Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
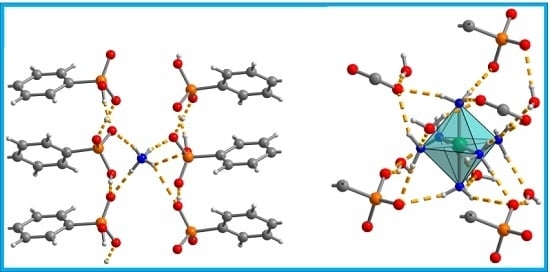
Single Crystal X-ray Structures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clearfield, A.; Demadis, K. Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: Oxford, UK, 2012; pp. 45–128. [Google Scholar]
- Deng, M.; Liu, X.; Zheng, Q.; Chen, Z.; Fang, C.; Yue, B.; He, H. Controllable preparation and structures of two zinc phosphonocarboxylate frameworks with MER and RHO zeolitic topologies. CrystEngComm 2013, 15, 7056–7061. [Google Scholar] [CrossRef]
- Gagnon, K.J.; Perry, H.P.; Clearfield, A. Conventional and unconventional metal-organic frameworks based on phosphonate ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034–1054. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2003, 2781–2804. [Google Scholar] [CrossRef]
- Taylor, J.M.; Vaidhyanathan, R.; Iremonger, S.S.; Shimizu, G.K.H. Enhancing water stability of metal-organic frameworks via phosphonate monoester linkers. J. Am. Chem. Soc. 2012, 134, 14338–14340. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.R.; Schmitt, W.; Evans, R.C. Lighting-Up Two-dimensional lanthanide phosphonates: Tunable structure-property relationships towards visible and near-infrared emitters. J. Phys. Chem. C 2014, 118, 10291–10301. [Google Scholar] [CrossRef]
- Jiménez-García, L.; Kaltbeitzel, A.; Pisula, W.; Gutmann, J.S.; Klapper, M.; Müllen, K. Phosphonated hexaphenylbenzene: A crystalline proton conductor. Angew. Chem. Int. Ed. 2009, 48, 9951–9953. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, G.K.H.; Vaidhyanathan, R.; Taylor, J.M. Phosphonate and sulfonate metal organic frameworks. Chem. Soc. Rev. 2009, 38, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Rojo, T.; Mesa, J.L.; Lago, J.; Bazan, B.; Pizarro, J.L.; Arriortua, M.I. Organically templated open-framework phosphite. J. Mater. Chem. 2009, 19, 3793–3818. [Google Scholar] [CrossRef]
- Hou, S.-Z.; Cao, D.-K.; Liu, X.-G.; Li, Y.-Z.; Zheng, L.-M. Metal phosphonates based on (4-carboxypiperidyl)-N-methylene-phosphonate: In situ ligand cleavage and metamagnetism in Co3(O3PCH2-NHC5H9-COO)2(O3PCH2-NC5H10)(H2O). Dalton Trans. 2009, 15, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Gil-Hernández, B.; Abu-Shandi, K.; Sanchiz, J.; Janiak, C. Iron, copper and zinc ammonium-1-hydroxyalkylidene-diphosphonates with zero-, one- and two-dimensional covalent metal-ligand structures extended into three-dimensional supramolecular networks by charge-assisted hydrogen-bonding. Polyhedron 2010, 29, 2537–2545. [Google Scholar] [CrossRef]
- Abu-Shandi, K.; Winkler, H.; Janiak, C. Structure and mössbauer study of the first mixed-valence iron diphosphonate. Z. Anorg. Allg. Chem. 2006, 632, 629–633. [Google Scholar] [CrossRef]
- Zhao, X.; Bell, J.G.; Tang, S.-F.; Li, L.; Thomas, K.M. Kinetic molecular sieving, thermodynamic and structural aspects of gas/vapor sorption on metal organic framework [Ni1.5(4,4′-bipyridine)1.5(H3L)-(H2O)3][H2O]7 where H6L = 2,4,6-trimethylbenzene-1,3,5-triyl tris(methylene)triphosphonic acid. J. Mater. Chem. A 2016, 4, 1353–1365. [Google Scholar] [CrossRef]
- Zhai, F.; Zheng, Q.; Chen, Z.; Ling, Y.; Liu, X.; Weng, L.; Zhou, Y. Crystal transformation synthesis of a highly stable phosphonate MOF for selective adsorption of CO2. CrystEngComm 2013, 15, 2040–2043. [Google Scholar] [CrossRef]
