Lithium Salt Screening for PEO-Based Solid Electrolytes of All Solid-State Li Ion Batteries Using Density Functional Theory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lithium Salt Properties and Screening Parameters
2.2. Theoretical Computation
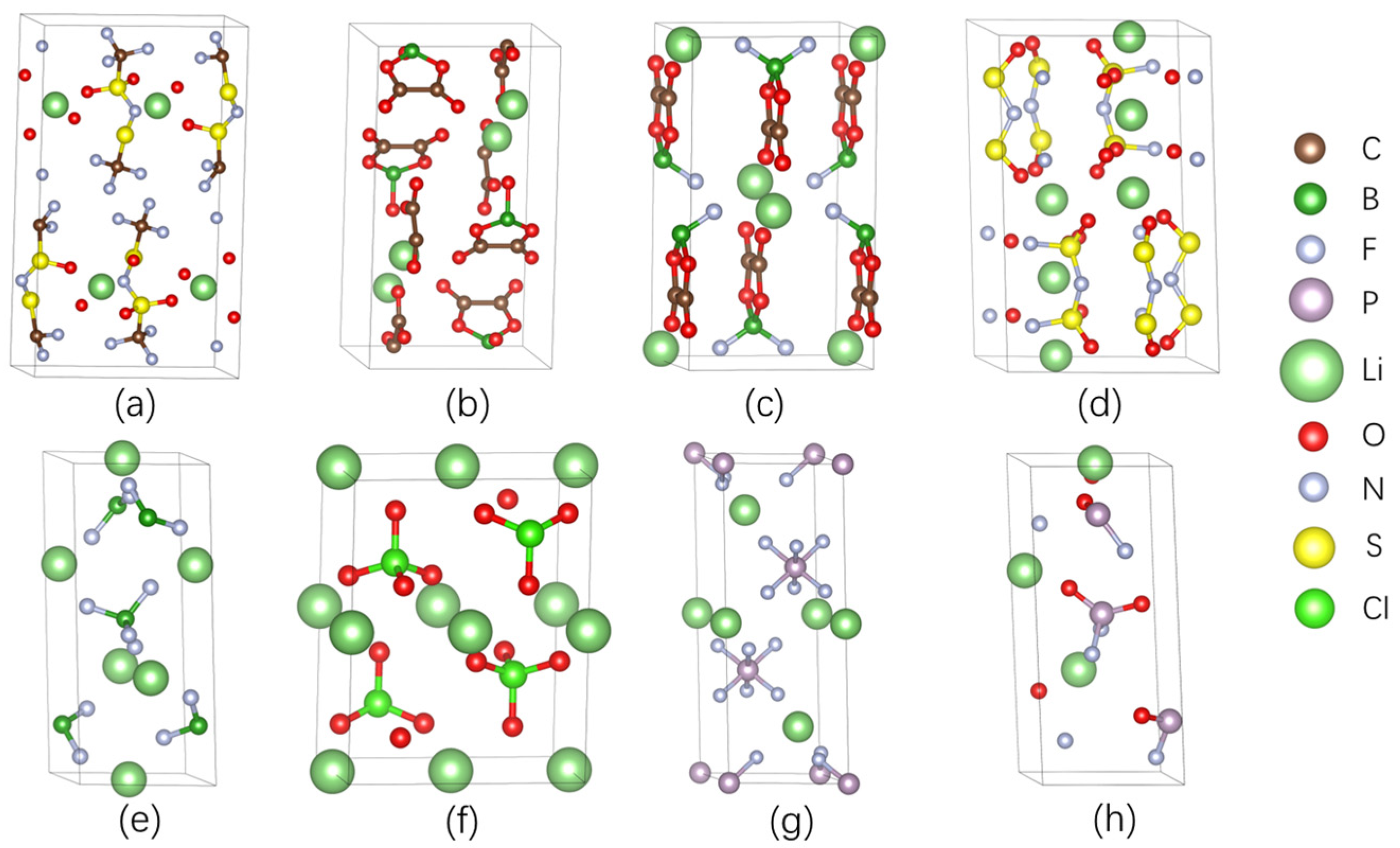
2.3. Structure of Lithium Salts and PEO
2.4. Screening Rules
| Algorithm 1: Normalize to [0,1] |
| Input: T[1:8] Output: N[1:8] for each T[i] do if (T[i] < 0) then T[i] = abs (T[i]); endif endfor T[9] = 0; Normalize(T[1:9]); N[1:8] = T[1:8] Return N[1:8] |
| Algorithm 2: Normalize to (−1,1] |
| Input: T[1:8] Output: N[1:8] Min = min(T[1:8]); for each T[i] do if (T[i] == 3.9) then T[i] = 0; else if (T[i] < 3.9) then j = i; T[i] = 3.9 + (3.9-T[i]); end if endfor Normalize(T[1:8]); T[j] = -T[j]; N[1:8] = T[1:8] Return N[1:8] |
3. Results
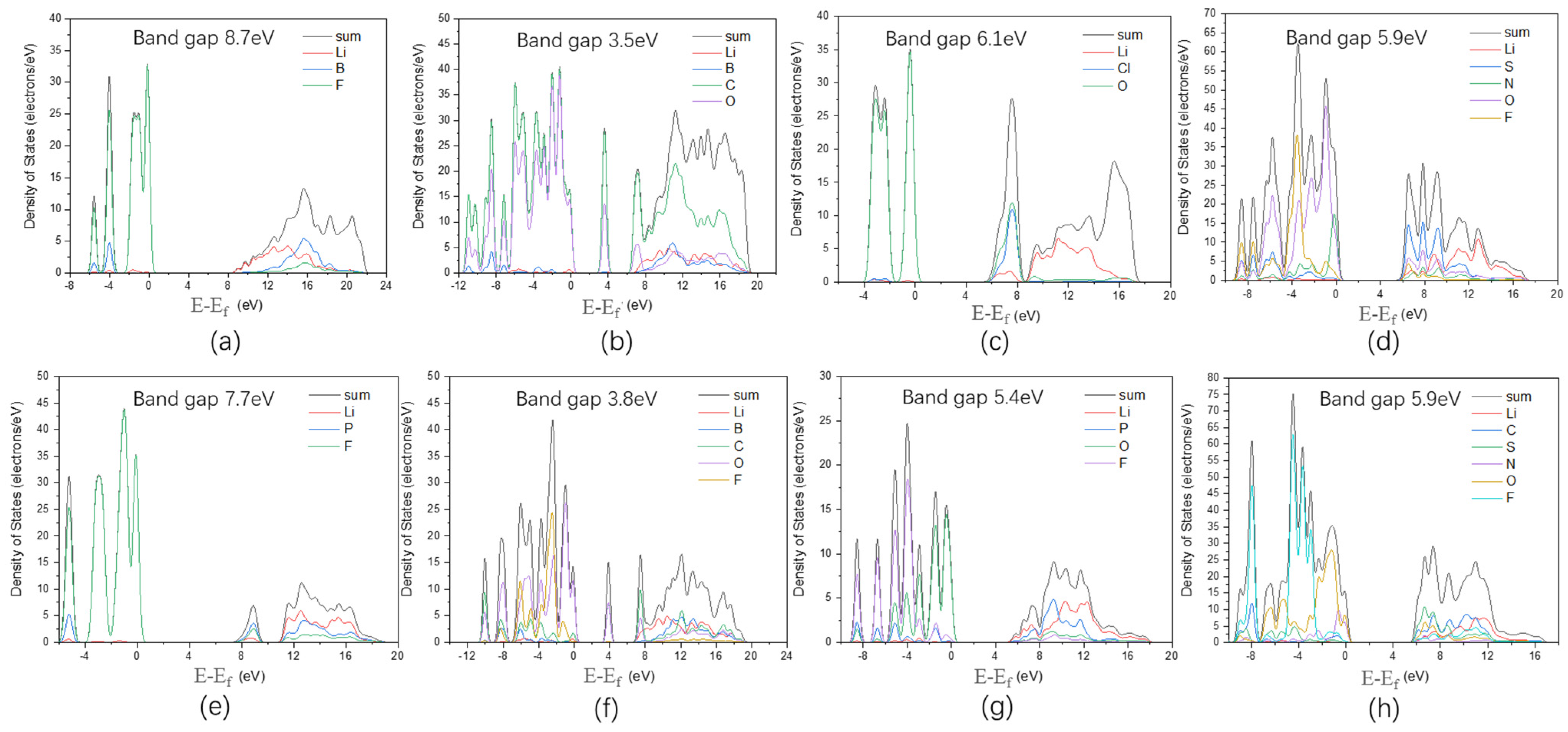
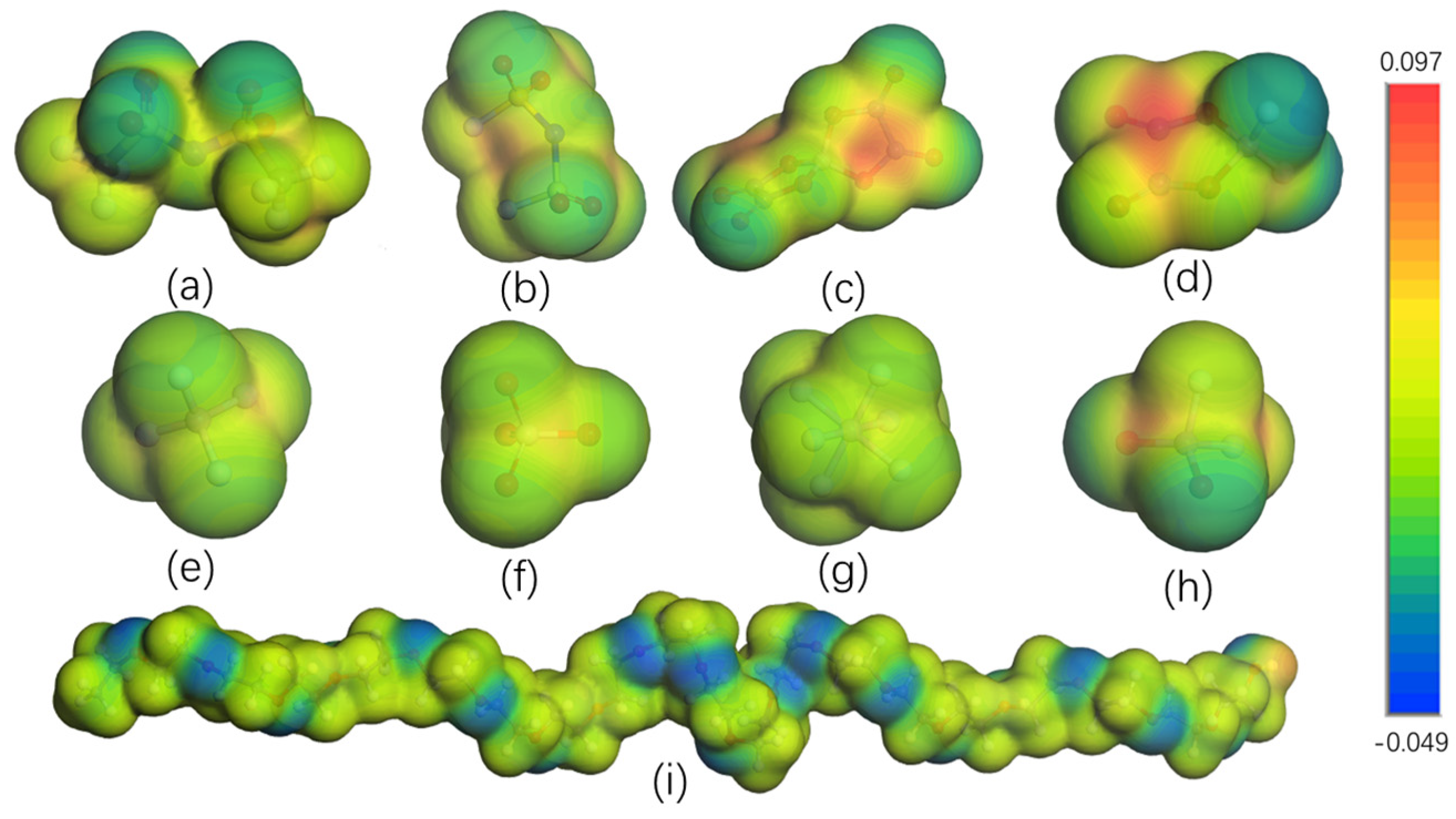
3.1. Electrochemical Stability
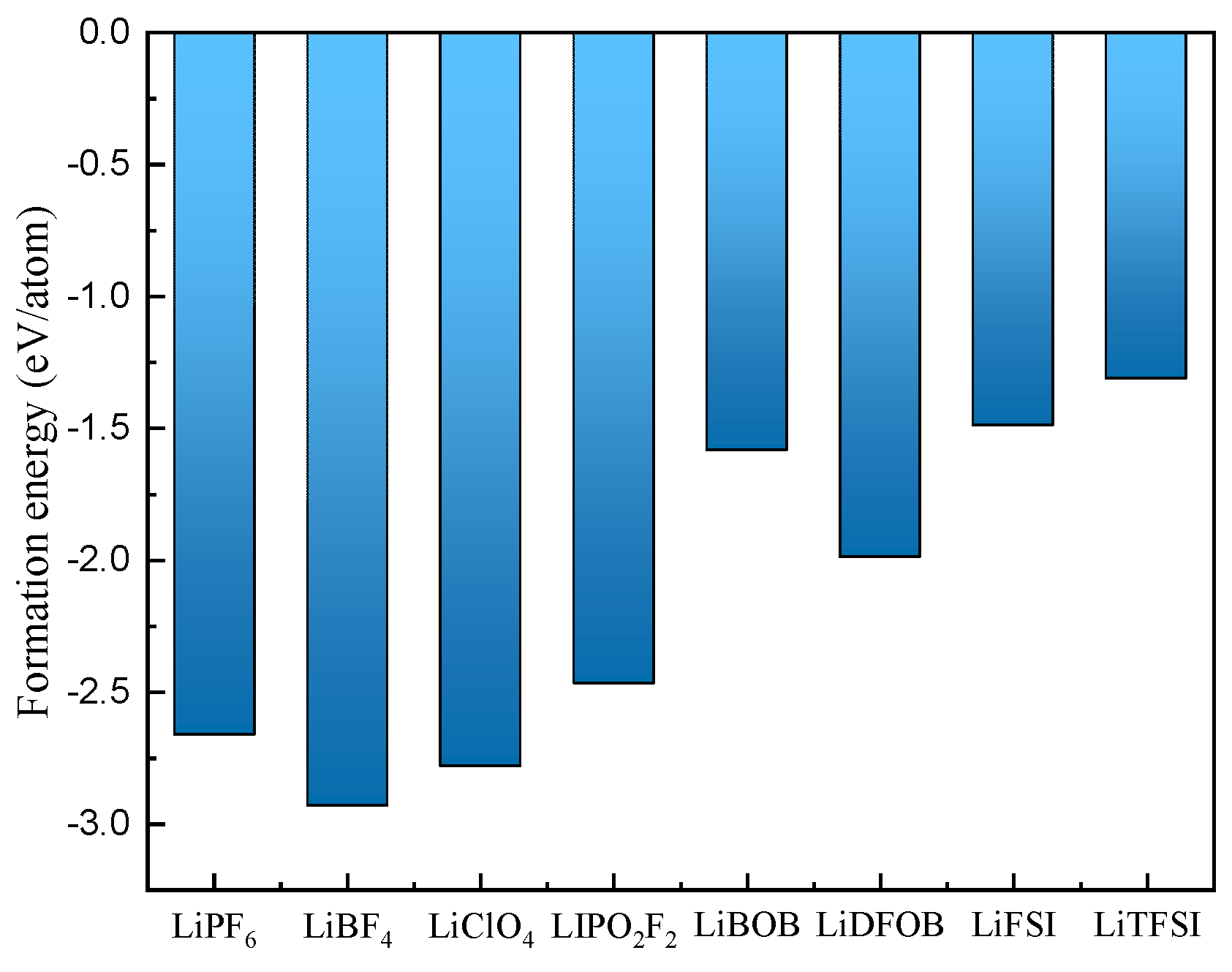
3.2. Lithium Salt Stability
3.3. Ionic Conductivity
3.4. Screening Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.R.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.C.; Huang, J.Q.; Yu, D.S.; Liu, Y.L.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. Infomat 2019, 1, 6–32. [Google Scholar]
- Su, X.; Xu, X.-P.; Ji, Z.-Q.; Wu, J.; Ma, F.; Fan, L.-Z. Polyethylene Oxide-Based Composite Solid Electrolytes for Lithium Batteries: Current Progress, Low-Temperature and High-Voltage Limitations, and Prospects. Electrochem. Energy Rev. 2024, 7, 2. [Google Scholar]
