Effect of Ammonium (NH4+) Impurity on the Crystallization of Cobalt Sulfate Hexahydrate from Aqueous Solutions Using Cooling Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
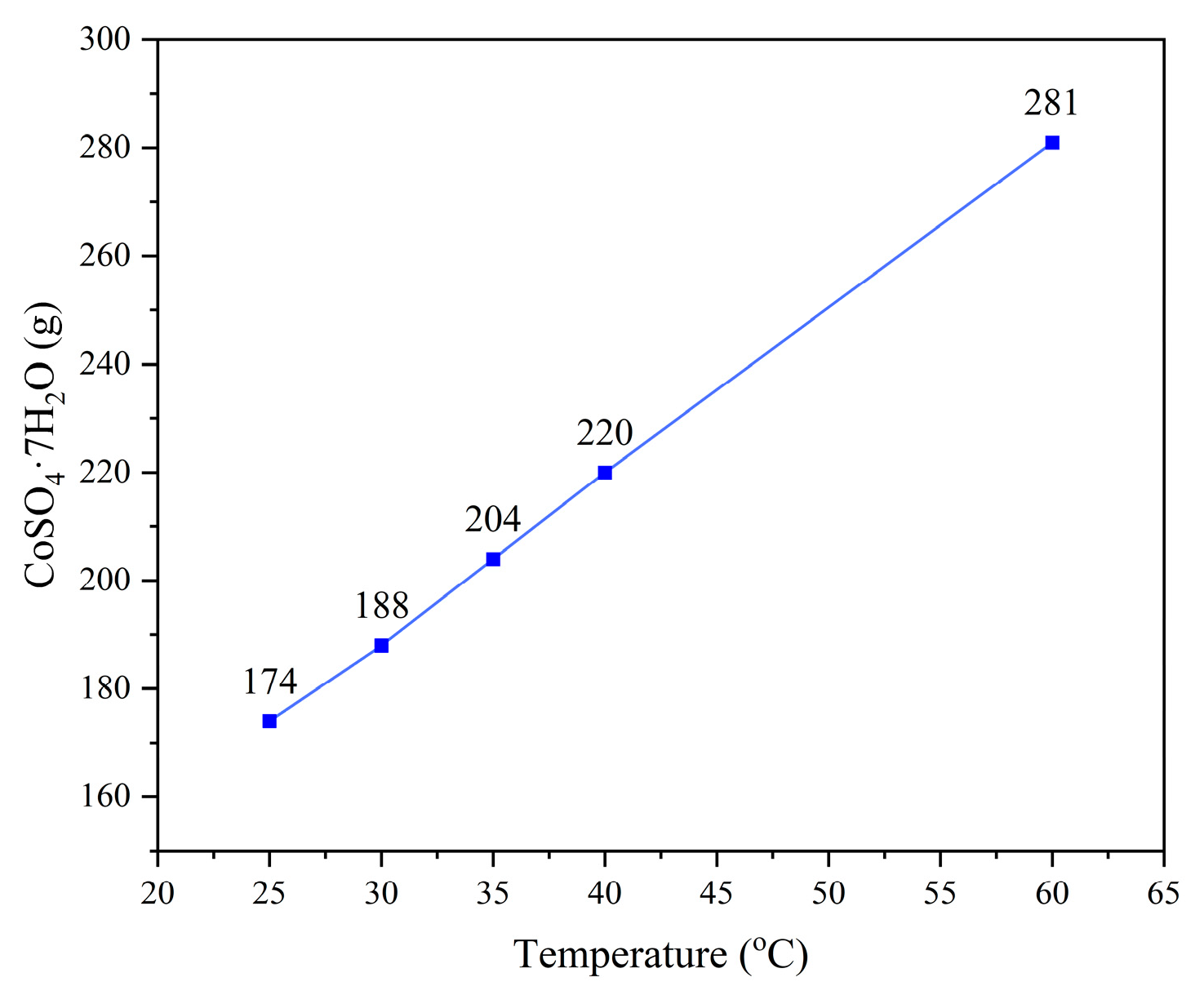
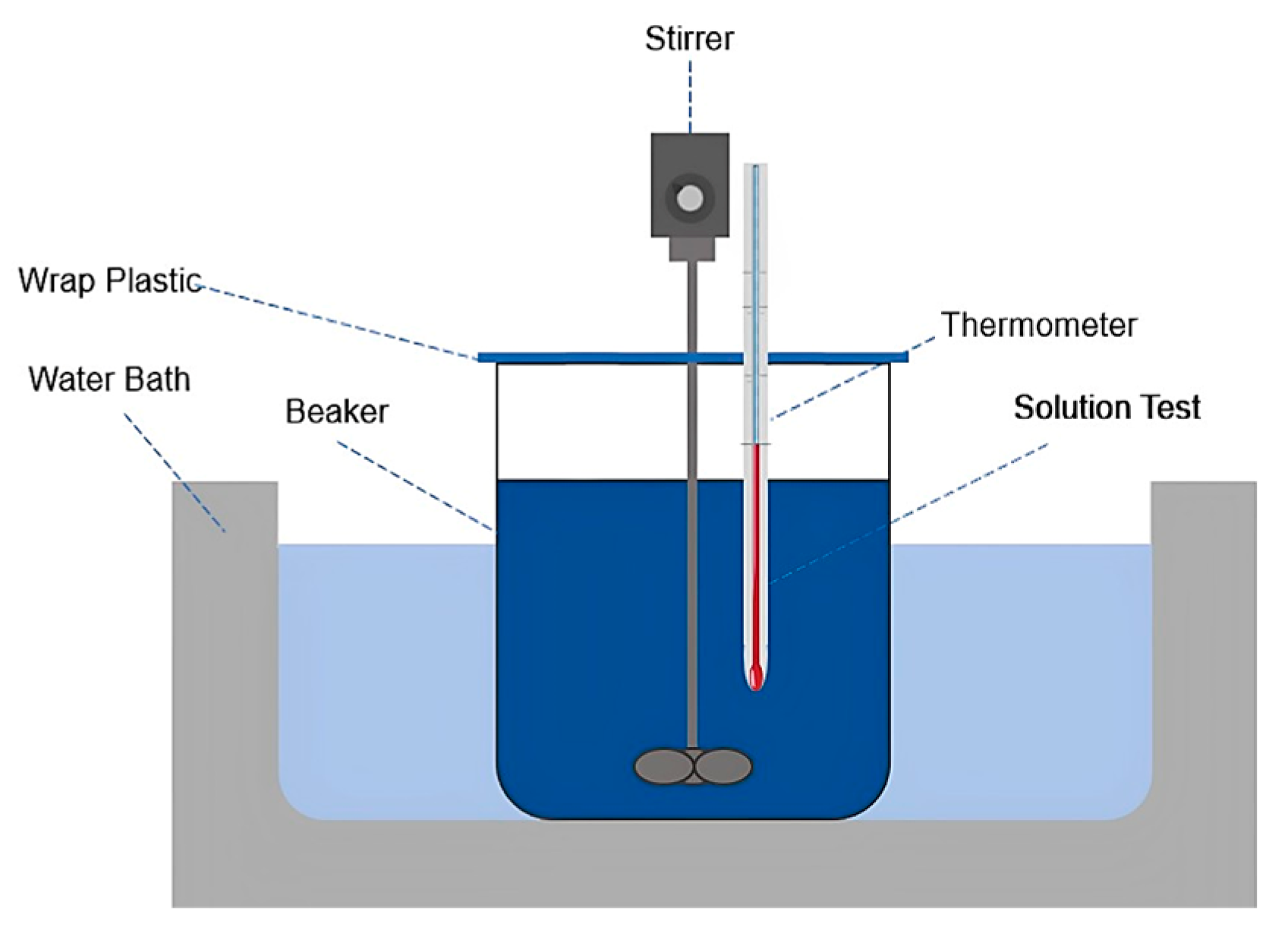
2.2. Experimental Methods
2.3. Characterization and Analysis Method
