The Diversity of Crystals, Microstructures and Texture That Form Ostreoidea Shells
Abstract
:1. Introduction
2. Methods
2.1. Samples
2.2. Sample Preparation for FE-SEM Imaging and EBSD Measurements
2.3. EBSD Measurements and Data Analysis
2.4. Terminology
3. Results
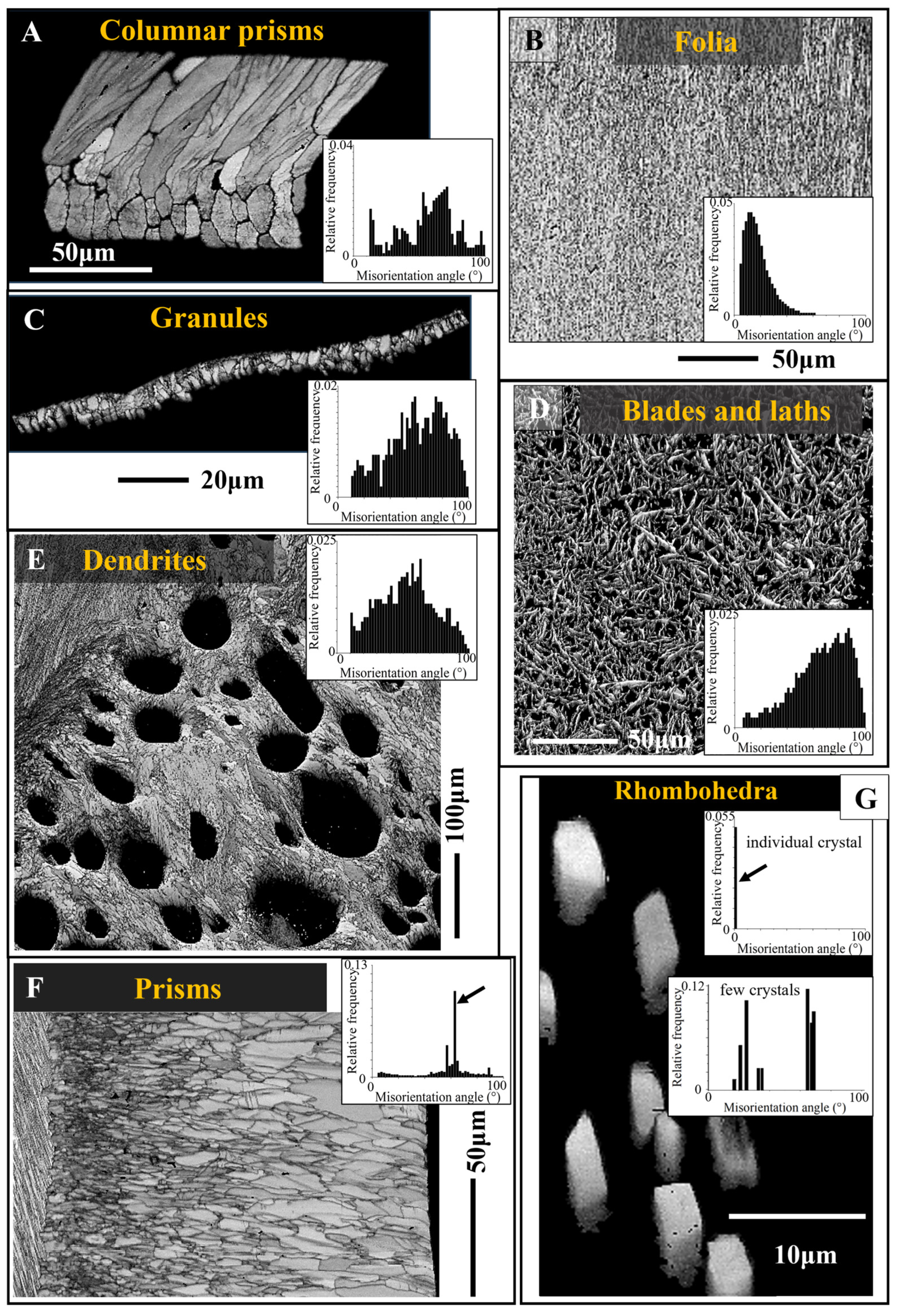
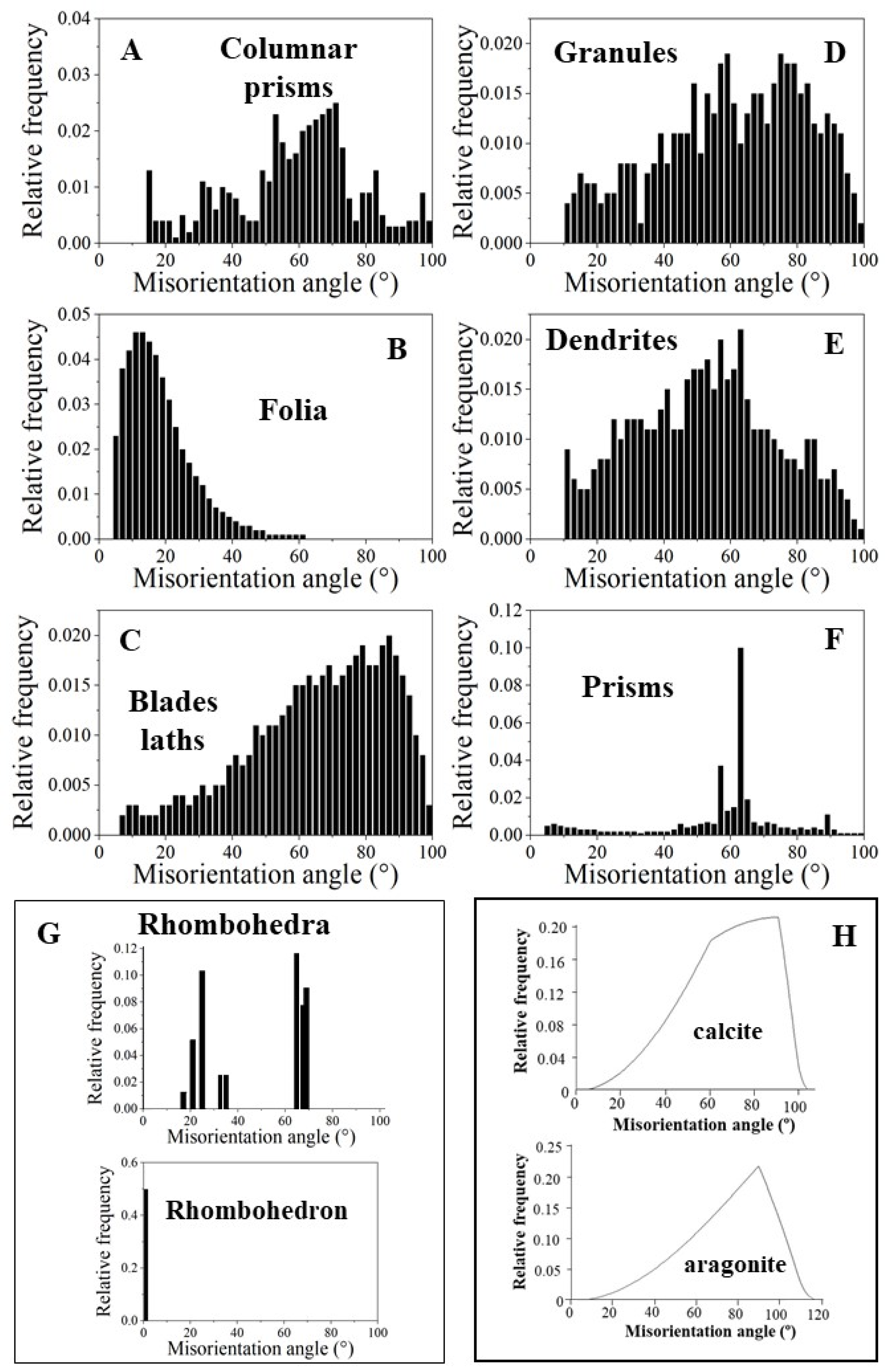
3.1. The Compact Shell Layers
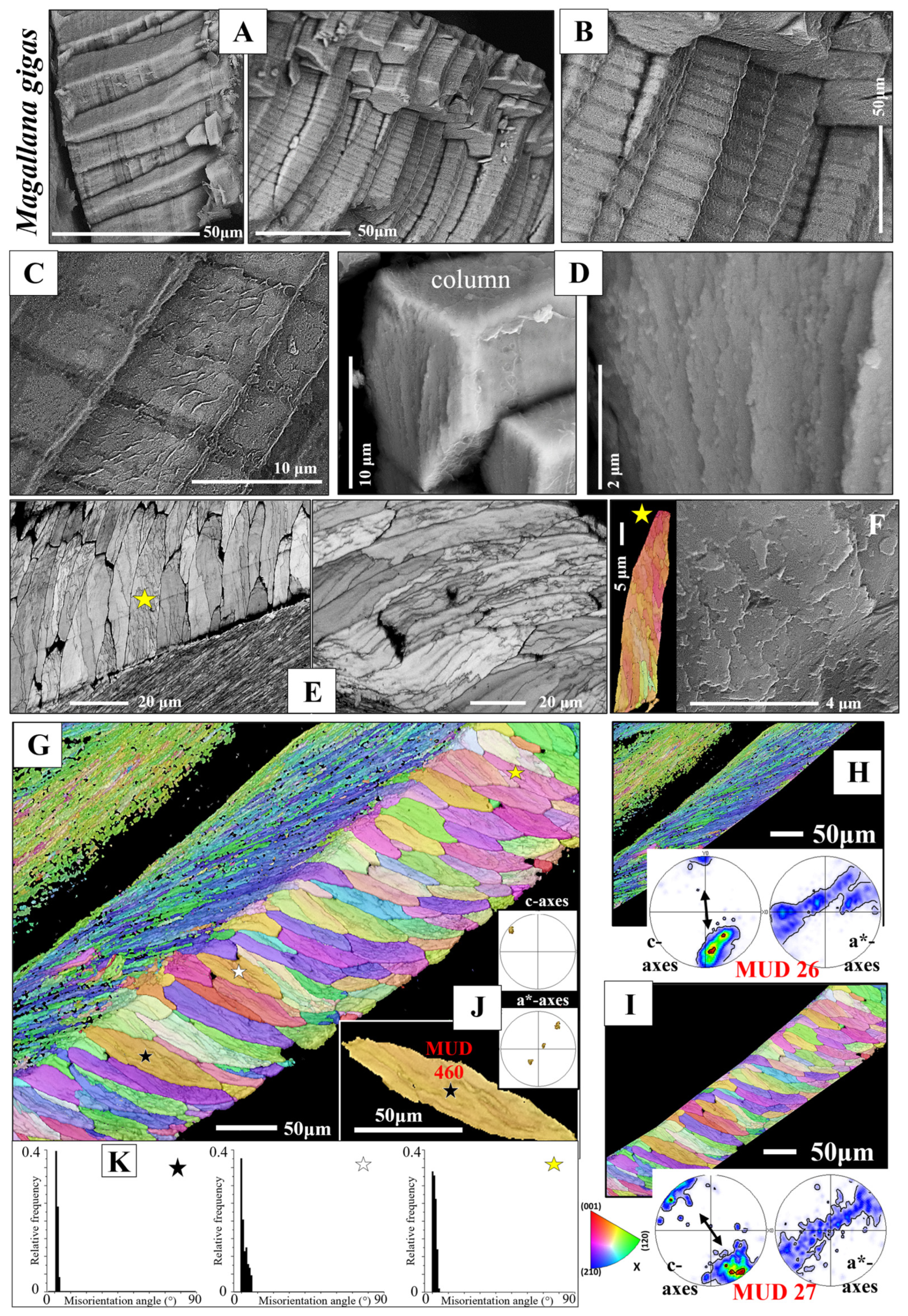
3.1.1. The Columnar Prisms
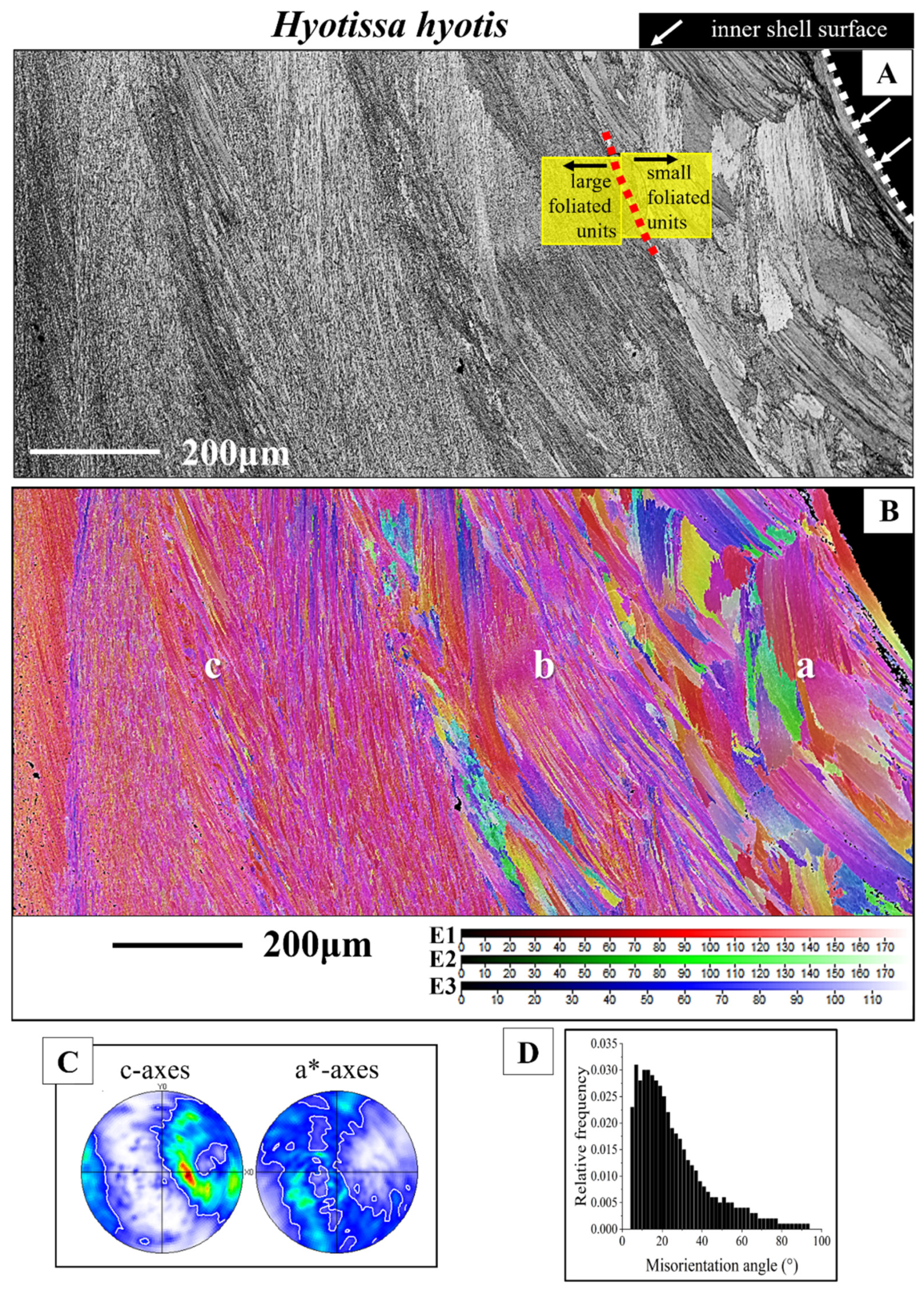
3.1.2. The Foliated Units

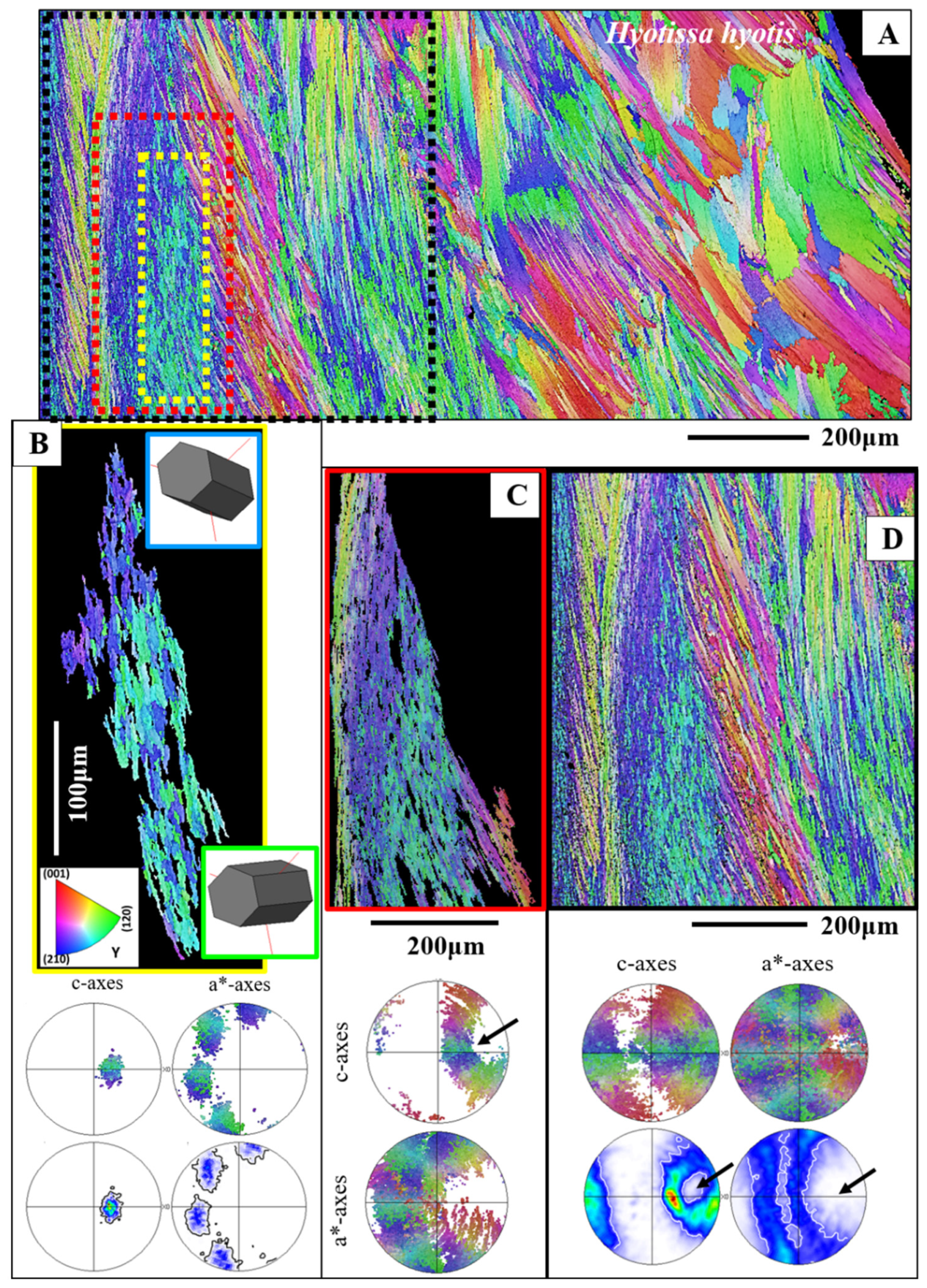
3.1.3. The Myostracal Prisms
3.1.4. The Granules of the Cement Layer

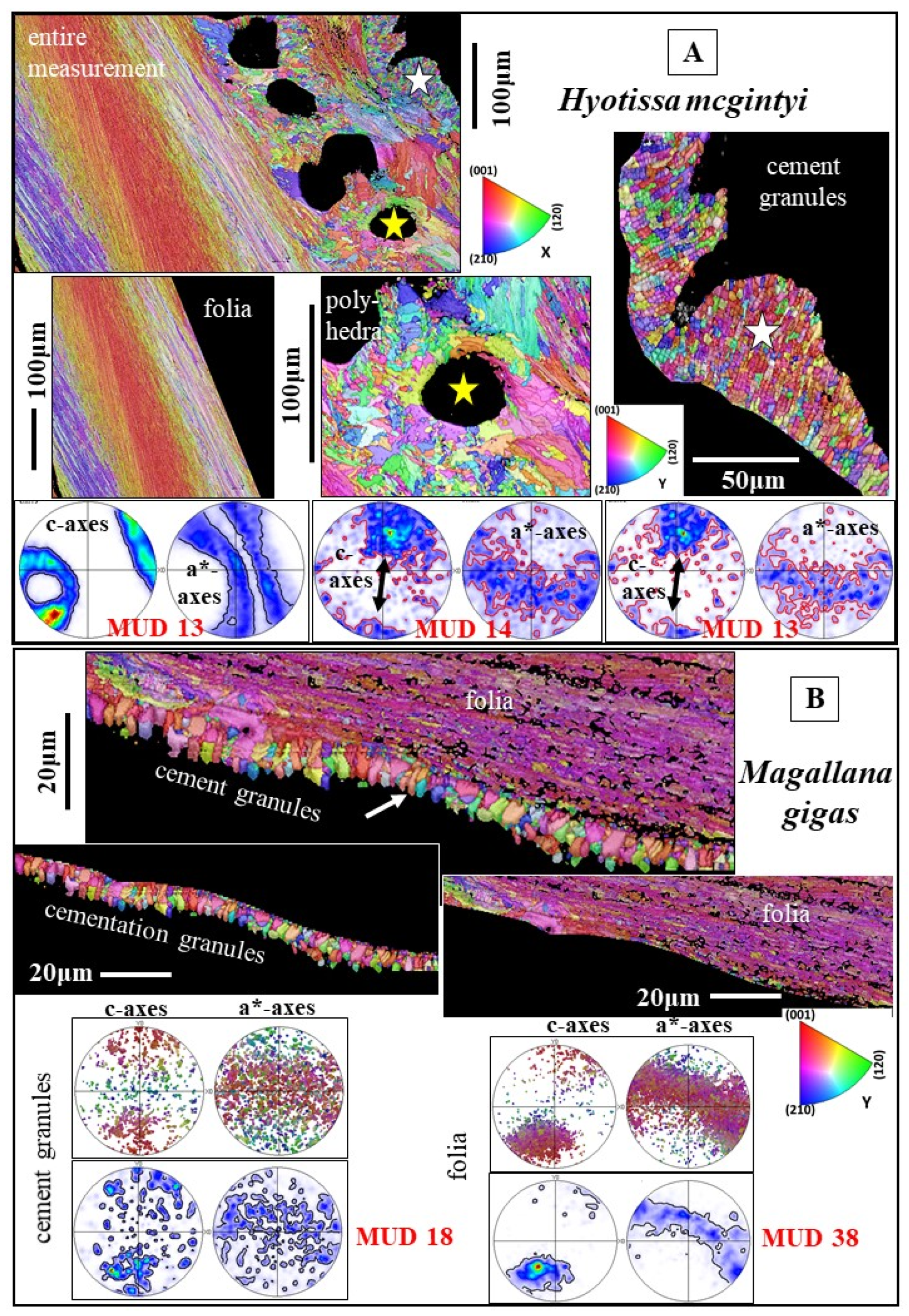
3.2. The Shell Layers Incorporating Voids and Pores
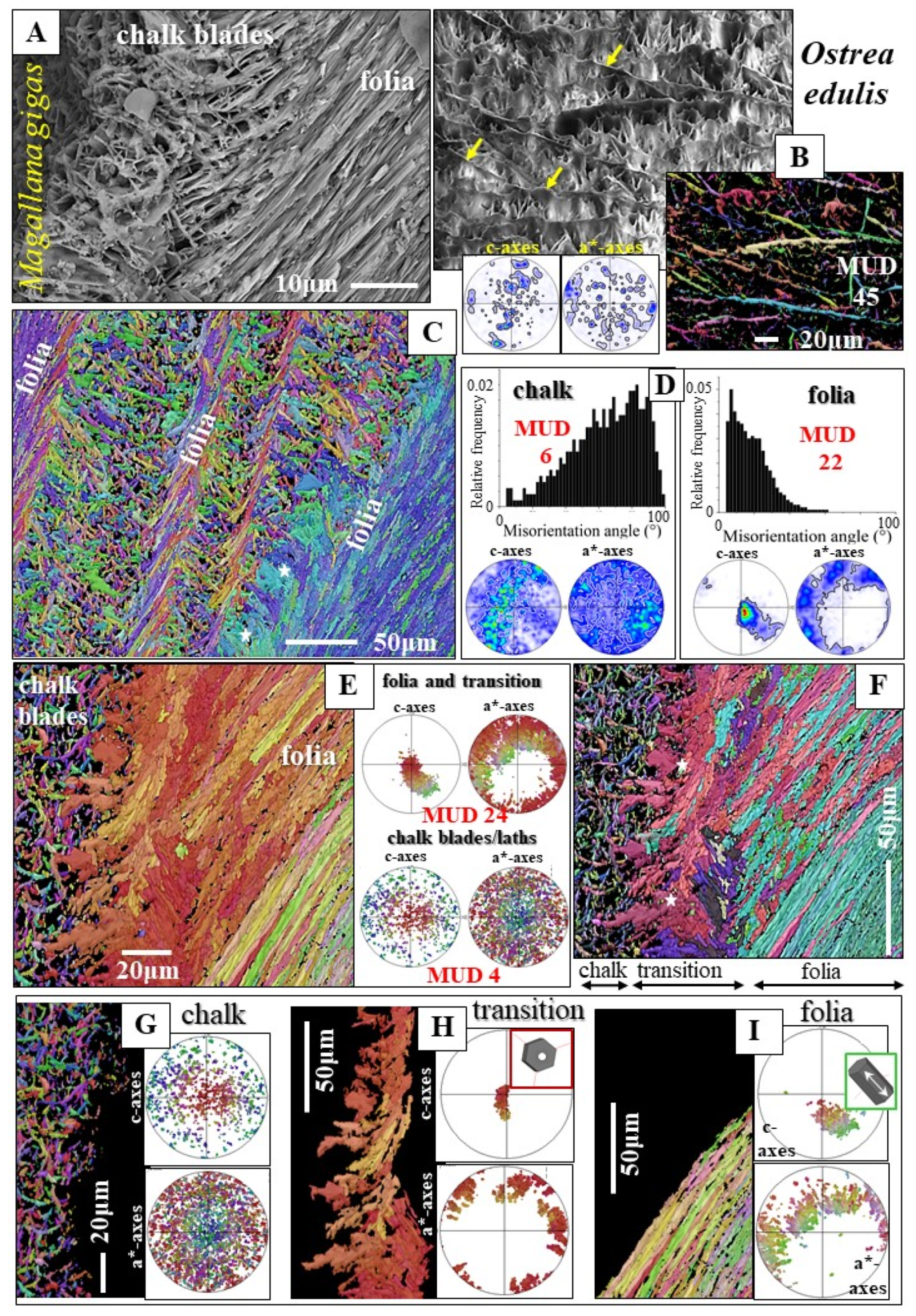
3.2.1. The Chalk Blades and Laths
3.2.2. The Vesicular Pores

3.2.3. The Rhombohedral Crystals
4. Discussion
4.1. The Microstructures
4.1.1. The Microstructures of the Compact Shell Layers
