Synthesis, Crystal Structure, and Magnetic Properties of Giant Unit Cell Intermetallics R117Co52+?Sn112+? (R = Y, La, Pr, Nd, Ho)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Analysis
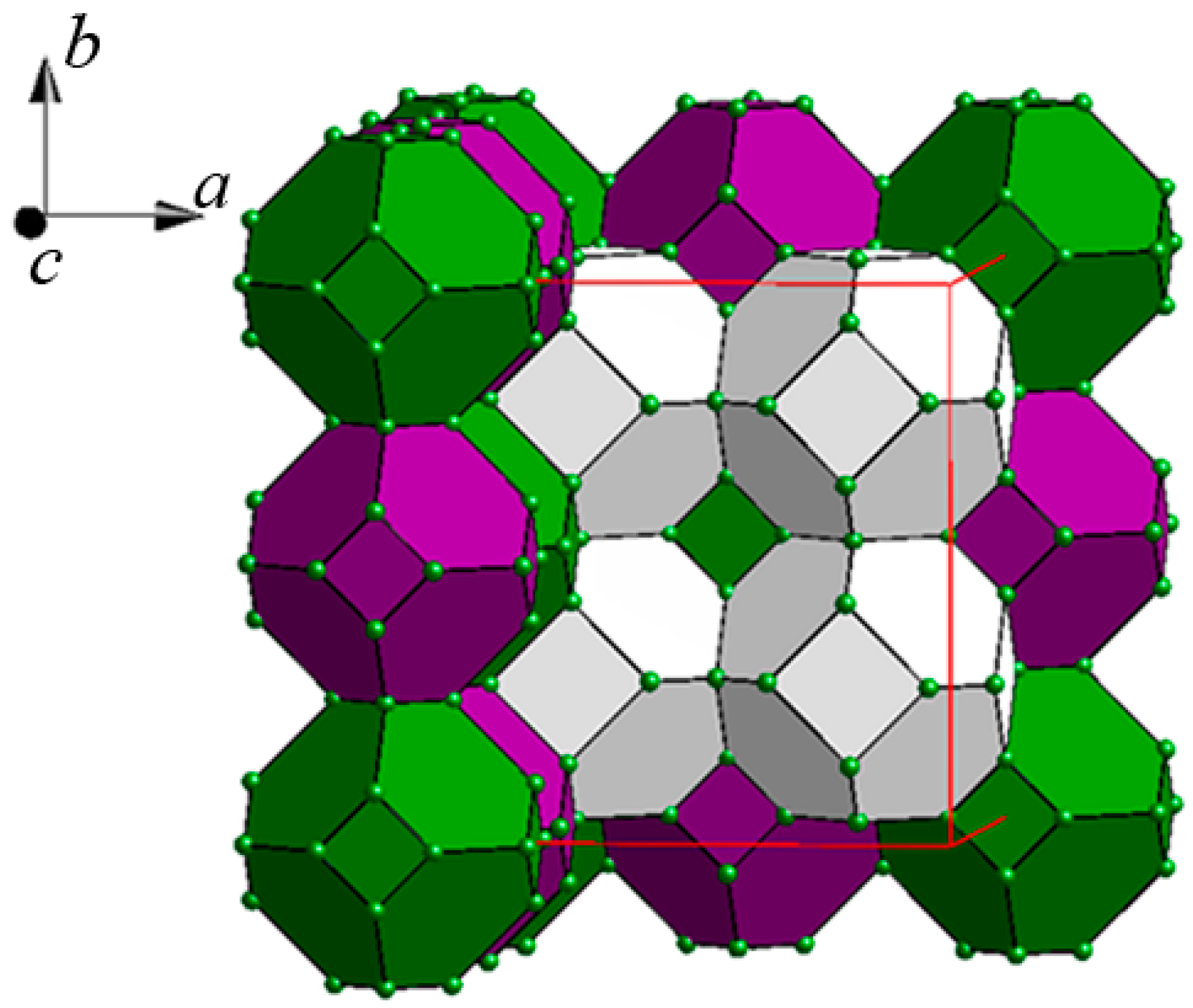
2.2. Crystal Structures
- (i)
- The Co2 and Co31/Co32 sites correspond to the Co2 and Co3 sites, respectively, in Y117Co56Sn115. The Co31/Co32 site splitting in La116Co63Sn115 can be explained by the necessity to maintain optimal interatomic distances while the size of the unit cell increases due to the larger size of La relative to Y. Thus, the Co32 position is located closer to the La8 atom (Figure 3c), resulting in an unreasonably short La-Co distance of 1.61 Å, which in turn causes the partial (83%) occupancy of the La8 site that correlates with the partial (12%) occupancy of the Co32 site.