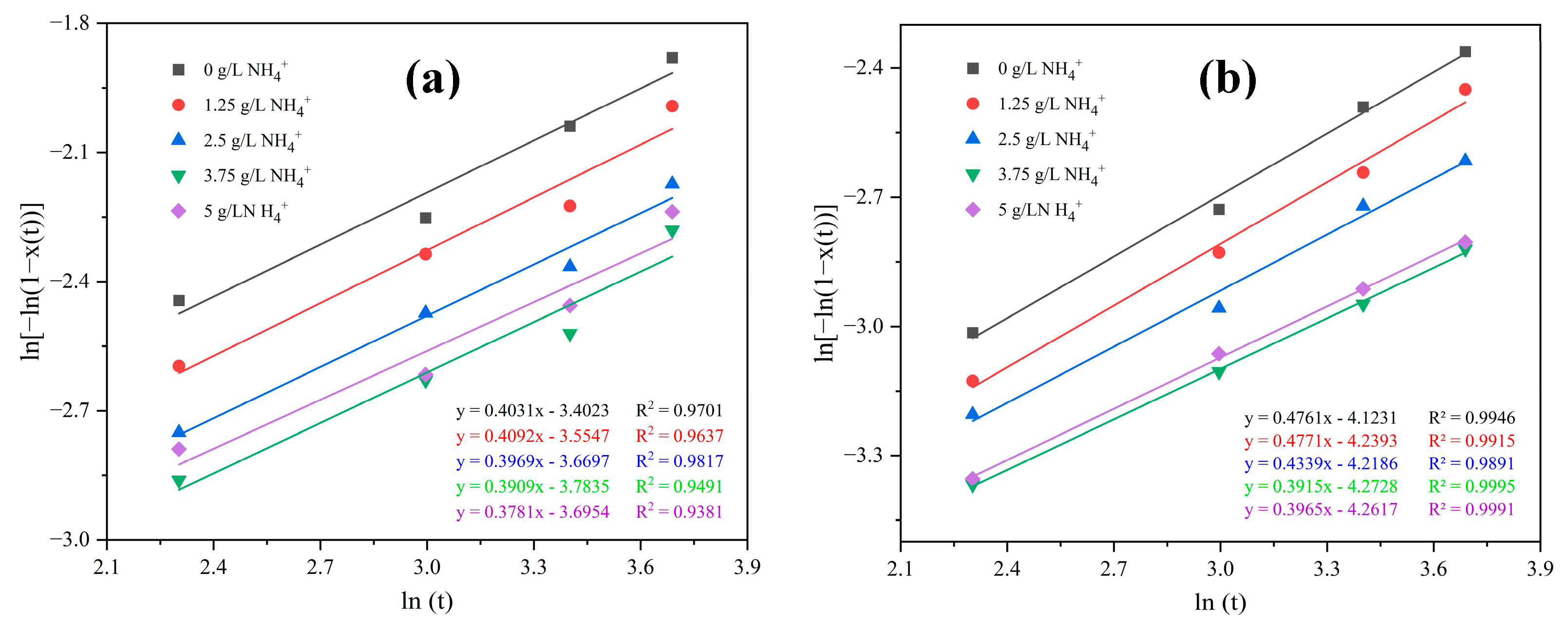
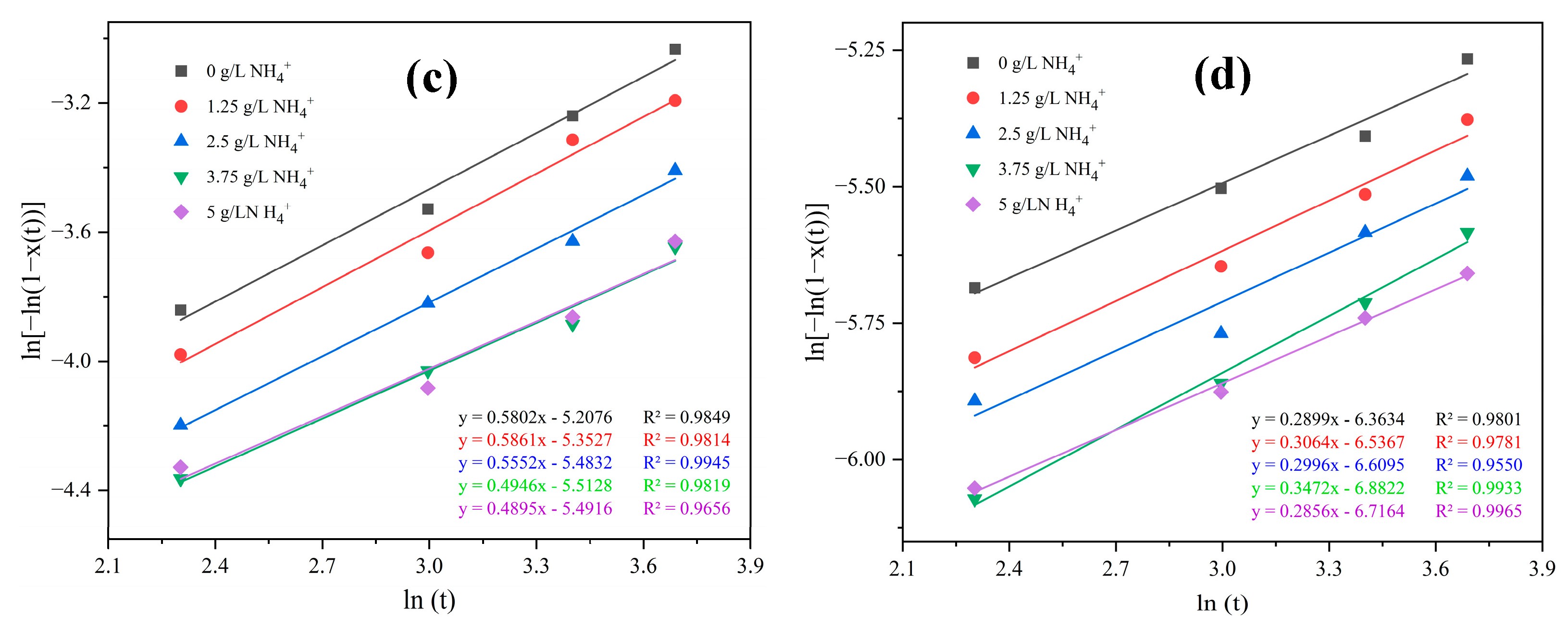
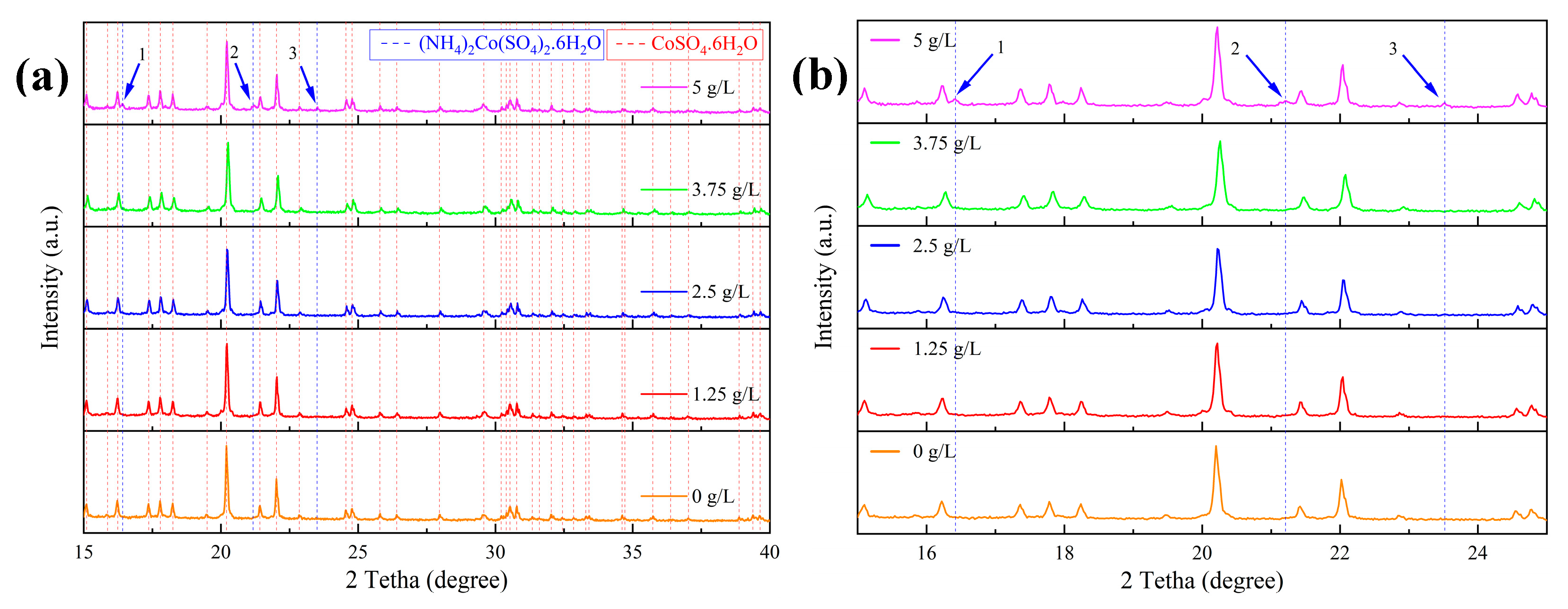
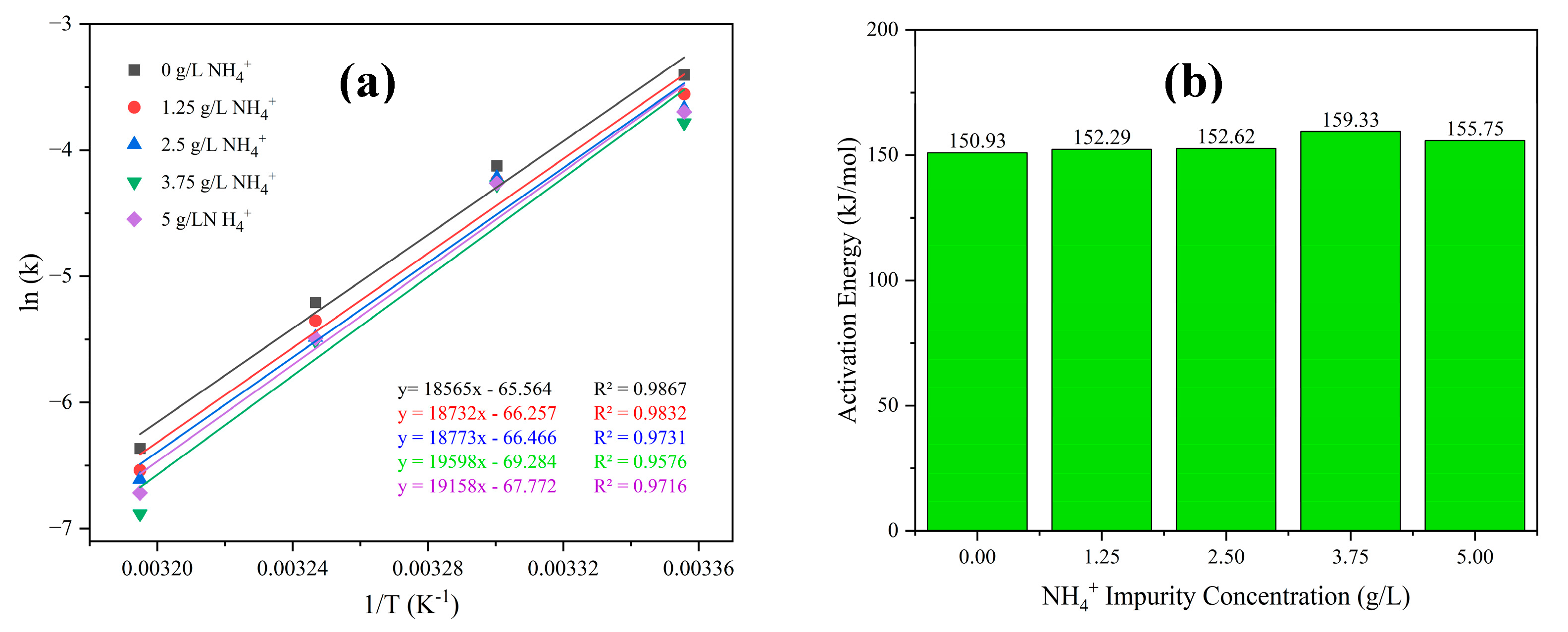
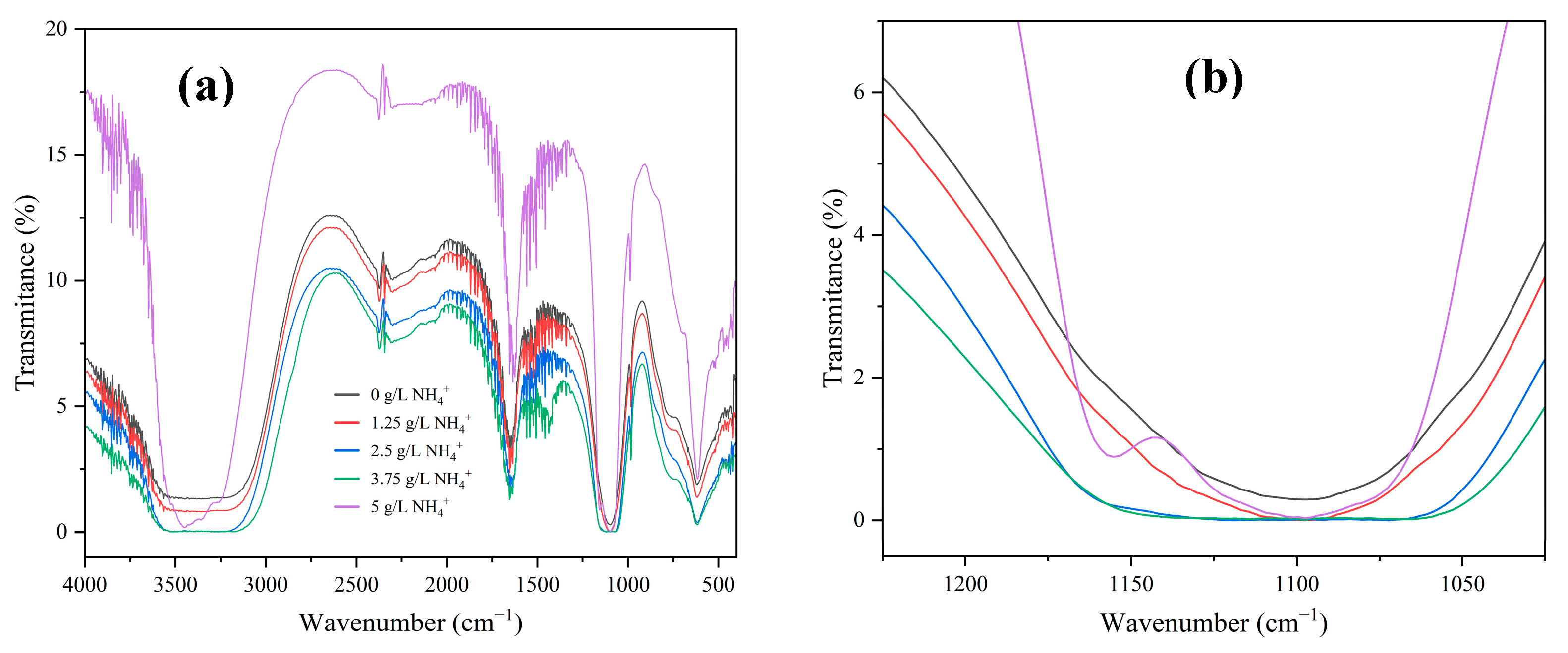
