Tailoring Al-Doped ZnO Nanoparticles via Scalable High-Energy Ball Milling–Solid-State Reaction: Structural, Optical, and Dielectric Insights for Light-Activated Antimicrobial Defense Against Medical Device Pathogens
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. Measurements
2.3. Screening for Antimicrobial Activity Tests
2.4. Statistical Analyses
3. Results and Discussion
3.1. Physical Characterizations
3.1.1. Results of X-Ray Diffraction Analysis
3.1.2. Structural and Microstructural Analysis
3.1.3. Fourier-Transform Infrared Analysis
3.1.4. Absorption Spectrum of Samples
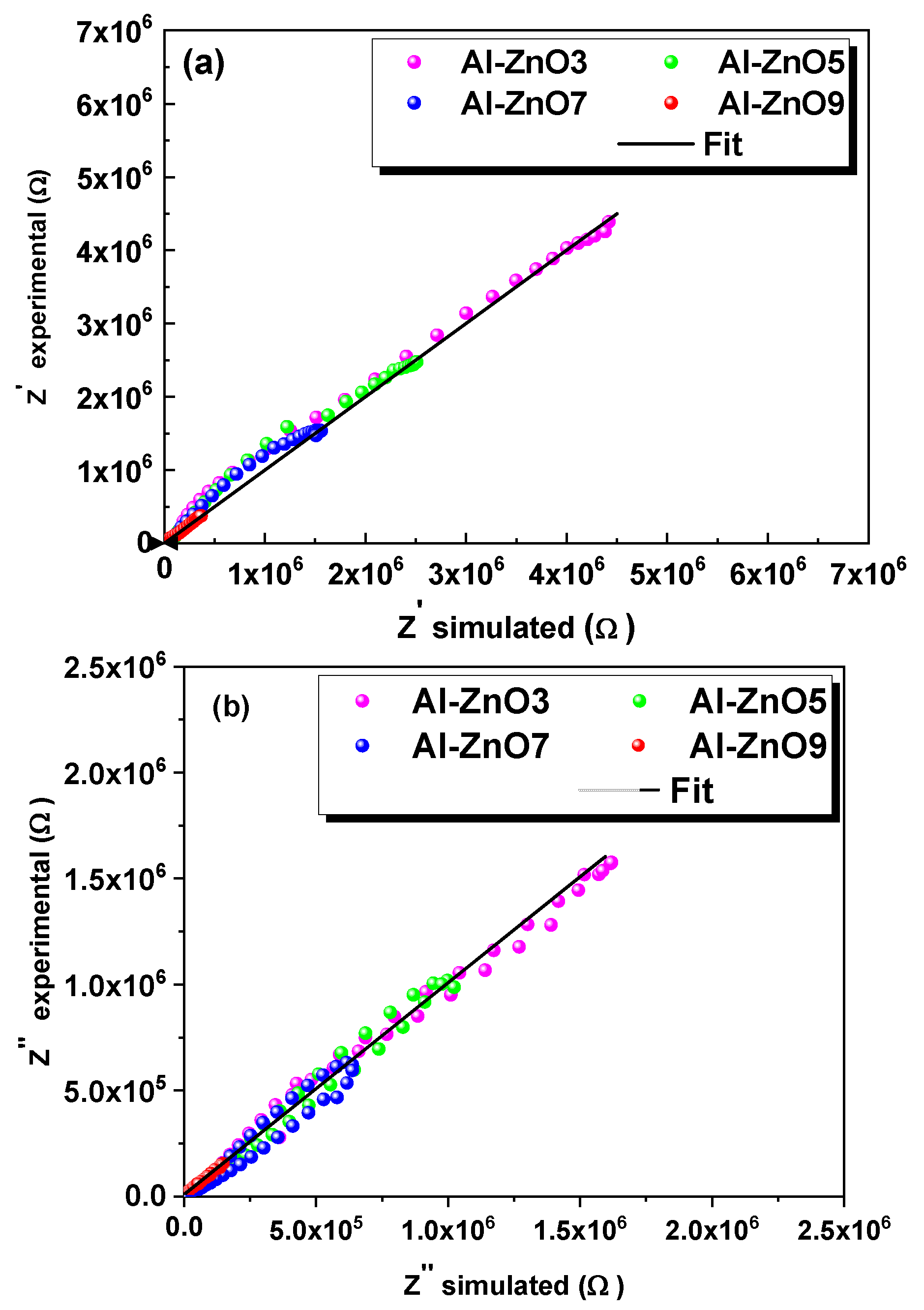
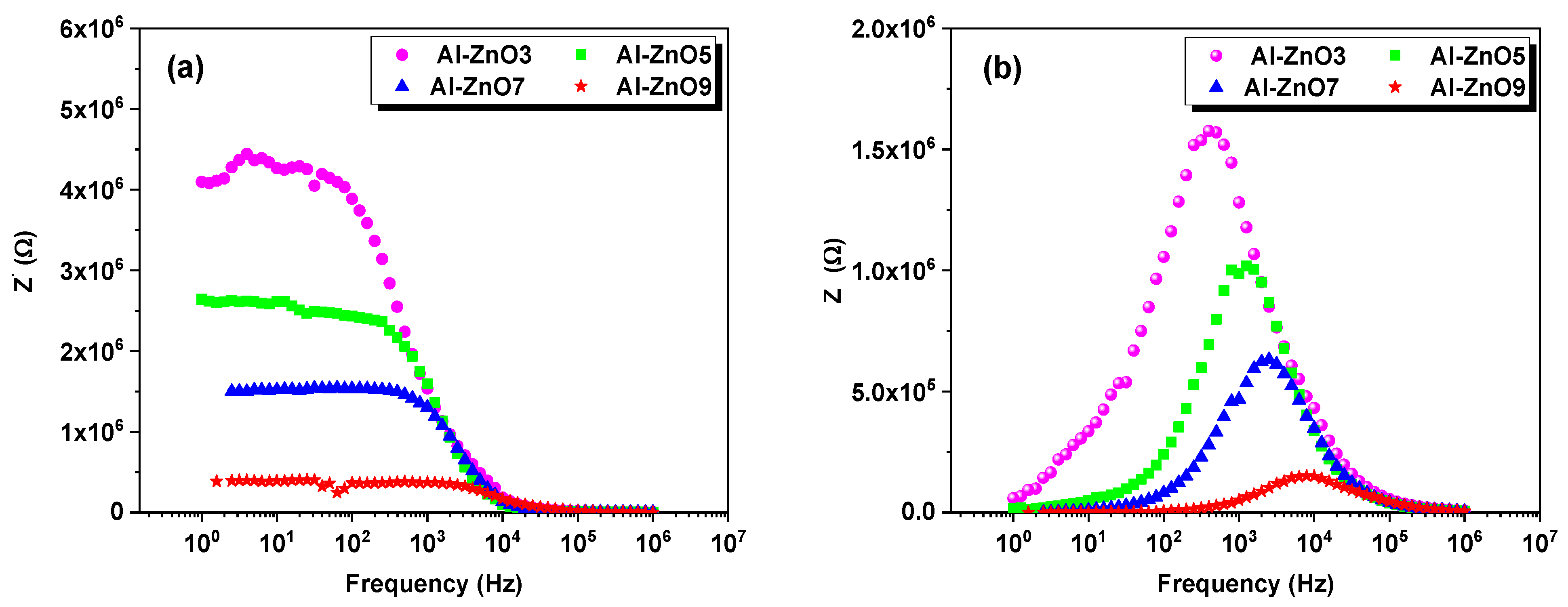
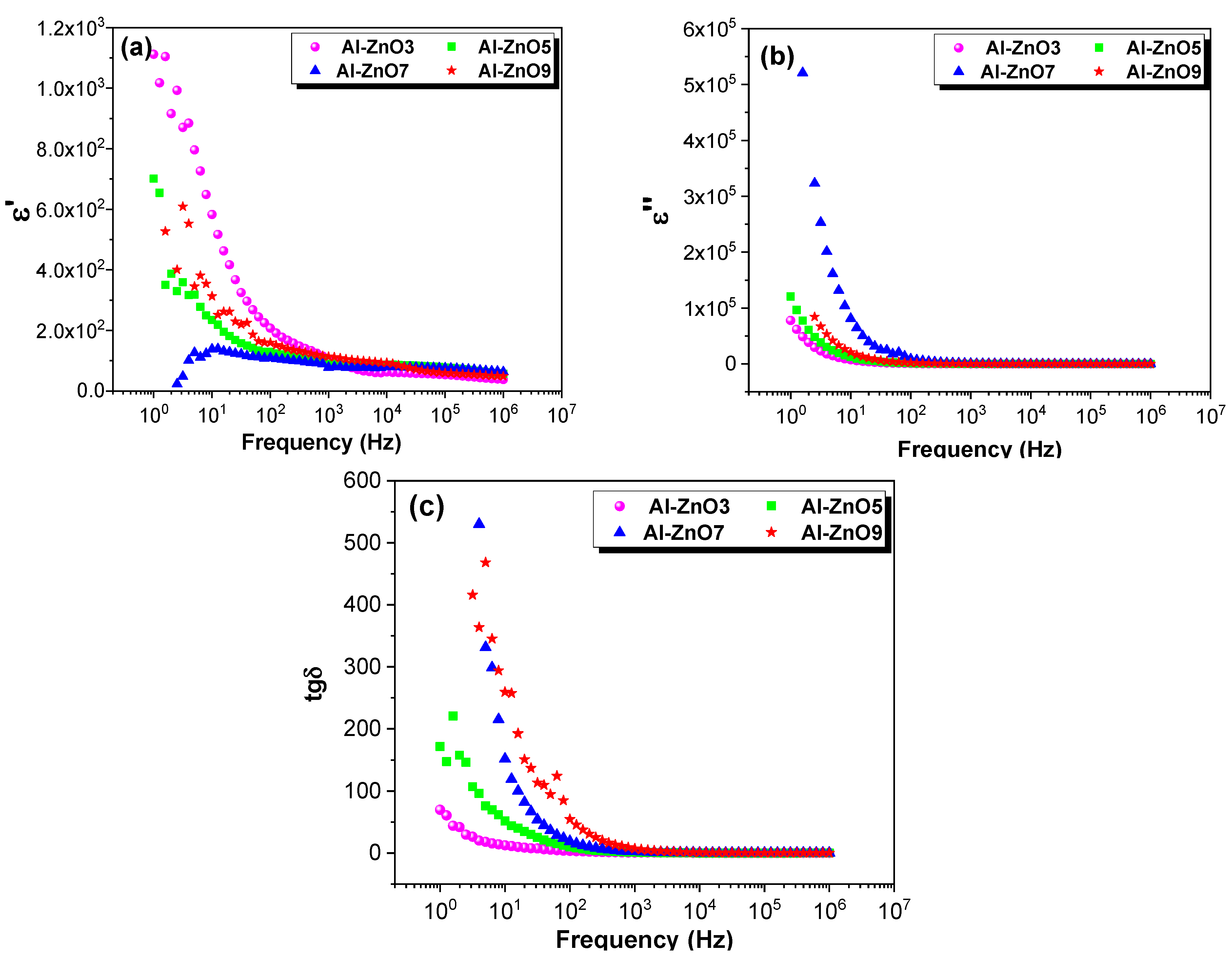
3.2. Dielectric and Electrical Properties
3.2.1. Electrical Analysis
3.2.2. Dielectric Constant
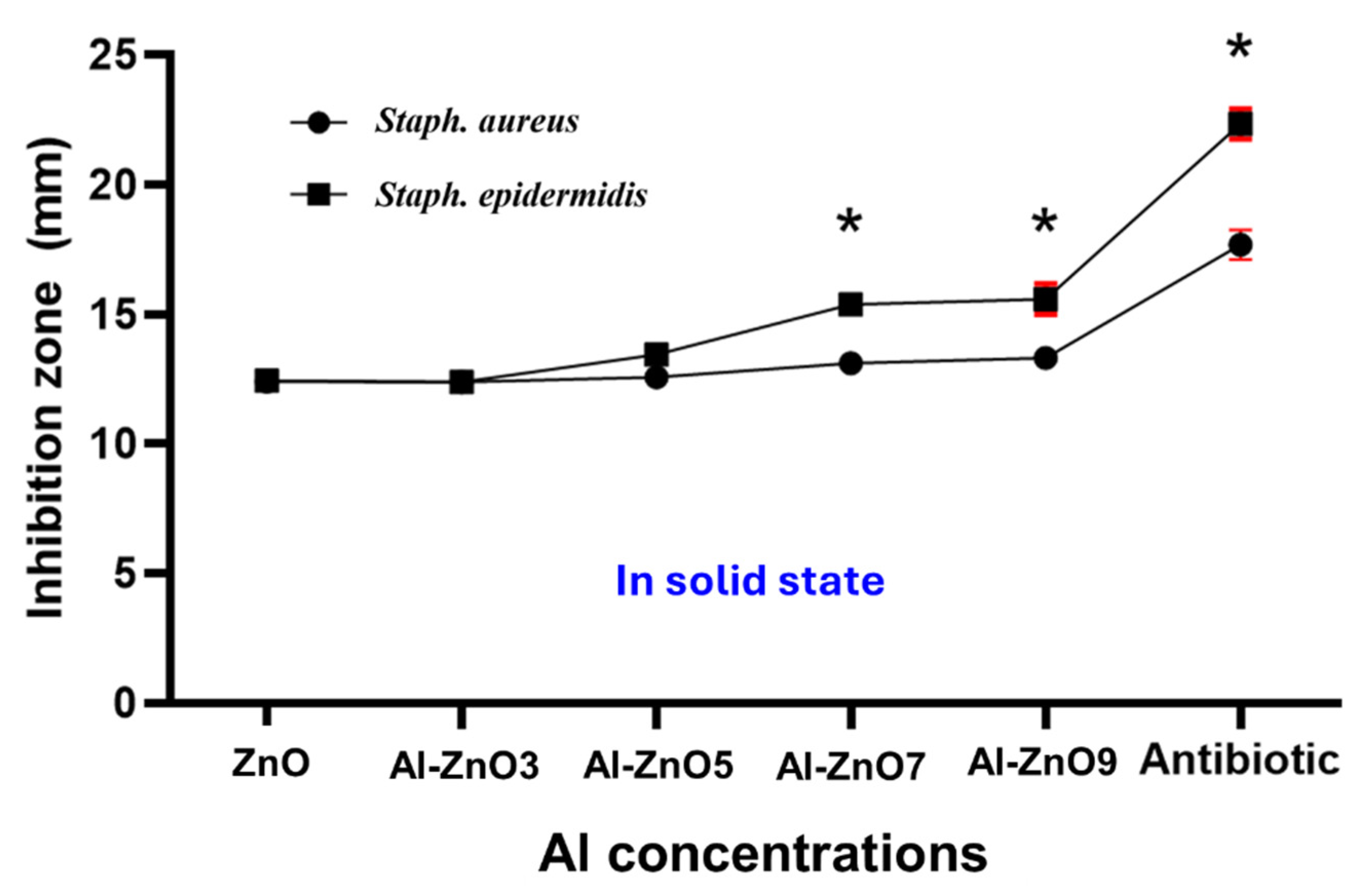
3.3. Screening for Antimicrobial Activity
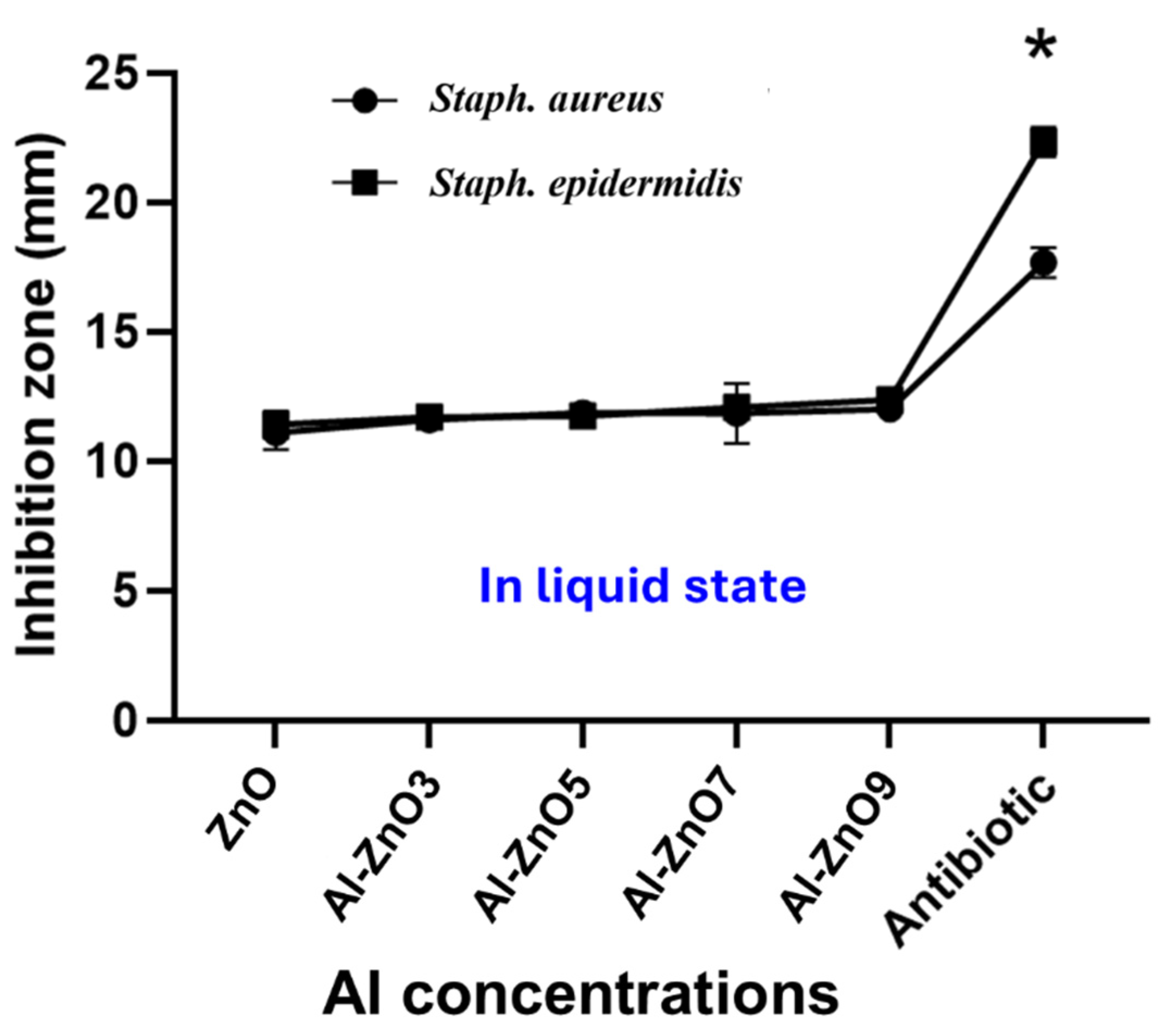
3.3.1. First Phase of Microbial Study: Doping Concentration, Microorganism Type, and Culture Medium State Parameters
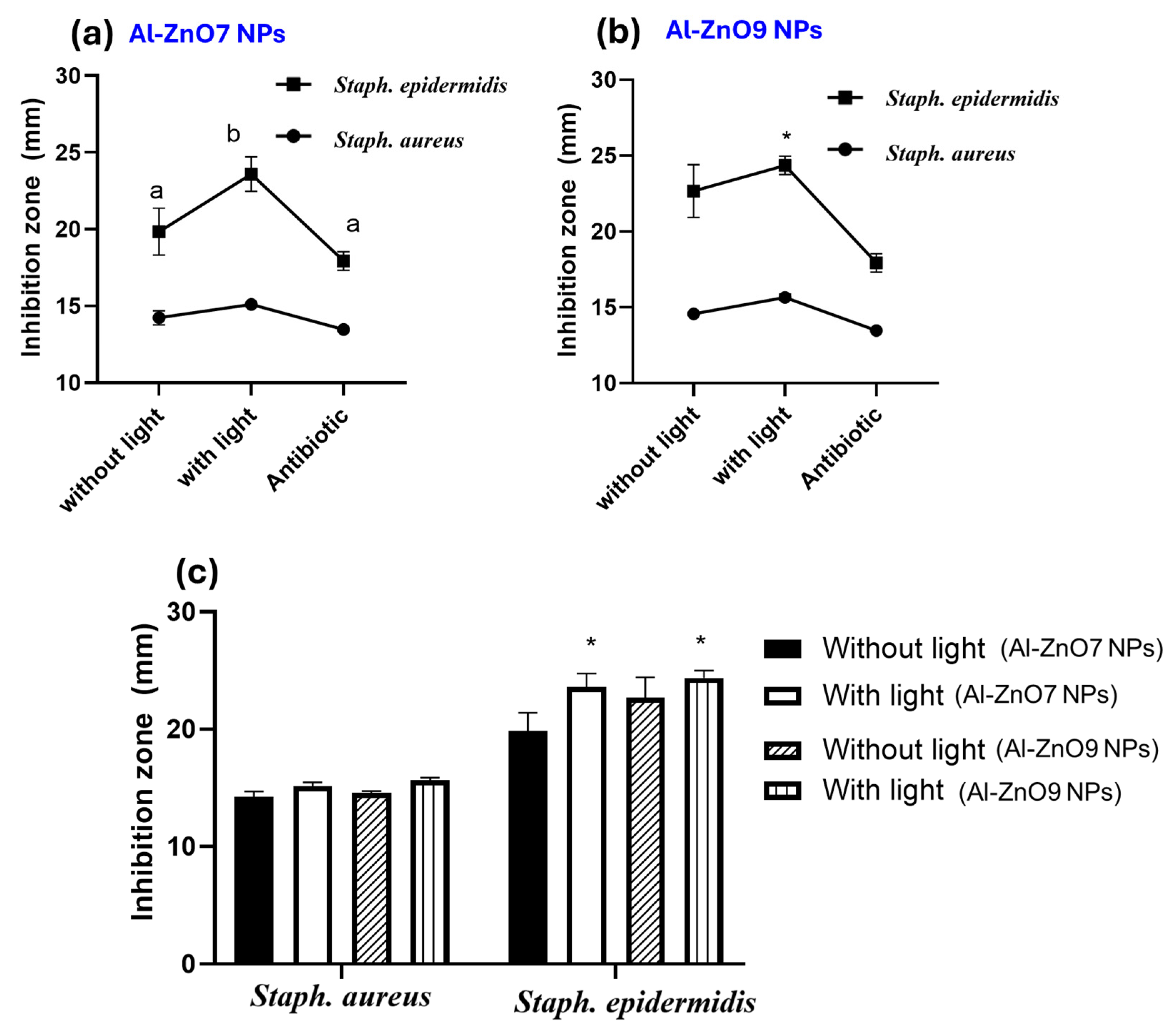
3.3.2. Second Phase of Microbial Study: Particle Size, Microorganism Type, and Exposure to Light/Dark Parameters
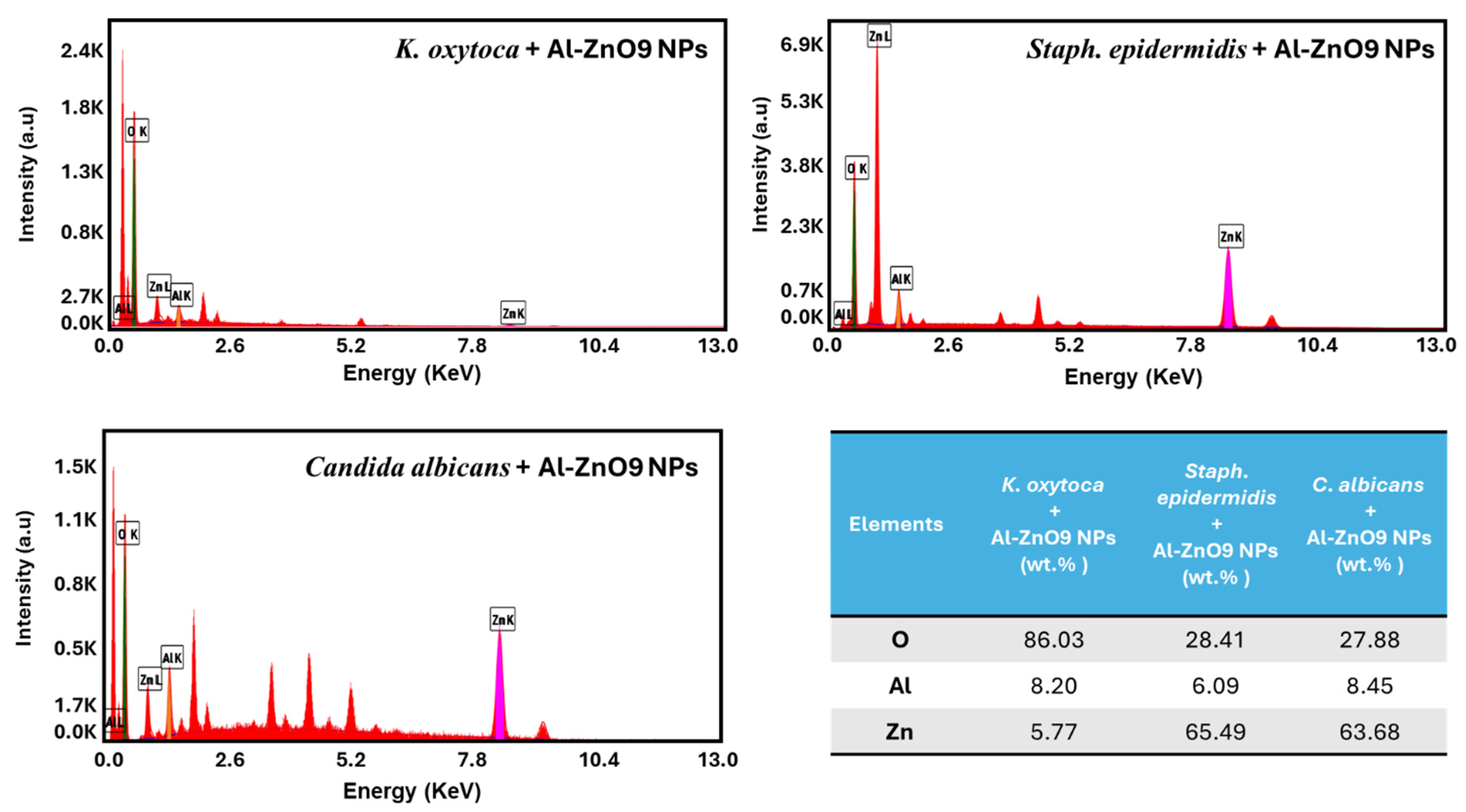