- Lotsch, B.V.; Maier, J. Relevance of solid electrolytes for lithium-based batteries: A realistic view. J. Electroceram 2017, 38, 128–141. [Google Scholar]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid. State Electr. 2017, 21, 1939–1964. [Google Scholar]
- Ghufira; Wu, Y.-S.; Wu, S.-h.; Chang, J.K.; Jose, R.; Yang, C. Solvent-free semi-interpenetrating composite polymer electrolyte based on dual Li-salt for solid-state lithium batteries. J. Energy Storage 2025, 111, 115335. [Google Scholar]
- Yan, S.S.; Liu, H.; Lu, Y.; Feng, Q.Q.; Zhou, H.Y.; Wu, Y.H.; Hou, W.H.; Xia, Y.C.; Zhou, H.Y.; Zhou, P.; et al. Selectively fluorinated aromatic lithium salts regulate the solvation structure and interfacial chemistry for all-solid-state batteries. Sci. Adv. 2025, 11, 4014. [Google Scholar]
- Zhang, Y.; Feng, W.; Zhen, Y.; Zhao, P.; Wang, X.; Li, L. Effects of lithium salts on PEO-based solid polymer electrolytes and their all-solid-state lithium-ion batteries. Ionics 2022, 28, 2751–2758. [Google Scholar]
- Tong, J.; Wu, S.; von Solms, N.; Liang, X.; Huo, F.; Zhou, Q.; He, H.; Zhang, S. The Effect of Concentration of Lithium Salt on the Structural and Transport Properties of Ionic Liquid-Based Electrolytes. Front. Chem. 2019, 7, 945. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar]
- Lee, S.S.; Park, H.-Y.; Jung, Y.-G.; Kim, J.-K.; Kim, Y. Effect of lithium salt on the properties of PEO based hybrid solid electrolyte for high safety lithium-ion batteries. Int. J. Nanotechnol. 2018, 15, 620–629. [Google Scholar] [CrossRef]
- Bonizzoni, S.; Ferrara, C.; Berbenni, V.; Anselmi-Tamburini, U.; Mustarelli, P.; Tealdi, C. NASICON-type polymer-in-ceramic composite electrolytes for lithium batteries. Phys. Chem. Chem. Phys. 2019, 21, 6142–6149. [Google Scholar] [CrossRef]
