Two-Dimensional Silver Bismuth Oxide/Bismuth Molybdate Z-Scheme Heterojunctions with Rich Oxygen Vacancies for Improved Pollutant Degradation and Bacterial Inactivation
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of Bi2MoO6
2.3. Synthesis of AgBiO3/Bi2MoO6
2.4. Characterization
2.5. Evaluation of Simulated Solar Driven Photocatalytic Performance
2.6. Bacterial Inactivation Test
3. Results and Discussion
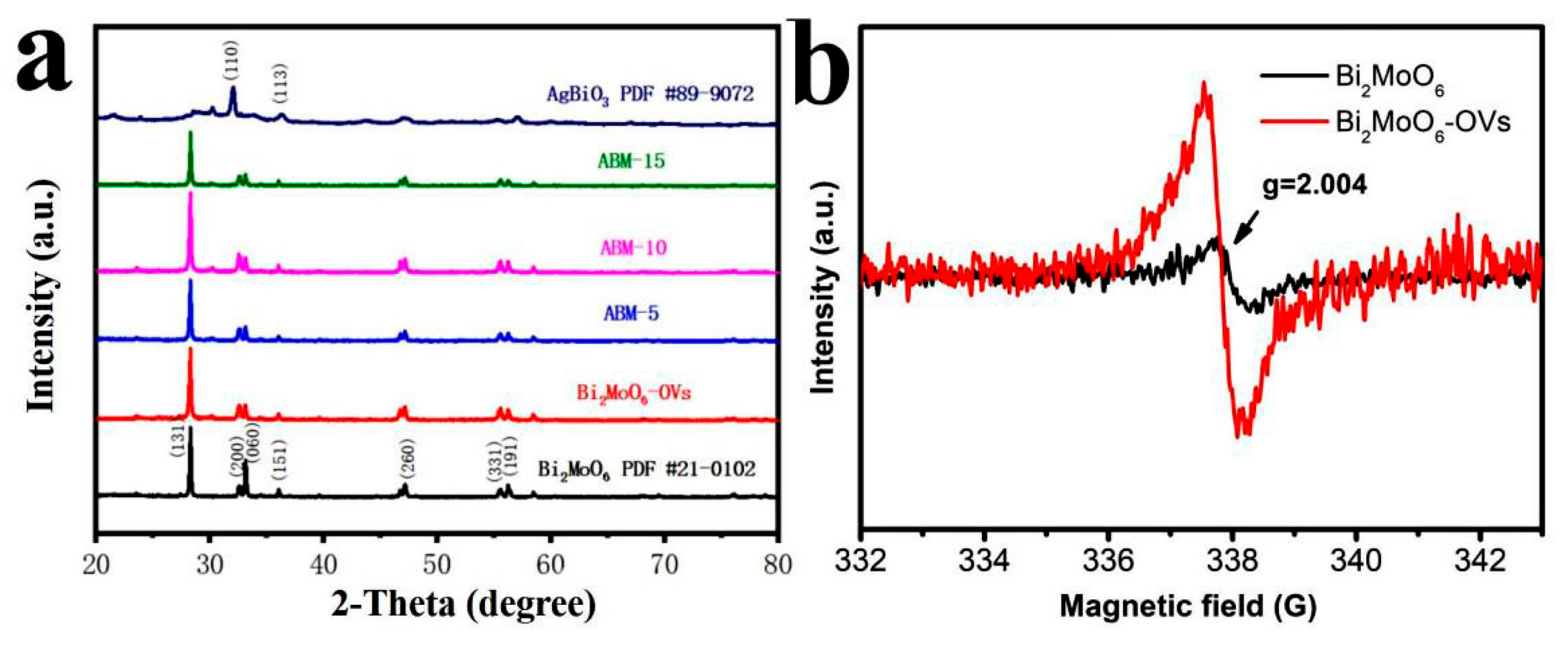
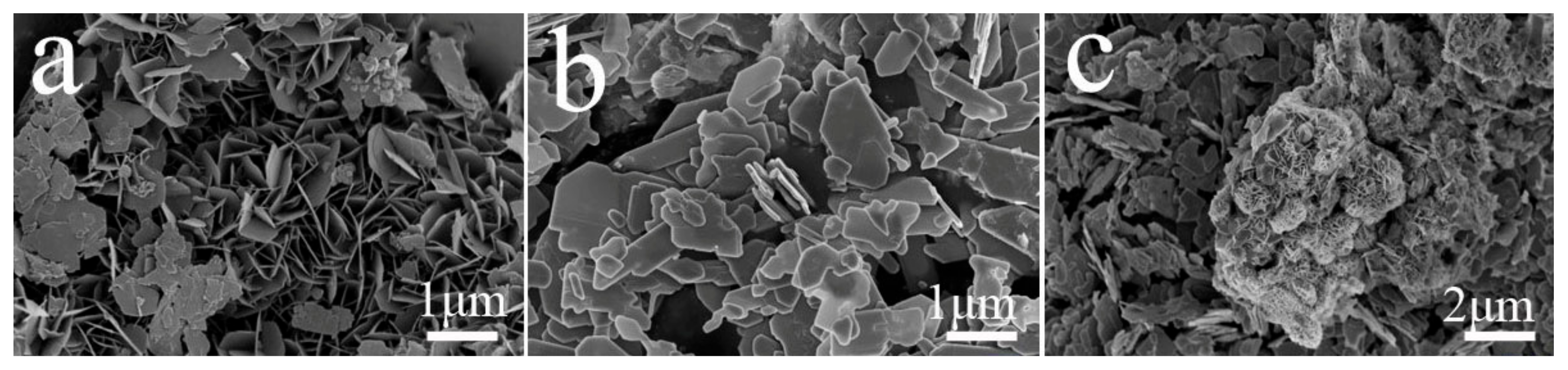
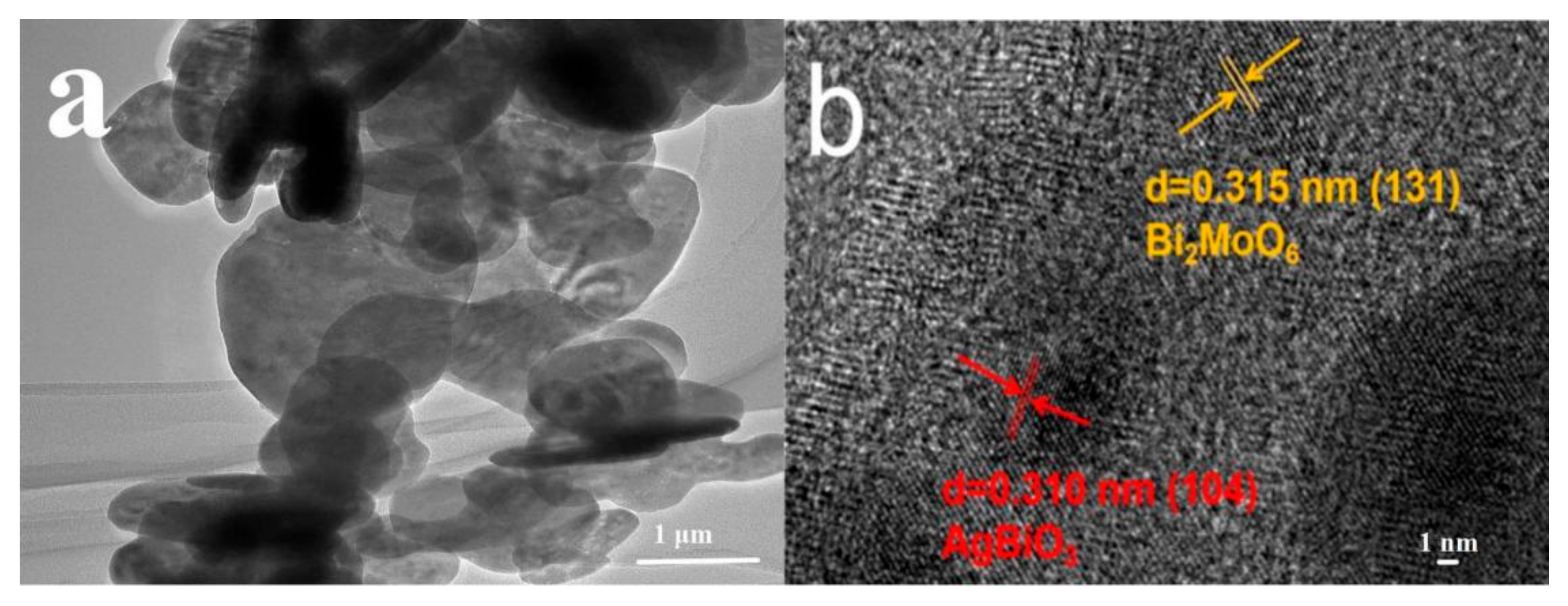
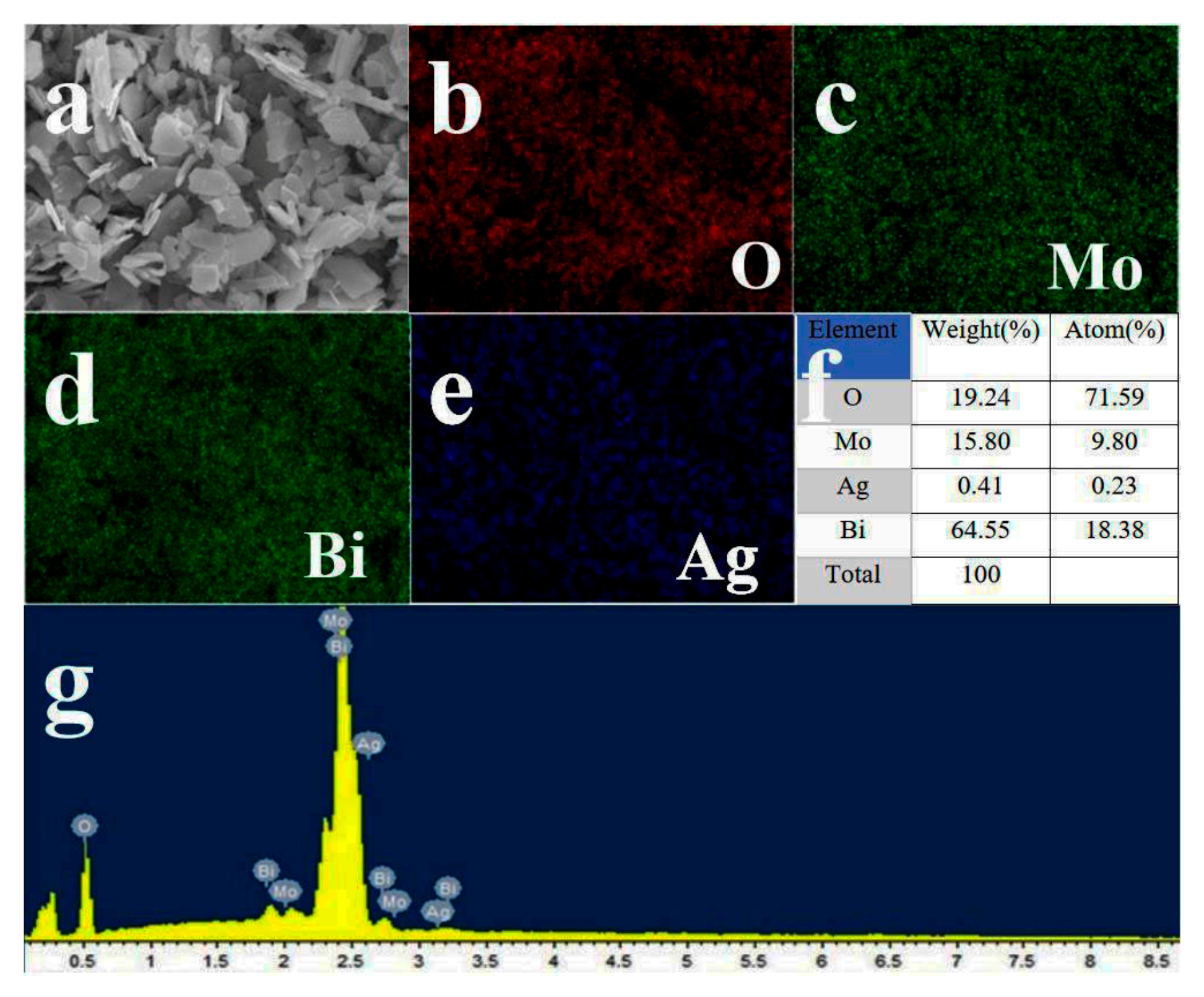
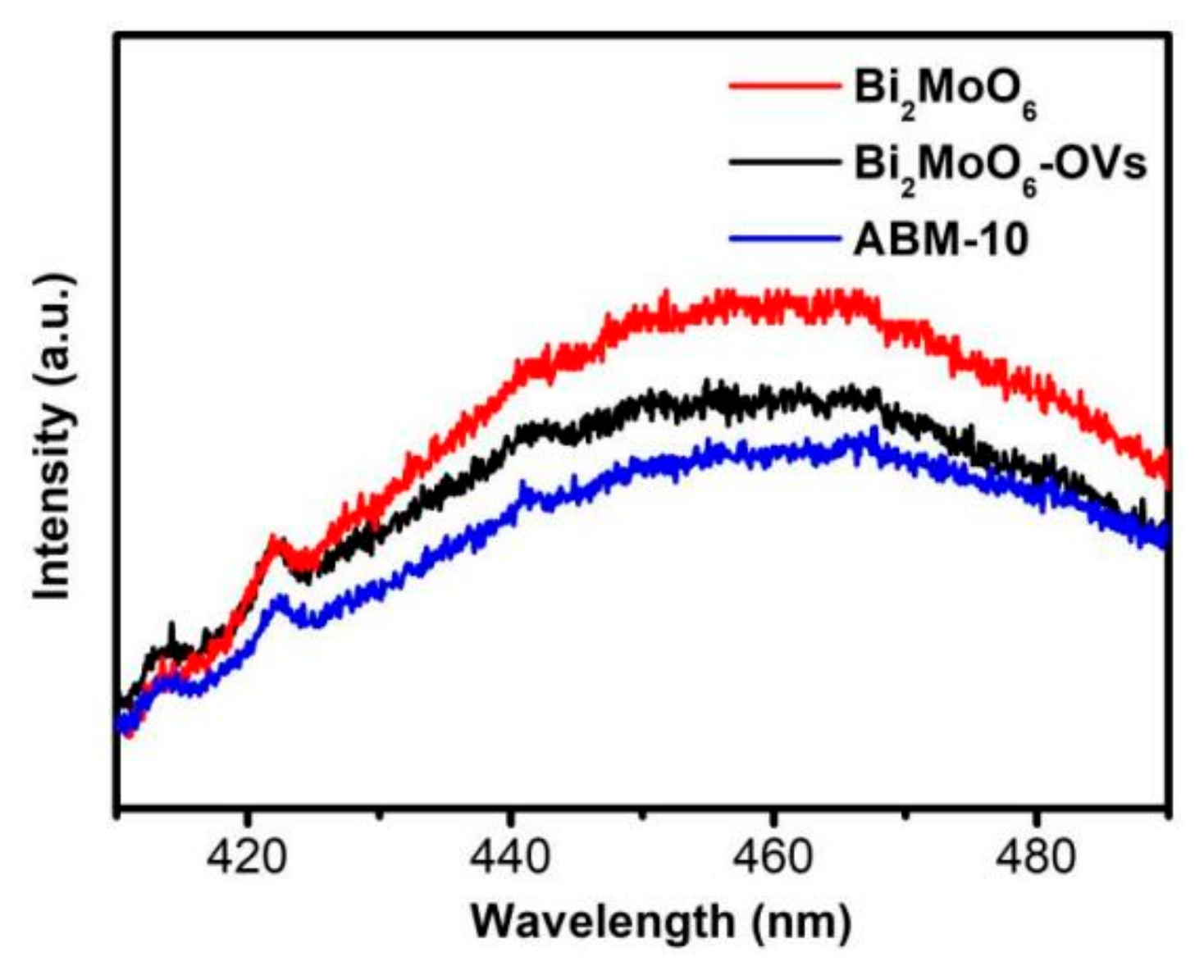
3.1. Physicochemical Properties
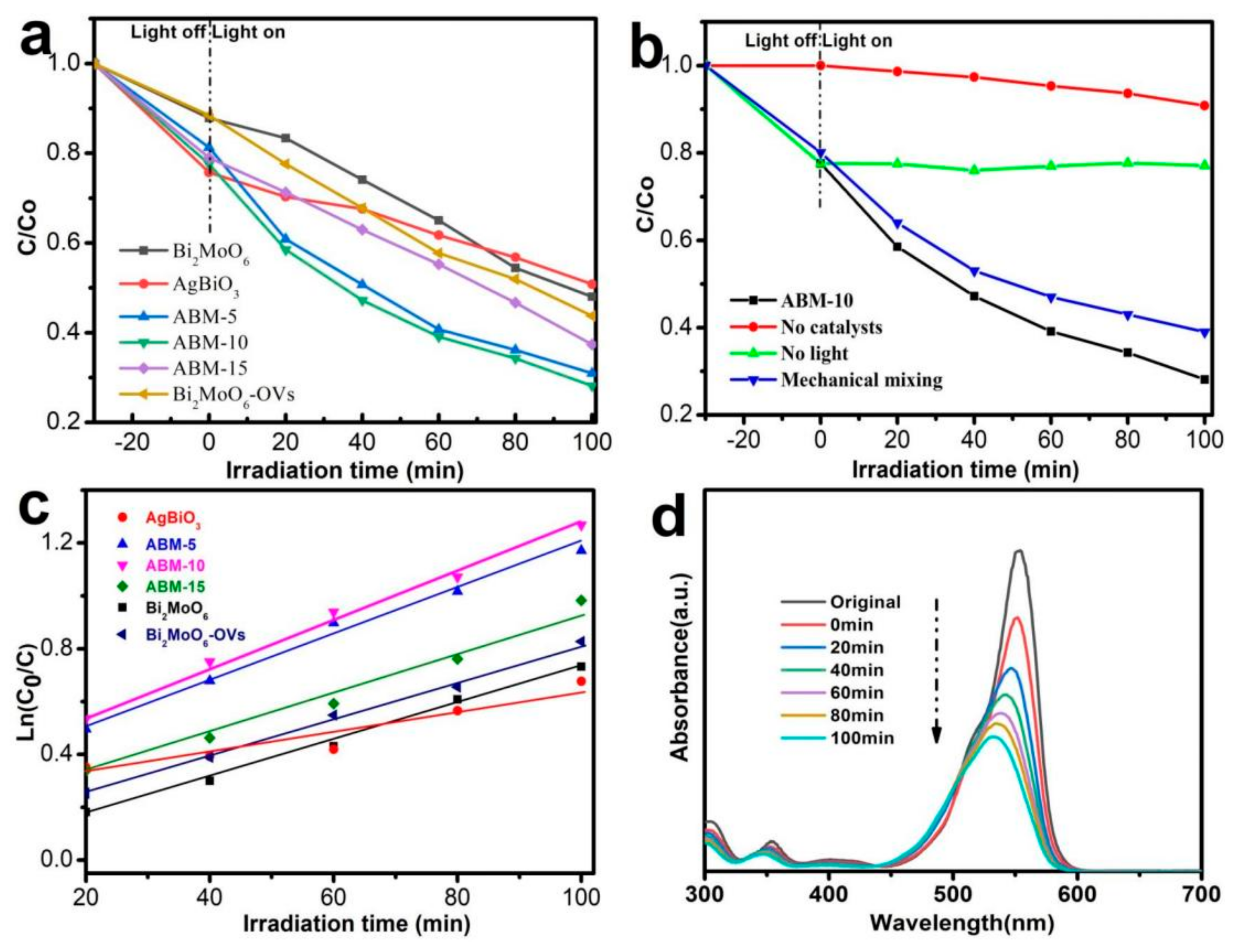
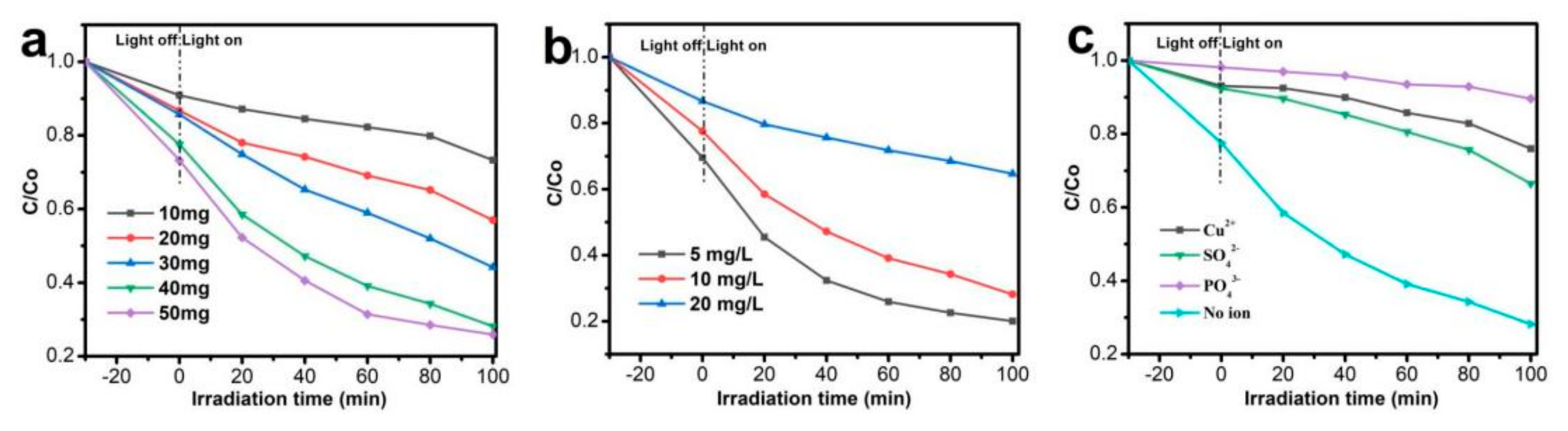
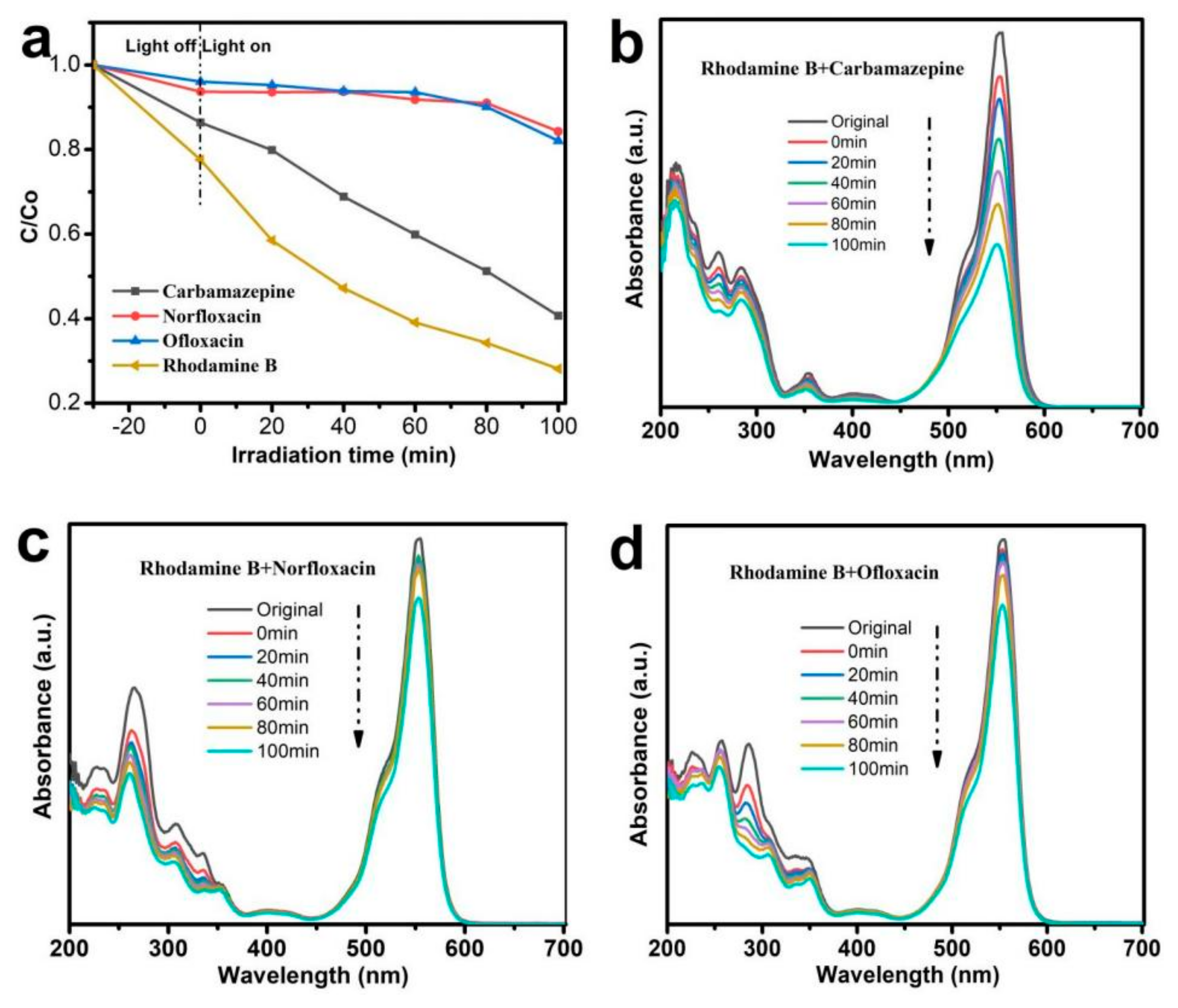
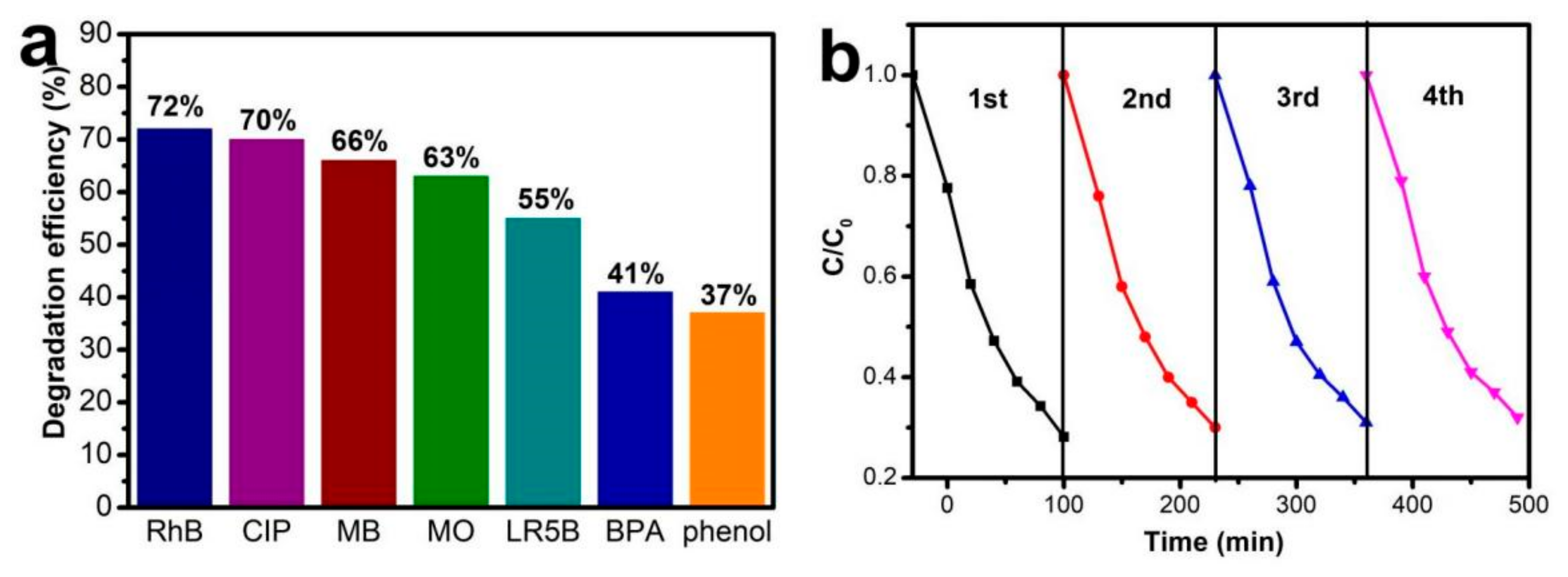
3.2. Photocatalytic Activity
3.3. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solayman, H.M.; Hossen, M.A.; Aziz, A.A.; Yahya, N.Y.; Hon, L.K.; Ching, S.L.; Monir, M.U.; Zoh, K.D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Villegas-Fuentes, A.; Garrafa-Gálvez, H.E.; Quevedo-Robles, R.V.; Luque-Morales, M.; Vilchis-Nestor, A.R.; Luque, P.A. Synthesis of semiconductor ZnO nanoparticles using Citrus microcarpa extract and the influence of concentration on their optical properties. J. Mol. Struct. 2023, 1281, 135067. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. Energy 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Meng, L.H.; Zhao, C.; Wang, T.Y.; Chu, H.Y.; Wang, C.C. Efficient ciprofloxacin removal over Z-scheme ZIF-67/V-BiOIO3 heterojunctions: Insight into synergistic effect between adsorption and photocatalysis. Sep. Purif. Technol. 2023, 313, 123511. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Fu, S.; Li, L.; Cui, J.; Gao, P.; Zhao, F.; Zhou, J. N doped carbon quantum dots modified defect-rich g-C3N4 for enhanced photocatalytic combined pollutions degradation and hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124552. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Parrino, F.; Gottuso, A.; Viganò, L.; Mariani, P.; Villa, I.; Cova, F.; Callone, E.; Dirè, S.; Palmisano, L.; Stredansky, M.; et al. Singlet Oxygen Photocatalytic Generation by Silanized TiO2 Nanoparticles. Angew. Chem. Int. Ed. 2025, 137, e202414445. [Google Scholar] [CrossRef]
- Li, L.; Cai, J.; Yan, Y.; Zhao, F.; Zhou, J. Flower-like direct Z-scheme WS2/Bi2O2CO3 photocatalyst with enhanced photocatalytic activity. J. Alloys Compd. 2019, 810, 151872. [Google Scholar] [CrossRef]
- Fu, S.; Du, Y.; Bie, J.; Huang, Z.; Hu, H.; Huang, Q.; Zhu, H.; Yuan, W.; Li, L.; Liu, B. Facile fabrication of Z-scheme Ag2WO4/BiOBr heterostructure with oxygen vacancies for improved visible-light photocatalytic performance. J. Sci. Adv. Mater. Devices 2023, 8, 100561. [Google Scholar] [CrossRef]