3.3.3. SEM and EDS Results
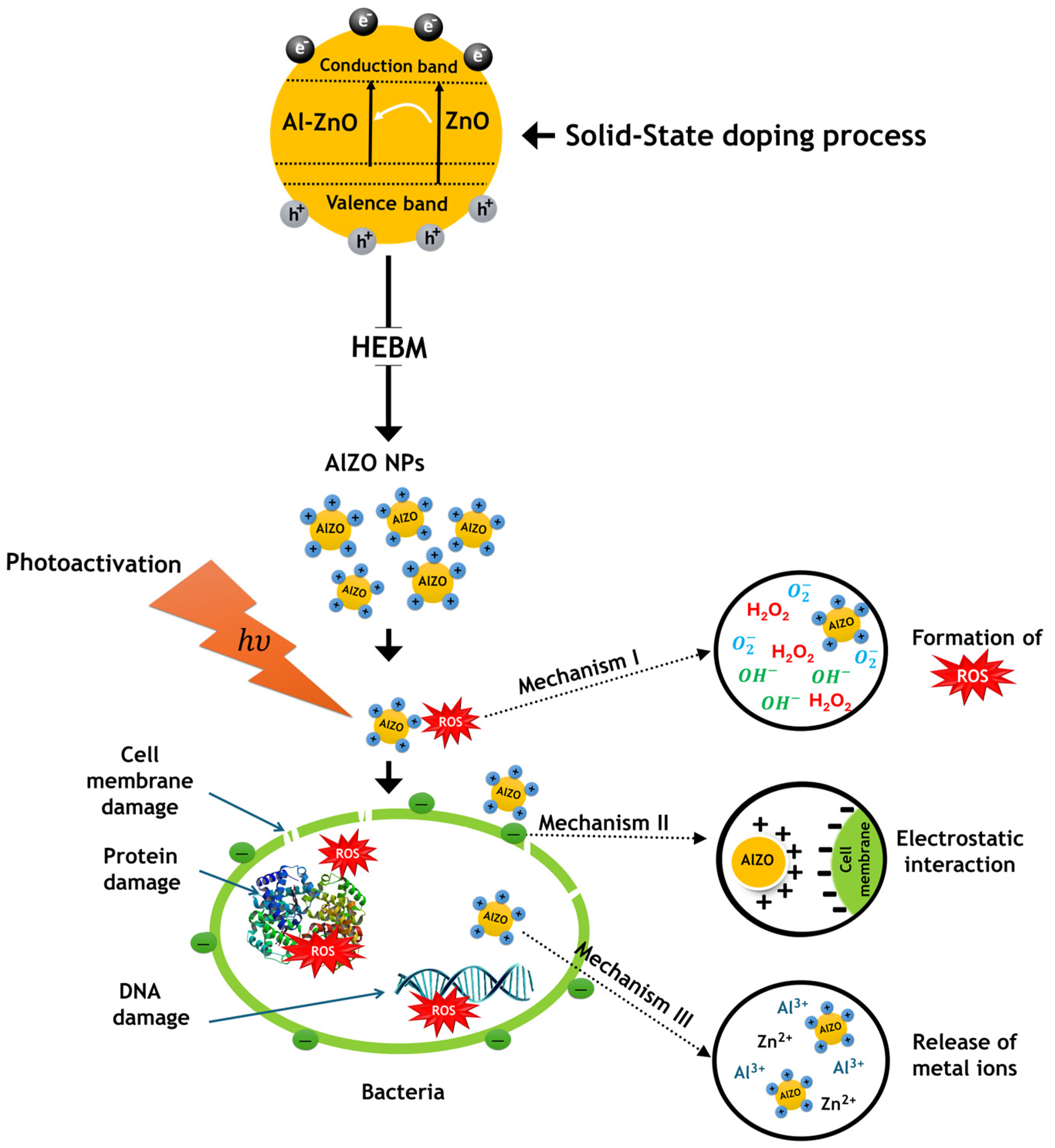
3.4. Elucidating the Relationship Between Physical Properties and Antimicrobial Efficacy via ROS Generation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M. The origins and molecular basis of antibiotic resistance. BMJ Br. Med. J. 1998, 317, 657. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Mohandoss, S.; Krishnamoorthi, R.; Bharathi, P.V.; Palanisamy, S.; Vinodhini, J. Enhanced bactericidal, antibiofilm and antioxidative response of Lawsonia inermis leaf extract synthesized ZnO NPs loaded with commercial antibiotic. Bioprocess. Biosyst. Eng. 2024, 47, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis, (n.d.). Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 1 August 2023).
- Franco-Paredes, C. Bloodstream Infections. In Core Concepts in Clinical Infectious Diseases (CCCID); Elsevier: Amsterdam, The Netherlands, 2016; pp. 9–15. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; Lens, P.N.L.; Balakrishnan, R.M. Microbial synthesis of chalcogenide semiconductor nanoparticles: A review. Microb. Biotechnol. 2016, 9, 11–21. [Google Scholar] [CrossRef]
