Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies
Abstract
1. Introduction
2. Characterization Methods of Nanocellulose
3. Types and Properties of Nanocellulose
- Cellulose nanofibers (CNFs): CNFs, also referred to as cellulose nanofibrils or fibrillated cellulose, are obtained by mechanically disintegrating cellulose fibers into nanoscale fibrils. These fibrils retain a significant portion of the cellulose’s original crystallinity and are characterized by their flexibility and high surface area, which makes them suitable for use in hydrogels, paper, and composite materials.
- Cellulose nanofibrils (CNFs): also known as nanofibrillated cellulose (NFC), CNFs are nanoscale, elongated fibers derived from cellulose. These fibrils are typically a few nanometers in diameter and several micrometers in length. They result from the mechanical disintegration of cellulose fibers, which can be achieved through methods such as high-pressure homogenization, grinding, or ultrasonication. Cellulose nanofibrils retain much of the cellulose’s crystalline structure, while the amorphous regions of the fiber are partially disrupted during processing. As a result, CNFs possess a high surface area, flexibility, and mechanical strength, making them ideal for a range of applications such as in nanocomposites, coatings, films, hydrogels, and as a reinforcing agent in various materials. They are renewable, biodegradable, and environmentally friendly, further enhancing their appeal for sustainable product development.
- Nanofibrillated cellulose (NFC): Also known as cellulose nanofibers (CNFs), NFC refers to cellulose fibers that have been mechanically disintegrated into nanoscale fibrils. These fibrils retain a significant portion of the cellulose’s crystalline structure, while the amorphous regions are partially broken down. The resulting material consists of flexible, thin, and elongated fibers that are typically a few nanometers in diameter and several micrometers in length. NFC is produced through mechanical processes such as high-pressure homogenization, grinding, or ultrasonication, which separate the cellulose fibers into individual nanofibrils. It has high surface area, excellent mechanical properties, and is biodegradable and renewable. Due to these characteristics, NFC has promising applications in various industries, including composites, films, coatings, hydrogels, and even as a reinforcement material in bioplastics and paper products.
- Cellulose nanocrystals (CNCs): Also known as cellulose nanowhiskers or nanocrystalline cellulose, CNCs are highly crystalline nanoparticles derived from the hydrolysis of cellulose using strong acids. They exhibit high mechanical strength, rigidity, and optical transparency, making them ideal for applications in nanocomposites, coatings, and drug delivery.
- Nanocrystalline cellulose (NCC): Also known as cellulose nanocrystals (CNCs), NCC refers to the crystalline portion of cellulose that has been isolated at the nanoscale through chemical methods, typically acid hydrolysis, using strong acids such as sulfuric or hydrochloric acid. During this process, the amorphous regions of cellulose are selectively broken down, leaving behind highly crystalline, stiff nanoparticles with a rod-like or whisker-like structure. NCC exhibits high mechanical strength, rigidity, and optical transparency, making it ideal for applications in nanocomposites, coatings, and drug delivery systems.
- Cellulose nanoparticles (CNPs): This is a general term used to describe any nanocellulose material that has been reduced to nanoparticle size. This category can encompass both CNCs and CNFs, depending on the specific isolation method used. CNPs are generally characterized by their small size and high surface area, making them suitable for a variety of applications.
- Cellulose nanowhiskers: These are similar to CNCs, but the term is often used to describe very small, rod-like particles isolated from cellulose sources through acid hydrolysis. Their distinct shape allows them to form highly ordered structures, making them ideal for reinforcing composites.
- Bacterial nanocellulose (BNC): BNC is produced by bacterial fermentation, primarily by species such as Gluconacetobacter and Acetobacter. Unlike plant-based cellulose, BNC is highly pure, has excellent water retention capacity, and exhibits high tensile strength. Its unique properties make it an attractive material for biomedical applications such as wound dressings, tissue scaffolds, and drug delivery systems.
- Cellulose nanoribbons: The term cellulose nanoribbon is specifically used to describe cellulose nanofibrils derived from bacterial sources, particularly bacterial nanocellulose (BNC). These nanoribbons are characterized by their flat, ribbon-like shape, and they retain a high degree of crystallinity and purity. Cellulose nanoribbons are typically produced through bacterial fermentation processes, where microorganisms like Gluconacetobacter or Acetobacter synthesize highly pure cellulose in the form of nanoscale fibrils. These nanoribbons possess excellent mechanical strength, high surface area, and significant water retention capacity, making them suitable for various applications, including biomedical uses, such as wound dressings and tissue scaffolds, as well as in the development of sustainable materials and nanocomposites.