- Luo, C.; Long, Q.; Cheng, B.; Zhu, B.C.; Wang, L.X. A DFT Study on S-Scheme Heterojunction Consisting of Pt Single Atom Loaded G-C3N4 and BiOCl for Photocatalytic CO2 Reduction. Acta Phys.-Chim. Sin. 2023, 39, 2212026. [Google Scholar] [CrossRef]
- Yu, H.B.; Jiang, L.B.; Wang, H.; Huang, B.B.; Yuan, X.Z.; Huang, J.H.; Zhang, J.; Zeng, G.M. Modulation of Bi2MoO6-based materials for photocatalytic water splitting and environmental application: A critical review. Small 2019, 15, 1901008. [Google Scholar]
- Gomez, E.; Cestaro, R.; Philippe, L.; Serra, A. Electrodeposition of nanostructured Bi2MoO6@Bi2MoO6-x homojunction films for the enhanced visible-light-driven photocatalytic degradation of antibiotics. Appl. Catal. B Environ. 2022, 317, 121703. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Ji, S.T.; Li, S.Y.; Zhou, X.Q.; Yin, J.H.; Liu, P.; Shi, W.N.; Wu, M.H.; Shen, L.X. Electrospinning visible light response Bi2MoO6/Ag3PO4 composite photocatalytic nanofibers with enhanced photocatalytic and antibacterial activity. Appl. Surf. Sci. 2021, 569, 150955. [Google Scholar]
- Liu, Z.; Tian, J.; Yu, C.L.; Fan, Q.Z.; Liu, X.Q. Solvothermal fabrication of Bi2MoO6 nanocrystals with tunable oxygen vacancies and excellent photocatalytic oxidation performance in quinoline production and antibiotics degradation. Chin. J. Catal. 2022, 43, 472–484. [Google Scholar] [CrossRef]