- (ii)
- The disordered Co61/Co62 site in La116Co63Sn115, corresponding to the Co6 site in Y117Co56Sn115, forms an octahedron capping the Sn8 cube, which in turns encloses the disordered Co7 site, as discussed above (Figure 4f). Thus, the site occupancies of the Co61, Co62, and Co7 sites in the structure of La116Co63Sn115 are correlated (Table 2).
- (iii)
- The La71 site (s.o.f. = 0.47) is located in the center of the multi-shell polyhedron TOH C. While in the Y-containing structure this central site is fully occupied, in the La-containing structure it is only partially occupied and surrounded by an additional tetrahedron of Co72 atoms (s.o.f. = 0.53) inserted between the La71 site and the rest of the shell (Figure 5a). Such Co72 site was not observed in the corresponding cluster in Y117Co56Sn115 (Figure 5b). The La116Co63Sn115 structure is the first example among the Tb117Fe52Ge112-type structures to exhibit such disorder in the TOH C.
2.3. Magnetic Properties
3. Materials and Methods
3.1. Synthesis
3.2. X-Ray Diffraction
3.3. Physical Measurements
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fredrickson, D.C.; Lee, S.; Hoffmann, R. Interpenetrating polar and nonpolar sublattices in intermetallics: The NaCd2 structure. Angew. Chem. Int. Ed. 2007, 46, 1958–1976. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.M.; Belin-Ferre, E. (Eds.) Complex Metallic Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2011.
- Feuerbacher, M.; Thomas, C.; Makongo, J.P.A.; Hoffmann, S.; Carrillo-Cabrera, W.; Cardoso, R.; Grin, Y.; Kreiner, G.; Joubert, J.M.; Schenk, T.; et al. The Samson phase, β-Mg2Al3, revisited. Z. Kristallogr. 2007, 222, 259–288. [Google Scholar]
- Shevelkov, A.V.; Kovnir, K. Zintl phases: Principles and recent developments. Struct. Bonding 2011, 139, 97–142. [Google Scholar]
- Samson, S. Crystal structure of NaCd2. Nature 1962, 195, 259–263. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. 1. Definitions and basic principles. Acta Crystallogr. 1958, 11, 184–190. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packing. 2. Analysis and classification of representative structures. Acta Crystallogr. 1959, 12, 483–499. [Google Scholar] [CrossRef]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R.; Lin, J.H. The Nowotny chimney ladder phases: Following the cpseudo clue toward an explanation of the 14 electron rule. Inorg. Chem. 2004, 43, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R. The Nowotny chimney ladder phases: Whence the 14 electron rule? Inorg. Chem. 2004, 43, 6159–6167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.S.; Corbett, J.D. A chemical approach to the discovery of quasicrystals and their approximant crystals. Struct. Bonding 2009, 133, 1–39. [Google Scholar]