- Li, M.; An, H.; Song, Y.; Liu, Q.; Wang, J.; Huo, H.; Lou, S.; Wang, J. Ion–Dipole-Interaction-Induced Encapsulation of Free Residual Solvent for Long-Cycle Solid-State Lithium Metal Batteries. J. Am. Chem. Soc. 2023, 145, 25632–25642. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Mo, J.; Li, C.; Li, H.; Zhang, Q.; Zeng, Z.; Xie, J.; Li, Y. Anion-Regulated Weakly Solvating Electrolytes for High-Voltage Lithium Metal Batteries. Energy Environ. Mater. 2022, 6, 12440. [Google Scholar] [CrossRef]
- Xue, Z.-M.; Ding, Y.-Z.; Chen, C.-H. A DFT study of electronic structures, energies, and molecular properties of lithium bis[croconato]borate and its derivatives. Electrochim. Acta 2007, 53, 990–997. [Google Scholar] [CrossRef]
- Zhao, D.; Song, L.; Wang, J.; Zhang, J.; Cui, X.; Wang, P.; Sun, J.; Cai, X.; Huang, J.; Zhang, N.; et al. Insight into the competitive reaction between LiDFP and LiFSI in lithium-ion battery at low temperature. J. Power Sources 2022, 549, 232147. [Google Scholar] [CrossRef]
- Pandian, S.; Adiga, S.P.; Tagade, P.; Hariharan, K.S.; Mayya, K.S.; Lee, Y.G. Electrochemical stability of ether based salt-in-polymer based electrolytes: Computational investigation of the effect of substitution and the type of salt. J. Power Sources 2018, 393, 204–210. [Google Scholar] [CrossRef]
- Ue, M.; Fujii, T.; Zhou, Z.-B.; Takeda, M.; Kinoshita, S. Electrochemical properties of Li[CnF2n+1BF3] as electrolyte salts for lithium-ion cells. Solid. State Ion. 2006, 177, 323–331. [Google Scholar] [CrossRef]