- Di, J.; Zhao, X.; Lian, C.; Ji, M.; Xia, J.; Xiong, J.; Zhou, W.; Cao, X.; She, Y.; Liu, H.; et al. Atomically-thin Bi2MoO6 nanosheets with vacancy pairs for improved photocatalytic CO2 reduction. Nano Energy 2019, 61, 54–59. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Chen, C.; Chen, A.; Zheng, W. High efficiency Bi2S3/Bi2MoO6/TiO2 photoanode for photoelectrochemical hydrogen generation. J. Alloys Compd. 2023, 965, 171139. [Google Scholar]
- Ma, Z.Q.; Li, J.; Wang, N.; Guo, W.; Zhang, K.Y. Antibacterial activity and the mechanism of the Z-Scheme Bi2MoO6/Bi5O7I heterojunction under visible light. Molecules 2023, 28, 6786. [Google Scholar] [CrossRef]
- Sun, Z.L.; Yang, X.L.; Yu, X.F.; Xia, L.H.; Peng, Y.H.; Li, Z.; Zhang, Y.; Cheng, J.B.; Zhang, K.S.; Yu, J.Q. Surface oxygen vacancies of Pd/Bi2MoO6−x acts as “Electron Bridge” to promote photocatalytic selective oxidation of alcohol. Appl. Catal. B Environ. 2021, 285, 119790. [Google Scholar]
- Chen, Y.; Yang, W.Y.; Gao, S.; Sun, C.X.; Li, Q. Synthesis of Bi2MoO6 nanosheets with rich oxygen vacancies by post synthesis etching treatment for enhanced photocatalytic performance. ACS Appl. Nano Mater. 2018, 1, 3565–3578. [Google Scholar]
- Hao, Z.Q.; Xu, L.L.; Wei, B.; Fan, L.L.; Liu, Y.; Zhang, M.Y.; Gao, H. Nanosize α-Bi2O3 decorated Bi2MoO6 via an alkali etching process for enhanced photocatalytic performance. RSC Adv. 2015, 5, 12346–12353. [Google Scholar]