4. Production and Isolation Techniques for Nanocellulose
5. Market Overview and Production Landscape of Nanocellulose
6. Strategies for Modification and Enhancement of Nanocellulose
7. Challenges and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, C.D.M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef] [PubMed]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Dissanayake, H.; Mansi, E.; Stancu, A. Eco Breakthroughs: Sustainable Materials Transforming the Future of Our Planet. Sustainability 2024, 16, 10790. [Google Scholar] [CrossRef]
- Young, R.A.; Rowell, R.M. Cellulose: Structure, Modification, and Hydrolysis; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous colloidal solutions of cellulose micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Mukherjee, S.M.; Woods, H.J. X-ray and electron microscope studies of the degradation of cellulose by sulphuric acid. Biochim. Biophys. Acta 1953, 10, 499–511. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose a new cellulose product: Properties uses commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Ngo, T.; Danumah, C.; Ahvazi, B. Production of cellulose nanocrystals at InnoTech Alberta. In Nanocellulose and Sustainability; CRC Press: Boca Raton, FL, USA, 2018; pp. 269–287. [Google Scholar] [CrossRef]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Effect of cellulose nanocrystal polymorphs on mechanical, barrier and thermal properties of poly (lactic acid) based bionanocomposites. RSC Adv. 2015, 5, 60426–60440. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Boix Sanfeliu, A.; Calzolai, L.; Emons, H.; Gaillard, C.; Gibson, P.; Holzwarth, U.; Koeber, R.; Linsinger, T.; et al. (Eds.) Towards a Review of the EC Recommendation for a Definition of the Term “Nanomaterial” Part 2: Assessment of Collected Information Concerning the Experience with the Definition; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef]
- Wu, X.; Chabot, V.L.; Kim, B.K.; Yu, A.; Berry, R.M.; Tam, K.C. Cost-effective and scalable chemical synthesis of conductive cellulose nanocrystals for high-performance supercapacitors. Electrochim. Acta 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Belosinschi, D.; Tofanica, B.-M. A new bio-material with 3D lightweight network for energy and advanced applications. Cellulose 2018, 25, 897–902. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels 2023, 9, 390. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Lobiuc, A.; Ungureanu, O.C.; Jităreanu, D.C. Design of Functional Polymer Systems to Optimize the Filler Retention in Obtaining Cellulosic Substrates with Improved Properties. Materials 2023, 16, 1904. [Google Scholar] [CrossRef]
- Ioelovich, M. Characterization of Various Kinds of Nanocellulose. In Handbook of Nanocellulose Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanoparticles: Structure–Morphology–Rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Matias, V.R.F.; Beveridge, T.J. Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol. Microbiol. 2005, 56, 240–251. [Google Scholar] [CrossRef]
- Ungureanu, E.; Samuil, C.; Țopa, D.C.; Ungureanu, O.C.; Tofanica, B.-M.; Fortună, M.E.; Brezuleanu, C.O. Adsorption of Ni(II) from Aqueous Media on Biodegradable Natural Polymers—Sarkanda Grass Lignin. Crystals 2024, 14, 381. [Google Scholar] [CrossRef]
- Martinez Garcia, F.D. Characterising the Elastic and Viscoelastic Interaction Between the Cell and Its Matrix in 3D: Because It Takes Two to Salsa Dance. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Chliveros, G.; Malamou, A. Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers. Fibers 2023, 11, 83. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Nizamuddin, S.; Xu, J.; Vancov, T.; Chen, C. Cellulose nanocrystals from agriculture and forestry biomass: Synthesis methods, characterization and industrial applications. Environ. Sci. Pollut. Res. 2024, 31, 58745–58778. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Van Der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital color in cellulose nanocrystal films. ACS Appl. Mater. Interfaces 2014, 6, 12302–12306. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Altgen, M.; Penttilӓ, P.; Rautkari, L. Review: Interaction of water vapour with wood and other hygro-responsive materials. J. Mater. Sci. 2024, 59, 7595–7635. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Almeida, R.O.; Maloney, T.C.; Gamelas, J.A.F. Production of functionalized nanocelluloses from different sources using deep eutectic solvents and their applications. Ind. Crops Prod. 2023, 199, 116583. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2015, 200, 1008–1018. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Habib, M.H.; Alassmy, Y.A.; Abduljawad, M.M.; Alshamrani, K.M.; Sebakhy, K.O. Solid State NMR a Powerful Technique for Investigating Sustainable/Renewable Cellulose-Based Materials. Polymers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Agarwal, U.P. Raman Spectroscopy of CNC- and CNF-Based Nanocomposites. In Handbook of Nanocellulose Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Khili, F.; Borges, J.; Almeida, P.L.; Boukherroub, R.; Omrani, A.D. Extraction of Cellulose Nanocrystals with Structure I and II and Their Applications for Reduction of Graphene Oxide and Nanocomposite Elaboration. Waste Biomass Valorizat. 2019, 10, 1913–1927. [Google Scholar] [CrossRef]
- Whba, F.; Mohamed, F.; Idris, M.I.; Yahya, M.S. Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Appl. Sci. 2023, 13, 6316. [Google Scholar] [CrossRef]
- Sodhi, R.N.S. Time-of-flight secondary ion mass spectrometry (TOF-SIMS):—Versatility in chemical and imaging surface analysis. Analyst 2004, 129, 483–487. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Koloor, S.S.R.; Ayatollahi, M.R.; Bushroa, A.R.; Yahya, M.Y. Assessment of Nano-Indentation method in mechanical characterization of heterogeneous nanocomposite materials using experimental and computational approaches. Sci. Rep. 2019, 9, 15763. [Google Scholar] [CrossRef]
- Wang, Z.; Kato, T.; Hirayama, T.; Kato, N.; Sasaki, K.; Saka, H. Surface damage induced by focused-ion-beam milling in a Si/Si p–n junction cross-sectional specimen. Appl. Surf. Sci. 2004, 241, 80–86. [Google Scholar] [CrossRef]
- Moud, A.A. Cellulose nanocrystals Examined by Atomic Force Microscopy: Applications and Fundamentals. ACS Food Sci. Technol. 2022, 2, 1789–1818. [Google Scholar] [CrossRef]
