Functionalisation of Colloidal Transition Metal Sulphides Nanocrystals: A Fascinating and Challenging Playground for the Chemist
Abstract
:1. Introduction
- efforts toward the unambiguous characterisation and assessment of metal sulphides surfaces, which is still an open issue, and a lively debate on it is present in the literature.
- the development of effective functionalisation strategies for metal sulphides surfaces which requires a careful consideration of the former point;
- survey the combination of experimental and computational tools to unravel the exact nature of functional groups and moieties present on the sulphide surface.
- -
- selected different substrates (i.e., molybdenum, iron, copper and zinc sulphide) were chosen as examples of functionalised metal sulphides;
- -
- selected functionalisation routes and moieties.
2. Transition Metal Sulphides: General Features and Surface Chemistry
2.1. Transition Metal Sulphides: Analogies and Differences with Respect to Transition Metal Oxides
2.2. The Surface of Transition Metal Sulphides: Bulk and Nanocrystals
2.3. Nanocrystal-Ligand Interactions and Ligands Exchange Dynamics
2.4. Applications-Oriented Functionalisation of Transition Metal Sulphides
- (i)
- (ii)
- (iii)
- formation of an interdigitated bilayer between amphiphilic molecules or polymers and the passivating surfactant layer on the NP surface.
3. Functionalisation of Transition Metal Sulphides: Case Studies
- relevance and wide applicability of the sulphide;
- broadest differences among the chemistry, the structural and electronic features of the selected sulphides;
- broadest differences among the chemistry and the structural features of the selected ligands as well as of their interaction with the sulphide surface.
3.1. Zinc Sulphide
3.2. Copper Sulphide
3.3. Iron Sulphide
3.4. Molybdenum Sulphide
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Niederberger, M. New developments in the nonaqueous and/or non-hydrolytic sol-gel synthesis of inorganic nanoparticles. Electrochim. Acta 2010, 55, 7717–7725. [Google Scholar] [CrossRef]
- Bowles, K.C.; Bell, R.A.; Ernste, M.J.; Kramer, J.R.; Manolopoulos, H.; Ogden, N. Synthesis and characterization of metal sulfide clusters for toxicological studies. Environ. Toxicol. Chem. 2002, 21, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Bruehwiler, D.; Seifert, R.; Calzaferri, G. Quantum-Sized Silver Sulfide Clusters in Zeolite A. J. Phys. Chem. B 1999, 103, 6397–6399. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Girao, A.V.; Trindade, T.; Costa, M.C.; Duarte, A.; Rocha-Santos, T. Biological synthesis of nanosized sulfide semiconductors: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 8283–8302. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.W.; Munn, A.S.; Starkey, C.L.; Huddle, T.A.; Lester, E.H. Continuous-flow hydrothermal synthesis for the production of inorganic nanomaterials. Philos. Trans. R. Soc. a-Math. Phys. Eng. Sci. 2015, 373, 20150015. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Sadighi, A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. 2013, 189, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and biomedical applications of copper sulfide nanoparticles: From sensors to theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Tang, J.C.; Zhao, D.Y. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, H.; Inoue, M.; Boggavarapu, S.; Calvert, P. Amorphous and crystalline copper sulfides, CuS. J. Mater. Chem. 1996, 6, 1157–1160. [Google Scholar] [CrossRef]
- Hendricks, M.P.; Campos, M.P.; Cleveland, G.T.; Jen-La Plante, I.; Owen, J.S. A tunable library of substituted thiourea precursors to metal sulfide nanocrystals. Science 2015, 348, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, M.P.; Campos, M.P.; Cleveland, G.T.; Owen, J.S. Rapid access to substituted thioureas: A tunable library of precursors for metal sulfide nanocrystals. In Abstracts of Papers of the American Chemical Society (vol. 248). 1155 16TH ST, NW; American Chemical Society: Washington, DC, USA.