- Zheng, Y.; Zhou, T.F.; Zhao, X.D.; Pang, W.K.; Gao, H.; Li, S.; Zhou, Z.; Liu, H.K.; Guo, Z.P. Atomic interface engineering and electric-field effect in ultrathin Bi2MoO6 nanosheets for superior lithium ion storage. Adv. Mater. 2017, 29, 1700396. [Google Scholar]
- Zhang, Y.Y.; Guo, L.; Wang, Y.X.; Wang, T.Y.; Ma, T.X.; Zhang, Z.Z.; Wang, D.J.; Xu, B.; Fu, F. In-situ anion exchange based Bi2S3/OV-Bi2MoO6 heterostructure for efficient ammonia production: A synchronized approach to strengthen NRR and OER reactions. J. Mater. Sci. Technol. 2022, 110, 152–160. [Google Scholar]
- Yang, H.; Li, J.; Su, Q.; Wang, B.; Zhang, Z.; Dai, Y.; Li, Y.; Hou, L. Construction of Bi2MoO6/CoWO4 Z-scheme heterojunction for high-efficiency photocatalytic degradation of norfloxacin under visible light: Performance and mechanism insights. Chem. Eng. J. 2023, 470, 144139. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Wang, B.; Li, Y.; Hou, L. Direct Z-scheme Bi2MoO6/UiO-66-NH2 heterojunctions for enhanced photocatalytic degradation of ofloxacin and ciprofloxacin under visible light. Appl. Catal. B Environ. 2022, 318, 121820. [Google Scholar]
- Boruah, B.; Gupta, R.; Modak, J.; Madras, G. Novel insights into the properties of AgBiO3 photocatalyst and its application in immobilized state for 4-nitrophenol degradation and bacteria inactivation. J. Photochem. Photobiol. A 2019, 373, 105–115. [Google Scholar]