- Weber, T.; Dshemuchadse, J.; Kobas, M.; Conrad, M.; Harbrecht, B.; Steurer, W. Large, larger, largest—A family of cluster-based tantalum copper aluminides with giant unit cells. I. Structure solution and refinement. Acta Crystallogr. Sect. B 2009, 65, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Harbrecht, B.; Weber, T.; Jung, D.Y.; Steurer, W. Large, larger, largest—A family of cluster-based tantalum copper aluminides with giant unit cells. II. The cluster structure. Acta Crystallogr. Sect. B 2009, 65, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.S.; Corbett, J.D. Development of the Ca-Au-In icosahedral quasicrystal and two crystalline approximants: Practice via pseudogap electronic tuning. J. Am. Chem. Soc. 2007, 129, 6789–6797. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.S.; Corbett, J.D. Approximant phases and an icosahedral quasicrystal in the Ca-Au-Ga system: The influence of size of gallium versus indium. Inorg. Chem. 2008, 47, 7651–7659. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.L.; Corbett, J.D. Development of an icosahedral quasicrystal and two approximants in the Ca-Au-Sn system: Syntheses and structural analyses. Inorg. Chem. 2010, 49, 10436–10444. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, R.T.; Guo, Y.; Fredrickson, D.C. Epitaxial stabilization between intermetallic and carbide domains in the structures of Mn16SiC4 and Mn17Si2C4. J. Am. Chem. Soc. 2016, 138, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Berns, V.M.; Fredrickson, D.C. Structural plasticity: How intermetallics deform themselves in response to chemical pressure, and the complex structures that result. Inorg. Chem. 2014, 53, 10762–10771. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Z.; Weber, T.; Deloudi, S.; Steurer, W. Multiple diffraction in an icosahedral Al-Cu-Fe quasicrystal. Philos. Mag. 2011, 91, 2528–2535. [Google Scholar] [CrossRef]
- Pehcarskii, V.K.; Bodak, O.I.; Belskii, V.K.; Starodub, P.K.; Mokra, I.R.; Gladyshevskii, E.I. Crystal structure of Tb117Fe52Ge112. Kristallografiya 1987, 32, 334–338. [Google Scholar]
- Mruz, O.Y. Ph.D. Thesis, L’viv State University, L’viv, Ukraine, 1988.
- Morozkin, A.V.; Seropegin, Y.D.; Portnoy, V.K.; Sviridov, I.A.; Leonov, A.V. New ternary compounds R117Fe52Ge112 (R = Gd, Dy, Ho, Er, Tm) and Sm117Cr52Ge112 of the Tb117Fe52Ge112-type structure. Mater. Res. Bull. 1998, 33, 903–908. [Google Scholar] [CrossRef]
- Salamakha, P.S. Crystal structures and crystal chemistry of ternary rare-earth germanides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 225–338. [Google Scholar]
- Kovnir, K.; Shatruk, M. Magnetism in giant unit cells—Crystal structure and magnetic properties of R117Co52+δSn112+γ (R = Sm, Tb, Dy). Eur. J. Inorg. Chem. 2011, 2011, 3955–3962. [Google Scholar] [CrossRef]