- Boluk, Y.; Danumah, C. Analysis of cellulose nanocrystal rod lengths by dynamic light scattering and electron microscopy. J. Nanopart. Res. 2014, 16, 2174. [Google Scholar] [CrossRef]
- Kumar, V.; Nazari, B.; Bousfield, D.; Toivakka, M. Rheology of microfibrillated cellulose suspensions in pressure-driven flow. Appl. Rheol. 2016, 26, 24–34. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Nanocellulose as a green and sustainable emerging material in energy applications: A review. Polym. Adv. Technol. 2017, 28, 1583–1594. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose–Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Kröger, M.; Badara, O.; Pääkkönen, T.; Schlapp-Hackl, I.; Hietala, S.; Kontturi, E. Efficient isolation method for highly charged phosphorylated cellulose nanocrystals. Biomacromolecules 2023, 24, 1318–1328. [Google Scholar] [CrossRef]
- Kirdant, S.P.; Biradar Tamboli, A.T.; Jadhav, V.H. Recent developments in the applications of biomass-derived sulfonated carbonaceous solid acid catalysts. Helv. Chim. Acta 2022, 105, e202200032. [Google Scholar] [CrossRef]
- Gui, X.; Wan, Z.; Zhang, H.; Niu, M.; Guo, Y.; Li, H. Preparation of cellulose nanocrystals by ultrasonication-assisted phosphotungstic acid method: An effective method of high yield and friendly environment. Ind. Crops Prod. 2024, 222, 119780. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.-L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R.; Chabot, B.; Loranger, E.; Naebe, M.; Shojaeiarani, J.; Gharehkhani, S.; Ahvazi, B.; Hu, J.; Thomas, S. Recent Advances in Cellulose Nanofibers Preparation through Energy-Efficient Approaches: A Review. Energies 2021, 14, 6792. [Google Scholar] [CrossRef]
- Serra, A.; González, I.; Oliver-Ortega, H.; Tarrès, Q.; Delgado-Aguilar, M.; Mutjé, P. Reducing the Amount of Catalyst in TEMPO-Oxidized Cellulose Nanofibers: Effect on Properties and Cost. Polymers 2017, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Mnasri, A.; Dhaouadi, H.; Khiari, R.; Halila, S.; Mauret, E. Effects of Deep Eutectic Solvents on cellulosic fibres and paper properties: Green “chemical” refining. Carbohydr. Polym. 2022, 292, 119606. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Xiao, H.; Liang, J.; Zhang, Y.; Chang, J.; Zhang, R.; Zhang, P. Conversion of Materials and Energy in Anaerobic Digestion of Sewage Sludge with High-Pressure Homogenization Pretreatment. Processes 2023, 11, 2467. [Google Scholar] [CrossRef]
- Bourmaud, A.; Morvan, C.; Bouali, A.; Placet, V.; Perré, P.; Baley, C. Relationships between micro-fibrillar angle, mechanical properties and biochemical composition of flax fibers. Ind. Crops Prod. 2012, 44, 343–351. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Drăgoi, E.N.; Danu, M.; Ailiesei, G.-L.; Balan, C.D.; Chicet, D.-L.; Nechita, M.T. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers 2023, 15, 1038. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Brezuleanu, C.O.; Ungureanu, V.I.; Chiruță, C.; Rotaru, R.; Tofanica, B.M.; Popa, V.I.; Jităreanu, D.C. Comparison Adsorption of Cd (II) onto Lignin and Polysaccharide-Based Polymers. Polymers 2023, 15, 3794. [Google Scholar] [CrossRef]
- Lewandowski, A.; Wilczyński, K. Modeling of Twin Screw Extrusion of Polymeric Materials. Polymers 2022, 14, 274. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial nanocellulose: Engineering, production, and applications. Bioengineered 2021, 12, 11463–11483. [Google Scholar] [CrossRef]
- Grand View Research. Nanocellulose Market Size, Share & Trends Analysis Report by Type (Cellulose Nanofibers, Bacterial Cellulose, Crystalline Nanocellulose), by Application, by Region, and Segment Forecasts, 2023–2030; Industry: Specialty & Chemicals; Grand View Research: San Francisco, CA, USA, 2022; p. 200. Available online: https://www.grandviewresearch.com/industry-analysis/nanocellulose-market (accessed on 15 January 2025).
- Markets and Markets. Nanocellulose Market by Type (MFC & NFC, CNC/NCC), Raw Material (Wood, Non-Wood), Application (Paper & Pulp, Composites, Paints & Coatings, Biomedical & Pharmaceuticals, Electronics & Sensors), and Region—Global Forecast to 2032; Markets and Markets: Northbrook, IL, USA, 2024; p. 220. Available online: https://www.marketsandmarkets.com/Market-Reports/nano-cellulose-market-56392090.html (accessed on 15 January 2025).
- Kings Research. Bioplastics Market Size, Share, Growth, and Industry Analysis by Product Type (Biodegradable, Non-Biodegradable), by Material Type (Polylactic Acid, Starch Blends, Polyethylene, Polyamide, Polyethylene Terephthalate, Polypropylene, Polyhydroxyalkanoates, Polybutylene Succinate), by Application (Packaging, Construction, Automotive and Transportation, Consumer Goods) and Regional Analysis, 2023–2030; Kings Research: Dubai, United Arab Emirates, 2024; p. 120. Available online: https://www.grandviewresearch.com/industry-analysis/bioplastics-industry (accessed on 15 January 2025).
- European Commission. The European Green Deal; European Commission: Luxembourg, 2019. [Google Scholar]
- European Commission. Circular Economy Action Plan; European Commission: Luxembourg, 2020. [Google Scholar]
- Heiskanen, I.; Axrup, L.; Norborg, M.-A.; Knoos, I. Process for Producing a Dispersion Comprising Nanoparticles and a Dispersion Produced According to the Process. U.S. Patent 9365978B2, 14 June 2016. Available online: https://patents.google.com/patent/US9365978B2/en (accessed on 15 January 2025).
- Heiskanen, I.; Backfolk, K.; Lyytikainen, K. Flexible Microfibrillated Film Formation. U.S. Patent 10501890B2, 10 December 2019. Available online: https://patents.google.com/patent/US10501890B2/en (accessed on 15 January 2025).
- Borregaard Exilva: Microfibrillated Cellulose at a Glance. Available online: https://info.borregaard.com/hubfs/Cellulose%20Fibrils/eBooks/Borregaard-Exilva_Microfibrillated-Cellulose-at-a-glance.pdf (accessed on 15 January 2025).
- Backa, S.O. Method and Device for Producing Dry Microfibrillated Cellulose. Patent WO2011095335A1, 11 August 2011. Available online: https://patents.google.com/patent/WO2011095335A1/en (accessed on 15 January 2025).
- Blell, R.; Vold, I.M.N.; Øvrebo, H.H.; Gonera, A. Skin Care Compositions Comprising Microfibrillated Cellulose. Patent EP3283052B1, 19 February 2020. Available online: https://patents.google.com/patent/EP3283052B1/en (accessed on 15 January 2025).
