Structural and Quantitative Investigation of Perovskite Pore Filling in Mesoporous Metal Oxides
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis
2.2. Device Fabrication
2.3. X-Ray Diffraction
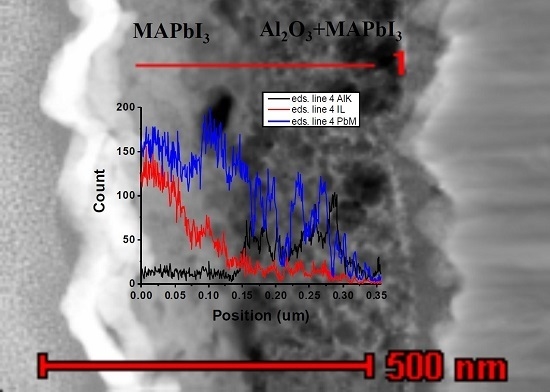
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gratzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–513. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.-H. Organolead halide perovskite: A rising player in high-efficiency solar cells. Chin. J. Catal. 2014, 35, 983–988. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Baker, R.-H.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratories NREL. Available online: http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed on 28 October 2016).
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Timothy, L. Kelly, perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photon. 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometaltrihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [PubMed]
- Etgar, L.; Peng, G.; Xue, Z.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cell. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef] [PubMed]
- Laben, W.A.; Etgar, L. Depleted hole conductor-free lead halide iodide heterojunction solar cell. Energy Environ. Sci. 2013, 6, 3249–3253. [Google Scholar] [CrossRef]
- Aharon, S.; Gamliel, S.; Cohen, B.; Etgar, L. Depletion region effect of highly efficient hole conductor free CH3NH3PbI3 perovskite solar cells. Phys. Chem. Chem. Phys. 2014, 16, 10512–10518. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Gamliel, S.; Etgar, L. Parameters influencing the deposition of methylammonium lead halide iodide in hole conductor free perovskite-based solar cells. Appl. Mater. 2014, 2, 081502. [Google Scholar] [CrossRef]
- Shi, J.; Dong, J.; Lv, S.; Xu, Y.; Zhu, L.; Xiao, J.; Xu, X.; Wu, H.; Li, D.; Luo, Y.; et al. Hole-conductor-free perovskite organic lead iodide heterojunction thin-film solar cells: High efficiency and junction property. Appl. Phys. Lett. 2014, 104, 063901. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Liul, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–399. [Google Scholar]
- Im, J.-H.; Jang, I.-H.; Pellet, N.; Grätzel, M.; Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Graetzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.T.; Pearson, A.J.; Kwak, C.K.; Dunbar, A.D.F.; Buckley, A.R.; Lidzey, D.G. Efficient planar heterojunction mixed-halide perovskite solar cell deposited via spray deposition. Energy Environ. Sci. 2014, 7, 2944–2950. [Google Scholar] [CrossRef]
- Gamliel, S.; Dymshits, A.; Aharon, S.; Terkieltaub, E.; Etgar, L. Micrometer Sized Perovskite Crystals in Planar Hole Conductor Free Solar Cells. J. Phys. Chem C 2015, 34, 19722–19728. [Google Scholar] [CrossRef]
- Leijtens, T.; Lauber, B.; Eperon, G.E.; Stranks, S.D.; Snaith, H.J. The Importance of Perovskite Pore Filling in Organometal Mixed Halide Sensitized TiO2-Based Solar Cells. J. Phys. Chem. Lett. 2014, 7, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Yue, Y.; Liu, J.; Bi, E.; Yang, X.; Islam, A.; Han, L. Consecutive Morphology Controlling Operations for Highly Reproducible Mesostructured Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 20707–20713. [Google Scholar] [CrossRef] [PubMed]
- Nanova, D.; Kast, A.K.; Pfannmller, M.; Muller, C.; Veith, L.; Wacker, I.; Agari, M.; Hermes, W.; Erk, P.; Kowalsky, W.; et al. Unraveling the Nanoscale Morphologies of Mesoporous Perovskite Solar Cells and Their Correlation to Device Performance. Nano Lett. 2014, 14, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jaehoon, C.; Seung, J.K.; Nam, G.P. Synthesis, structure, and photovoltaic property of a nanocrystalline 2H perovskite-type novel sensitizer (CH3CH2NH3)PbI3. Nanoscale Res. Lett. 2012, 7, 353. [Google Scholar]
- Dymshits, A.; Rotem, A.; Etgar, L. High voltage in hole conductor free organo metal halide perovskite solar cells. J. Mater. Chem. A 2014, 2, 20776–20781. [Google Scholar] [CrossRef]
- Lilliu, S.; Dane, T.G.; Alsari, M.; Griffin, J.; Barrows, A.T.; Dahlem, M.S.; Friend, R.H.; Lidzey, D.G.; Macdonald, J.E. Mapping morphological and structural properties of lead halide perovskites by scanning nanofocus XRD. Adv. Funct. Mater. 2016. [Google Scholar] [CrossRef]
- Barrows, A.T.; Lilliu, S.; Pearson, A.J.; Babonneau, D.; Dunbar, A.D.F.; Lidzey, D.G. Monitoring the Formation of a CH 3NH3PbI3–xClx Perovskite during Thermal Annealing Using X-ray Scattering. Adv. Funct. Mater. 2016, 26, 4934–4942. [Google Scholar] [CrossRef]
- Kawamura, Y.; Mashiyama, H.; Hasebe, K. Structural Study on Cubic–Tetragonal Transition of CH3NH3PbI3. J. Phys. Soc. Jpn. 2012, 71, 1694–1697. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitized solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamliel, S.; Popov, I.; Cohen, B.-E.; Uvarov, V.; Etgar, L. Structural and Quantitative Investigation of Perovskite Pore Filling in Mesoporous Metal Oxides. Crystals 2016, 6, 149. https://doi.org/10.3390/cryst6110149
Gamliel S, Popov I, Cohen B-E, Uvarov V, Etgar L. Structural and Quantitative Investigation of Perovskite Pore Filling in Mesoporous Metal Oxides. Crystals. 2016; 6(11):149. https://doi.org/10.3390/cryst6110149
Chicago/Turabian StyleGamliel, Shany, Inna Popov, Bat-El Cohen, Vladimir Uvarov, and Lioz Etgar. 2016. "Structural and Quantitative Investigation of Perovskite Pore Filling in Mesoporous Metal Oxides" Crystals 6, no. 11: 149. https://doi.org/10.3390/cryst6110149
APA StyleGamliel, S., Popov, I., Cohen, B.-E., Uvarov, V., & Etgar, L. (2016). Structural and Quantitative Investigation of Perovskite Pore Filling in Mesoporous Metal Oxides. Crystals, 6(11), 149. https://doi.org/10.3390/cryst6110149