- The columnar layer is strongly enriched in organic substance. The latter is developed as thick membranes delineating adjacent columns ([48] and Figures 35 and 37 in [49]). In addition, we observed that much organic substance is present within the columns, e.g., Figure 3B,C. Surprisingly, the deposition of organic substance in the columns has a banded appearance (Figure 3A–C). Thus, we find within a column an alternation of organic-rich and slightly less organic-rich bands (Figure 3B,C). Organic substance distribution in a column is rather inhomogeneous, as is not the case for other bivalve aragonitic columnar prisms (e.g., Elliptio crassidens (Lamarck, 1819) [31]). The growth bands in ostreoid columnar layers are episodic growth bands. Harper and Checa [48] investigated the organic content of the shell layers of pearl oysters (very many species of 29 taxa). These form their shells of columnar calcite and nacreous aragonite. The authors detected a significant difference in organic substance content between the columnar and the nacreous shell layers and suggested that the high percentage of organic substance of the columnar layer, relative to the nacreous shell layer, might be regarded as a functional trait. The more organic substance-rich and, thus, more ductile the columnar layer is, the better is the structural flexibility of the columnar shell layer. The authors suggested that a tighter closure of the valves could be achieved and, with this, a better protection of the soft tissue and the organs [48].
- The other structural characteristic that we observed for the columnar layer of the investigated species is the strong structuring of the columns (Figure 3E,F and Figure S3). An individual column consists of many subunits, domains. These have very irregular shapes and sizes (Figure 3E and Figure S3A) and are misoriented to each other by about 5° to 10° (Figure 3K). The subunits or domains of the columns are formed of platy calcite crystals (Figure S2). These have strongly irregular morphologies and vary highly in size (Figure 3F and Figure S3B,C). It should be noted that, for the species investigated in this study, neither the structuring of the columns into subunits/domains nor the crystals that comprise the domains resemble the structures that we find for the corresponding foliated shells. It should be kept in mind that we observed very different microstructures and textures for the columnar-prismatic and for the foliated shell layers. Despite the latter, three structural characteristics are similar for these shell layers: (i) their very hierarchical architecture, (ii) the high co-orientation strength of calcite crystals in the subunits/domains (Figure 3J and Figure 4E,F) and (iii) the very low co-orientation strength of individual units (columns, foliated units) in the relevant shell layer (Figure 3H,I).
4.1.2. The Microstructures Hosting the Cavities and Pores
4.1.3. Microstructures Generating Curved Surfaces
4.2. The Textures
- (i)
- For the Ostreoidea, the diversity of crystal texture patterns is increased, relative to the diversity of texture patterns that we detect for the shells of other bivalved, biocarbonate-forming, marine organism orders.
- (ii)
- We observe, for Ostreoidea shells, a larger diversity in crystal microstructures, relative to what we find for their crystal textures.