- Manasreh, O. Introduction to Nanomaterials and Devices; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 466. Available online: https://books.google.com/books/about/Introduction_to_Nanomaterials_and_Device.html?id=v5srD63HPMMC (accessed on 3 February 2024).
- Mhetre, H.V.; Kanse, Y.K.; Patil, S.S. Nanomaterials: Applications in Electronics. Int. J. Adv. Eng. Nano Technol. 2021, 4, 7–19. [Google Scholar] [CrossRef]
- Nguyen, L.; Kho, R.; Bae, W.; Mehra, K. Glutathione as a matrix for the synthesis of CdS nanocrystallites. Chemosphere 1999, 38, 155–173. [Google Scholar] [CrossRef]
- Bawendi, M.G.; Steigerwald, M.L.; Brus, L.E. The Quantum Mechanics of Larger Semiconductor Clusters (“Quantum Dots”), Annu. Rev. Phys. Chem. 1990, 41, 477–496. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Rani, S.; Kumar, V.; Gupta, U. Zinc oxide nanoparticles for bioimaging and drug delivery. Nanostructured Zinc Oxide: Synthesis. Prop. Appl. 2021, 483–509. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- De La Rosa, E.; Yacaman, M.; Sun, L.; Downer, M.C.; Torres, L.A.D.; Salas, P. Second-harmonic imaging of ZnO nanoparticles. In Proceedings of the Conference on Lasers and Electro-Optics, Baltimore, MD, USA, 6–11 May 2007; Available online: https://opg.optica.org/abstract.cfm?uri=CLEO-2007-CTuA2 (accessed on 17 April 2025).