- Jia, Z.; Liu, J.; Li, R.; Fan, C.; Wang, Y. Bi4O7 modified AgBiO3 to construct Z-scheme heterojunction for photocatalytic removing phenol. Mater. Sci. Semicond. Process. 2024, 177, 108359. [Google Scholar]
- Zhao, Z.H.; Wang, Y.; Yu, Q.; Lin, X.C.; Xu, X.W.; Zhang, J.; Lu, H.J.; Chen, X.X.; Zhang, D.P. Construction of AgBiO3/g-C3N4 nanocomposites with enhanced photocatalytic activity and their application in the degradation of bisphenol A. Desalination Water Treat. 2021, 227, 228–237. [Google Scholar]
- Dutta, V.; Sonu; Raizada, P.; Verma, P.K.; Ahamad, T.; Thakur, S.; Hussain, C.M.; Singh, P. Constructing carbon nanotubes@CuBi2O4/AgBiO3 all solid-state mediated Z-scheme photocatalyst with enhanced photocatalytic activity. Mater. Lett. 2022, 320, 132374. [Google Scholar]
- Lu, J.; Bie, J.; Fu, S.; Wu, J.; Huang, Q.; He, P.; Yang, Z.; Zhang, X.; Zhu, H.; Deng, P. Construction of a novel two-dimensional AgBiO3/BiOBr step-scheme heterojunction for enhanced broad-spectrum photocatalytic performance. RSC Adv. 2022, 12, 20628–20639. [Google Scholar]
- Chen, Y.; Han, Z.; Liu, Z.; Liu, Z.; Feng, P. Fabrication of flower-like BiO2-x/AgBiO3 photocatalysts with excellent visible light driven photocatalytic degradation. J. Phys. Chem. Solids 2022, 165, 110692. [Google Scholar] [CrossRef]