- Kinnibrugh, T.L.; Ayi, A.A.; Bakhmutov, V.I.; Zon, J.; Clearfield, A. Reversible dehydration behavior reveals coordinatively unsaturated metal sites in microporous aluminum phosphonates. Cryst. Growth Des. 2013, 13, 2973–2981. [Google Scholar] [CrossRef]
- Menelaou, M.; Dakanali, M.; Raptopoulou, C.P.; Drouza, C.; Lalioti, N.; Salifoglou, A. pH-Specific synthetic chemistry, and spectroscopic, structural, electrochemical and magnetic susceptibility studies in binary Ni(II)-(carboxy)phosphonate systems. Polyhedron 2009, 28, 3331–3339. [Google Scholar] [CrossRef]
- Ling, Y.; Deng, M.; Chen, Z.; Xia, B.; Liu, X.; Yang, Y.; Zhou, Y.; Weng, L. Enhancing CO2 adsorption of a Zn-phosphonocarboxylate framework by pore space partitions. Chem. Commun. 2013, 49, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Breeze, B.A.; Shanmugam, M.; Tuna, F.; Winpenny, R.E.P. A series of nickel phosphonate-carboxylate cages. Chem. Commun. 2007, 48, 5185–5187. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.-M.; Perez, O.; Leclaire, A.; Couthon-Gourvès, H.; Jaffrès, P.-A. Lead(II) Hybrid Materials from 3- or 4-Phosphonobenzoic Acid. Eur. J. Inorg. Chem. 2009, 4870–4876. [Google Scholar] [CrossRef]
- Liao, T.-B.; Ling, Y.; Chen, Z.-X.; Zhou, Y.-M.; Weng, L.-H. A rutile-type porous zinc(II)-phosphonocarboxylate framework: Local proton transfer and size-selected catalysis. Chem. Commun. 2010, 46, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.; Zima, V.; Beneš, L.; Melánová, K.; Trchová, M.; Vlček, M. New barium 4-carboxyphenylphosphonates: Synthesis, characterization and interconversions. Solid State Sci. 2008, 10, 1533–1542. [Google Scholar] [CrossRef]
- Pütz, A.-M.; Carrella, L.M.; Rentschler, E. A distorted honeycomb motive in divalent transition metal compounds based on 4-Phosphonbenzoic acid and exchange coupled Co(II) and Cu(II): Synthesis, structural description and magnetic properties. Dalton Trans. 2013, 42, 16194–16199. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.-M.; Barrier, N.; Boudin, S.; Dorcet, V.; Caignaert, V.; Boullay, P.; Hix, G.B.; Jaffrès, P.-A. Remarkable thermal stability of Eu(4-phosphonobenzoate): Structure investigations and luminescence properties. Dalton Trans. 2009, 47, 10614–10620. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Guo, L.-R.; Shen, Y.; Zheng, L.-M. LiF-assisted crystallization of zinc 4-carboxyphenylphosphonates with pillared layered structures. CrystEngComm 2009, 11, 1674–1678. [Google Scholar] [CrossRef]
- Rueff, J.-M.; Perez, O.; Caignaert, V.; Hix, G.; Berchel, M.; Quentel, F.; Jaffrès, P.-A. Silver-based hybrid materials from meta- or para-phosphonobenzoic acid: Influence of the topology on silver release in water. Inorg. Chem. 2015, 54, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Zima, V.; Svoboda, J.; Beneš, L.; Melánová, K.; Trchová, M.; Dybal, J. Synthesis and characterization of new strontium 4-carboxyphenylphosphonates. J. Solid State Chem. 2007, 180, 929–939. [Google Scholar] [CrossRef]
- Adelani, P.O.; Albrecht-Schmitt, T.E. Comparison of thorium (IV) and uranium (VI) carboxyphosphonates. Inorg. Chem. 2010, 49, 5701–5705. [Google Scholar] [CrossRef] [PubMed]