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 2010, 51, 799–890. [Google Scholar] [CrossRef]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro. Lett. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Putri, N.A.; Dwiputra, M.A.; Winata, S.M.; Isnaeni; Ginting, R.T.; Nugroho, F.A.A.; Fauzia, V. MoSe 2 Quantum Dots Enhance the Performance of ZnO Nanostructure-Based UV Photodetectors. ACS Appl. Nano Mater. 2024, 7, 3835–3842. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Jaballah, S.; Alaskar, Y.; Alshunaifi, I.; Ghiloufi, I.; Neri, G.; Bouzidi, C.; Dahman, H.; El Mir, L. Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles. Chemosensors 2021, 9, 300. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chang, S.J.; Ji, L.W.; Hsiao, Y.J.; Tang, I.T. Fast Detection and Flexible Microfluidic pH Sensors Based on Al-Doped ZnO Nanosheets with a Novel Morphology. ACS Omega 2019, 4, 19847. [Google Scholar] [CrossRef]
- Yoon, D.H.; Choi, G.M. Microstructure and CO gas sensing properties of porous ZnO produced by starch addition. Sens. Actuators. B Chem. 1997, 45, 251–257. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Sodium alanate system for efficient hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 8798–8812. [Google Scholar] [CrossRef]
- Massoudi, I.; Hamdi, R.; Ababutain, I.; Alhussain, E.; Kharma, A. HSBM-Produced Zinc Oxide Nanoparticles: Physical Properties and Evaluation of Their Antimicrobial Activity against Human Pathogens. Scientifica 2022, 2022, 9989282. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Memic, A.; Azam, A.; Alarfaj, E.; Zahed, N.; Al-Hamedi, S. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 2011, 6, 863–869. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B. Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin. J. Compos. Sci. 2022, 6, 188. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Anandh, B.A.; Sakthivel, R.; Ganesh, A.S.; Subramani, S.; Rajamanickam, A.T. Impact of aluminium on ZnO thin films for antimicrobial activity. Asian J. Chem. 2021, 33, 2393–2399. [Google Scholar] [CrossRef]
- Ali, D.; Butt, M.Z.; Muneer, I.; Bashir, F.; Hanif, M.; Khan, T.M.; Abbasi, S.A. Synthesis, characterization and antibacterial performance of transparent c-axis oriented Al doped ZnO thin films. Surf. Interfaces 2021, 27, 101452. [Google Scholar] [CrossRef]
- Vijayaraj, C.; Nedunchezhian, G.; Sozhaveni, S. Antibacterial Activity, Structural, Optical And Morphological Properties Of Al Doped Zno Nanoparticles Synthesized By Sol-Gel Method. Int. J. Sci. Technol. Res. 2020, 9, 2. Available online: www.ijstr.org (accessed on 17 April 2025).
- Khashan, K.S.; Sulaiman, G.M.; Hussain, S.A.; Marzoog, T.R.; Jabir, M.S. Synthesis, Characterization and Evaluation of Anti-bacterial, Anti-parasitic and Anti-cancer Activities of Aluminum-Doped Zinc Oxide Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3677–3693. [Google Scholar] [CrossRef]
- Chidhambaram, N. Augmented antibacterial efficacies of the aluminium doped ZnO nanoparticles against four pathogenic bacteria. Mater. Res. Express 2019, 6, 075061. [Google Scholar] [CrossRef]
- Saxena, V.; Pandey, L.M. Synthesis, characterization and antibacterial activity of aluminum doped zinc oxide. Mater. Today Proc. 2019, 18, 1388–1400. [Google Scholar] [CrossRef]
- Brintha, S.R.; Ajitha, M. Synthesis, Structural and Antibacterial Activity of Aluminium and Nickel Doped ZnO Nanoparticles by Sol-gel Method. Asian J. Chem. Sci. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Wirunchit, S.; Gansa, P.; Koetniyom, W. Synthesis of ZnO nanoparticles by Ball-milling process for biological applications. Mater. Today Proc. 2021, 47, 3554–3559. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97. [Google Scholar] [CrossRef]
- Li, N.; Yadav, S.K.; Wang, J.; Liu, X.Y.; Misra, A. Growth and Stress-induced Transformation of Zinc blende AlN Layers in Al-AlN-TiN Multilayers. Sci. Rep. 2015, 5, 18554. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Fullprof A Program for Rietveld Refinement and Pattern Matching Analysis. In Proceedings of the Abstract of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 16–19 July 1990; p. 127. Available online: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=868636 (accessed on 10 April 2025).
