Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds
Abstract
:1. Introduction
2. Results and Discussion
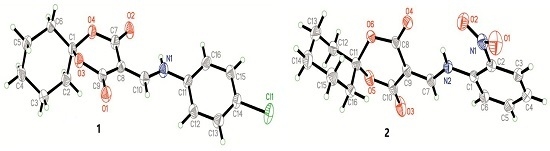
2.1. Crystal Structures
2.2. Spectroscopic Properties
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of Two Spiro Compounds
3.3. Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sundaram, R.; Ganesan, R.; Murugesan, G. In vitro antiplasmodial activity of spiro benzofuran compound from mangrove plant of southern india. Asian Pac. J. Trop. Med. 2012, 5, 358–361. [Google Scholar] [CrossRef]
- Liu, R.D.; Kong, D.L.; Wu, M.S.; Li, G.N.; Li, G.Z. Synthesis, structure and antimicrobial activity of 2-phenyl-4H-spiro[benzo[e][1,4,2] oxa-zaphosphinine-3,3′-indolin]-2′-one 2-oxide. Chin. J. Struct. Chem. 2014, 33, 204–208. [Google Scholar]
- Ding, Y.; Tian, Z.; Zhu, N. Research progress of antibacterial spiro-compounds. Chin. J. Org. Chem. 2010, 30, 1156–1163. [Google Scholar]
- Van Hes, R.; Smit, A.; Kralt, T.; Peters, A. Synthesis and antiviral activities of adamantane spiro compounds. J. Med. Chem. 1972, 15, 132–136. [Google Scholar] [CrossRef]
- Anikina, L.V.; Vikharev, Y.B.; Rozhkova, Y.S.; Shklyaev, V. Synthesis and biological activity of 3-spiro[adamantane-2,3′-isoquinolines]. Pharm. Chem. J. 2013, 46, 707–710. [Google Scholar] [CrossRef]
- Doggett, N.S.; Bailey, D.J.; Qazi, T. Estrogen potentiating activity of two spiro compounds having approximately similar molecular dimensions to stilbestrol. J. Med. Chem. 1977, 20, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.S.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of a-santonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Bhattacharya, S.; Kundu, A. Rationalized design, synthesis and pharmacological screening of amino acid linked spiro pyrrolldlno oxylndole analogs through environment friendly reaction. J. Adv. Pharm. Technol. Res. 2013, 4, 198–205. [Google Scholar] [PubMed]
- Arya, K.; Rawat, D.H.; Dandia, A.; Sasai, H. Bronsted acidic ionic liquids: Green, efficient and reusable catalyst for synthesis of fluorinated spiro [indole-thiazinones/hiazolidinones] as antihistamic agents. J. Fluor. Chem. 2012, 137, 117–122. [Google Scholar] [CrossRef]
- Yu, B.; Shi, X.J.; Qi, P.P.; Yu, D.Q.; Liu, H.M. Design, synthesis and biological evaluation of novel steroidal spiro-oxindoles as potent antiproliferative agents. J. Steroid Biochem. Mol. Biol. 2014, 141, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, J.B.; Matsuura, T.; Wang, Y.M. DNA modification with photochromic spiro compounds. Chin. Chem. Lett. 2002, 13, 299–302. [Google Scholar]
- Crossley, D.L.; Gabbutt, C.D.; Mark Heron, B.; Kay, P.; Mogstad, M. Synthesis and photochromic properties of spiro[naphthopyra-7′H-benzocyclohepta-5′,8′-dienes]. Dyes Pigment. 2012, 95, 62–68. [Google Scholar] [CrossRef]