- Kaymaksiz, S.; Wilhelm, F.; Wachtler, M.; Wohlfahrt-Mehrens, M.; Hartnig, C.; Tschernych, I.; Wietelmann, U. Electrochemical stability of lithium salicylato-borates as electrolyte additives in Li-ion batteries. J. Power Sources 2013, 239, 659–669. [Google Scholar] [CrossRef]
- Xue, Z.-M.; Ding, J.; Zhou, W.; Chen, C.-H. Density functional theory study on LBDOB and its derivatives: Electronic structures, energies, and molecular properties. Electrochim. Acta 2010, 55, 3838–3844. [Google Scholar] [CrossRef]
- Xue, Z.-M.; Zhou, W.; Ding, J.; Chen, C.-H. Electronic structures and molecular properties of FLBDOB and its derivatives: A combined experimental and theoretical study. Electrochim. Acta 2010, 55, 5342–5348. [Google Scholar] [CrossRef]
- Xue, Z.-M.; Zhou, W.; Sun, B.-B.; Chen, C.-H. Density functional theory study on LDFBDB and its derivatives: Electronic structures, energies, and molecular properties. Electrochim. Acta 2011, 56, 8770–8775. [Google Scholar]
- Osborne, D.A.; Breedon, M.; Rüther, T.; Spencer, M. Towards higher electrochemical stability of electrolytes: Lithium salt design through in silico screening. J. Mater. Chem. A 2022, 10, 13254–13265. [Google Scholar]
- Sarkar, R.; Kundu, T.K. Combined density functional theory and molecular dynamics analyses on Lithium/Sodium ion interaction and diffusion in gel polymer electrolytes with poly-vinylidene fluoride scaffold, propylene carbonate/Ionic Liquid solvent, and perchlorate salt. Mater. Today Commun. 2023, 37, 107311. [Google Scholar]