- Melánová, K.; Klevcov, J.; Beneš, L.; Svoboda, J.; Zima, V. New layered functionalized titanium (IV) phenylphosphonates. J. Phys. Chem. Solids 2012, 73, 1452–1455. [Google Scholar] [CrossRef]
- Li, J.-T.; Cao, D.-K.; Akutagawa, T.; Zheng, L.M. Zn3(4-OOCC6H4PO3)2: A polar metal phosphonate with pillared layered structure showing SHG-activity and large dielectric anisotropy. Dalton Trans. 2010, 39, 8606–8608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, Y.; Weng, L.; Zhao, D. Mixed-solvothermal syntheses and structures of six new zinc phosphonocarboxylates with zeolite-type and pillar-layered frameworks. Cryst. Growth Des. 2008, 8, 4045–4053. [Google Scholar] [CrossRef]
- Merkushev, E.B.; Shvartzberg, M.S. Organoiodine Compounds and Syntheses Based on Them; Tomsk Gos. Pedagogicheskii Institut: Tomsk, Soviet Union, 1982; pp. 2598–2601. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Lin, Z.; Lei, X.-Q.; Bai, S.-D.; Ng, S.W. Ammonium benzenephosphonate. Acta Crystallogr. Sect. E.—Struct Rep. Online 2008, 64, o1607. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. On Noncovalent Interactions in Crystals: Supramolecular Chemistry: From Molecules to Nanomaterials; Steed, J., Gale, P.A., Eds.; Wiley: Chichester, UK, 2012; Volume 6, pp. 2829–2868. [Google Scholar]
- Góra, R.W.; Maj, M.; Grabowski, S.J. Resonance-assisted hydrogen bonds revisited. Resonance stabilization vs. charge delocalization. Phys. Chem. Chem. Phys. 2013, 15, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Mó, O.; Yáñez, M.; Elguero, J. Resonance-assisted hydrogen bonds: A critical examination. structure and stability of the enols of β-diketones and β-enaminones. J. Phys. Chem. A 2007, 111, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Bertolasi, V.; Pretto, L.; Ferretti, V.; Gilli, G. Covalent versus electrostatic nature of the strong hydrogen bond: Discrimination among single, double, and asymmetric single-well hydrogen bonds by variable-temperature X-ray crystallographic methods in β-diketone enol RAHB systems. J. Am. Chem. Soc. 2004, 126, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for intramolecular N–H···O resonance-assisted hydrogen bonding in enaminones and related heterodienes. A combined crystal-structural, IR and NMR spectroscopic, and quantum-mechanical investigation. J. Am. Chem. Soc. 2000, 122, 10405–10417. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Synergistic effect of a Novel triazine Charring Agent and ammonium polyphosphate on the flame retardant properties of Halogen-Free Flame Retardant Polypropylene composites. Thermochim. Acta 2016. [Google Scholar] [CrossRef]
- Wilk, M.; Janczak, J.; Videnova-Adrabinska, V. Hexaaquacobalt(II) bis[hydrogen bis(4-carboxyphenyl-phosphonate)] dihydrate. Acta Crystallogr. Sect. C—Cryst. Struct. Commun. 2011, 67, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Justice, R.; Sevov, S.C. Hydrogen-bonded metal-complex sulfonate (MCS) inclusion compounds: Effect of the guest molecule on the host framework. Inorg. Chem. 2007, 46, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Morral, F.R. Alfred werner and cobalt complexes. Adv. Chem. 2009, 6, 70–77. [Google Scholar]