4.3. The Changeover Between Adjacent Microstructures
4.3.1. Oriented Nucleation Related Changeover
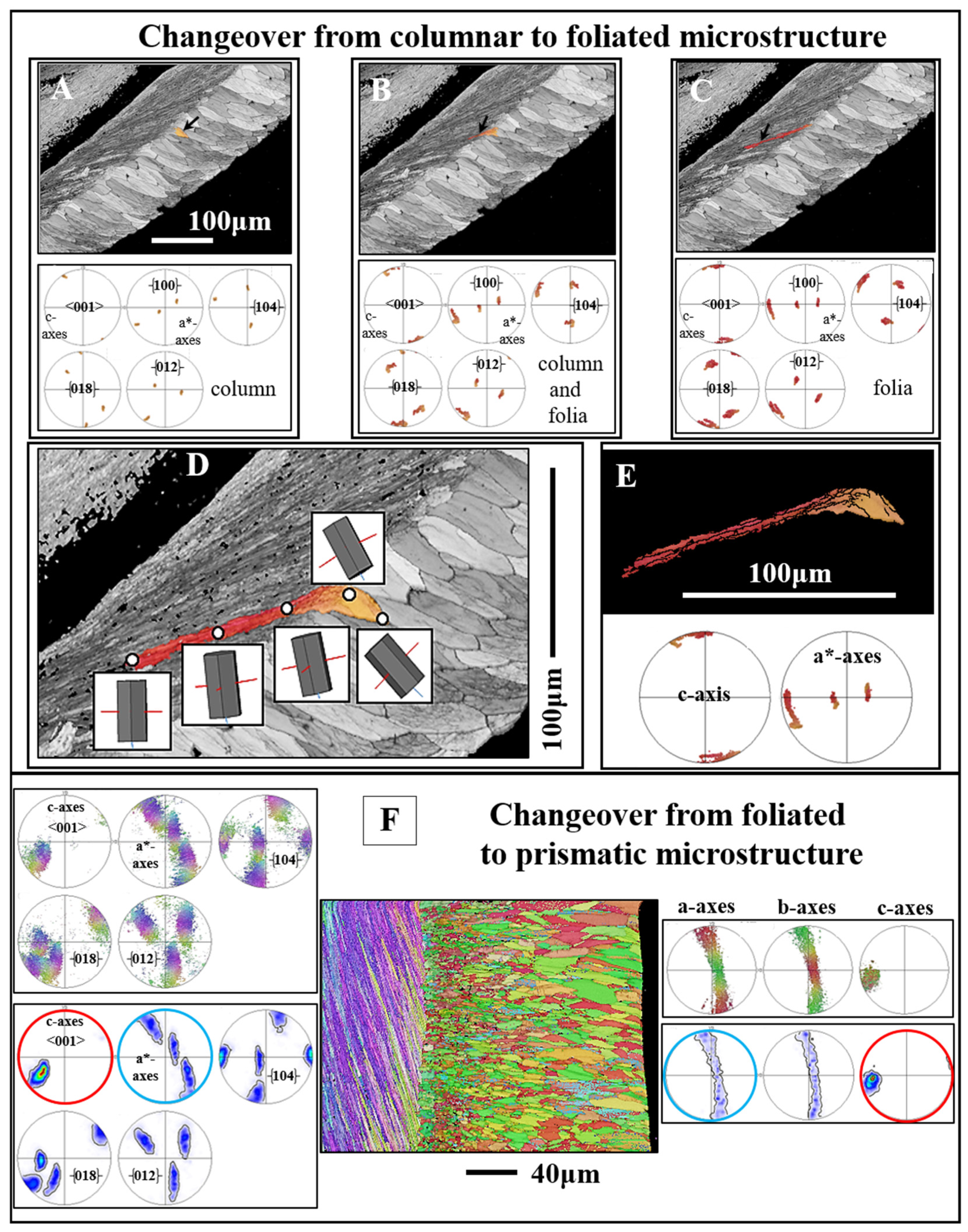
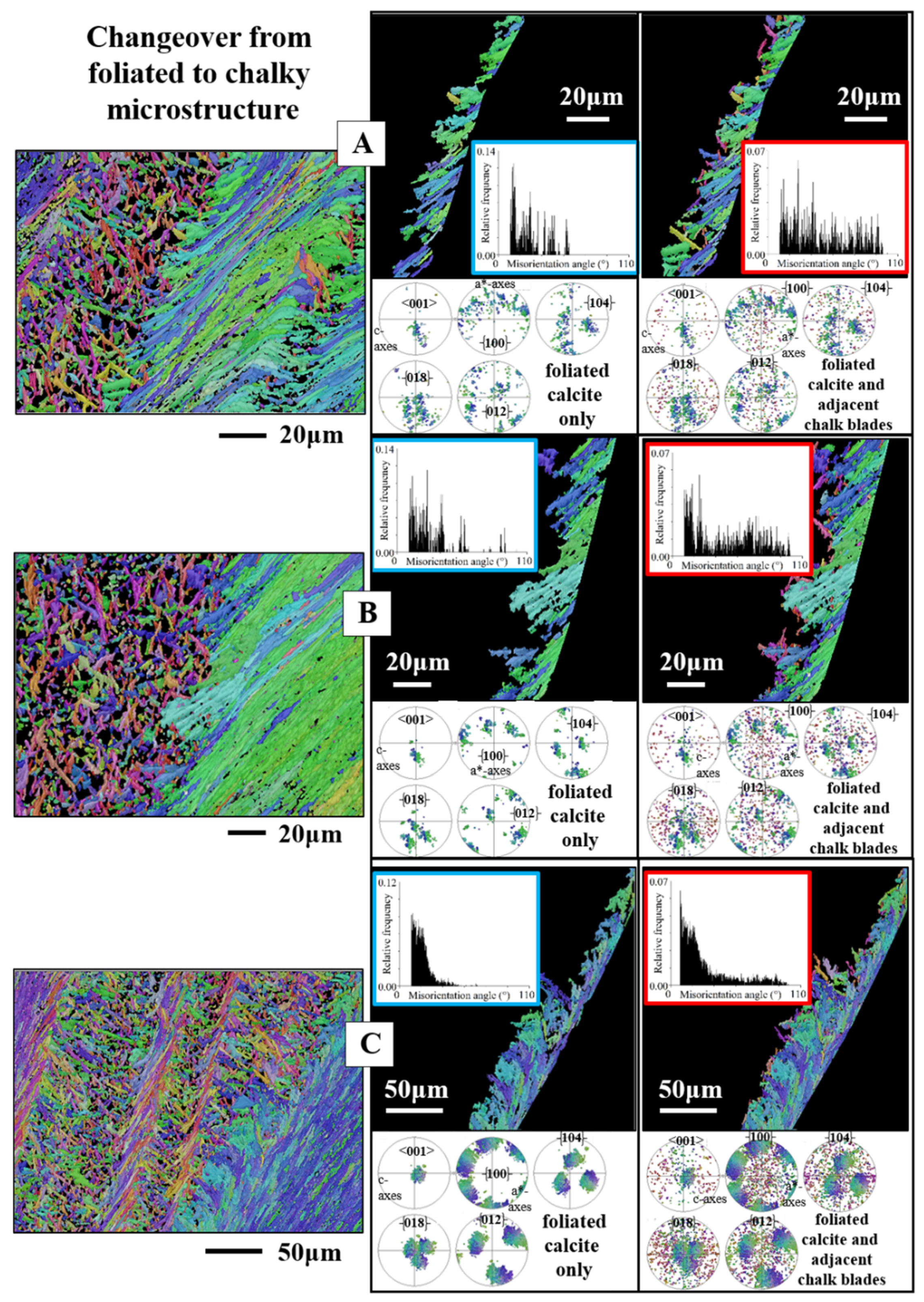
4.3.2. Crystal Twin-Related Changeover
- (i)
- Relative frequency versus misorientation angle diagrams;
- (ii)
- Degree of misorientation versus distance diagrams (Figure S10).
- (i)
- The misorientation angles and their frequencies (Figure S11B);
- (ii)
- The crystallographic indices of the misorientation axes and the frequencies of these misorientation axes (Figure S11C).
5. Conclusions
- The diversity in Ostreoidea shell crystal morphology is extensive. The microstructures that are formed out of these crystals are numerous. The crystal textures that are generated by the microstructures are large in number.
- It is most interesting that the same ostreoid organism secretes crystals with many morphologies and generates many microstructures and textures for shell formation. As ostreoid organisms are, in general, short lived, this structural diversity in crystal formation is secreted in a short time period.
- The calcite of the individual crystals (the laths of a folium, the laths and blades of the chalk, rhombohedra, dendrites, granules) is very co-oriented. It is single crystalline or is very close to being single crystalline. In contrast, when these crystals form a crystal unit, the assembly of the crystal units in the shell layer is very little co-oriented. The assembly of crystal units for some microstructures is close to being random.
- The foliated microstructure and the foliated texture are very specific. Both are rooted in the nature and mode of assembly on different hierarchical levels of the foliated crystal morphology, the lath-shaped arrow-headed crystals. The foliated assembly of these crystals evokes gradedness in c- and a*-axes orientation distribution and formation of a ring-shaped c- and a*-axes orientation distribution for the foliated texture. In addition, the arrowhead ending of the foliated crystals and their orientation, relative to inner shell surface, results in the fact that the foliated texture axis is not the calcite c-axis.
- The structure of the shell layer that hosts the vesicular pores of the Gryphaeidae is very particular. This shell layer is formed by fractal-shaped dendrites. These interlink strongly in 3D, generate a compact layer of calcite and host a multitude of pores.
- Twinned crystals were only observed for myostracal aragonite. There is no twin-related changeover between adjacent calcitic shell layers nor between adjacent calcite columns.
- We did not observe any clear-cut co-orientation of crystallographic axes for the crystals between adjacent shell layers.