- Hosseini, M.R.; Sarvi, M.N. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mat. Sci. Semicond. Proc. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Kadlag, K.P.; Rao, M.J.; Nag, A. Ligand-Free, Colloidal, and Luminescent Metal Sulfide Nanocrystals. J. Phys. Chem. Lett. 2013, 4, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kristl, M.; Drofenik, M. Sonochemical synthesis of nanocrystalline mercury sulfide, selenide and telluride in aqueous solutions. Ultrason. Sonochem. 2008, 15, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kristl, M.; Hojnik, N.; Gyergyek, S.; Drofenik, M. Sonochemical preparation of copper sulfides with different phases in aqueous solutions. Mater. Res. Bull. 2013, 48, 1184–1188. [Google Scholar] [CrossRef]
- Kuzyniak, W.; Adegoke, O.; Sekhosana, K.; D’Souza, S.; Tshangana, S.C.; Hoffmann, B.; Ermilov, E.A.; Nyokong, T.; Hopfner, M. Synthesis and characterization of quantum dots designed for biomedical use. Int. J. Pharm. 2014, 466, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhou, M.; Li, W.R.; Weng, Q.H.; Li, C.L.; Xue, Y.M.; Jiang, X.F.; Zeng, X.H.; Bando, Y.; Golberg, D. Engineering sulfur vacancies and impurities in NiCo2S4 nanostructures toward optimal supercapacitive performance. Nano Energy 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Pileni, M.P. Colloidal assemblies used as templates to control the size, shape and self organization of nanoparticles. PCCP 1997, 101, 1578–1587. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.F.; Zhang, X.M.; Wang, C.; Wang, W.Z.; Xie, Y.; Qian, Y.T. Solvent-thermal preparation of nanocrystalline tin chalcogenide. J. Phys. Chem. Solids 1999, 60, 415–417. [Google Scholar] [CrossRef]
- Ramasamy, K.; Malik, M.A.; Revaprasadu, N.; O’Brien, P. Routes to Nanostructured Inorganic Materials with Potential for Solar Energy Applications. Chem. Mater. 2013, 25, 3551–3569. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.H.; Tan, H.T.; Yan, Q.Y. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikov, S.I.; Rempel, A.A. Synthesis of nanocrystalline silver sulfide. Inorg. Mater. 2015, 51, 759–766. [Google Scholar] [CrossRef]
- Shiri, L.; Ghorbani-Choghamarani, A.; Kazemi, M. Sulfides Synthesis: Nanocatalysts in C-S Cross-Coupling Reactions. Aust. J. Chem. 2016, 69, 585–600. [Google Scholar] [CrossRef]
- Teranishi, T.; Saruyama, M.; Kanehara, M. Seed-mediated synthesis of metal sulfide patchy nanoparticles. Nanoscale 2009, 1, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Tolia, J.; Chakraborty, M.; Murthy, Z.V.P. Synthesis and characterization of semiconductor metal sulfide nanocrystals using microemulsion technique. Cryst. Res. Technol. 2012, 47, 909–916. [Google Scholar] [CrossRef]
- Van der Stam, W.; Berends, A.C.; Donega, C.D. Prospects of Colloidal Copper Chalcogenide Nanocrystals. Chemphyschem 2016, 17, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Huang, H.T.; Liang, B.; Liu, Z.; Chen, D.; Shen, G.Z. ZnS Nanostructures: Synthesis, Properties, and Applications. Crit. Rev. Solid State Mater. Sci. 2013, 38, 57–90. [Google Scholar] [CrossRef]
- Zhang, F.; Wong, S.S. Controlled synthesis of semiconducting metal sulfide nanowires. Chem. Mater. 2009, 21, 4541–4554. [Google Scholar] [CrossRef]
- Wang, D.-S.; Zheng, W.; Hao, C.-H.; Peng, Q.; Li, Y.-D. A Synthetic Method for Transition-Metal Chalcogenide Nanocrystals. Chem. Eur. J. 2009, 15, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Chemseddine, A. Metal-Oxide and -Sulfide Nanocrystals and Nanostructures; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; pp. 315–352. [Google Scholar]