- Reddy, D.S.; Duncan, S.; Shimizu, G.K.H. A Family of supramolecular inclusion solids based upon second-sphere interactions. Angew. Chem. Int. Ed. 2003, 42, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Dorn, T.; Chamayou, A.-C.; Janiak, C. Hydrophilic interior between hydrophobic regions in inverse bilayer structures of cation–1,1′-binaphthalene- 2,2′-diyl phosphate salts. New J. Chem. 2006, 30, 156–167. [Google Scholar] [CrossRef]
- Maclaren, J.K.; Sanchiz, J.; Gili, P.; Janiak, C. Hydrophobic-exterior layer structures and magnetic properties of trinuclear copper complexes with chiral amino alcoholate ligands. New J. Chem. 2012, 36, 1596–1609. [Google Scholar] [CrossRef]
- Enamullah, M.; Vasylyeva, V.; Janiak, C. Chirality at metal and helical ligand folding in optical isomers of chiral bis(naphthaldiminato)nickel(II) complexes. Inorg. Chim. Acta 2013, 408, 109–119. [Google Scholar] [CrossRef]
- Ward, M.D. Design of crystalline molecular networks with charge-assisted hydrogen bonds. Chem. Commun. 2005, 47, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Gil-Hernández, B.; Maclaren, J.K.; Höppe, H.A.; Pasan, J.; Sanchiz, J.; Janiak, C. Homochiral lanthanoid(III) mesoxalate metal-organic frameworks: Synthesis, crystal growth, chirality, magnetic and luminescent properties. CrystEngComm 2012, 14, 2635–2644. [Google Scholar] [CrossRef]
- Maclaren, J.K.; Janiak, C. Amino-acid based coordination polymers. Inorg. Chim. Acta 2012, 389, 183–190. [Google Scholar]
- Chamayou, A.-C.; Neelakantan, M.A.; Thalamuthu, S.; Janiak, C. The first vitamin B6 zinc complex, pyridoxinato-zinc acetate: A 1D coordination polymer with polar packing through strong inter-chain hydrogen bonding. Inorg. Chim. Acta 2011, 365, 447–450. [Google Scholar] [CrossRef]
- Drašković, B.M.; Bogdanović, G.A.; Neelakantan, M.A.; Chamayou, A.-C.; Thalamuthu, S.; Avadhut, Y.S.; Schmedt auf der Günne, J.; Banerjee, S.; Janiak, C. N-o-Vanillylidene-l-histidine: Experimental charge density analysis of a double zwitterionic amino acid Schiff-base compound. Cryst. Growth Des. 2010, 10, 1665–1676. [Google Scholar] [CrossRef]
- Collins, L.W.; Wendlandt, W.W.; Gibson, E.K. The thermal dissociation of the [Co(NH3)5Cl]Cl2 and [Co(NH3)5Br]Br2 complexes in vacuo. Thermochim. Acta 1974, 8, 303–306. [Google Scholar] [CrossRef]
- Saito, A. Thermal decomposition of the complexes [Co(NH3)6][Nd(SO4)3]·nH2O. Thermochim. Acta 1986, 102, 373–386. [Google Scholar] [CrossRef]
- Dines, M.B.; DiGiacomo, P.M. Derivatized lamellar phosphates and phosphonates of M(IV) ions. Inorg. Chem. 1981, 20, 92–97. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Welzel, U. Modern Diffraction Methods; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Pecharsky, V.; Zavalij, P. Fundamentals of Powder Diffraction and Structural Characterization of Materials, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Cryst. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Heering, C.; Francis, B.; Nateghi, B.; Makhloufi, G.; Janiak, C. Syntheses, structures and properties of group 12 element (Zn, Cd, Hg) coordination polymers with a phosphonate-biphenyl-carboxylate linker. CrystEngComm 2016. (to be submitted). [Google Scholar]
- APEX2. SAINT, Data Reduction and Frame Integration Program for the CCD Area-Detector System, Bruker Analytical X-ray Systems; Data Collection program for the CCD Area-Detector System: Madison, WI, USA, 1997–2006. [Google Scholar]