- Wang, C.; Liu, H.; Liang, Y.; Li, D.; Zhao, X.; Chen, J.; Huang, W.; Gao, L.; Fan, L.Z. Molecular-level Designed Polymer Electrolyte for High-Voltage Lithium–Metal Solid-State Batteries. Adv. Funct. Mater. 2022, 33, 2209828. [Google Scholar]
- Liu, L.; Wang, T.; Sun, L.; Song, T.; Yan, H.; Li, C.; Mu, D.; Zheng, J.; Dai, Y. Stable Cycling of All-Solid-State Lithium Metal Batteries Enabled by Salt Engineering of PEO-Based Polymer Electrolytes. Energy Environ. Mater. 2023, 7, e12580. [Google Scholar]
- Pan, Y.; Yu, H.; Zhang, Y.; Wang, Z.; Wang, S.; Li, C.; Ma, Y.; Shi, X.; Zhang, H.; Song, D.; et al. In-depth exploration of the effect mechanisms of various lithium salt anions in solid-state and liquid lithium metal batteries. J. Mater. Chem. A 2024, 12, 16447–16456. [Google Scholar] [CrossRef]
- Xu, G.; Shangguan, X.; Dong, S.; Zhou, X.; Cui, G.J.A.C. Formulierung von Elektrolyten mit gemischten Lithiumsalzen für Lithium-Batterien. Angew. Chem. 2020, 132, 3426–3442. [Google Scholar] [CrossRef]
- Clark, S.J.; Segallii, M.D.; Pickardii, C.J.; Hasnipiii, P.J.; Probertiv, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Burke, K.; Perdew, J.P.; Langreth, D.C. Is the local density approximation exact for short wavelength fluctuations? Phys. Rev. Lett. 1994, 73, 1283. [Google Scholar]
- Prasada Rao, R.; Chen, H.; Adams, S. Stable Lithium Ion Conducting Thiophosphate Solid Electrolytes Lix(PS4)yXz (X = Cl, Br, I). Chem. Mater. 2019, 31, 8649–8662. [Google Scholar] [CrossRef]