- Windebank, M.; Skuse, D.; Motsi, T.; Tellier, G. Microfibrillated Cellulose with Enhanced Properties and Methods of Making the Same. U.S. Patent 11242651B2, 8 February 2022. Available online: https://patents.google.com/patent/US11242651B2/en (accessed on 15 January 2025).
- Gane, P.A.C.; Schoelkopf, J.; Gantenbein, D.; Schenker, M. Process for the Production of Nano-Fibrillar Cellulose Gels. U.S. Patent 10294371B2, 21 May 2019. Available online: https://patents.google.com/patent/US10294371B2/en (accessed on 15 January 2025).
- Gane, P.A.C.; Schoelkopf, J.; Gantenbein, D.; Schenker, M.; Pohl, M.; Kübler, B. Process for the Production of Nano-Fibrillar Cellulose Suspensions. U.S. Patent 20210262164A1, 26 August 2021. Available online: https://patents.google.com/patent/US20210262164A1/en (accessed on 15 January 2025).
- Gane, P.A.C.; Schenker, M.; Subramanian, R.; Schoelkopf, J. Process for the Manufacture of Structured Materials Using Nano-Fibrillar Cellulose Gels. U.S. Patent 10053817B2, 21 August 2018. Available online: https://patents.google.com/patent/US10053817B2/en (accessed on 15 January 2025).
- Nakatani, T.; Sato, S.; Kimura, K. Method for Evaluating Cellulose Nanofiber Dispersion. U.S. Patent 20210349072A1, 11 November 2021. Available online: https://patents.google.com/patent/US20210349072A1/en (accessed on 15 January 2025).
- Nakatani, T.; Kimura, K.; Sato, S. Dry Solids of Anionically Modified Cellulose Nanofibers and Processes for Preparing Them. U.S. Patent 20230203205A1, 29 June 2023. Available online: https://patents.google.com/patent/US20230203205A1/en (accessed on 15 January 2025).
- Hua, X.; Laleg, M.; Owston, T. Cellulose Nanofilaments and Method to Produce Same. Patent EP2569468B1, 25 January 2017. Available online: https://patents.google.com/patent/EP2569468B1/en (accessed on 15 January 2025).
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.F.; Guimarães, M.A.; Rubini, B.R. Process to Produce Microfibrillated Cellulose by Impacts. U.S. Patent 20250003151A1, 2 January 2025. Available online: https://patents.google.com/patent/US20250003151A1/en (accessed on 15 January 2025).
- Yousefi, H.; Azari, V.; Khazaeian, A. Direct Mechanical Production of Wood Nanofibers from Raw Wood Microparticles with No Chemical Treatment. Ind. Crops Prod. 2018, 115, 26–31. [Google Scholar] [CrossRef]
- Ghalehno, M.D.; Yousefi, H. Toward Wheat Straw Valorization by Its Downsizing to Five Types of cellulose Nanomaterials and Nanopapers Thereof. Waste Biomass Valor 2023, 14, 2885–2896. [Google Scholar] [CrossRef]
- Van Engelen, G.P.F.M.; Van Ingen, G.A.; Meeuwissen, C. Plant derived cellulose compositions for use as drilling muds. Patent WO2014017911A1, 30 January 2014. Available online: https://patents.google.com/patent/WO2014017911A1/en (accessed on 15 January 2025).
- Bourassa, P.; Methot, M.; Berry, R. Preparation of Solvent and Polymer Redispersible Formulations of Dried Cellulose Nanocrystals (CNC). U.S. Patent 11926714B2, 12 March 2024. Available online: https://patents.google.com/patent/US11926714B2/pt (accessed on 15 January 2025).
- Berry, R.M.; Aklaghi, S.P.; Tam, K.C. Surface Modified Nanocrystalline Cellulose and Uses Thereof. Patent CA2880555C, 31 August 2021. Available online: https://patents.google.com/patent/CA2880555C/en (accessed on 15 January 2025).
- Berry, R.; Granger, A. Polyurethane Composites Comprising Nanocrystalline Cellulose and Method for Improving Properties of Polyurethanes Thereof. Patent CA2913359A1, 4 December 2014. Available online: https://patents.google.com/patent/CA2913359A1/en (accessed on 15 January 2025).
- Hamad, W.Y.; Su, S. NCC-Based Supramolecular Materials for Thermoplastic and Thermoset Polymer Composites. U.S. Patent 9751969B2, 5 September 2017. Available online: https://patents.google.com/patent/US9751969B2/en (accessed on 15 January 2025).
- Guarilloff, P. Powdery Cosmetic Composition Comprising Nanocrystalline Cellulose. Patent WO2018100061A1, 7 June 2018. Available online: https://patents.google.com/patent/WO2018100061A1/en (accessed on 15 January 2025).
- Andrews, M.P.; Morse, T. Method for Producing Functionalized Nanocrystalline Cellulose and Functionalized Nanocrystalline Cellulose Thereby Produced. U.S. Patent 10968283B2, 6 April 2021. Available online: https://patents.google.com/patent/US10968283B2/en (accessed on 15 January 2025).
- Lapidot, S.; Shalev, S.R.; Slattegard, R.; Shoseyov, O.; Azerraf, C.; Braslavsky, I.; Yashunsky, V. Porous Nanocrystalline Cellulose Structures. U.S. Patent 10669390B2, 2 June 2020. Available online: https://patents.google.com/patent/US10669390B2/en (accessed on 15 January 2025).
- Slattegard, R.; Shavev, S.R.; Azerraf, C.; Nevo, Y. NCC Films and Products Based Thereon. Patent WO2017046798A1, 23 March 2017. Available online: https://patents.google.com/patent/WO2017046798A1/ja (accessed on 15 January 2025).