- Jantrasee, S.; Moontragoon, P.; Pinitsoontorn, S. Thermoelectric properties of Al-doped ZnO: Experiment and simulation. J. Semicond. 2016, 37, 092002. [Google Scholar] [CrossRef]
- Gherab, K.; Al-Douri, Y.; Voon, C.H.; Hashim, U.; Ameri, M.; Bouhemadou, A. Aluminium nanoparticles size effect on the optical and structural properties of ZnO nanostructures synthesized by spin-coating technique. Results Phys. 2017, 7, 1190–1197. [Google Scholar] [CrossRef]
- Dwibedi, D.; Murugesan, C.; Leskes, M.; Barpanda, P. Role of annealing temperature on cation ordering in hydrothermally prepared zinc aluminate (ZnAl2O4) spinel. Mater. Res. Bull. 2018, 98, 219–224. [Google Scholar] [CrossRef]
- Ghrib, T.; Ali, A.B.; Ercan, F.; Mhareb, M.H.A.; Al-muteliq, S.Y.; Ercan, I.; Kaygili, O.; Hamad, M.K.; Sfina, N.; Mhiri, M.; et al. Structural, optical, photoluminescence and radiation-shielding investigation of magnesium doped barium zirconate (BaZrO3) ceramics. Radiat. Phys. Chem. 2024, 223, 111976. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Ahammed, N.; Hassan, M.D.S.; Hassan, M. Effects of aluminum (Al) incorporation on structural, optical and thermal properties of ZnO nanoparticles. Mater. Sci.-Pol. 2018, 36, 419–426. [Google Scholar] [CrossRef]
- Ajala, F.; Hamrouni, A.; Houas, A.; Lachheb, H.; Megna, B.; Palmisano, L.; Parrino, F. The influence of Al doping on the photocatalytic activity of nanostructured ZnO: The role of adsorbed water. Appl. Surf. Sci. 2018, 445, 376–382. [Google Scholar] [CrossRef]
- Baradaran, M.; Ghodsi, F.E. Highly efficient visible photocatalytic degradation of MB organic dye by heteromorphic ZnO/AZO/ZnO nanocatalysts: Effect of AZO thickness. J. Solgel. Sci. Technol. 2019, 92, 25–39. [Google Scholar] [CrossRef]
- Chen, K.J.; Fang, T.H.; Hung, F.Y.; Ji, L.W.; Chang, S.J.; Young, S.J.; Hsiao, Y.J. The crystallization and physical properties of Al-doped ZnO nanoparticles. Appl. Surf. Sci. 2008, 254, 5791–5795. [Google Scholar] [CrossRef]
- Himabindu, B.; Devi, N.S.M.P.L.; Nagaraju, P.; Kanth, B.R. A nanostructured Al-doped ZnO as an ultra-sensitive room-temperature ammonia gas sensor. J. Mater. Sci. Mater. Electron. 2023, 34, 1014. [Google Scholar] [CrossRef]
- Srinatha, N.; Kumar, K.J.R.; Kumar, M.R.S.; Pattar, K.V.; Madhu, A.; Angadi, B. Combustion synthesis of Al-doped ZnO nanoparticles using a new fuel and the study of its structural, micro-structural and optical properties. Appl. Nanosci. 2021, 11, 1197–1209. [Google Scholar] [CrossRef]
- Mouzaia, F.; Djouadi, D.; Chelouche, A.; Hammiche, L.; Touam, T. Structural and optical investigations of pure and Al-doped ZnO nano-aerogels: Effects of supercritical organic solvent. Appl. Phys. A Mater. Sci. Process 2019, 125, 637. [Google Scholar] [CrossRef]
- Sankar Ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Vadivel, M.; Babu, R.R.; Ramamurthi, K. Studies on the Structural, Optical and Magnetic Properties of Al doped ZnO Nanoparticles. Int. J. Chem. Tech. Res. 2014, 7, 1206–1211. [Google Scholar]
- Vanaja, A.; Ramaraju, G.V.; Rao, K.S. Structural and optical investigation of Al doped ZnO nanoparticles synthesized by Sol-gel process. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Kim, K.H.; Umakoshi, T.; Abe, Y.; Kawamura, M.; Kiba, T. Morphological properties of Al-doped ZnO nano/microstructures. Superlattices Microstruct. 2016, 91, 188–192. [Google Scholar] [CrossRef]
- Mallika, A.N.; Ramachandrareddy, A.; Sowribabu, K.; Reddy, K.V. Synthesis and optical characterization of aluminum doped ZnO nanoparticles. Ceram. Int. 2014, 40, 12171–12177. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Kumar, S.; Khan, M.N.; Ahamed, M.; Al Dwayyan, A.S. Microstructure and blueshift in optical band gap of nanocrystalline Al xZn1-xO thin films. J. Lumin. 2014, 155, 275–281. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, M.A.M.; Ahamed, M. Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci. Rep. 2015, 5, 13876. [Google Scholar] [CrossRef] [PubMed]
- Ambedkar, A.K.; Singh, M.; Kumar, V.; Kumar, V.; Singh, B.P.; Kumar, A.; Gautam, Y.K. Structural, optical and thermoelectric properties of Al-doped ZnO thin films prepared by spray pyrolysis. Surf. Interfaces 2020, 19, 100504. [Google Scholar] [CrossRef]
- Manohar, A.; Krishnamoorthi, C.; Naidu, K.C.B.; Narasaiah, B.P. Dielectric, Magnetic Hyperthermia and Photocatalytic Properties of Mg.Zn.FeO Nanocrystals. IEEE Trans. Magn. 2020, 56, 1–7. [Google Scholar] [CrossRef]