- Arifiadi, A.; Wichmann, L.; Brake, T.; Lechtenfeld, C.; Buchmann, J.; Demelash, F.; Yan, P.; Brunklaus, G.; Cekic-Laskovic, I.; Wiemers-Meyer, S.; et al. Evaluation of Alternative Lithium Salts for High-Voltage Lithium Ion Batteries: Higher Relevance of Plated Li Morphology than the Amount of Electrode Crosstalk. Small 2024, 5, e2410762. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Persson, K.A. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. Apl. Mater. 2013, 1, 011002. [Google Scholar]
- Unge, M.; Gudla, H.; Zhang, C.; Brandell, D. Electronic conductivity of polymer electrolytes: Electronic charge transport properties of LiTFSI-doped PEO. Phys. Chem. Chem. Phys. 2020, 22, 7680–7684. [Google Scholar] [CrossRef] [PubMed]
- Fortuin, B.A.; Meabe, L.; Peña, S.R.; Zhang, Y.; Qiao, L.; Etxabe, J.; Garcia, L.; Manzano, H.; Armand, M.; Martínez-Ibañez, M.; et al. Molecular-Level Insight into Charge Carrier Transport and Speciation in Solid Polymer Electrolytes by Chemically Tuning Both Polymer and Lithium Salt. J. Phys. Chem. C 2023, 127, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The Critical Role of Fillers in Composite Polymer Electrolytes for Lithium Battery. Nano-Micro Lett. 2023, 15, 74. [Google Scholar]
- Nachimuthu, S.; Cheng, H.J.; Lai, H.J.; Cheng, Y.H.; Kuo, R.-T.; Zeier, W.G.; Hwang, B.J.; Jiang, J.C. First-principles study on selenium-doped Li10GeP2S12 solid electrolyte: Effects of doping on moisture stability and Li-ion transport properties. Mater. Today Chem. 2022, 26, 101223. [Google Scholar]
- Chen, W.; Li, Y.; Feng, D.; Lv, C.; Li, H.; Zhou, S.; Jiang, Q.; Yang, J.; Gao, Z.; He, Y.; et al. Recent progress of theoretical research on inorganic solid state electrolytes for Li metal batteries. J. Power Sources 2023, 561, 232720. [Google Scholar] [CrossRef]