- The rounded shape of inner shell surfaces is generated in both the Ostreidae and the Gryphaeidae by forming a little co-oriented assembly of small, foliated units along the innermost shell surface. The size of the foliated units increases gradually, away from the inner shell surface.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenzel, H.B. Systematic Descriptions. In Treatise on Invertebrate Paleontology: Part N Mollusca 6: Bivalvia: Oysters; Moore, R.C., Ed.; The Geological Society of America: Boulder, CO, USA, 1971; Volume 3, pp. 1096–1172. ISBN 0-8137-3026-0. [Google Scholar]
- Márquez-Aliaga, A.; Jiménez-Jiménez, A.P.; Checa, A.G.; Hagdorn, H. Early Oysters and Their Supposed Permian Ancestors. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 229, 127–136. [Google Scholar] [CrossRef]
- Mouchi, V.; Andrus, C.F.T.; Checa, A.G.; Elliot, M.; Griesshaber, E.; Hausmann, N.; Huyghe, D.; Lartaud, F.; Peharda, M.; de Winter, N.J. Oyster Shells as Archives of Present and Past Environmental Variability and Life History Traits: A Multi-Disciplinary Review of Sclerochronology Methods and Applications. Limnol. Oceanogr. Lett. 2025. [Google Scholar] [CrossRef]
- Yonge, C.M. Oysters; Collins: New York, NY, USA, 1960. [Google Scholar]
- Bricteux-Gregoire, S.; Duchateau-Bosson, G.; Jeuniaux, C.; Florkin, M. Constituants Osmotiquement Actifs des Muscles Adducteurs d’Ostrea edulis Adaptée a L’eau de Mer Ou a L’eau Saumatre. Arch. Int. Physiol. Biochim. 1964, 72, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, D.; De Mol, L.; Le Guilloux, E.; Wisshak, M.; Huvenne VA, I.; Moeremans, R.; Henriet, J.P. Environmental Setting of Deep-Water Oysters in the Bay of Biscay. Deep Sea Res. 2010, 57, 1561–1572. [Google Scholar] [CrossRef]
- Checa, A.G.; Linares, F.; Maldonado-Valderrama, J.; Harper, E.M. Foamy Oysters: Vesicular Microstructure Production in the Gryphaeidae via Emulsification. J. R. Soc. Interface 2020, 17, 20200505. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Kong, L.; Yu, H.; Zheng, X. Identifying the True Oysters (Bivalvia: Ostreidae) with Mitochondrial Phylogeny and Distance-Based DNA Barcoding. Mol. Ecol. Resour. 2011, 11, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, S.; Hu, Y.; Yu, H.; Kong, L.; Xu, C.; Li, Q. Multi-Omic Insights into the Formation and Evolution of a Novel Shell Microstructure in Oysters. BMC Biol. 2023, 21, 204. [Google Scholar] [CrossRef]
- Nakahara, H.; Bevelander, G. The Formation and Growth of the Prismatic Layer of Pinctada radiata. Calcif Tissue Res. 1971, 7, 31–45. [Google Scholar] [CrossRef]
- MacDonald, J.; Freer, A.; Cusack, M. Alignment of Crystallographic C-Axis throughout the Four Distinct Microstructural Layers of the Oyster Crassostrea gigas. Cryst. Growth Des. 2010, 10, 1243–1246. [Google Scholar] [CrossRef]
- Checa, A.G.; Macías-Sánchez, E.; Harper, E.M.; Cartwright, J.H.E. Organic Membranes Determine the Pattern of the Columnar Prismatic Layer of Mollusc Shells. Proc. R Soc. B 2016, 283, 20160032. [Google Scholar] [CrossRef]
- Dauphin, Y.; Ball, A.D.; Castillo-Michel, H.; Chevallard, C.; Cuif, J.-P.; Farre, B.; Pouvreau, S.; Salomé, M. In Situ Distribution and Characterization of the Organic Content of the Oyster Shell Crassostrea gigas (Mollusca, Bivalvia). Micron 2013, 44, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Sancho Vaquer, A.; Checa, A.G.; Griesshaber, E.; Fernández-Díaz, L.; Salas, C.; Schmahl, W.W. Layers Formed of Rhombohedral Calcite and Biopolymers in Oyster Shells. Crystals 2025, submitted. [Google Scholar]
- Taylor, J.D.; Kennedy, W.J.; Hall, A. The Shell Structure and Mineralogy of the Bivalvia Introduction. Nuculacea—Trigonacea. Bull Br. Mus. Nat. Hist. Zool. Suppl. 1969, 1–125. [Google Scholar] [CrossRef]
- Carriker, M.R.; Swann, C.P.; Prezant, R.S.; Counts, C.L. Chemical Elements in the Aragonitic and Calcitic Microstructural Groups of Shell of the Oyster Crassostrea virginica: A Proton Probe Study. Mar. Biol. 1991, 109, 287–297. [Google Scholar] [CrossRef]
- Dungan, C.F. Aragonite-Fiber Calcification of the Hinge Ligament Mechanical Antagonist to Valve Adduction in Oysters. J. Shellfish. Res. 2008, 27, 1004–1005. [Google Scholar]
- Carriker, M.R.; Palmer, R.E.; Prezant, R. Functional Ultramorphology of the Dissoconch Valves of the Oyster Crassostrea virginica. J. Shellfish Res. 1981, 70, 139–183. [Google Scholar]
- Mouchi, V.; Lartaud, F.; Guichard, N.; Immel, F.; de Rafélis, M.; Broussard, C.; Crowley, Q.G.; Marin, F. Chalky versus Foliated: A Discriminant Immunogold Labelling of Shell Microstructures in the Edible Oyster Crassostrea gigas. Mar. Biol. 2016, 163, 256. [Google Scholar] [CrossRef]
- Runnegar, B. Crystallography of the Foliated Calcite Shell Layers of Bivalve Molluscs. Alcheringa 1984, 8, 273–290. [Google Scholar] [CrossRef]
- Checa, A.G.; Esteban-Delgado, F.J.; Rodríguez-Navarro, A.B. Crystallographic Structure of the Foliated Calcite of Bivalves. J. Struct. Biol. 2007, 157, 393–402. [Google Scholar] [CrossRef]
- Checa, A.G.; Harper, E.M.; González-Segura, A. Structure and Crystallography of Foliated and Chalk Shell Microstructures of the Oyster Magallana: The Same Materials Grown under Different Conditions. Sci. Rep. 2018, 8, 7507. [Google Scholar] [CrossRef]
- Esteban-Delgado, F.J.; Harper, E.M.; Checa, A.G.; Rodríguez-Navarro, A.B. Origin and Expansion of Foliated Microstructure in Pteriomorph Bivalves. Biol. Bull. 2008, 214, 153–165. [Google Scholar] [CrossRef]
- Checa, A.G.; Esteban-Delgado, F.J.; Ramírez-Rico, J.; Rodríguez-Navarro, A.B. Crystallographic Reorganization of the Calcitic Prismatic Layer of Oysters. J. Struct. Biol. 2009, 167, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.M. Post-Larval Cementation in the Ostreidae and Its Implications for Other Cementing Bivalvia. J. Molluscan Stud. 1992, 58, 37–47. [Google Scholar] [CrossRef]
- Harper, E.M. Attachment of Mature Oysters (Saccostrea cucullata) to Natural Substrata. Mar. Biol. 1997, 127, 449–453. [Google Scholar] [CrossRef]
- MacDonald, J.; Freer, A.; Cusack, M. Attachment of Oysters to Natural Substrata by Biologically Induced Marine Carbonate Cement. Mar. Biol. 2010, 157, 2087–2095. [Google Scholar] [CrossRef]
- Checa, A.G. Physical and Biological Determinants of the Fabrication of Molluscan Shell Microstructures. Front. Mar. Sci. 2018, 5, 353. [Google Scholar] [CrossRef]
- Banker, R.M.W.; Sumner, D.Y. Structure and Distribution of Chalky Deposits in the Pacific Oyster Using X-Ray Computed Tomography (CT). Sci. Rep. 2020, 10, 12118. [Google Scholar] [CrossRef]
- Hedegaard, C.; Wenk, H.-R. Microstructure and Texture Patterns of Mollusc Shells. J. Molluscan Stud. 1998, 64, 133–136. [Google Scholar] [CrossRef]