- Salamakha, P.; Sologub, O.; Bocelli, G.; Otani, S.; Takabatake, T. Dy117Co57Sn112, a new structure type of ternary intermetallic stannides with a giant unit cell. J. Alloys Compd. 2001, 314, 177–180. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Yan, J.; Fu, Y.; Zeng, L. Crystal structure and magnetic properties of the new ternary compound Pr117Co57Sn112. J. Alloys Compd. 2010, 491, 49–52. [Google Scholar] [CrossRef]
- Cirafici, S.; Canepa, F.; Manfrinetti, P.; Napoletano, M. Physical properties of Ce2CoSn2. J. Alloys Compd. 2001, 317–318, 550–555. [Google Scholar] [CrossRef]
- Schmitt, D.C.; Haldolaarachchige, N.; Xiong, Y.; Young, D.P.; Jin, R.; Chan, J.Y. Probing the lower limit of lattice thermal conductivity in an ordered extended solid: Gd117Co56Sn112, a phonon glass-electron crystal system. J. Am. Chem. Soc. 2012, 134, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Mudryk, Y.; Manfrinetti, P.; Smetana, V.; Liu, J.; Fornasini, M.L.; Provino, A.; Pecharsky, V.K.; Miller, G.J.; Gschneidner, K.A., Jr. Structural disorder and magnetism in rare-earth (R) R117Co54+xSn112±y. J. Alloys Compd. 2013, 557, 252–260. [Google Scholar] [CrossRef]
- Liu, J.; Smetana, V.; Gschneidner, K.A., Jr.; Miller, G.J.; Pecharsky, V.K. The crystal structure and magnetic properties of Pr117Co56.7Ge112. J. Appl. Phys. 2013, 113, 17E120. [Google Scholar] [CrossRef]
- Bradley, A.J.; Jones, P. An X-ray investigation of the copper-aluminium alloys. J. Inst. Met. 1933, 51, 131–162. [Google Scholar]
- Chabot, B.; Cenzual, K.; Parthé, E. Nested polyhedra units: A geometrical concept for describing complicated cubic structures. Acta Crystallogr. Sect. A 1981, 37, 6–11. [Google Scholar] [CrossRef]
- Heusler, O. Crystal structure and the iron magnetism of manganese-aluminium-copper alloys. Ann. Phys. 1934, 19, 155–201. [Google Scholar] [CrossRef]
- Semitelou, J.P.; Yakinthos, J.K. Commensurate and incommensurate phases in Ho5Sn3 compound. J. Magn. Magn. Mater. 2003, 261, 392–398. [Google Scholar] [CrossRef]
- HighScorePlus; PANalytical B.V.: Almelo, The Netherlands, 2006.
- SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Sheldrick, G.M. SADABS; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]







| Nominal Composition | EDX Analysis | Lattice Constant a (Å) | Impurities |
|---|---|---|---|
| Y117Co55Sn115 | Y117(9)Co52(11)Sn110(8) | 29.930(2) | none |
| La117Co61Sn115 | La117(7)Co67(9)Sn113(6) | 31.334(2) | La2CoSn4 + unidentified |
| Pr117Co56Sn112 | Pr117(7)Co54(8)Sn112(7) | 30.8233(5) | Pr2CoSn4 |
| Nd117Co56Sn116 | Nd117(7)Co54(10)Sn111(6) | 30.6550(4) | Nd2Co0.8Sn4 |
| Ho117Co57Sn112 | Ho117(6)Co55(12)Sn108(8) | 29.7449(5) | Ho5Sn3 |
| Atom | Site | s.o.f. | x | y | z | Ueq |
|---|---|---|---|---|---|---|