- Jebli, M.; Rayssi, C.; Dhahri, J.; Khirouni, K. Investigation of electrical properties and conduction mechanism using CBH model of Ba0.97La0.02Ti1−xNb4x/5O3 (x = 0.00 and 0.02) compounds. ApPhA 2020, 126, 109. [Google Scholar] [CrossRef]
- Chtourou, R.; Louati, B.; Guidara, K. Structural and ac conductivity studies of sodium tetralead triphosphate compound. J. Alloys Compd. 2018, 732, 286–292. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H. Effect of Sb doping on the electrical and dielectric properties of ZnO nanocrystals. Ceram. Int. 2019, 45, 8000–8007. [Google Scholar] [CrossRef]
- Larbi, T.; Ouni, B.; Boukachem, A.; Boubaker, K.; Amlouk, M. Electrical measurements of dielectric properties of molybdenum-doped zinc oxide thin films. Mater. Sci. Semicond. Process 2014, 22, 50–58. [Google Scholar] [CrossRef]
- Rhimi, T.; Toumi, M.; Khirouni, K.; Guermazi, S. AC conductivity, electric modulus analysis of KLi(H2PO4)2 compound. J. Alloys Compd. 2017, 714, 546–552. [Google Scholar] [CrossRef]
- Salah, M.; Azizi, S.; Boukhachem, A.; Khaldi, C.; Amlouk, M.; Lamloumi, J.; Salah, M.; Azizi, S.; Boukhachem, A.; Khaldi, C.; et al. Rietveld refinement of X-ray diffraction, impedance spectroscopy and dielectric relaxation of Li-doped ZnO-sprayed thin films. ApPhA 2019, 125, 615. [Google Scholar] [CrossRef]
- Jebli, M.; Rayssi, C.; Hamdaoui, N.; Rabaoui, S.; Dhahri, J.; Henda, M.B.; Shaarany, I. Effect of Nb-doping on the structural and electrical properties of Ba0.97La0.02Ti1−xNb4x/5O3 ceramics at room temperature synthesized by molten-salt method. J. Alloys Compd. 2019, 784, 204–212. [Google Scholar] [CrossRef]
- Selvi, K.T.; Mangai, K.A.; Priya, M.; Sagadevan, S. Investigation of the dielectric and impedance properties of ZnO/MgO nanocomposite. Phys. B Condens. Matter 2020, 594, 412355. [Google Scholar] [CrossRef]
- Kaur, D.; Bharti, A.; Sharma, T.; Madhu, C. Dielectric Properties of ZnO-Based Nanocomposites and Their Potential Applications. Int. J. Opt. 2021, 2021, 9950202. [Google Scholar] [CrossRef]
- Mujahid, M. Synthesis, characterization and electrical properties of visible-light-driven Pt-ZnO/CNT. Bull. Mater. Sci. 2015, 38, 995–1001. [Google Scholar] [CrossRef]
- Kilinç, N.; Arda, L.; Öztürk, S.; Öztürk, Z.Z. Structure and electrical properties of Mg-doped ZnO nanoparticles. Cryst. Res. Technol. 2010, 45, 529–538. [Google Scholar] [CrossRef]
- Naik, C.C.; Salker, A.V. Tailoring magnetic and dielectric properties of Co0.9Cu0.1Fe2O4 with substitution of small fractions of Gd3+ ions. J. Mater. Sci. Mater. Electron. 2018, 29, 5380–5390. [Google Scholar] [CrossRef]
- Tabib, A.; Sdiri, N.; Elhouichet, H.; Férid, M. Investigations on electrical conductivity and dielectric properties of Na doped ZnO synthesized from sol gel method. J. Alloys Compd. C 2015, 622, 687–694. [Google Scholar] [CrossRef]
- Horne, J.E.; Brockwell, D.J.; Radford, S.E. Role of the lipid bilayer in outer membrane protein folding in Gram-negative bacteria. J. Biol. Chem. 2020, 295, 10340. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Smitha, M.S.; Singh, S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef]
- Jan, T.; Azmat, S.; Rahman, A.U.; Ilyas, S.Z.; Mehmood, A. Experimental and DFT study of Al doped ZnO nanoparticles with enhanced antibacterial activity. Ceram. Int. 2022, 48, 20838–20847. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, X.; Ma, L.; Shi, Y.; Zhang, D.; Qu, K. Analytical methods for assessing antimicrobial activity of nanomaterials in complex media: Advances, challenges, and perspectives. J. Nanobiotechnol. 2023, 21, 97. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M.U. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Zeid, S.A.; Perez, A.; Bastide, S.; Le Pivert, M.; Rossano, S.; Remita, H.; Hautière, N.; Leprince-Wang, Y. Antibacterial and Photocatalytic Properties of ZnO Nanostructure Decorated Coatings. Coatings 2023, 14, 41. [Google Scholar] [CrossRef]
- Rigby, K.M.; DeLeo, F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2011, 34, 237–259. [Google Scholar] [CrossRef]