- Griesshaber, E.; Yin, X.; Ziegler, A.; Kelm, K.; Checa, A.; Eisenhauer, A.; Schmahl, W.W. Patterns of Mineral Organization in Carbonate Biological Hard Materials. In Highlights in Applied Mineralogy; Heuss-Aßbichler, S., Amthauer, G., John, M., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 245–272. ISBN 978-3-11-049734-2. [Google Scholar]
- Chateigner, D.; Hedegaard, C.; Wenk, H.-R. Mollusc Shell Microstructures and Crystallographic Textures. J. Struct. Geol. 2000, 22, 1723–1735. [Google Scholar] [CrossRef]
- Bieler, R.; Mikkelsen, P.M.; Collins, T.M.; Glover, E.A.; González, V.L.; Graf, D.L.; Harper, E.M.; Healy, J.; Kawauchi, G.Y.; Sharma, P.P.; et al. Investigating the Bivalve Tree of Life—An Exemplar-Based Approach Combining Molecular and Novel Morphological Characters. Invertebr. Syst. 2014, 28, 32–115. [Google Scholar] [CrossRef]
- Hull, D.; Bacon, D.J. Introduction to Dislocations, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2011; ISBN 978-0-08-096672-4. [Google Scholar]
- Seto, J.; Ma, Y.; Davis, S.A.; Meldrum, F.; Gourrier, A.; Kim, Y.Y.; Schilde, U.; Sztucki, M.; Burghammer, M.; Maltsev, S.; et al. Structure-Property Relationships of a Biological Mesocrystal in the Adult Sea Urchin Spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef]
- Bergstrom, L.; Sturm, E.V.; Salazar-Alvarez, G.; Cölfen, H. Mesocrystals in Biominerals and Colloidal Arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef]
- Ma, M.-G.; Cölfen, H. Mesocrystals—Applications and Potential. Curr. Opin. Colloid Interface Sci. 2014, 19, 56–65. [Google Scholar] [CrossRef]
- Mackenzie, J.K. Second Paper on Statistics Associated with the Random Disorientation of Cubes. Biometrika 1958, 45, 229–240. [Google Scholar] [CrossRef]
- Yin, X.; Griesshaber, E.; Fernández-Díaz, L.; Ziegler, A.; García-García, F.J.; Schmahl, W.W. Influence of Gelatin–Agarose Composites and Mg on Hydrogel-Carbonate Aggregate Formation and Architecture. Cryst. Growth Des. 2019, 19, 5696–5715. [Google Scholar] [CrossRef]
- Yin, X.; Weitzel, F.; Griesshaber, E.; Fernández-Díaz, L.; Jiménez-López, C.; Ziegler, A.; Rodríguez-Navarro, A.B.; Schmahl, W.W. Bacterial EPS in Agarose Hydrogels Directs Mineral Organization in Calcite Precipitates: Species-Specific Biosignatures of Bacillus subtilis, Mycobacterium phley, Mycobacterium smagmatis, and Pseudomonas putida EPS. Cryst. Growth Des. 2020, 20, 4402–4417. [Google Scholar] [CrossRef]
- Gao, H.; Ji, B.; Jäger, I.L.; Arzt, E.; Fratzl, P. Materials Become Insensitive to Flaws at Nanoscale: Lessons from Nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef]
- Hoerl, S.; Le Moine, T.; Peter, N.J.; Amini, S.; Griesshaber, E.; Wang, J.; Harper, E.M.; Salas, C.; Checa, A.G.; Schwaiger, R.; et al. Crystal Organisation and Material Properties of Chama and Glycymeris Myostraca and Shells. Materialia 2024, 36, 102149. [Google Scholar] [CrossRef]
- Hoerl, S.; Griesshaber, E.; Checa, A.G.; Schmahl, W.W. The Biological Crystals in Chamid Bivalve Shells: Diversity in Morphology and Crystal Arrangement Pattern. Crystals 2024, 14, 649. [Google Scholar] [CrossRef]
- Gim, J.; Schnitzer, N.; Otter, L.M.; Cui, Y.; Motreuil, S.; Marin, F.; Wolf, S.E.; Jacob, D.E.; Misra, A.; Hovden, R. Nanoscale Deformation Mechanics Reveal Resilience in Nacre of Pinna nobilis Shell. Nat. Commun. 2019, 10, 4822. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Lu, K. Stabilizing Nanostructures in Metals Using Grain and Twin Boundary Architectures. Nat. Rev. Mater. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Hierarchical Structure and Mechanical Properties of Nacre: A Review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Harper, E.M.; Checa, A.G. Tightly Shut: Flexible Valve Margins and Microstructural Asymmetry in Pterioid Bivalves. Mar Biol 2020, 167, 78. [Google Scholar] [CrossRef]
- Carriker, M.R. The Shell and Ligament. In The Eastern Oyster: Crassostrea virginica; Kennedy, V.S., Newell, R.I.E., Eble, A.F., Eds.; Maryland Sea Grant College: College Park, MD, USA, 1996; pp. 1–8. [Google Scholar]
- Carter, J.G. Chapter 10. Evolutionary Significance of Shell Microstructure in the Palaeotaxodonta, Pteriomorphia and Isofilibranchia (Bivalvia: Mollusca). In Skeletal Biomineralization, Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 135–296. [Google Scholar]
- Griesshaber, E.; Sancho Vaquer, A.; Checa, A.G.; Salas, C.; Harper, E.M.; Schmahl, W.W. The Textural Motif of Foliated Calcite in Ostreoidea (Mollusca). Crystals 2025, 15, 244. [Google Scholar] [CrossRef]
- Grenier, C.; Griesshaber, E.; Schmahl, W.; Berning, B.; Checa, A.G. Skeletal Microstructures of Cheilostome Bryozoans (Phylum Bryozoa, Class Gymnolaemata): Crystallography and Secretion Patterns. Mar. Life Sci. Technol. 2024, 6, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Grenier, C.; Griesshaber, E.; Schmahl, W.W.; Checa, A.G. Microstructure and Crystallographic Characteristics of Stenolaemate Bryozoans (Phylum Bryozoa and Class Stenolaemata). Cryst Growth Des 2023, 23, 965–979. [Google Scholar] [CrossRef]
- Aylmore, L.a.G.; Quirk, J.P. Swelling of Clay–Water Systems. Nature 1959, 183, 1752–1753. [Google Scholar] [CrossRef]
- Lutterotti, L. Total Pattern Fitting for the Combined Size–Strain–Stress–Texture Determination in Thin Film Diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Yamaguchi, K. Shell Structure and Behaviour Related to Cementation in Oysters. Mar. Biol. 1994, 118, 89–100. [Google Scholar] [CrossRef]
- Simonet Roda, M.; Griesshaber, E.; Angiolini, L.; Rollion-Bard, C.; Harper, E.M.; Bitner, M.A.; Milner Garcia, S.; Ye, F.; Henkel, D.; Häussermann, V.; et al. The Architecture of Recent Brachiopod Shells: Diversity of Biocrystal and Biopolymer Assemblages in Rhynchonellide, Terebratulide, Thecideide and Craniide Shells. Mar. Biol. 2021, 169, 4. [Google Scholar] [CrossRef]
- Casella, L.A.; He, S.; Griesshaber, E.; Fernández-Díaz, L.; Greiner, M.; Harper, E.M.; Jackson, D.J.; Ziegler, A.; Mavromatis, V.; Dietzel, M.; et al. Hydrothermal Alteration of Aragonitic Biocarbonates: Assessment of Micro- and Nanostructural Dissolution–Reprecipitation and Constraints of Diagenetic Overprint from Quantitative Statistical Grain-Area Analysis. Biogeosciences 2018, 15, 7451–7484. [Google Scholar] [CrossRef]
- Almagro, I.; Drzymała, P.; Berent, K.; Sainz-Díaz, C.I.; Willinger, M.G.; Bonarski, J.; Checa, A.G. New Crystallographic Relationships in Biogenic Aragonite: The Crossed-Lamellar Microstructures of Mollusks. Cryst. Growth Des. 2016, 16, 2083–2093. [Google Scholar]
- Carriker, M.R.; Palmer, R.E.; Sick, L.V.; Johnson, C.C. Interaction of Mineral Elements in Sea Water and Shell of Oysters (Crassostrea virginica (Gmelin)) Cultured in Controlled and Natural Systems. J. Exp. Mar. Biol. Ecol. 1980, 46, 279–296. [Google Scholar] [CrossRef]