| Y117Co55.8(1)Sn115.11(6)/La115.78(4)Co63.1(2)Sn114.95(6) | ||||||
| Y1/La1 | 96k | 1 | 0.06778(2)/0.06702(2) | 0.06778(2)/0.06702(2) | 0.15444(3)/0.15441(2) | 0.0073(2)/0.00909(9) |
| Y2/La2 | 96k | 1 | 0.17896(2)/0.18021(2) | 0.17896(2)/0.18021(2) | 0.40537(3)/0.40423(2) | 0.0086(2)/0.0101(1) |
| Y3/La3 | 96k | 1 | 0.20045(2)/0.20032(2) | 0.20045(2)/0.20032(2) | 0.06684(3)/0.06818(2) | 0.0068(2)/0.00713(9) |
| Y4/La4 | 96j | 1 | 0/0 | 0.24630(3)/0.24595(2) | 0.39490(3)/0.39566(2) | 0.0068(2)/0.00729(9) |
| Y5/La5 | 48i | 1 | 0.5/0.5 | 0.11983(3)/0.11945(2) | 0.11983(3)/0.11945(2) | 0.0118(3)/0.0138(1) |
| Y6/La6 | 24e | 1 | 0/0 | 0/0 | 0.33771(8)/0.34290(3) | 0.0187(4)/0.0139(2) |
| Y7/La71 | 8c | 1/0.470(3) | 0.25/0.25 | 0.25/0.25 | 0.25/0.25 | 0.0083(6)/0.0067(5) |
| –/Co72 | 32f | –/0.530(3) | –/0.22218(6) | –/0.22218(6) | –/0.22218(6) | –/0.0067(5) |
| Y8/La8 | 4a | 1/0.84(1) | 0/0 | 0/0 | 0/0 | 0.0067(8)/0.0104(9) |
| Sn1/Sn1 | 96k | 1 | 0.07276(2)/0.07488(2) | 0.07276(2)/0.07488(2) | 0.32334(2)/0.32299(2) | 0.0059(1)/0.0072(1) |
| Sn2/Sn2 | 96k | 1 | 0.10775(2)/0.10868(2) | 0.10775(2)/0.10868(2) | 0.24000(2)/0.24033(2) | 0.0061(1)/0.0078(1) |
| Sn3/Sn3 | 48i | 1 | 0.5/0.5 | 0.20792(2)/0.20859(2) | 0.20792(2)/0.20859(2) | 0.0075(2)/0.0108(2) |
| Sn4/Sn4 | 48h | 1 | 0/0 | 0.14539(2)/0.14537(2) | 0.14539(2)/0.14537(2) | 0.0060(2)/0.0074(1) |
| Sn5/Sn5 | 48g | 1 | 0.25/0.25 | 0.25/0.25 | 0.14034(3)/0.14353(2) | 0.0068(2)/0.0087(2) |
| Sn6/Sn6 | 32f | 1 | 0.14614(2)/0.14779(2) | 0.14614(2)/0.14779(2) | 0.14614(2)/0.14779(2) | 0.0069(2)/0.0097(2) |
| Sn7/Sn7 | 24e | 1 | 0/0 | 0/0 | 0.10910(4)/0.10619(3) | 0.0061(2)/0.0081(2) |
| Sn8/Sn8 | 24e | 1 | 0/0 | 0/0 | 0.21602(4)/0.21631(4) | 0.0074(2)/0.0119(2) |
| Sn9/Sn91 | 32f | 1/0.88(3) | 0.44652(2)/0.4420(2) | 0.44652(2)/0.4420(2) | 0.44652(2)/0.4420(2) | 0.0191(3)/0.026(1) |
| –/Sn92 | 32f | 1/0.12(3) | –/0.4340(9) | –/0.4340(9) | –/0.4340(9) | –/0.026(1) |
| Co1/Co1 | 96k | 1 | 0.16876(3)/0.16870(2) | 0.16876(3)/0.16870(2) | 0.23130(4)/0.22973(3) | 0.0083(2)/0.0112(2) |
| Co2/Co2 | 96k | 0.514(4)/0.501(4) | 0.07974(5)/0.07930(6) | 0.07974(5)/0.07930(6) | 0.01571(8)/0.01391(7) | 0.0102(7)/0.0218(8) |
| Co3/Co31 | 32f | 0.521(8)/0.592(8) | 0.05746(7)/0.05506(7) | 0.05746(7)/0.05506(7) | 0.05746(7)/0.05506(7) | 0.0073(12)/0.022(1) |
| –/Co32 | 32f | –/0.110(5) | –/0.0286(4) | –/0.0286(4) | –/0.0286(4) | –/0.022(1) |
| Co4/Co4 | 32f | 1 | 0.39315(4)/0.39159(4) | 0.39315(4)/0.39159(4) | 0.39315(4)/0.39159(4) | 0.0108(4)/0.0191(4) |
| Co51 | 32f | 0.67(1)/0.630(8) | 0.30786(3)/0.2994(1) | 0.30786(3)/0.2994(1) | 0.30786(3)/0.2994(1) | 0.0074(5)/0.0083(10) |
| Sn52 | 32f | 0.33(1)/0.370(8) | 0.30786(3)/0.3077(1) | 0.30786(3)/0.3077(1) | 0.30786(3)/0.3077(1) | 0.0074(5)/0.0083(10) |