- Nelson, K.; Retsina, T. Processes for Producing Nanocellulose, and Nanocellulose Compositions Produced Therefrom. U.S. Patent 12091818B2, 17 September 2024. Available online: https://patents.google.com/patent/US12091818B2/en (accessed on 15 January 2025).
- Nelson, K.; Retsina, T.; Pylkkanen, V.; Iakovlev, M. Nanocellulose Production Co-Located at a Pulp and Paper Mill. Patent CA2996423A1, 2 March 2017. Available online: https://patents.google.com/patent/CA2996423A1/en (accessed on 15 January 2025).
- Mcalpine, S.; Nakoneshny, J. Production of Crystalline Cellulose. Patent WO2017127938, 8 March 2017. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017127938 (accessed on 15 January 2025).
- Jannatamani, H.; Motamedzadegan, A.; Farsi, M.; Yousefi, H. Rheological Properties of Wood/Bacterial Cellulose and Chitin Nano-Hydrogels as a Function of Concentration and Their Nano-Films Properties. IET Nanobiotechnol. 2022, 16, 158–169. [Google Scholar] [CrossRef]
- Keane, J.K. Yarn Reinforced Bacterial Cellulose Hybrid Materials. U.S. Patent 12084700B2, 10 September 2024. Available online: https://patents.google.com/patent/US12084700B2/en (accessed on 15 January 2025).
- von Szadkowski, K. The Path to Sustainable Mobility; Volkswagen Group: Wolfsburg, Germany, 2021; p. 50. Available online: https://ethz.ch/content/dam/ethz/special-interest/mavt/csfm-dam/events/2023/symposium/presentations/vw-szadkowski-the-path.pdf (accessed on 15 January 2025).
- Oettel, C. Fabrication of Leather-like Biomaterial Derived from Bacterial Nanocellulose by Using an Optimized Casting Process. In Proceedings of the Program, Cologne, Germany, 12–13 March 2025; Available online: https://cellulose-fibres.eu/wp-content/uploads/2024/12/Volkswagen_OettelCindy_CFC25_Abstract.pdf (accessed on 15 January 2025).
- Mikheeva, E.I.; Kuranova, M.A.; Gorokhova, A.A. Downstream Process for Modified Bacterial Cellulose Production with Increased Functional Properties for the Textile Industry. In Proceedings of the XI International Conference of Young Scientists: Bioinformaticians, Biotechnologists, Biophysicists, Virologists, Molecular Biologists, and Fundamental Medicine Specialists—2024, Novosibirsk, Russia, 24–27 September 2024; p. 92. [Google Scholar]
- Research and Markets. The Global Market for Micro- and Nanocellulose 2025–2035; Research and Markets: Dublin, Ireland, 2024; p. 578. Available online: https://www.researchandmarkets.com/reports/5878970/the-global-market-micro-nanocellulose (accessed on 15 January 2025).
- Uşurelu, C.D.; Panaitescu, D.M.; Oprică, G.M.; Nicolae, C.-A.; Gabor, A.R.; Damian, C.M.; Ianchiş, R.; Teodorescu, M.; Frone, A.N. Effect of Medium-Chain-Length Alkyl Silane Modified Nanocellulose in Poly(3-hydroxybutyrate) Nanocomposites. Polymers 2024, 16, 3069. [Google Scholar] [CrossRef]
- Heise, K.; Koso, T.; King, A.W.T.; Nypelö, T.; Penttilä, P.; Tardy, B.L.; Beaumont, M. Spatioselective surface chemistry for the production of functional and chemically anisotropic nanocellulose colloids. J. Mater. Chem. A 2022, 10, 23413–23432. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal based composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Penfold, N.J.W.; Yeow, J.; Boyer, C.; Armes, S.P. Emerging trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are natural compounds a promising alternative to synthetic Cross-Linking agents in the preparation of hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Suryanto, H.; Muhajir, M.; Susilo, B.D.; Pradana YR, A.; Wijaya, H.W.; Ansari, A.S.; Yanuhar, U. Nanofibrillation of bacterial cellulose using High-Pressure homogenization and its films characteristics. J. Renew. Mater. 2021, 9, 1717–1728. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Vizireanu, S.I.; Oprică, G.M.; Uşurelu, C.D.; Stancu, C.; Sătulu, V.; Ghiurea, M.; Nicolae, C.-A.; Raduly, M.F.; Frone, A.N. Influence of Cold Plasma Treatment on Cellulose Modification with Different Oxidizing Agents. Materials 2025, 18, 1066. [Google Scholar] [CrossRef]