- Higuera-Ruiz, R.; Elorza, J. Biometric, Microstructural, and High-Resolution Trace Element Studies in Crassostrea gigas of Cantabria (Bay of Biscay, Spain): Anthropogenic and Seasonal Influences. Estuar. Coast. Shelf Sci. 2009, 82, 201–213. [Google Scholar] [CrossRef]
- Korringa, P. On the Nature and Function of “Chalky” Deposits in the Shell of Ostrea edulis Linnaeus. In Proceedings of the California Academy of Sciences, San Francisco, CA, USA, 1 May–31 August 1951; 4th Series. Creative Media Partners, LLC: Sacramento, CA, USA, 1951; Volume 27, pp. 133–158. [Google Scholar]
- Pelseneer, P. Hermaphrodite Oysters. Nature 1929, 124, 14. [Google Scholar] [CrossRef]
- Ranson, G. Les Espèces Actuelles et Fossils Du Genre Pycnodonta F. de W.: 1. Pycnodonta hyotis (L.). Bull. Mus. Natl. Hist. Nat. Paris 1941, 13, 82–92. [Google Scholar]
- Galtsoff, P.S. The American Oyster, Crassostrea virginica (Gmelin). Chesap. Sci. 1965, 6, 199. [Google Scholar] [CrossRef]
- Margolis, S.V.; Carver, R.E. Microsculpture of Chalky Deposits Found in Shells of the Oyster Crassostrea virginica. Nautilus 1974, 88, 62–65. [Google Scholar]
- Ullmann, C.V.; Böhm, F.; Rickaby, R.E.M.; Wiechert, U.; Korte, C. The Giant Pacific Oyster (Crassostrea gigas) as a Modern Analog for Fossil Ostreoids: Isotopic (Ca, O, C) and Elemental (Mg/Ca, Sr/Ca, Mn/Ca) Proxies. Geochem. Geophys. Geosystems 2013, 14, 4109–4120. [Google Scholar] [CrossRef]
- de Winter, N.J.; Daemmer, L.K.; Falkenroth, M.; Reichart, G.-J.; Moretti, S.; Martinez-Garcia, A.; Hoeche, N.; Schoene, B.R.; Rodiouchkina, K.; Goderis, S.; et al. Multi-Isotopic and Trace Element Evidence against Different Formation Pathways for Oyster Microstructures. Geochim. Cosmochim. ACTA 2021, 308, 326–352. [Google Scholar] [CrossRef]
- Simonet Roda, M.; Griesshaber, E.; Angiolini, L.; Harper, D.A.T.; Jansen, U.; Bitner, M.A.; Henkel, D.; Manzanero, E.; Müller, T.; Tomašových, A.; et al. The Evolution of Thecideide Microstructures and Textures: Traced from Triassic to Holocene. Lethaia 2021, 54, 558–577. [Google Scholar] [CrossRef]
- Hahn, T.; Klapper, H. Twinning of Crystals. In International Tables for Crystallography, Online MRW; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 393–448. ISBN 978-0-470-68575-4. [Google Scholar]
- Richards, R.P. The Four Twin Laws of Calcite and How to Recognize Them. Rocks Miner. 1999, 75, 308–317. [Google Scholar] [CrossRef]
- Yin, X.; Griesshaber, E.; Checa, A.G.; Nindiyasari-Behal, F.; Sánchez-Almazo, I.; Ziegler, A.; Schmahl, W.W. Calcite Crystal Orientation Patterns in the Bilayers of Laminated Shells of Benthic Rotaliid Foraminifera. J. Struct. Biol. 2021, 231, 107707. [Google Scholar] [CrossRef]
- Lastam, J.; Griesshaber, E.; Yin, X.; Rupp, U.; Sánchez-Almazo, I.; Heß, M.; Walther, P.; Checa, A.; Schmahl, W.W. Patterns of Crystal Organization and Calcite Twin Formation in Planktonic, Rotaliid, Foraminifera Shells and Spines. J. Struct. Biol. 2022, 215, 107898. [Google Scholar] [CrossRef]
- Lastam, J.; Griesshaber, E.; Yin, X.; Rupp, U.; Sánchez-Almazo, I.; Heß, M.; Walther, P.; Checa, A.; Schmahl, W.W. The Unique Fibrilar to Platy Nano- and Microstructure of Twinned Rotaliid Foraminiferal Shell Calcite. Sci. Rep. 2023, 13, 2189. [Google Scholar] [CrossRef]
- Pokroy, B.; Kapon, M.; Marin, F.; Adir, N.; Zolotoyabko, E. Protein-Induced, Previously Unidentified Twin Form of Calcite. Proc. Natl. Acad. Sci. USA 2007, 104, 7337–7341. [Google Scholar] [PubMed]
- Schmahl, W.W.; Yin, X.; Lastam, J.; Griesshaber, E.; Hörl, S.; Sancho Vaquer, A. Statistical Analysis of EBSD Data and Geometrical Theory of Classical and Non-Classical Twinning in Foraminiferal Shell Calcite. Sci. Rep. 2025, accepted. [Google Scholar] [CrossRef]
- Suzuki, M.; Kim, H.; Mukai, H.; Nagasawa, H.; Kogure, T. Quantitative XRD Analysis of {1 1 0} Twin Density in Biotic Aragonites. J. Struct. Biol. 2012, 180, 458–468. [Google Scholar] [CrossRef]
- Kogure, T.; Suzuki, M.; Kim, H.; Mukai, H.; Checa, A.G.; Sasaki, T.; Nagasawa, H. Twin Density of Aragonite in Molluscan Shells Characterized Using X-Ray Diffraction and Transmission Electron Microscopy. J. Cryst. Growth 2014, 397, 39–46. [Google Scholar] [CrossRef]
- Griesshaber, E.; Schmahl, W.W.; Ubhi, H.S.; Huber, J.; Nindiyasari, F.; Maier, B.; Ziegler, A. Homoepitaxial Meso- and Microscale Crystal Co-Orientation and Organic Matrix Network Structure in Mytilus edulis Nacre and Calcite. Acta Biomater. 2013, 9, 9492–9502. [Google Scholar] [CrossRef] [PubMed]
- Mutvei, H. Ultrastructural Characteristics of the Nacre in Some Gastropods. Zool. Scr. 1978, 7, 287–296. [Google Scholar] [CrossRef]
- Kobayashi, I.; Akai, J. Twinned Aragonite Crystals Found in the Bivalvian Crossed Lamellar Shell Structure. J. Geol. Soc. Jpn. 1994, 100, 177–180. [Google Scholar] [CrossRef]
- Crippa, G.; Griesshaber, E.; Checa, A.G.; Harper, E.M.; Simonet Roda, M.; Schmahl, W.W. SEM, EBSD, Laser Confocal Microscopy and FE-SEM Data from Modern Glycymeris Shell Layers. Data Brief 2020, 33, 106547. [Google Scholar] [CrossRef]
| MUD Value of an EBSD Scan Covering Many Crystals | MUD Value of an EBSD Scan Over an Individual Crystal | |
|---|---|---|
| Columnar crystals | 25–30 | 250–460 |
| Foliated units | 18–67 | Folium: 591, 626 Foliated unit: 174, 545 |
| Pallial prisms | 125–130 | 650–680 |
| Adductor prisms | 85–90 | 650–700 |
| Chalk blades/laths | 4–45 | 450–500 |
| Dendritic crystals | 12–27 | 350–600 |
| Cementation granules | 13–18 | 600–650 |
| Rhombohedral crystals | - | 700, >700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho Vaquer, A.; Griesshaber, E.; Salas, C.; Harper, E.M.; Checa, A.G.; Schmahl, W.W. The Diversity of Crystals, Microstructures and Texture That Form Ostreoidea Shells. Crystals 2025, 15, 286. https://doi.org/10.3390/cryst15030286
Sancho Vaquer A, Griesshaber E, Salas C, Harper EM, Checa AG, Schmahl WW. The Diversity of Crystals, Microstructures and Texture That Form Ostreoidea Shells. Crystals. 2025; 15(3):286. https://doi.org/10.3390/cryst15030286
Chicago/Turabian StyleSancho Vaquer, Anna, Erika Griesshaber, Carmen Salas, Elizabeth M. Harper, Antonio G. Checa, and Wolfgang W. Schmahl. 2025. "The Diversity of Crystals, Microstructures and Texture That Form Ostreoidea Shells" Crystals 15, no. 3: 286. https://doi.org/10.3390/cryst15030286
APA StyleSancho Vaquer, A., Griesshaber, E., Salas, C., Harper, E. M., Checa, A. G., & Schmahl, W. W. (2025). The Diversity of Crystals, Microstructures and Texture That Form Ostreoidea Shells. Crystals, 15(3), 286. https://doi.org/10.3390/cryst15030286