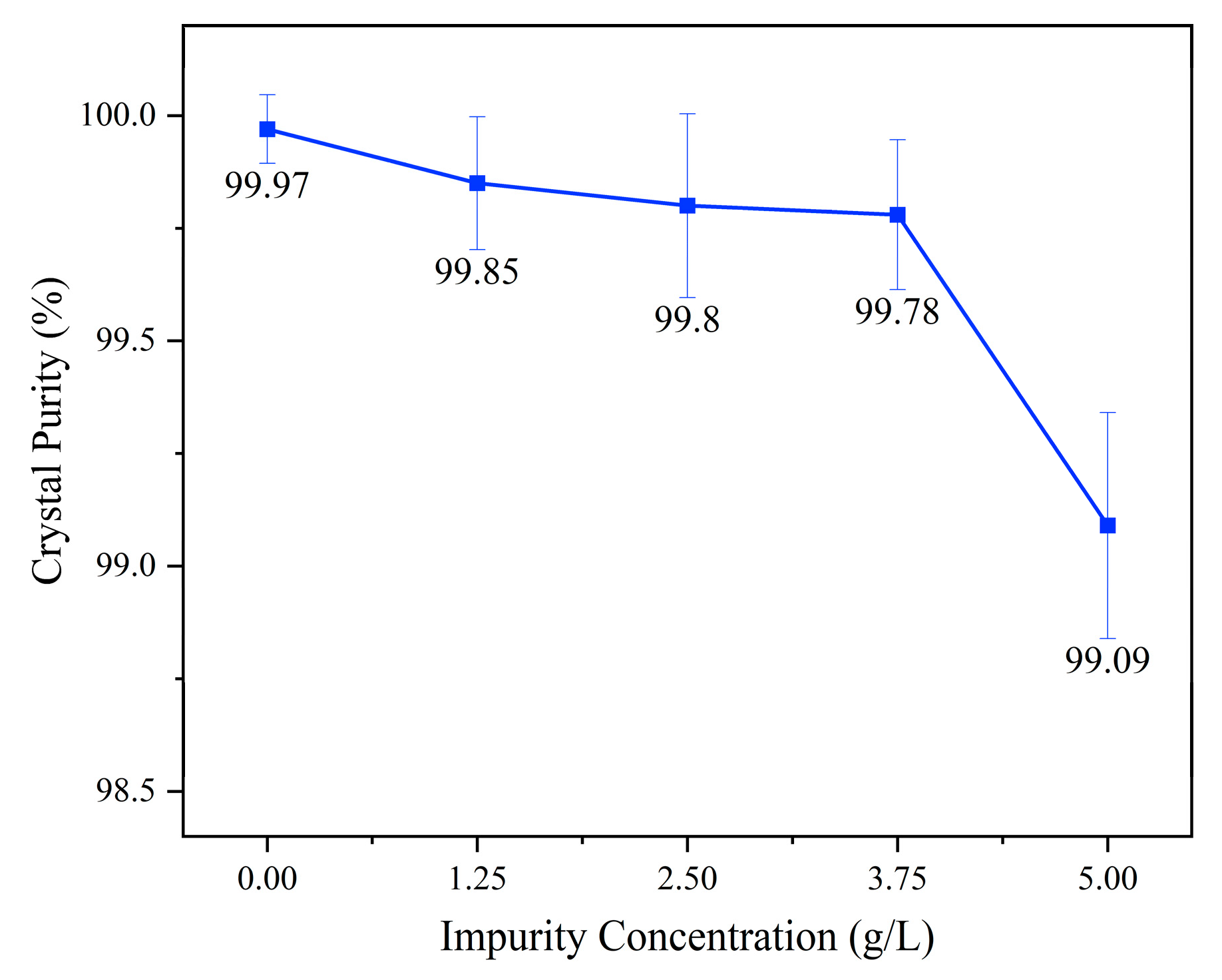
3. Results and Discussions
4. Conclusions
- Competitive interaction between ammonium ions and the cobalt aquo complex for binding with SO42− ions reduces the crystal growth rate and promotes an elevation in the crystallization activation energy. The incorporation of ammonium ions into the crystal structure produces ammonium cobalt sulfate hexahydrate crystals (Tutton’s salt), hence increases the crystal growth rate and reduces the crystallization activation energy.