- Sheldrick, G. SADABS: Area-Detector Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, version 3.2; Crystal and Molecular Structure Visualization; Crystal Impact—K. Brandenburg & H. Putz Gbr: Bonn, Germany, 2009. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]










| D–H···A | D–H [Å] | H···A [Å] | D···A [Å] | D–H···A [°] | Symmetry Transformations |
|---|---|---|---|---|---|
| N1–H1A···O8 | 0.94 (3) | 1.90 (3) | 2.840 (3) | 177 (3) | |
| N1–H1B···O10 vi | 0.86 (3) | 2.25 (3) | 2.945 (3) | 138 (3) | vi = 2 − x, 2 − y, −z |
| N1–H1C···O3 v | 0.84 (3) | 2.00 (3) | 2.817 (3) | 165 (3) | v = 1 + x, 1 + y, z |
| N1–H1D···O9 vii | 0.95 (3) | 1.92 (3) | 2.845 (3) | 164 (2) | vii = 1 − x, 2−y, −z |
| O2–H2···O1 i | 0.94 (5) | 1.71 (5) | 2.623 (3) | 164 (4) | i = −1 − x, −y, 1 − z |
| O4–H4···O8 iv | 0.78 (3) | 1.78 (3) | 2.563 (2) | 175 (3) | iv = x, −1 + y, z |
| O7–H7···O6 ii | 0.99 (5) | 1.66 (5) | 2.642 (3) | 172 (4) | ii = −x, 1 − y, 1 − z |
| O9–H9···O5 | 1.17 (3) | 1.26 (3) | 2.428 (2) | 180 (3) | |
| O10–H10···O3 iii | 0.83 (3) | 1.72 (3) | 2.537 (2) | 170 (3) | iii = 1 + x, y, z |
| P1–O3 | 1.4975 (18) | P2–O8 | 1.5045 (18) |
| P1–O5 | 1.5174 (18) | P2–O9 | 1.5190 (18) |
| P1–O4 | 1.5583 (19) | P2–O10 | 1.5553 (19) |
| P1–C10 | 1.787 (3) | P2–C23 | 1.796 (3) |
| C13–O1 | 1.235 (4) | C26–O6 | 1.230 (4) |
| C13–O2 | 1.283 (4) | C26–O7 | 1.275 (4) |
| O3–P1–O5 | 115.63 (11) | O8–P2–O9 | 112.38 (11) |
| O3–P1–O4 | 107.83 (10) | O8–P2–O10 | 109.16 (10) |
| O5–P1–O4 | 108.78 (11) | O9–P2–O10 | 110.04 (10) |
| O3–P1–C10 | 109.35 (11) | O8–P2–C23 | 109.32 (11) |
| O5–P1–C10 | 107.54 (11) | O9–P2–C23 | 108.76 (11) |
| O4–P1–C10 | 107.44 (11) | O10–P2–C23 | 107.04 (11) |
| Co1–N1 | 1.957 (2) | P1–O1 | 1.5235 (18) |
| Co1–N2 | 1.965 (2) | P1–O2 | 1.5283 (18) |
| Co1–N3 | 1.951 (2) | P1–O3 | 1.5247 (18) |
| Co1–N4 | 1.959 (2) | P1–C1 | 1.820 (2) |
| Co1–N5 | 1.976 (2) | ||
| Co1–N6 | 1.961 (2) | ||
| N5–Co1–N1 | 87.17 (9) | N3–Co1–N4 | 89.51 (9) |
| N4–Co1–N1 | 91.46 (9) | N3–Co1–N2 | 90.46 (9) |
| N4–Co1–N5 | 90.01 (9) | N6–Co1–N1 | 91.82 (9) |
| N2–Co1–N1 | 88.66 (9) | N6–Co1–N5 | 178.81 (9) |
| N2–Co1–N5 | 92.53 (9) | N6–Co1–N2 | 88.07 (9) |
| N2–Co1–N4 | 177.47 (9) | N6–Co1–N3 | 90.31 (9) |
| N3–Co1–N1 | 177.66 (9) | N6–Co1–N4 | 89.40 (9) |
| N3–Co1–N5 | 90.71 (9) |
| D–H···A | D–H [Å] | H···A [Å] | D···A [Å] | D–H···A [°] | Symmetry Transformations |
|---|---|---|---|---|---|
| N1–H1A···O8 i | 0.84 (5) | 2.71 (4) | 3.327 (3) | 132 (4) | i = x, y, z − 1 |
| N1–H1B···O2 | 0.84 (4) | 2.14 (4) | 2.976 (3) | 173 (3) | |
| N1–H1C···O8 ii | 0.87 (4) | 2.15 (4) | 2.971 (3) | 156 (3) | ii = −x + 1, −y, −z + 1 |
| N2–H2A···O4 vi | 0.86 (4) | 2.28 (4) | 3.086 (3) | 157 (3) | vi = x + 1/2, −y + 1/2, z − 1/2 |
| N2–H2B···O6 | 0.86 (4) | 2.07 (4) | 2.909 (4) | 163 (3) | |
| N2–H2C···O1 | 0.89 (3) | 2.00 (3) | 2.864 (3) | 165 (3) | |
| N3–H3A···O6 | 0.82 (5) | 2.60 (5) | 3.177 (4) | 129 (4) | |
| N3–H3B···O2 iii | 0.90 (4) | 1.90 (4) | 2.791 (3) | 174 (3) | iii = x + 1, y, z |
| N3–H3C···O5 viii | 0.69 (5) | 2.56 (4) | 3.048 (3) | 130 (4) | viii = x + 3/2, −y + 1/2, z − 1/2 |
| N3–H3C···O4 viii | 0.69 (5) | 2.56 (4) | 3.180 (3) | 152 (4) | viii = x + 3/2, −y + 1/2, z − 1/2 |
| N4–H4A···O9 iv | 0.90 (4) | 2.05 (4) | 2.937 (3) | 171 (3) | iv = x + 1, y, z − 1 |