- Fu, S.; Chu, Z.; Huang, Z.; Dong, X.; Bie, J.; Yang, Z.; Zhu, H.; Pu, W.; Wu, W.; Liu, B. Construction of Z-scheme AgCl/BiOCl heterojunction with oxygen vacancies for improved pollutant degradation and bacterial inactivation. RSC Adv. 2024, 14, 3888. [Google Scholar] [PubMed]
- Fu, S.; Liu, X.; Yan, Y.; Li, L.; Liu, H.; Zhao, F.; Zhou, J. Few-layer WS2 modified BiOBr nanosheets with enhanced broad-spectrum photocatalytic activity towards various pollutants removal. Sci. Total Environ. 2019, 694, 133756. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lim, T.-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar]
- Fu, S.; Yuan, W.; Liu, X.; Yan, Y.; Liu, H.; Li, L.; Zhao, F.; Zhou, J. A novel 0D/2D WS2/BiOBr heterostructure with rich oxygen vacancies for enhanced broad-spectrum photocatalytic performance. J. Colloid Interface Sci. 2020, 569, 150–163. [Google Scholar]
- Yang, L.; Wang, P.; Yin, J.; Wang, C.; Dong, G.; Wang, Y.; Ho, W. Engineering of reduced graphene oxide on nanosheet–g-C3N4/perylene imide heterojunction for enhanced photocatalytic redox performance. Appl. Catal. B Environ. 2019, 250, 42–51. [Google Scholar]
- Zeng, X.; Wang, Z.; Wang, G.; Gengenbach, T.R.; McCarthy, D.T.; Deletic, A.; Yu, J.; Zhang, X. Highly dispersed TiO2 nanocrystals and WO3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 2017, 218, 163–173. [Google Scholar]