- Jiang, Y.; Xue, S.; Li, Z.; Deng, J.; Mi, A.; Chan, A.S.C. Asymmetric hydroformylation of styrene catalyzed by chiral spiro diphosphite-rhodium(I) complexes. Tetra. Asymm. 1998, 9, 3185–3189. [Google Scholar] [CrossRef]
- Chu, Z.Z.; Wang, D.; Zhang, C.; Wang, F.Z.; Wu, H.W.; Lv, Z.B.; Hou, S.C.; Fan, X.; Zou, D.H. Synthesis of spiro[fluorene-9,9′-xanthene] derivatives and their application as hole-transporting materials for organic light-emitting devices. Synth. Met. 2012, 162, 614–620. [Google Scholar] [CrossRef]
- Zeng, W.L.; Li, Y.F.; Guo, H.M. Syntheses and Crystal Structures of 1,5-dioxaspiro[5.5]undecane-2,4-dione Derivatives. J. Chem. Crystallogr. 2013, 43, 223–227. [Google Scholar] [CrossRef]
- Zeng, W.L.; Meng, Q.G.; Guo, H.M.; Zhang, L.; Jian, F.F. Syntheses and crystal structures of two novel spiro compounds containing 1,5-Dioxaspiro[5.5]undecane-2,4-dione. Chin. J. Struct. Chem. 2010, 29, 696–699. [Google Scholar]
- Zeng, W.L.; Cai, X.; Guo, H.M. Synthesis, experimental and theoretical characterization of 3-(4-(dimethylamino)benzylidene)-1,5-dioxaspiro[5.5]undecane-2,4-dione. Chin. J. Struct. Chem. 2013, 32, 1603–1610. [Google Scholar]
- Cremer, D.J.; Pople, A. A general definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Özdemir, M.; Sönmez, M.; Sen, F.; Dincer, M.; Özdemir, N. A novel one-pot synthesis heterocyclic compound (4-benzoyl-5-phenyl-2(pyridin-2-yl)-3,3a-dihydropyrazolo[1,5-c] pyrimidin-7 (6H)-one): Structural (X-ray and DFT) and spectroscopic (FI-IR, NMR, UV-Vis and Mass) characterization studies. Spectrochim. Acta Part A 2015, 137, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- SMART and SAINT. Area Detector Control and Integration Software; Siemens Analytical X-Ray Systems Inc.: Madison, WI, USA, 1996. [Google Scholar]




| Selected Bond Lengths for 1 | Selected Bond Lengths for 2 | ||
| C(11)–N(1) | 1.422(6) | N(2)–C(7) | 1.3248(18) |
| N(1)–C(10) | 1.308(7) | N(2)–C(1) | 1.4023(18) |
| C(10)–C(8) | 1.371(7) | C(9)–C(7) | 1.366(2) |
| C(8)–C(9) | 1.437(8) | C(8)–C(9) | 1.445(2) |
| C(8)–C(7) | 1.448(6) | C(9)–C(10) | 1.456(2) |
| Cl(1)–C(14) | 1.735(5) | C(2)–N(1) | 1.4634(19) |
| Selected Bond Angles for 1 | Selected Bond Angles for 2 | ||
| C(10)–N(1)–C(11) | 124.4(4) | C(7)–N(2)–C(1) | 125.57(12) |
| N(1)–C(10)–C(8) | 127.0(5) | N(2)–C(7)–C(9) | 125.05(14) |
| C(16)–C(11)–C(12) | 119.3(5) | C(7)–C(9)–C(10) | 117.25(14) |
| C(16)–C(11)–N(1) | 119.3(4) | C(8)–C(9)–C(10) | 120.39(14) |
| C(10)–C(8)–C(9) | 116.7(4) | C(6)–C(1)–C(2) | 116.79(13) |
| C(10)–C(8)–C(7) | 122.4(5) | C(6)–C(1)–N(2) | 121.34(13) |
| C(9)–C(8)–C(7) | 119.6(5) | C(2)–C(1)–N(2) | 121.87(12) |
| D–H···A | Symmetry | D–H (Å) | H···A (Å) | D···A (Å) | ∠D–H···A (°) |
|---|---|---|---|---|---|
| N(1)–H(1A)···O(2) 1 | intra | 0.86 | 2.168 | 2.783(6) | 128.3 |
| N(1)–H(1A)···O(2) 1 | −2−x, 1−y, 1−z | 0.86 | 2.539 | 3.311(7) | 149.8 |
| C(10)–H(10A)···O(1) 1 | intra | 0.93 | 2.420 | 2.767(7) | 102.0 |