| N4–H4B···O7 v | 0.83 (4) | 2.77 (3) | 3.270 (4) | 120 (3) | v = −x + 1, −y, −z |
| N4–H4C···O7 iii | 0.86 (4) | 2.00 (4) | 2.852 (3) | 171 (3) | iii = x + 1, y, z |
| N5–H5A···O8 ii | 0.90 (4) | 2.19 (4) | 3.035 (3) | 157 (3) | ii = −x + 1, −y, −z + 1 |
| N5–H5B···O2 iii | 0.88 (4) | 2.63 (4) | 3.437 (3) | 153 (3) | iii = x + 1, y, z |
| N5–H5C···O1 | 0.83 (4) | 2.13 (4) | 2.939 (3) | 162 (3) | |
| N6–H6A···O3 i | 0.82 (4) | 2.09 (5) | 2.904 (3) | 175 (3) | i = x, y, z − 1 |
| N6–H6B···O5 viii | 0.93 (4) | 2.26 (4) | 3.170 (3) | 167 (3) | viii = x + 3/2, −y + 1/2 |
| N6–H6C···O4 vi | 0.85 (4) | 2.13 (4) | 2.948 (3) | 160 (3) | vi = x + 1/2, −y + 1/2, z − 1/2 |
| O6–H6E···O5 viii | 0.87 | 1.82 (1) | 2.657 (4) | 161 (4) | viii = x + 3/2, −y + 1/2, z − 1/2 |
| O7–H7A···O3 ii | 0.62 (5) | 2.12 (5) | 2.740 (3) | 171 (6) | ii = −x + 1, −y, −z + 1 |
| O7–H7B···O2 | 0.85 (4) | 1.89 (5) | 2.690 (3) | 156 (4) | |
| O8–H8A···O9 | 0.82 (5) | 1.99 (5) | 2.802 (3) | 175 (4) | |
| O8–H8B···O3 | 0.73 (5) | 1.97 (5) | 2.693 (3) | 175 (5) | |
| O9–H9A···O1 vii | 0.74 (4) | 1.96 (4) | 2.704 (3) | 176 (4) | vii = x − 1, y, z |
| O9–H9B···O4 ix | 0.78 (4) | 1.95 (4) | 2.728 (3) | 173 (4) | ix = x + 1/2, −y + 1/2, z + 1/2 |
| 1 | 2 | |
|---|---|---|
| Chemical formula | C26H21O10P2·H4N | C13H8O5P·CoH18N6·4(H2O) |
| Mr | 573.41 | 508.36 |
| Crystal system, space group | Triclinic, P¯1 | Monoclinic, P21/n |
| Temperature (K) | 150 | 173 |
| a (Å) | 5.9358(5) | 7.0193(5) |
| b (Å) | 7.5309(5) | 35.454(3) |
| c (Å) | 27.781(2) | 9.2797(7) |
| α (°) | 95.413(4) | 90 |
| β (°) | 90.768(5) | 111.921(4) |
| γ (°) | 92.816(5) | 90 |
| V (Å3) | 1234.65(16) | 2142.4 (3) |
| Z | 2 | 4 |
| μ (mm−1) | 0.24 | 0.93 |
| Crystal size (mm) | 0.20 × 0.15 × 0.01 | 0.33 × 0.3 × 0.15 |
| Absorption correction | Multi-scan, wR2(int) was 0.0937 before and 0.0571 after correction. The Ratio of minimum to maximum transmission is 0.9165. The l/2 correction factor is 0.0000. | Multi-scan, wR2(int) was 0.1520 before and 0.0844 after correction. The Ratio of minimum to maximum transmission is 0.6784. The l/2 correction factor is 0.0015. |
| Tmin, Tmax | 0.683, 0.746 | 0.507, 0.748 |
| No. of measured, independent and observed reflections | 20157, 4937, 3104 [I > 2σ(I)] | 89006, 4214, 4150 [I > 2σ(I)] |
| Rint | 0.066 | 0.073 |
| (sin θ/λ)max (Å−1) | 0.617 | 0.617 |
| R[F2 > 2σ (F2)], wR(F2), S | 0.049, 0.121, 1.02 | 0.038, 0.100, 1.04 |
| No. of reflections | 4937 | 4214 |
| No. of parameters | 379 | 374 |
| Δρmax, Δρmin (e·Å−3) | 0.30, –0.34 | 1.10, –0.85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heering, C.; Nateghi, B.; Janiak, C. Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid. Crystals 2016, 6, 22. https://doi.org/10.3390/cryst6030022
Heering C, Nateghi B, Janiak C. Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid. Crystals. 2016; 6(3):22. https://doi.org/10.3390/cryst6030022
Chicago/Turabian StyleHeering, Christian, Bahareh Nateghi, and Christoph Janiak. 2016. "Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid" Crystals 6, no. 3: 22. https://doi.org/10.3390/cryst6030022
APA StyleHeering, C., Nateghi, B., & Janiak, C. (2016). Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid. Crystals, 6(3), 22. https://doi.org/10.3390/cryst6030022