- Wang, D.; Shen, H.; Guo, L.; Wang, C.; Fu, F.; Liang, Y. La and F co-doped Bi2MoO6 architectures with enhanced photocatalytic performance via synergistic effect. RSC Adv. 2016, 6, 71052–71060. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, H.; He, P.; Li, M.; Cao, Y.; Du, Y.; Wen, Y.; Zhao, Y.; Liu, X.; Song, Y. Two-Dimensional Silver Bismuth Oxide/Bismuth Molybdate Z-Scheme Heterojunctions with Rich Oxygen Vacancies for Improved Pollutant Degradation and Bacterial Inactivation. Crystals 2025, 15, 318. https://doi.org/10.3390/cryst15040318
Wang Y, Zhu H, He P, Li M, Cao Y, Du Y, Wen Y, Zhao Y, Liu X, Song Y. Two-Dimensional Silver Bismuth Oxide/Bismuth Molybdate Z-Scheme Heterojunctions with Rich Oxygen Vacancies for Improved Pollutant Degradation and Bacterial Inactivation. Crystals. 2025; 15(4):318. https://doi.org/10.3390/cryst15040318
Chicago/Turabian StyleWang, Yanhong, Huijie Zhu, Pengli He, Mingyu Li, Yinhuan Cao, Yanqiang Du, Yun Wen, Yixiang Zhao, Xiaowen Liu, and Yonglong Song. 2025. "Two-Dimensional Silver Bismuth Oxide/Bismuth Molybdate Z-Scheme Heterojunctions with Rich Oxygen Vacancies for Improved Pollutant Degradation and Bacterial Inactivation" Crystals 15, no. 4: 318. https://doi.org/10.3390/cryst15040318
APA StyleWang, Y., Zhu, H., He, P., Li, M., Cao, Y., Du, Y., Wen, Y., Zhao, Y., Liu, X., & Song, Y. (2025). Two-Dimensional Silver Bismuth Oxide/Bismuth Molybdate Z-Scheme Heterojunctions with Rich Oxygen Vacancies for Improved Pollutant Degradation and Bacterial Inactivation. Crystals, 15(4), 318. https://doi.org/10.3390/cryst15040318