- Mandell, G.L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J. Clin. Investig. 1975, 55, 561–566. [Google Scholar] [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR study of visible light-induced ros generation by nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišic, A.B.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; de Moraes Ruy Sapata, V.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Jiang, L.; Loo, S.C.J. Intelligent Nanoparticle-Based Dressings for Bacterial Wound Infections. ACS Appl. Bio Mater. 2021, 4, 3849–3862. [Google Scholar] [CrossRef]
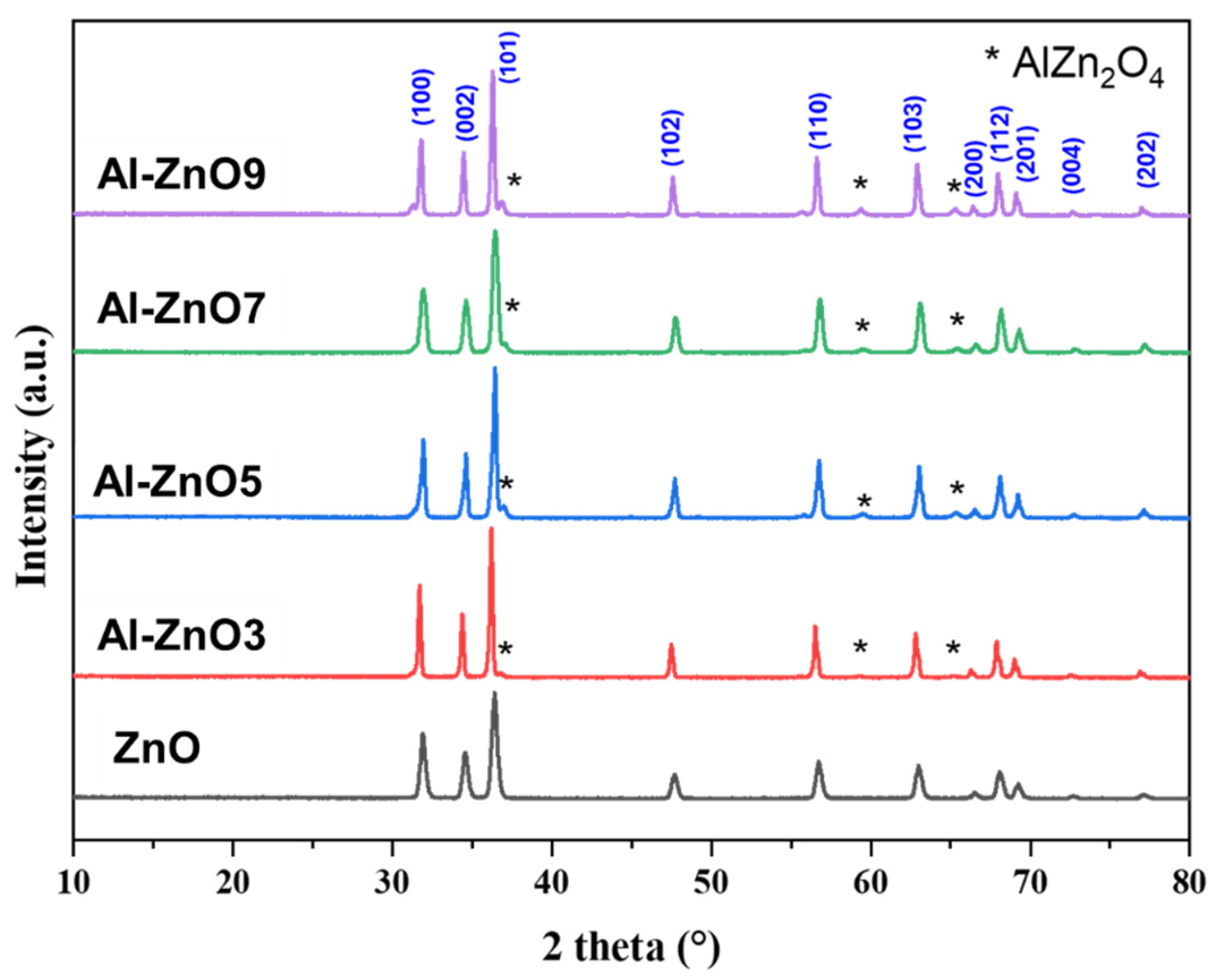

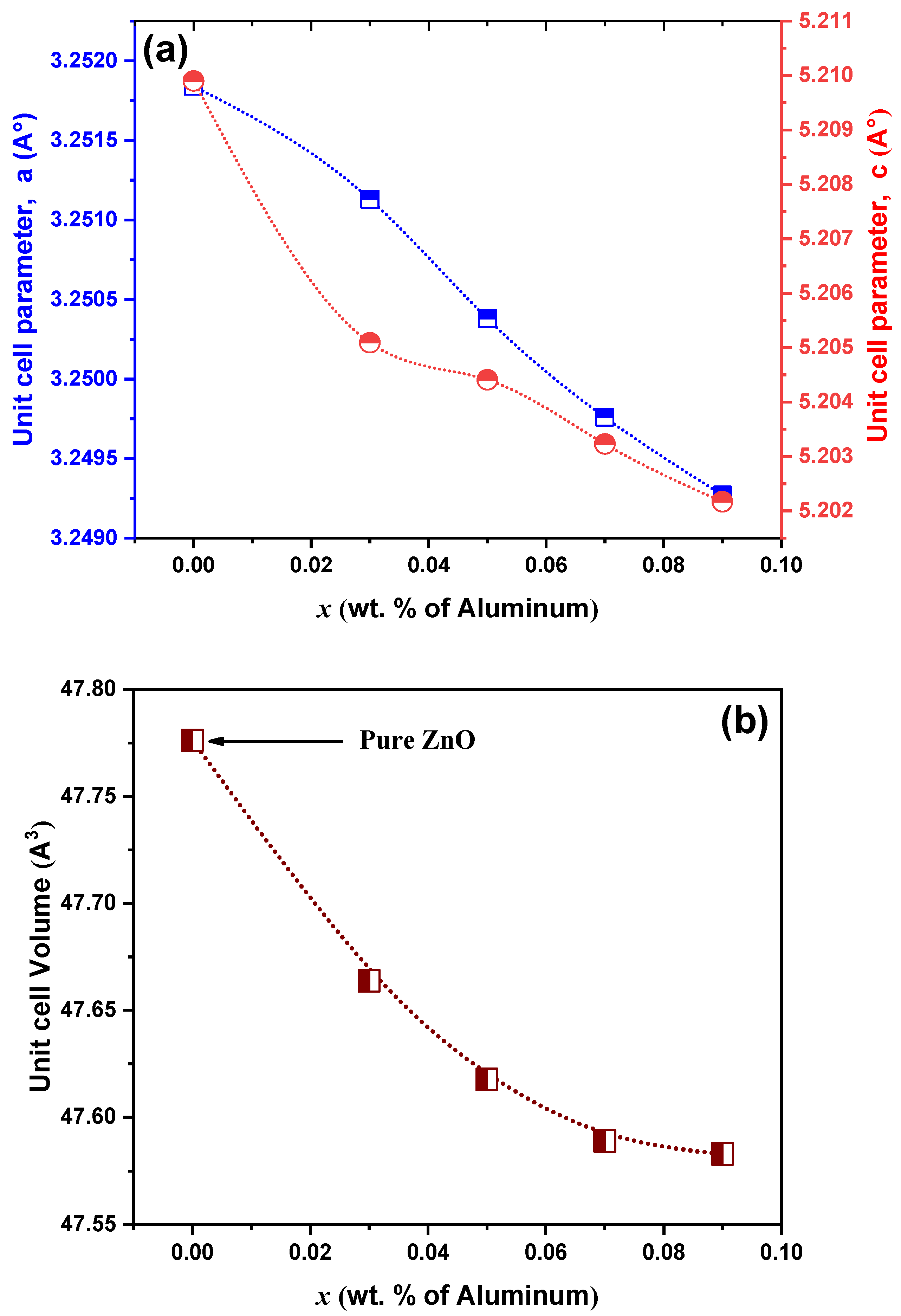
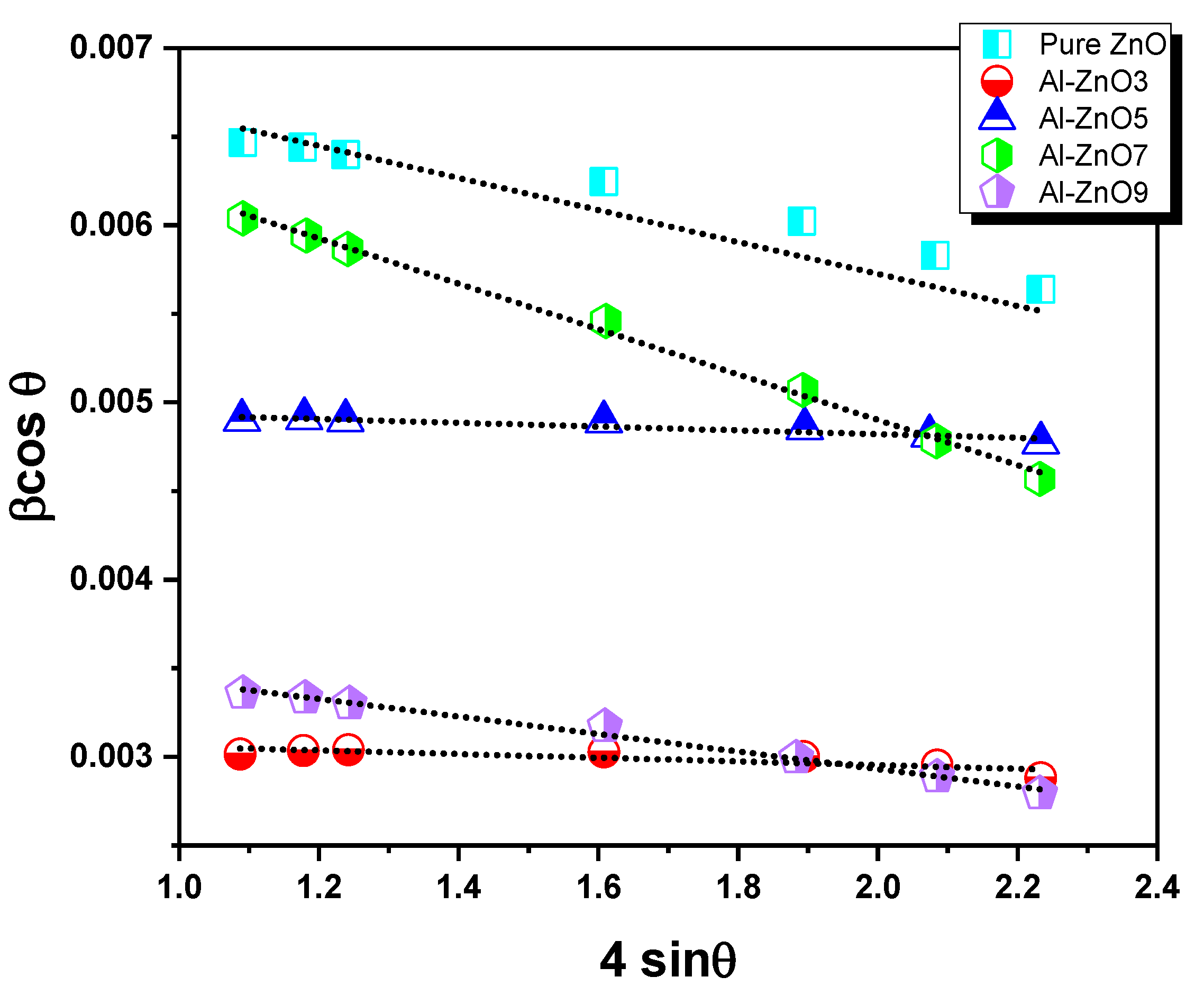


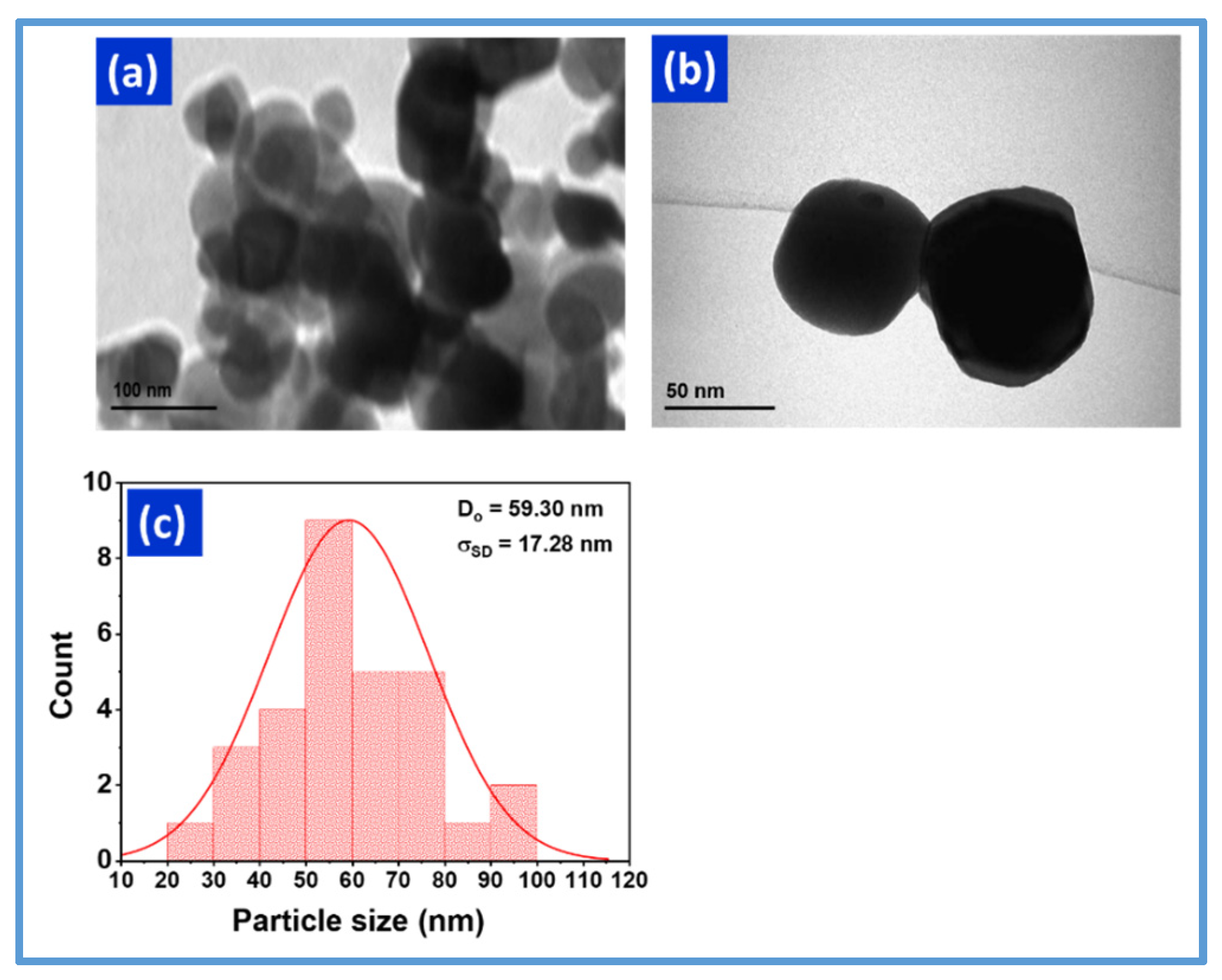
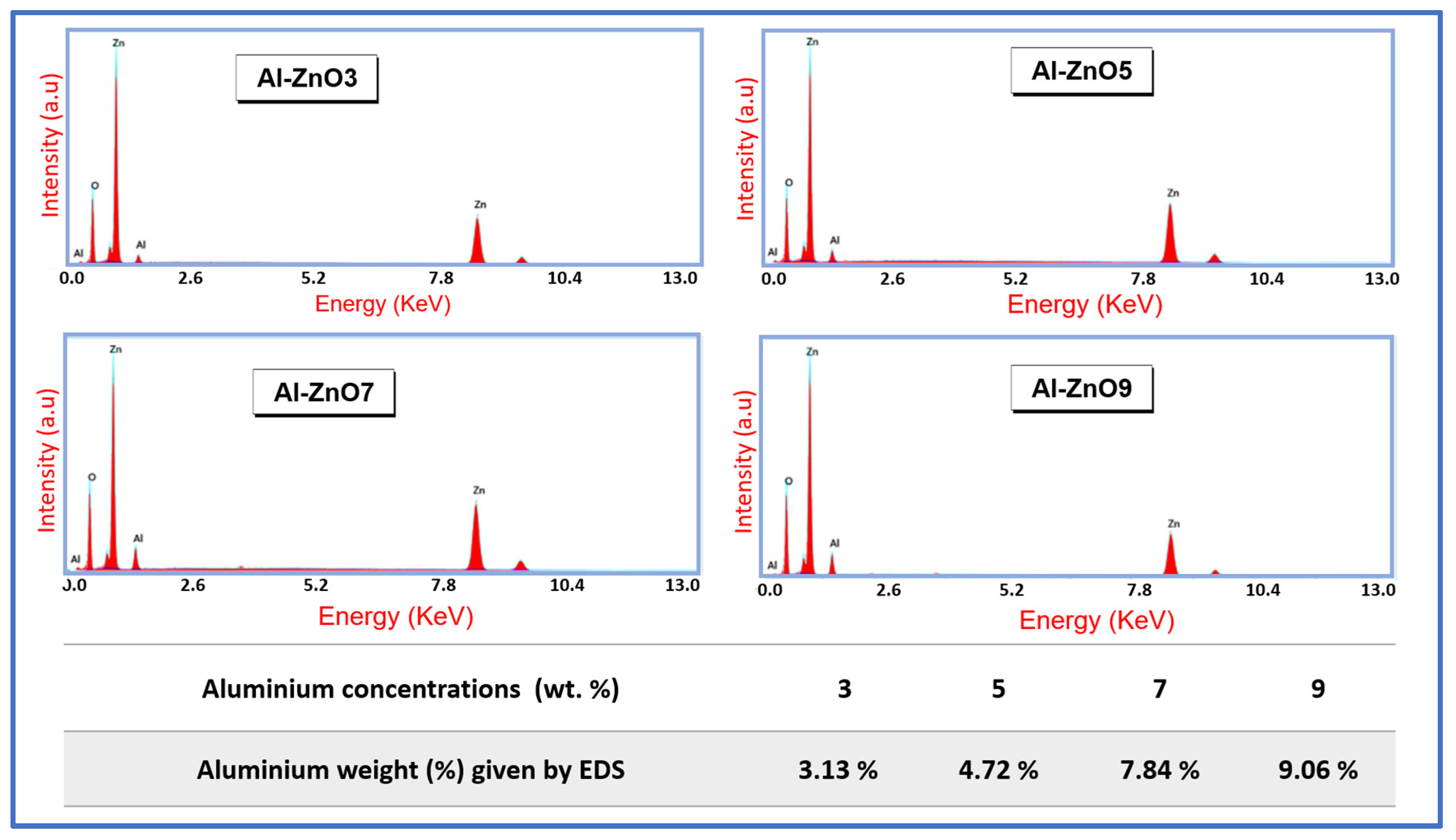
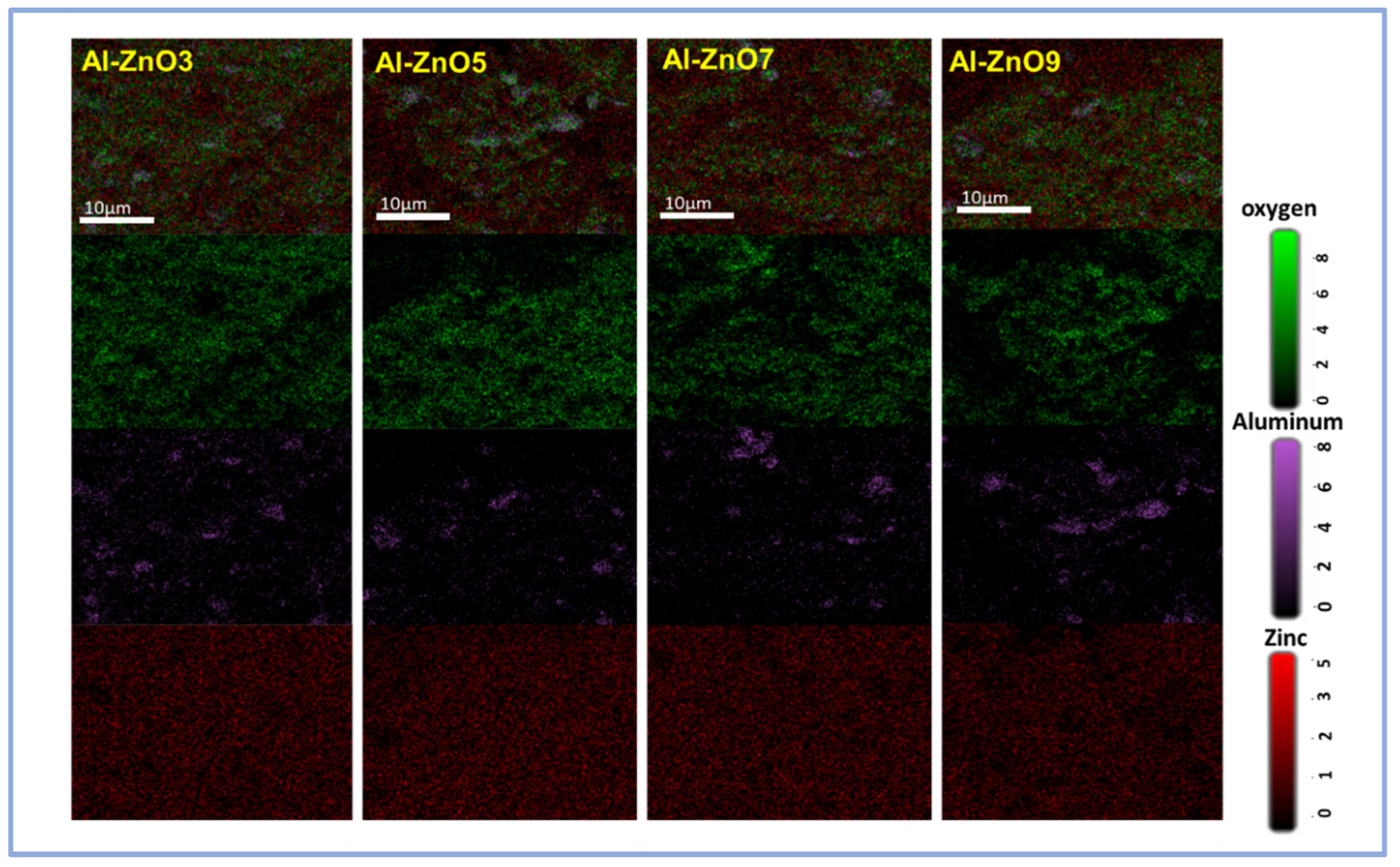

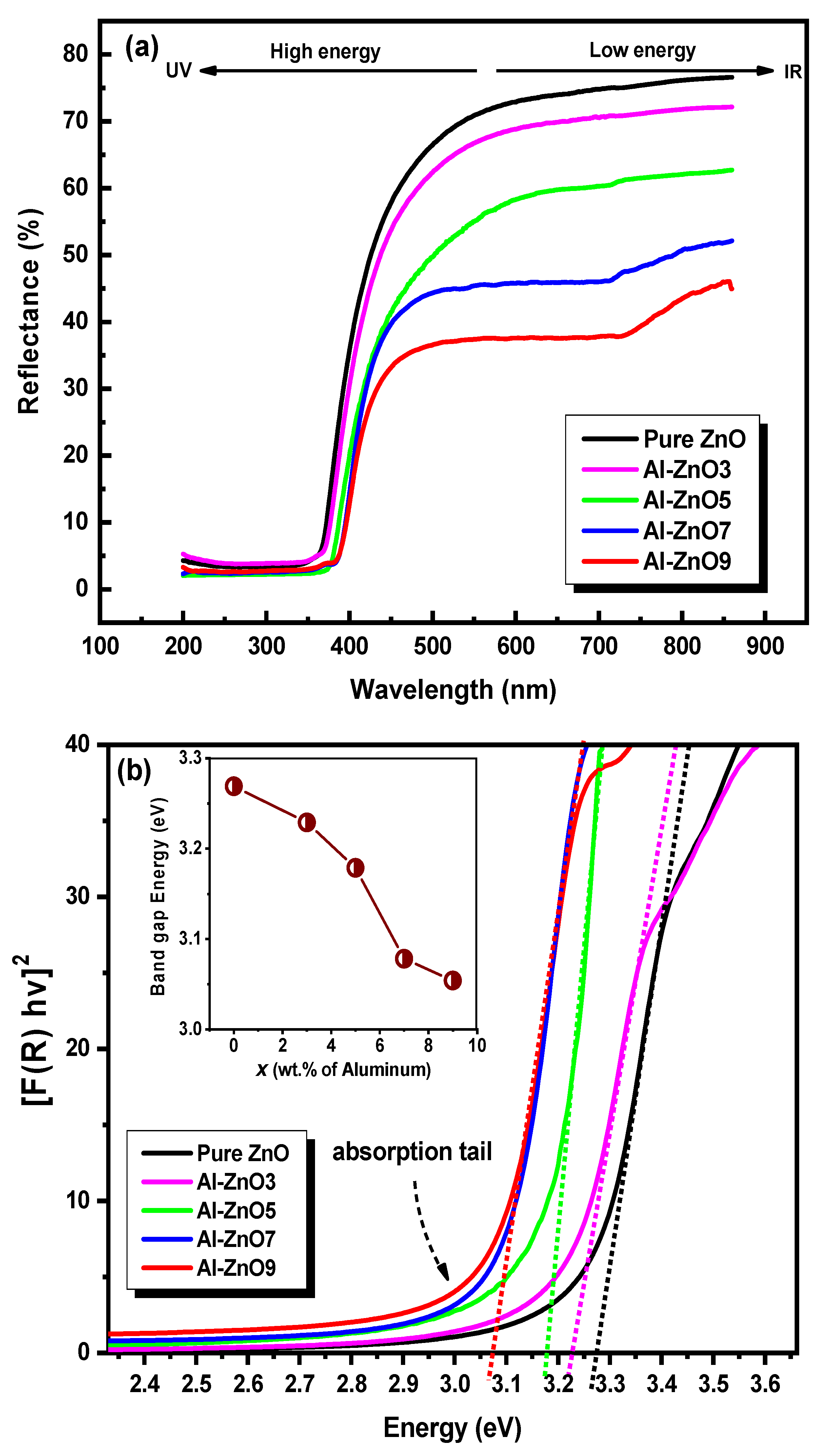
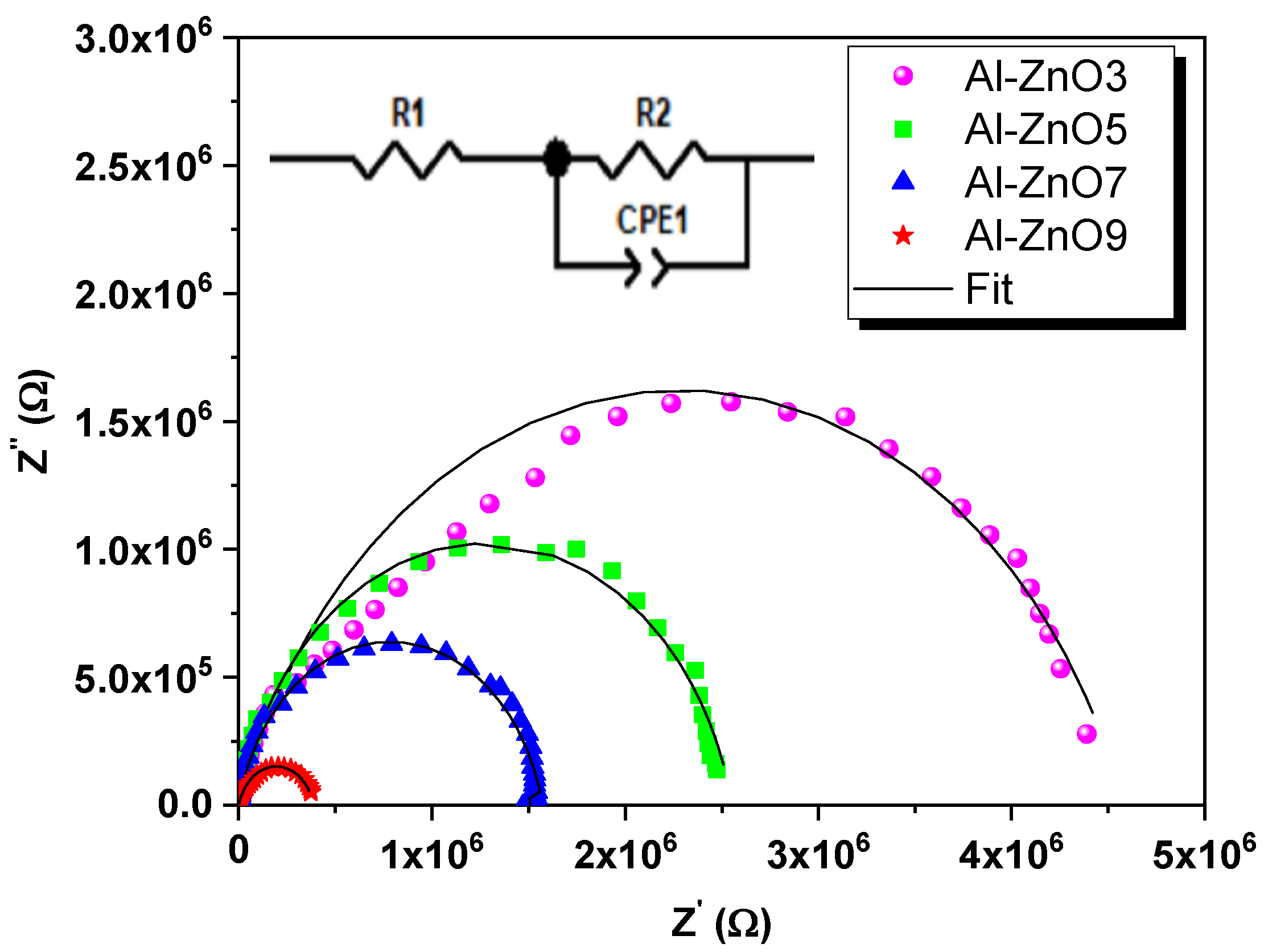


| Year | Reference | Synthesis Method | Doping Concentration | Microbes | Type of Tested Microbes | Studied Parameters |
|---|---|---|---|---|---|---|
| 2022 | Anandh et al. [30] | SILAR technique | 1 wt.% 3 wt.% 5 wt.% | Escherichia coli Staphylococcus aureus Aspergillus niger | Gram-negative bacterium Gram-positive bacterium A filamentous fungus | Doping concentration Type of microbe |
| 2021 | Ali et al. [31] | Spray pyrolysis technique | 0 wt.% 0.5 wt.% 1.25 wt.% 2.25 wt.% 7.5 wt.% | Escherichia coli | Gram-negative bacterium | Doping concentration |
| 2020 | Vijayaraj et al. [32] | Sol–gel method | 1 wt.% 2 wt.% 3 wt.% 4 wt.% | Escherichia coli Staphylococcus aureus Aspergillus flavus Aspergillus niger | Gram-negative bacterium Gram-positive bacterium A filamentous fungus A filamentous fungus | Doping concentration Type of microbe |
| 2020 | Khashan et al. [33] | Laser ablation method | 0.2 wt.% 0.27 wt.% 0.33 wt.% 0.42 wt.% | Escherichia coli Staphylococcus aureus | Gram-negative bacterium Gram-positive bacterium | Doping concentration Type of microbe |
| 2019 | Chidhambaram et al. [34] | Wet chemical route | 1 wt.% 3 wt.% 5 wt.% | Escherichia coli Proteus mirabilis Enterococcus faecalis Staphylococcus aureus | Gram-negative bacterium Gram-negative bacterium Gram-positive bacterium Gram-positive bacterium | Doping concentration Type of microbe |
| 2019 | Saxena et al. [35] | Co-precipitation method | 15 wt.% | Escherichia coli Enterococcus hirae | Gram-negative bacterium Gram-positive bacterium | Type of microbe |
| 2016 | Brintha et al. [36] | Sol–gel method | 7 wt.% | Escherichia coli Klebsiella pneumoniae Bacillus cereus Staphylococcus aureus | Gram-negative bacterium Gram-negative bacterium Gram-positive bacterium Gram-positive bacterium | Doping elements Type of microbe |
| Sample Code | Do ping Level | Mass of Al2O3 (g) | wt.% Al Doping | Atomic Fraction (x) | ) |
|---|---|---|---|---|---|
| Al-ZnO3 | 3 wt.% Al | 0.0928 | 0.05 | 2.5% | |
| Al-ZnO5 | 5 wt.% Al | 0.1579 | 0.08 | 4.0% | |
| Al-ZnO7 | 7 wt.% Al | 0.2258 | 0.11 | 5.5% | |
| Al-ZnO9 | 9 wt.% Al | 0.2967 | 0.14 | 7.0% |
| Type | Microorganism | Strain/ATCC Number |
|---|---|---|
| Gram-negative bacteria | Escherichia coli (E. coli) | ATCC 25922 |
| Klebsiella oxytoca (K. oxytoca) | ATCC 700324 | |
| Gram-positive bacteria | Staphylococcus aureus (Staph. aureus) | ATCC 25923 |
| Staphylococcus epidermidis (Staph. epidermidis) | ATCC 12228 | |
| Fungi | Candida tropicalis (C. tropicalis) | ATCC 13803 |
| Candida albicans (C. albicans) | ATCC 14053 |
| Sample | ZnO (wt.%) | ZnAl2O4 (wt.%) |
|---|---|---|
| ZnO | 100 | 0 |
| Al-ZnO3 | 98.5 | 1.5 |
| Al-ZnO5 | 97.2 | 2.8 |
| Al-ZnO7 | 95.8 | 4.2 |
| Al-ZnO9 | 94.1 | 5.9 |
| Sample | Crystallite Size (nm) | Strain |
|---|---|---|
| ZnO | 38 | −5.01 × 10−4 |
| Al-ZnO3 | 29 | 1.10 × 10−4 |
| Al-ZnO5 | 20 | −1.35 × 10−4 |
| Al-ZnO7 | 47 | −9.79 × 10−4 |
| Al-ZnO9 | 19 | −7.08 × 10−4 |
| Sample | ) | ) | C (F) | A |
|---|---|---|---|---|
| Al-ZnO3 | 405 | 4.58 106 | 4.4 10−10 | 0.784 |
| Al-ZnO5 | 300 | 2.54 106 | 2.52 10−10 | 0.85 |
| Al-ZnO7 | 210 | 1.58 106 | 2.29 10−10 | 0.855 |
| Al-ZnO9 | 180 | 3.9 105 | 2.127 10−10 | 0.86 |
| Tested Microorganisms | Mean of the Inhibition Area in mm of the Six Nanoparticles in Solid and Liquid State ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnO | Al-ZnO3 | Al-ZnO5 | Al-ZnO7 | Al-ZnO9 | Positive Control * | ||||||
| Solid | Liquid | Solid | Liquid | Solid | Liquid | Solid | Liquid | Solid | Liquid | ||
| Gram-Negative Bacteria | |||||||||||
| E. coli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16.7 ± 0.58 |