| C(10)–H(10A)···O(1) 1 | −1−x, −y, 1−z | 0.93 | 2.550 | 3.469(7) | 169.7 |
| C(12)–H(12A)···O(1) 1 | −1−x, −y, 1−z | 0.93 | 2.346 | 3.188(7) | 150.4 |
| C(16)–H(16A)···O(2) 1 | −2−x, 1−y, 1−z | 0.93 | 2.463 | 3.291(7) | 148.3 |
| C(5)–H(5B)···Cg(3) a 1 | −2−x, −y, 1−z | 0.97 | 3.254 | 3.790 | 116.6 |
| N(2)–H(2A)···O(2) 2 | intra | 0.86 | 2.041 | 2.6809(19) | 130.6 |
| N(2)–H(2A)···O(4) 2 | intra | 0.86 | 2.103 | 2.7429(17) | 130.8 |
| N(2)–H(2A)···N(1) 2 | intra | 0.86 | 2.582 | 2.911(2) | 125.2 |
| C(2)–H(7A)···O(3) 2 | intra | 0.93 | 2.462 | 2.798(2) | 101.4 |
| C(4)–H(4A)···O(1) 2 | x, 5/2−y, −1/2+z | 0.93 | 2.589 | 3.368(2) | 141.7 |
| C(14)–H(4A)···O(3) 2 | 1−x, 1−y, −z | 0.97 | 2.562 | 3.465(3) | 154.9 |
| Ring | Symmetry | Dihedral angels (°) | π···π (Å) | Centroid to centroid distances (Å) | |
| Cg(2)···Cg(2) b 2 | 2−x, −y, −z | 0 | 3.701 | 3.465 | |
| Compounds | 1 | 2 |
|---|---|---|
| Empirical formula | C16H16ClNO4 | C16H16N2O6 |
| Color/shape | yellow/block | yellow/block |
| Formula weight | 321.75 | 332.31 |
| Crystal system | triclinic | monoclinic |
| Space group | P-1 | P21/c |
| Unit cell dimensions | a = 5.9448(12) Å α = 100.28(3)° | a = 12.472(3) Å α = 90° |
| b = 9.782(2) Å β = 100.66(3)° | b = 11.856(2) Å β = 99.83(3)° | |
| c = 13.480(3) Å γ = 97.83(3)° | c = 10.643(2) Å γ = 90° | |
| Volume | 746.3(3) Å3 | 1550.7(5) Å3 |
| Z | 2 | 4 |
| Density (calculated) | 1.432 g·cm−3 | 1.423 g·cm−3 |
| Absorption coefficient | 0.274 mm−1 | 0.110 mm−1 |
| F(000) | 336 | 696 |
| Crystal size | 0.15 × 0.10 × 0.08 mm3 | 0.25 × 0.22 × 0.12 mm3 |
| Theta range for data collection | 3.15°–25.25° | 3.25°–27.48° |
| Limiting indices | −6 ≤ h ≤ 7, −11 ≤ k ≤ 11, −16 ≤ l ≤ 1 | −16 ≤ h ≤ 16, −15 ≤ k ≤ 15, −13 ≤ l ≤ 13 |
| Reflections collected | 6027 | 14428 |
| Independent reflections (Rint) | 2701 (0.0497) | 3552 (0.0379) |
| Reflections observed (I > 2σ(I)) | 1007 | 2471 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2701/0/199 | 3552/0/217 |
| Goodness-of-fit on F2 | 1.041 | 1.059 |
| R1/wR2 (I > 2σ(I)) R1/wR2 (all data) | 0.0544 / 0.1538 0.1661 / 0.2587 | 0.0444 / 0.1187 0.0677 / 0.1345 |
| Largest diff. peak and hole | 0.421 and −0.359 e Å−3 | 0.273 and −0.227 e Å−3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Jiang, J. Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds. Crystals 2016, 6, 69. https://doi.org/10.3390/cryst6060069
Zeng W, Jiang J. Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds. Crystals. 2016; 6(6):69. https://doi.org/10.3390/cryst6060069
Chicago/Turabian StyleZeng, Wulan, and Jinhe Jiang. 2016. "Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds" Crystals 6, no. 6: 69. https://doi.org/10.3390/cryst6060069
APA StyleZeng, W., & Jiang, J. (2016). Synthesis and Crystal Structures of Two Novel O, N-Containing Spiro Compounds. Crystals, 6(6), 69. https://doi.org/10.3390/cryst6060069