- Ballinas-Casarrubias, L.; Villanueva-Solís, L.; Espinoza-Hicks, C.; Camacho-Dávila, A.; Castillo, H.A.P.; Pérez, S.B.; Villa, E.D.; Hernández, M.D.D.; González-Sánchez, G. Effect of Laccase-Mediated Biopolymer Grafting on Kraft Pulp Fibers for Enhancing Paper’s Mechanical Properties. Polymers 2017, 9, 570. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, L.; Li, J.; Dong, J.; Cheng, Z. Properties of cellulose nanofibril produced from wet ball milling after enzymatic treatment vs. mechanical grinding of bleached softwood kraft fibers. BioRes 2020, 15, 3809–3820. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
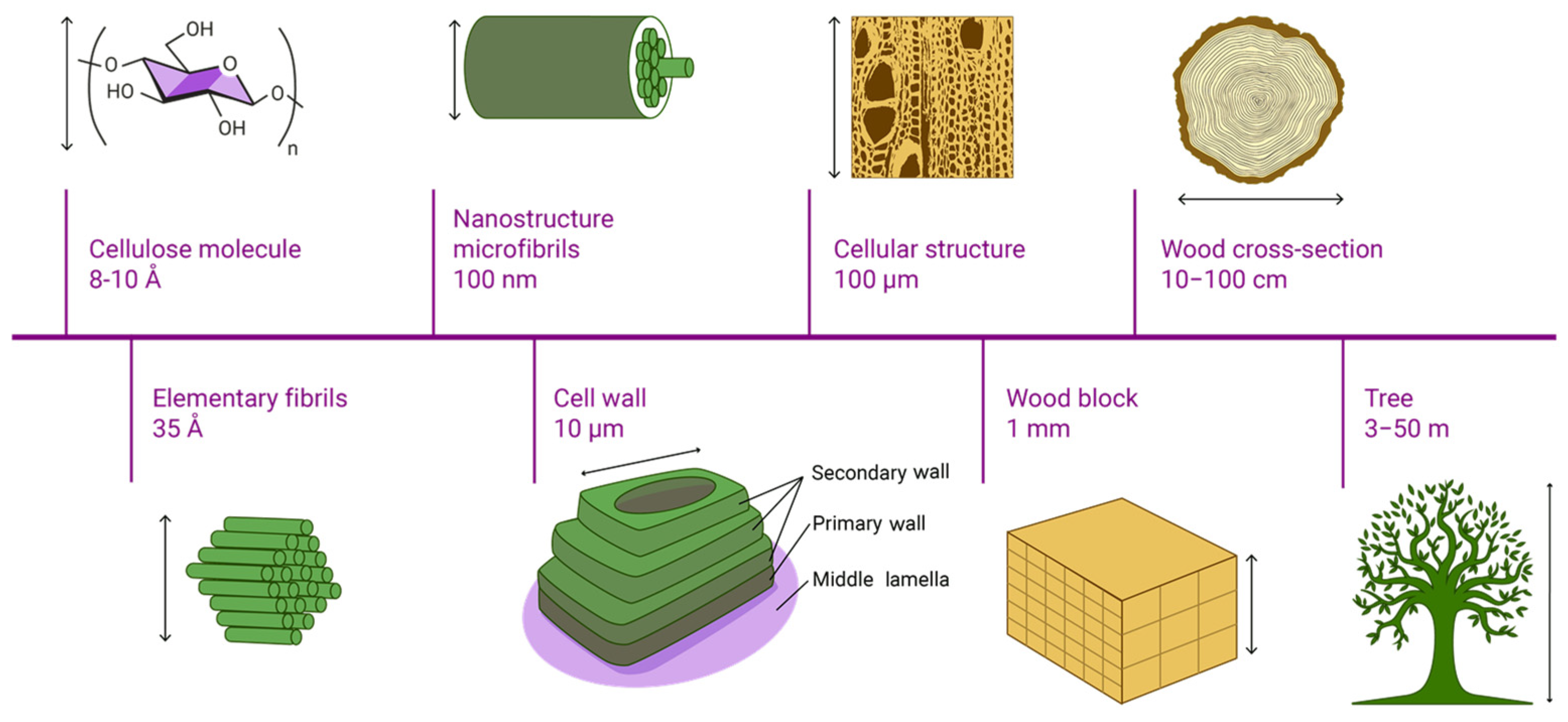
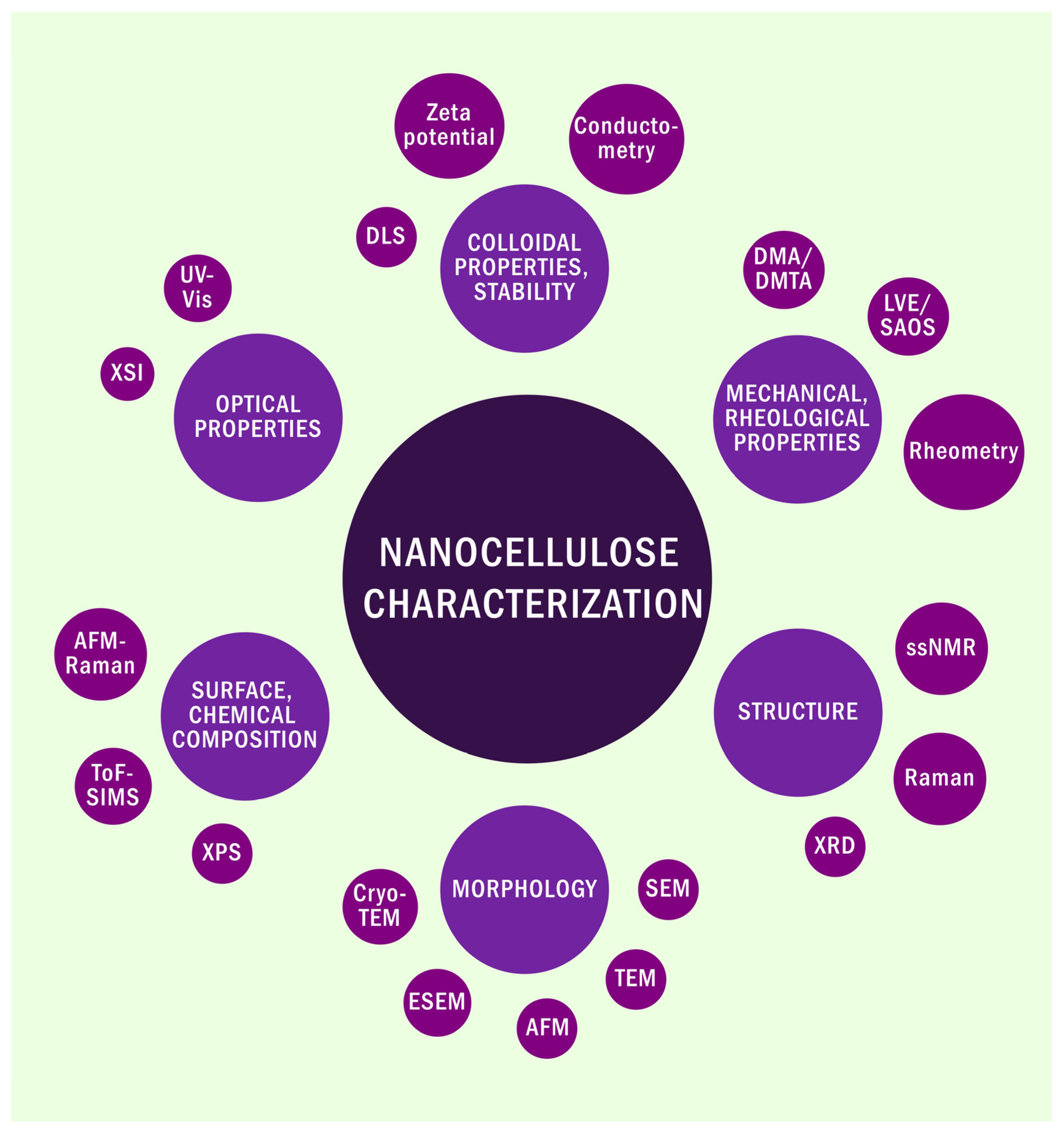
| Method | Primary Application | Information Provided |
|---|---|---|
| Scanning electron microscopy (SEM) | Morphology visualization | Surface morphology, fibril dimensions, and distribution |
| Transmission electron microscopy (TEM) | Morphology visualization | Dimensions, shape, crystalline structure |
| Atomic force microscopy (AFM) | Surface topography | Fibril dimensions, surface roughness |
| X-ray diffraction (XRD) | Crystallinity analysis, crystal structure | Crystallinity index (CrI), crystal phases, distinguishes amorphous/crystalline regions |
| X-ray photoelectron spectroscopy (XPS) | Surface chemistry analysis | Elemental composition, chemical bonding, functional groups |
| Infrared spectroscopy (FTIR) | Chemical composition | Identifies surface modifications/impurities |
| Nuclear magnetic resonance (NMR) | Molecular structure analysis | Crystalline vs. amorphous regions, surface chemistry |
| Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) | Thermal stability | Decomposition temperatures, thermal resistance |
| Type of Nanocellulose | Cellulose Nanofibers (CNFs) | Cellulose Nanocrystals (CNCs) | Bacterial Nanocellulose (BNC) |
|---|---|---|---|
| Synonyms | Cellulose nanofibrils (CNF), nanofibrillated cellulose (NFC) | Nanocrystalline cellulose (NCC), cellulose nanoparticles (CNPs), cellulose nanowhiskers (CNWs) | Cellulose nanoribbon (CNR) |
| Characteristics |
|
|
|
| Isolation Method |
|
|
|
| Yield | 70–90% | 30–50% | <60 g/L |
| Energy Demand | High (20–30 MWh/ton) | Low (chemical-driven) | Moderate (fermentation) |
| Main Application |
|
|
|
| Scalability | Industrial (CNF) | Pilot-scale | Lab-to-pilot |
| Type of Nanocellulose | Cellulose Nanofibrils (CNFs) | Cellulose Nanocrystals (CNCs) | Bacterial Nanocellulose (BNC) |
|---|---|---|---|
| Crystallinity | Low to moderate (20–60%) | High (60–90%) | High (80–90%) |
| Diameter | 5–100 nm | 3–20 nm | 20–100 nm |
| Length | Several micrometers | 100–500 nm | Continuous, web-like structure |
| Surface Area | High | ||
| Aspect Ratio | High | High | Low (web-like structure) |
| Flexibility | High (flexible and entangled) | Rigid (rod-shaped particles) | High (flexible, can form films) |
| Mechanical Strength | Moderate | High (rigid and strong) | Moderate to high |
| Water Holding Capacity | High | Moderate | Very high |
| Biodegradability | Biodegradable | ||
| Purity | Varies (depends on isolation method) | High (depending on hydrolysis conditions) | Very high (due to biosynthesis process) |
| Type of Nanocellulose | Cellulose Nanofibrils (CNFs) | Cellulose Nanocrystals (CNCs) | Bacterial Nanocellulose (BNC) |
|---|---|---|---|
| Isolation Methods |
|
|
|
| Characterization Methods |
|
|
|
| Product | Company | Country | Description | Implementation Stage | References |
|---|---|---|---|---|---|
| CNF | |||||
| Papira® | Stora Enso | Finland–Sweden | Wood-based foam for packaging was initially made from CNF, but due to challenges, the R&D team switched to pulp fibers, refining the Papira® formulation by 2019. | Commercial production (capacity is not mentioned in the open sources) | storaenso.com [62,63] |