| K. oxytoca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16.3 ± 0.58 |
| Gram-Positive Bacteria | |||||||||||
| Staph. aureus | 12.4 ± 0.1 | 11.1 ± 0.38 | 12.37 ± 0.24 | 11.6 ± 0.12 | 12.57 ± 0.07 | 11.87 ± 0.09 | 13.1 ± 0.1 | 11.83 ± 0.66 | 13.3 ± 0.15 | 12.0 ± 0.001 | 17.67 ± 0.33 |
| Staph. epidermidis | 12.43 ± 0.12 | 11.4 ± 0.31 | 12.37 ± 0.20 | 11.7 ± 0.17 | 13.43 ± 0.23 | 11.73 ± 0.15 | 15.37 ± 0.09 | 12.07 ± 0.07 | 15.57 ± 0.35 | 12.37 ± 0.19 | 22.33 ± 0.33 |
| Fungi | |||||||||||
| C. tropicalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.3 ± 0.58 |
| C. albicans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15.0 ± 1.0 |
| Microorganism | ZnO | Al-ZnO3 | Al-ZnO5 | Al-ZnO7 | Al-ZnO9 | Reference Antibiotics |
|---|---|---|---|---|---|---|
| Staph. aureus | 12.4 ± 0.1 a | 12.37 ± 0.24 a | 12.57 ± 0.07 a | 13.1 ± 0.1 b | 13.3 ± 0.15 b | 17.67 ± 0.33 d |
| Staph. epidermidis | 12.43 ± 0.12 a | 12.37 ± 0.20 ab | 13.43 ± 0.23 b | 15.37 ± 0.09 c | 15.57 ± 0.35 abc | 22.33 ± 0.33 d |
| Microorganism | ZnO | Al-ZnO3 | Al-ZnO5 | Al-ZnO7 | Al-ZnO9 | Reference Antibiotics |
|---|---|---|---|---|---|---|
| Staph. aureus | 11.1 ± 0.38 a | 11.6 ± 0.12 a | 11.87 ± 0.09 a | 11.83 ± 0.66 a | 12.0 ± 0.001 a | 17.67 ± 0.33 b |
| Staph. epidermidis | 11.4 ± 0.31 a | 11.7 ± 0.17 a | 11.73 ± 0.15 a | 12.07 ± 0.07 a | 12.37 ± 0.19 a | 22.33 ± 0.33 b |
| Tested Microorganisms | Measurement of the Inhibition Area in mm Using Well Diffusion Method ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In Dark | Under Light | |||||||||
| Not Subjected to Additional HEBM | Subjected to Additional HEBM | Positive Control | Not Subjected to Additional HEBM | Subjected to Additional HEBM | Positive Control | |||||
| Al-ZnO7 | Al-ZnO9 | Al-ZnO7 | Al-ZnO9 | Al-ZnO7 | Al-ZnO9 | Al-ZnO7 | Al-ZnO9 | |||
| Gram-Negative Bacteria | ||||||||||
| E. coli | 0 | 0 | 11.03 ± 0.25 | 11.3 ± 0.45 | 11.6 ± 0.32 | 0 | 0 | 12.7 ± 0.6 | 12.7 ± 0.2 | 12.3 ± 0.3 |
| K. oxytoca | 0 | 0 | 12.5 ± 0.2 | 13.2 ± 0.28 | 15 ± 1.09 | 0 | 0 | 13.1 ± 0.26 | 13.8 ± 0.2 | 15.03 ± 0.06 |
| Gram-Positive Bacteria | ||||||||||
| Staph. aureus | 13.1 ± 0.17 | 13.3 ± 0.26 | 14.2 ± 0.46 | 14.7 ± 0.15 | 13.4 ± 1.02 | 13.7 ± 0.17 | 13.9 ± 0.26 | 15.1 ± 0.36 | 15.7 ± 0.2 | 13.5 ± 0.057 |
| Staph. epidermidis | 15.3 ± 0.15 | 15.6 ± 0.6 | 19.9 ± 1.5 | 22.7 ± 1.7 | 17.7 ± 0.25 | 20.0 ± 0.50 | 18.5 ± 0.4 | 23.6 ± 1.1 | 24.4 ± 0.61 | 17.9 ± 0.60 |
| Fungi | ||||||||||
| C. tropicalis | 0 | 0 | 0 | 0 | 18.3 ± 0.58 | 0 | 0 | 0 | 0 | 21 ± 1.73 |
| C. albicans | 0 | 0 | 0 | 0 | 20 ± 2 | 21.6 ± 1.65 | 22.9 ± 0.7 | 24.1 ± 0.5 | 24.5 ± 0.26 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shammari, Z.; Massoudi, I.; Rached, A.; Ababutain, I.; Alghamdi, A.; Aldakheel, R.; Amin, K.A.; Kotb, E.; Ben Ali, A. Tailoring Al-Doped ZnO Nanoparticles via Scalable High-Energy Ball Milling–Solid-State Reaction: Structural, Optical, and Dielectric Insights for Light-Activated Antimicrobial Defense Against Medical Device Pathogens. Crystals 2025, 15, 397. https://doi.org/10.3390/cryst15050397
Al-Shammari Z, Massoudi I, Rached A, Ababutain I, Alghamdi A, Aldakheel R, Amin KA, Kotb E, Ben Ali A. Tailoring Al-Doped ZnO Nanoparticles via Scalable High-Energy Ball Milling–Solid-State Reaction: Structural, Optical, and Dielectric Insights for Light-Activated Antimicrobial Defense Against Medical Device Pathogens. Crystals. 2025; 15(5):397. https://doi.org/10.3390/cryst15050397
Chicago/Turabian StyleAl-Shammari, Zurayfah, Imen Massoudi, Amani Rached, Ibtisam Ababutain, Azzah Alghamdi, Reem Aldakheel, Kamel Adel Amin, Essam Kotb, and Amor Ben Ali. 2025. "Tailoring Al-Doped ZnO Nanoparticles via Scalable High-Energy Ball Milling–Solid-State Reaction: Structural, Optical, and Dielectric Insights for Light-Activated Antimicrobial Defense Against Medical Device Pathogens" Crystals 15, no. 5: 397. https://doi.org/10.3390/cryst15050397
APA StyleAl-Shammari, Z., Massoudi, I., Rached, A., Ababutain, I., Alghamdi, A., Aldakheel, R., Amin, K. A., Kotb, E., & Ben Ali, A. (2025). Tailoring Al-Doped ZnO Nanoparticles via Scalable High-Energy Ball Milling–Solid-State Reaction: Structural, Optical, and Dielectric Insights for Light-Activated Antimicrobial Defense Against Medical Device Pathogens. Crystals, 15(5), 397. https://doi.org/10.3390/cryst15050397






