---|---|---|---|---|
| Figure 5a | 15.70 | 0.36 | 0.32 | 8.70 | 9.06 | 1.23 | 61.89 | 2.75 |
| Figure 5c | 15.53 | 0.26 | 0.40 | 8.56 | 9.73 | 1.24 | 62.32 | 1.96 |
| Figure 5e | 19.24 | 1.04 | 0.10 | 6.08 | 11.09 | 1.92 | 52.12 | 8.42 |
| Figure 5g | 27.45 | 1.19 | 0.24 | 5.86 | 10.20 | 2.05 | 44.23 | 8.79 |
| Figure 5i | 13.72 | 1.65 | 0.08 | 5.60 | 13.39 | 3.48 | 58.64 | 3.44 |
| A | 30.25 | 0.37 | 0.54 | 8.80 | 7.01 | 0.96 | 49.21 | 2.86 |
| B | 23.85 | 5.38 | 0.35 | 2.90 | 6.99 | 16.34 | 33.96 | 10.24 |
| C | 21.92 | 5.62 | 0.34 | 2.15 | 8.45 | 14.82 | 32.53 | 14.18 |
| D | 26.97 | 5.68 | 0.18 | 2.71 | 6.85 | 16.28 | 32.12 | 9.21 |
| Reaction | ΔG (kJ/mol) |
|---|---|
| Fe + Cl2(g) = FeCl2 | −221.23 |
| Cr + Cl2(g) = CrCl2 | −275.97 |
| Ni + Cl2(g) = NiCl2 | −159.71 |
| 4Fe + 3O2(g) = 2Fe2O3 | −1131.89 |
| 4Cr + 3O2(g) = 2Cr2O3 | −1756.17 |
| 2Ni + O2(g) = 2NiO | −301.81 |
| Fe + 2HCl(g) = FeCl2 + H2(g) | −19.9 |
| Cr + 2HCl(g) = CrCl2 + H2(g) | −74.6 |
| Ni + 2HCl(g) = NiCl2 + H2(g) | 41.6 |
| 4FeCl2 + 3O2(g) = 2Fe2O3 + 4Cl2(g) | −246.98 |
| 4CrCl2 + 3O2(g) = 2Cr2O3 + 4Cl2(g) | −652.28 |
| 2NiCl2 + O2(g) = 2NiO + 2Cl2(g) | 17.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Tang, X.; Ying, Z.; Ai, H.; Sun, H.; Zhang, W.; Wang, Y.; Cheng, J.; Yan, L. Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl-KCl Molten Salt at High Temperatures. Crystals 2025, 15, 280. https://doi.org/10.3390/cryst15030280
Lv R, Tang X, Ying Z, Ai H, Sun H, Zhang W, Wang Y, Cheng J, Yan L. Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl-KCl Molten Salt at High Temperatures. Crystals. 2025; 15(3):280. https://doi.org/10.3390/cryst15030280
Chicago/Turabian StyleLv, Ruimin, Xian Tang, Zhemian Ying, Hua Ai, Hua Sun, Wei Zhang, Ying Wang, Jinjuan Cheng, and Long Yan. 2025. "Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl-KCl Molten Salt at High Temperatures" Crystals 15, no. 3: 280. https://doi.org/10.3390/cryst15030280
APA StyleLv, R., Tang, X., Ying, Z., Ai, H., Sun, H., Zhang, W., Wang, Y., Cheng, J., & Yan, L. (2025). Corrosion Mechanism and Properties of 316L Stainless Steel in NaCl-KCl Molten Salt at High Temperatures. Crystals, 15(3), 280. https://doi.org/10.3390/cryst15030280