| Exilva® | Borregaard | Norway | The product is made from microfibrillated cellulose derived from wood fibers and serves as an additive in resins and adhesives. It improves viscosity and substrate wetting, enhancing adhesive strength and sustainability, and provides resistance to harsh conditions (pH, temperature, and shear forces). | Commercial production of 50,000 tons of MFC dispersion per annum (or 1000 tons in dry matter) | borregaard.com [64,65,66] |
| FiberLean® | FiberLean Technologies | United Kingdom | Microfibrillated cellulose derived from wood pulp, used as a performance-enhancing additive in paper, packaging, coatings, and composites. | Commercial production of 1000–10,000 tons per annum | fiberlean.com [67,68,69,70] |
| Cellulose nanofiber | NIPPON PAPER INDUSTRIES CO., LTD. | Japan | Produced from wood pulp using the TEMPO catalytic oxidation method. Applications include reinforced plastics, food and cosmetics additives, and packaging materials. | Commercial production (capacity is not mentioned in the open sources) | nipponpapergroup.com [71,72] |
| FiloCell™ | Kruger Biomaterials Inc. | Canada | CNFs are derived from kraft wood pulp through a mechanical process and released in wet pulp, dry and dispersed fluff, or water suspension for packaging and coatings. | Commercial production of 6000 tons per annum for all the products | biomaterials.kruger.com [73] |
| Re:ancel™ T-CNF | ANPOLY Inc. | South Korea | TEMPO-modified cellulose nanofiber (CNF) at 2% consistency in water. Transparent, high-viscosity hydrogel material used for packaging, filtration, and medical biomaterials. | Commercial production (capacity is not mentioned in the open sources) | anpolyinc.com (based on the Isogai method [74]) |
| Suzano biofiber | Suzano | Brazil | CNF is made from eucalyptus cellulose pulp for personal care products, textiles, different types of paper, and other industries. | Commercial production (capacity is not mentioned in the open sources) | suzano.com.br [75] |
| Mechanical cellulose (lignocellulose, wood) nanofiber gels, oxidized cellulose nanofiber gel | Nano Novin Polymer Co. | Iran | Chemo-mechanically produced nanofiber gels (5–8%) are derived from wood and agricultural residues according to the customer’s order. | Custom-order production | nanonovin.com [76,77] |
| Curran® | CelluComp Ltd. | Scotland | CNF is derived from the residual pulp of root vegetables, such as sugar beets, through a proprietary extraction process. It is used in packaging and composite materials, offering performance characteristics comparable to carbon fiber. Curran®-based biocomposites can be formulated with conventional resins such as epoxy, polyurethane, and polyester. Its platelet structure enhances rheology in applications like paints. | Scaling production with ongoing collaboration | https://www.cellucomp.com [78] |
| CNC | |||||
| CelluRods ® | CelluForce Inc. | Canada | CNC is produced from wood pulp using mechanochemical processes for application in the oil and gas industry, coatings, packaging, and biopolymers. | Commercial production, 300 tons per annum | celluforce.com [79,80,81,82] |
| DextraCel | Anomera Inc. | Canada | CNC is available as an aqueous suspension or as dispersible powders. The surface contains carboxylate and hydroxyl groups. Applications include bioplastics, coatings, and adhesives. | Commercial production, 150+ tons per annum for all the products | anomera.ca [83,84] |
| MelOx™ VBcoat™ | Melodea Ltd. | Israel | A CNC-based coating that provides a sustainable barrier against oxygen and oil for packaging applications. | Commercial production (capacity is not mentioned in the open sources) | melodea.eu [85,86] |