- The addition of ammonium up to a concentration of 3.75 g/L does not alter the resulting crystal structure, whereas the addition of 5 g/L ammonium leads to the formation of a new crystal structure (Tutton’s salt). The addition of ammonium at any concentration does not alter the crystal shape.
- The reduction in crystal purity upon adding a low concentration of ammonium was mainly caused by the adsorption of ammonium onto the crystal.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariponnammal, S.; Anusha, S.; Shalini, S. Structural and Spectroscopic Investigations on Cobalt Sulphate. Mater. Today Proc. 2020, 27, 336–339. [Google Scholar] [CrossRef]
- Pacheco, T.S.; Ghosh, S.; de Oliveira, M.; Barbosa, A.A.; Perpétuo, G.J.; Franco, C.J. Growth and Characterization of Potassium Cobalt Nickel Sulfate Hexahydrate Crystals: A New UV Light Filter. J. Sci. Adv. Mater. Devices 2017, 2, 354–359. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Sui, X.; Karahan, H.E.; Wang, X.; Chen, Y. Selective Synthesis of Single Walled Carbon Nanotubes on Metal (Iron, Nickel or Cobalt) Sulfate-Based Catalysts. Carbon 2018, 129, 128–136. [Google Scholar] [CrossRef]
- Ma, Y.; Svärd, M.; Xiao, X.; Gardner, J.M.; Olsson, R.T.; Forsberg, K. Precipitation and Crystallization Used in the Production of Metal Salts for Li-Ion Battery Materials: A Review. Metals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Fisher, H.; Rawles, C. Cobalt Market Report 2022, 1st ed.; Cobalt Institute: Guildford, UK, 2023; Volume 1. [Google Scholar]
- Zhao, Z.; Kagi, H.; Komatsu, K.; Yamashita, K.; Nakano, S. Pressure-Induced Phase Transitions of Cobalt Sulfate Hydrates and Discovery of a New High-Pressure Phase, CoSO4·5H2O. J. Solid State Chem. 2022, 308, 122904. [Google Scholar] [CrossRef]
- Zhang, J.; Said, A.; Han, B.; Louhi-Kultanen, M. Semi-Batch Evaporative Crystallization and Drying of Cobalt Sulphate Hydrates. Hydrometallurgy 2022, 208, 105821. [Google Scholar] [CrossRef]
- Wu, K.L.; Wang, H.Y.; Ward, J.D. Economic Comparison of Crystallization Technologies for Different Chemical Products. Ind. Eng. Chem. Res. 2018, 57, 12444–12457. [Google Scholar] [CrossRef]
- Zhang, J.; Louhi-Kultanen, M.; Zhang, J. Determination of Nucleation Kinetics of Cobalt Sulfate by Measuring Metastable Zone Width and Induction Time in Pure and Sulfuric Acid Solution. Powder Technol. 2023, 422, 118463. [Google Scholar] [CrossRef]
- Ma, Y.; Akbarkermani, M.; Svärd, M.; Xiao, X.; Sahadevan, S.A.; Gardner, J.; Olsson, R.T.; Forsberg, K. Phase Diagrams of CoSO4-H2O and CoSO4-H2SO4-H2O Systems for CoSO4·nH2O (n = 6,7) Recovery by Cooling and Eutectic Freeze Crystallization. Hydrometallurgy 2024, 227, 106332. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Barnard, K.R.; Zhang, W.; Robinson, D.J. Synergistic Solvent Extraction of Nickel and Cobalt: A Review of Recent Developments. Solvent Extr. Ion Exch. 2011, 29, 719–754. [Google Scholar] [CrossRef]
- Pahla, G.; Ntuli, F.; Magwa, N. New Experimental Findings on the Separation of Cobalt and Nickel from an Ammonia-Ammonium-Based Leach Liquor Using Ammonium-Saponified Cyanex 272. S. Afr. J. Chem. Eng. 2024, 49, 1–10. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Xue, Z.; Yang, J.; Du, X. Efficient Separation and Recovery of Co and Ni from Ammonium Sulfate Roasting-Water Leaching Solutions of Oceanic Cobalt-Rich Crusts by Sulfide Precipitation and P507–Cyanex 272 Synergistic Extraction. Asia-Pac. J. Chem. Eng. 2024, 19, e3033. [Google Scholar] [CrossRef]