- Chapman, N.; Borodin, O.; Yoon, T.; Nguyen, C.C.; Lucht, B.L. Spectroscopic and Density Functional Theory Characterization of Common Lithium Salt Solvates in Carbonate Electrolytes for Lithium Batteries. J. Phys. Chem. C 2017, 121, 2135–2148. [Google Scholar]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–21. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Fang, W.; Ding, J.; Yuan, D.; Luo, S.; Zhang, H.; Ji, X. Impact of Fluorine-Based Lithium Salts on SEI for All-Solid-State PEO-Based Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2303718. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yin, J.; Chen, M.; Shen, J.; Zhao, X.; Liu, Y. Lithium Salt Screening for PEO-Based Solid Electrolytes of All Solid-State Li Ion Batteries Using Density Functional Theory. Crystals 2025, 15, 333. https://doi.org/10.3390/cryst15040333
Liu Q, Yin J, Chen M, Shen J, Zhao X, Liu Y. Lithium Salt Screening for PEO-Based Solid Electrolytes of All Solid-State Li Ion Batteries Using Density Functional Theory. Crystals. 2025; 15(4):333. https://doi.org/10.3390/cryst15040333
Chicago/Turabian StyleLiu, Qian, Jinghua Yin, Minghua Chen, Jialong Shen, Xinhao Zhao, and Yulong Liu. 2025. "Lithium Salt Screening for PEO-Based Solid Electrolytes of All Solid-State Li Ion Batteries Using Density Functional Theory" Crystals 15, no. 4: 333. https://doi.org/10.3390/cryst15040333
APA StyleLiu, Q., Yin, J., Chen, M., Shen, J., Zhao, X., & Liu, Y. (2025). Lithium Salt Screening for PEO-Based Solid Electrolytes of All Solid-State Li Ion Batteries Using Density Functional Theory. Crystals, 15(4), 333. https://doi.org/10.3390/cryst15040333