| BioPlus® with AVAP® and BioPlus® with GreenBox® | GranBio Technologies (USA), a subsidiary of GranBio Investimentos S.A. (Brazil) | USA–Brazil | NC is extracted from wood, hemp, and agricultural residues. CNF and CNC have adjustable particle size and composition, ranging from hydrophilic pure cellulose to hydrophobic cellulose coated with lignin. The GreenBox process results in particularly low production costs. GranBio’s NC can be surface-modified to enhance compatibility with non-aqueous media, such as plastics and oils, and is offered in water-free masterbatch formulations. | Commercial production, ½ tons per day | granbio.com.br [87,88] |
| BGB Ultra ™ | Blue Goose Biorefineries Inc. | Canada | CNC is produced using a transition metal-catalyzed oxidation process on viscose-grade hardwood pulp and lignocellulosic biomass such as wood, hemp, flax, and wheat straw. The product is an aqueous suspension of type I cellulose nanocrystals that forms a gel at 8.0% w/w. | Pilot plant | bluegoosebiorefineries.com [89] |
| Nanolinter® | Nanolinter | Turkey | Lignocellulosic materials, including cotton plant-derived linter and paper pulp, are used for CNC production. It is applied as reinforcement material in composites, pharmaceuticals, cosmetics, chemicals, materials, and paints. | Custom-order production | nanolinter.com |
| Nanocrystacell | Navitas | Slovenia | Produced from tree cellulose, this material is available as either an aqueous suspension (2–5 wt.%) or a powder for adhesives, paper production, cement, and composite industries. | Custom-order production | nanocrystacell.eu |
| Bacterial nanocellulose | |||||
| Bacterial cellulose nanofiber sheet, oxidized bacterial cellulose nanofiber gel | Nano Novin Polymer Co. | Iran | The sheets are produced through bacterial synthesis in proper culture mediums followed by treating with chemical treatments. The oxidized gel contains 2% of the solid matter. | Custom-order production | nanonovin.com [90] |
| Biomaterial based on bacterial nanocellulose | Modern Synthesis Ltd. | United Kingdom | Nanocellulose materials are produced using bacteria; the product demonstrates excellent performance characteristics for applications in the fashion industry. | Scaling production, validated product–market fit, successful integration of materials into real products | modernsynthesis.com [91] |
| Biomaterial based on bacterial nanocellulose | Volkswagen Group Innovation | Germany | Biotechnological production of bacterial nanocellulose as a sustainable leather-like material for interior applications, using a fermentation-based process with bacterial cultures, yeast, and carbohydrates, followed by post-processing treatments. | Material optimization and experimental validation | volkswagen-group.com [92,93] |
| Biovolokno (biofiber) | byScoby | Russia | Biotechnological production of bacterial cellulose with post-cultivation processing to enhance mechanical properties, creating a sustainable alternative to leather and synthetic materials. | Limited commercial release; product introduced through brand partnerships, with initial market sales and further validation before scaling to full production | vk.com/byscoby [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofanica, B.-M.; Mikhailidi, A.; Fortună, M.E.; Rotaru, R.; Ungureanu, O.C.; Ungureanu, E. Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies. Crystals 2025, 15, 352. https://doi.org/10.3390/cryst15040352
Tofanica B-M, Mikhailidi A, Fortună ME, Rotaru R, Ungureanu OC, Ungureanu E. Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies. Crystals. 2025; 15(4):352. https://doi.org/10.3390/cryst15040352
Chicago/Turabian StyleTofanica, Bogdan-Marian, Aleksandra Mikhailidi, Maria E. Fortună, Răzvan Rotaru, Ovidiu C. Ungureanu, and Elena Ungureanu. 2025. "Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies" Crystals 15, no. 4: 352. https://doi.org/10.3390/cryst15040352
APA StyleTofanica, B.-M., Mikhailidi, A., Fortună, M. E., Rotaru, R., Ungureanu, O. C., & Ungureanu, E. (2025). Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies. Crystals, 15(4), 352. https://doi.org/10.3390/cryst15040352