- Parhi, P.K.; Panigrahi, S.; Sarangi, K.; Nathsarma, K.C. Separation of Cobalt and Nickel from Ammoniacal Sulphate Solution Using Cyanex 272. Sep. Purif. Technol. 2008, 59, 310–317. [Google Scholar] [CrossRef]
- Mielniczek-Brzóska, E.; Sangwal, K.; Chocyk, D.; Kluziak, K. Effect of Al(III) Impurity on the Crystallization of Ammonium Dihydrogen Phosphate (ADP) from Aqueous Solutions by Cooling Method. J. Cryst. Growth 2022, 595, 126816. [Google Scholar] [CrossRef]
- Shirzad, K.; Viney, C. A Critical Review on Applications of the Avrami Equation beyond Materials Science. J. R. Soc. Interface 2023, 20, 20230242. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yokota, M.; Mullin, J.W. The Combined Influence of Supersaturation and Impurity Concentration on Crystal Growth. J. Cryst. Growth 2000, 212, 480–488. [Google Scholar] [CrossRef]
- Sangwal, K. Additives and Crystallization Processes: From Fundamentals to Applications; John Wiley and Sons: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Zalkin, A.; Ruben, H.; Templeton, D.H. The Crystal Structure of Cobalt Sulfate Hexahydrate. Acta Crystallogr. 1962, 15, 1219–1224. [Google Scholar] [CrossRef]
- Montgomery, H.; Morosin, B.; Natt, J.J.; Witkowska, A.M.; Lingafelter, E.C. The Crystal Structure of Tutton’s Salts. VI. Vanadium(II), Iron(II) and Cobalt(II) Ammonium Sulfate Hexahydrates. Acta Crystallogr. 1967, 22, 775–780. [Google Scholar] [CrossRef]
- Manomenova, V.L.; Rudneva, E.B.; Komornikov, V.A.; Lyasnikova, M.S.; Vasilyeva, N.A.; Voloshin, A.E. The Ammonium Cobalt Sulfate Hexahydrate (ACSH) Crystal Growth from Aqueous Solutions and Some Properties of Solutions and Crystals. J. Cryst. Growth 2020, 532, 125416. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; John Wiley and Sons: Oxford, UK, 2008; pp. 1872–1892. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriyan, M.W.; Yang, J.; Xu, K.; Hu, Y.; Nan, T.; Liu, L.; Yu, Q. Effect of Ammonium (NH4+) Impurity on the Crystallization of Cobalt Sulfate Hexahydrate from Aqueous Solutions Using Cooling Method. Crystals 2025, 15, 295. https://doi.org/10.3390/cryst15040295
Andriyan MW, Yang J, Xu K, Hu Y, Nan T, Liu L, Yu Q. Effect of Ammonium (NH4+) Impurity on the Crystallization of Cobalt Sulfate Hexahydrate from Aqueous Solutions Using Cooling Method. Crystals. 2025; 15(4):295. https://doi.org/10.3390/cryst15040295
Chicago/Turabian StyleAndriyan, Mohammad Wahyu, Jianguang Yang, Kaihua Xu, Yi Hu, Tianxiang Nan, Lian Liu, and Qing Yu. 2025. "Effect of Ammonium (NH4+) Impurity on the Crystallization of Cobalt Sulfate Hexahydrate from Aqueous Solutions Using Cooling Method" Crystals 15, no. 4: 295. https://doi.org/10.3390/cryst15040295
APA StyleAndriyan, M. W., Yang, J., Xu, K., Hu, Y., Nan, T., Liu, L., & Yu, Q. (2025). Effect of Ammonium (NH4+) Impurity on the Crystallization of Cobalt Sulfate Hexahydrate from Aqueous Solutions Using Cooling Method. Crystals, 15(4), 295. https://doi.org/10.3390/cryst15040295