| Co6/Co61 | 24e | 0.25/0.200(9) | 0/0 | 0/0 | 0.4184(4)/0.4195(7) | 0.026(3)/0.018(3) |
| –/Co62 | 24e | –/0.31(1) | –/0 | –/0 | –/0.4394(4) | –/0.018(3) |
| –/Co7 | 24e | –/0.185(4) | –/0 | –/0 | –/0.4816(3) | –/0.029(4) |
| Atomic Pair a | Interatomic Distance (Å) b | |||||
|---|---|---|---|---|---|---|
| R = Y c | R = Dy [24] | R = Tb [23] | R = Gd [28] d | R = Sm [23] | R = La c | |
| R–Coouter | 2.41(2) | – | 2.370 | 2.452 | 2.575 | 2.71(1) |
| R–Sn | 3.964(4) | 3.921 | 3.900 | 3.878 | 3.856 | 4.041(2) |
| Sn–Sn | 3.201(1) | 3.335 | 3.228 | 3.328 | 3.511 | 3.682(1) |
| Coinner–Sn | – | 2.888 | 2.795 | 2.557 | 2.756 | 2.855(12) |
| Coouter–Sn | 2.416(5) | – | 2.416 | 2.436 | 2.511 | 2.782(2) |
| Coouter–Sn(cub.face) | 0.846 | – | 0.792 | 0.630 | 0.375 | 0.274 |
| Compound | TN/TC (K) | TSRO (K) | θ (K) | μeff (μB) | Mmax (μB) at 7 T |
|---|---|---|---|---|---|
| Y117Co56Sn115 | – | – | –4.3(2) | 1.62(2)/Co | 0.21(1)/Co |
| Pr117Co54Sn112 | 10.5(3) | 5.5(4) | 17(3) | 4.9(3)/Pr | 1.49(1)/Pr |
| Nd117Co54Sn111 | 18.3(1) | 11.1(2) | 24.7(3) | 3.80(4)/Nd | 1.78(1)/Nd |
| Ho117Co55Sn108 | 10.6(2) | – | 10.0(3) | 10.20(3)/Ho | 6.51(2)/Ho |
| Composition | Y117Co55.8(1)Sn115.11(6) | La115.78(4)Co63.1(2)Sn114.95(6) |
|---|---|---|
| T, K | 230 | 230 |
| Space group | Fmm (No. 225) | Fmm (No. 225) |
| a, Å | 29.930(2) | 31.334(2) |
| V, Å3 | 26810(5) | 30763(6) |
| Z | 4 | 4 |
| ρcalc, g·cm–3 | 6.762 | 7.232 |
| µ, mm–1 | 38.736 | 28.138 |
| 2θmax, deg | 60.72 | 54.75 |
| Reflections collected | 55002 | 79250 |
| Unique reflections | 2027 | 1788 |
| Parameters refined | 101 | 113 |
| R1, wR2 [Fo > 4σ(Fo)] | 0.029, 0.054 | 0.017, 0.031 |
| Goodness-of-fit | 1.09 | 1.17 |
| Diff. peak and hole, e·Å–3 | 1.58 and −3.12 | 3.33 and −2.13 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, P.; Abramchuk, M.; Shatruk, M. Synthesis, Crystal Structure, and Magnetic Properties of Giant Unit Cell Intermetallics R117Co52+?Sn112+? (R = Y, La, Pr, Nd, Ho). Crystals 2016, 6, 165. https://doi.org/10.3390/cryst6120165
Chai P, Abramchuk M, Shatruk M. Synthesis, Crystal Structure, and Magnetic Properties of Giant Unit Cell Intermetallics R117Co52+?Sn112+? (R = Y, La, Pr, Nd, Ho). Crystals. 2016; 6(12):165. https://doi.org/10.3390/cryst6120165
Chicago/Turabian StyleChai, Ping, Mykola Abramchuk, and Michael Shatruk. 2016. "Synthesis, Crystal Structure, and Magnetic Properties of Giant Unit Cell Intermetallics R117Co52+?Sn112+? (R = Y, La, Pr, Nd, Ho)" Crystals 6, no. 12: 165. https://doi.org/10.3390/cryst6120165
APA StyleChai, P., Abramchuk, M., & Shatruk, M. (2016). Synthesis, Crystal Structure, and Magnetic Properties of Giant Unit Cell Intermetallics R117Co52+?Sn112+? (R = Y, La, Pr, Nd, Ho). Crystals, 6(12), 165. https://doi.org/10.3390/cryst6120165