- Hiratani, T.; Konishi, K. Functionalization of inorganic nanoclusters based on the molecular recognition events at the heterogeneous interface. J. Synth. Org. Chem. Jpn. 2008, 66, 239–248. [Google Scholar] [CrossRef]
- Hertl, W. Surface chemical properties of zinc sulfide. Langmuir 1988, 4, 594–598. [Google Scholar] [CrossRef]
- Lee, C.M.; Jang, D.; Cheong, S.J.; Kim, E.M.; Jeong, M.H.; Kim, S.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Surface engineering of quantum dots for in vivo imaging. Nanotechnology 2010, 21, 285102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Loh, K.P.; Sow, C.H.; Gu, H.R.; Su, X.D.; Huang, C.; Chen, Z.K. Surface modification studies of edge-oriented molybdenum sulfide nanosheets. Langmuir 2004, 20, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Buonsanti, R.; Milliron, D.J. Chemistry of Doped Colloidal Nanocrystals. Chem. Mater. 2013, 25, 1305–1317. [Google Scholar] [CrossRef]
- Chen, J.-H.; Long, X.-H.; Zhao, C.-H.; Kang, D.; Guo, J. DFT calculation on relaxation and electronic structure of sulfide minerals surfaces in presence of H2O molecule. J. Cent. South Univ. 2014, 21, 3945–3954. [Google Scholar] [CrossRef]
- Luther, G.W.; Rickard, D.T. Metal sulfide cluster complexes and their biogeochemical importance in the environment. J. Nanop. Res. 2005, 7, 389–407. [Google Scholar] [CrossRef]
- Mal, J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Metal chalcogenide quantum dots: Biotechnological synthesis and applications. RSC Adv. 2016, 6, 41477–41495. [Google Scholar] [CrossRef]
- Rosso, K.M. Reactivity of Sulfide Mineral Surfaces. Rev. Mineral. Geochem. 2006, 61, 557–607. [Google Scholar] [CrossRef]
- Smet, P.F.; Moreels, I.; Hens, Z.; Poelman, D. Luminescence in sulfides: A rich history and a bright future. Materials 2010, 3, 2834–2883. [Google Scholar] [CrossRef]
- Zhang, H.; Hyun, B.-R.; Wise, F.W.; Robinson, R.D. A Generic Method for Rational Scalable Synthesis of Monodisperse Metal Sulfide Nanocrystals. Nano Lett. 2012, 12, 5856–5860. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, V.; Pan, H.; Shi, H.; Quek, S.Y.; Zhang, Y.W. Nanoscale Transition Metal Dichalcogenides: Structures, Properties, and Applications. Crit. Rev. Solid State Mater. Sci. 2014, 39, 319–367. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Cuenya, B.R.; Behafarid, F. Nanocatalysis: Size- and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 2015, 70, 135–187. [Google Scholar] [CrossRef]
- Frank, M.; Baumer, M. From atoms to crystallites: Adsorption on oxide-supported metal particles. Phys. Chem. Chem. Phys. 2000, 2, 3723–3737. [Google Scholar] [CrossRef]
- Hebie, S.; Napporn, T.W.; Morais, C.; Kokoh, K.B. Size-Dependent Electrocatalytic Activity of Free Gold Nanoparticles for the Glucose Oxidation Reaction. Chemphyschem 2016, 17, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Gao, P.F.; Huang, C.Z.; Li, Y.F. Shape- and size-dependent catalysis activities of iron-terephthalic acid metal-organic frameworks. Sci. China-Chem. 2015, 58, 1553–1560. [Google Scholar] [CrossRef]
- Mondal, J.; Trinh, Q.T.; Jana, A.; Ng, W.K.H.; Borah, P.; Hirao, H.; Zhao, Y.L. Size-Dependent Catalytic Activity of Palladium Nanoparticles Fabricated in Porous Organic Polymers for Alkene Hydrogenation at Room Temperature. ACS Appl. Mater. Interfaces 2016, 8, 15307–15319. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, X.; Fortrie, R.; Sauer, J. Size-Dependent Catalytic Activity of Supported Vanadium Oxide Species: Oxidative Dehydrogenation of Propane. J. Am. Chem. Soc. 2014, 136, 7751–7761. [Google Scholar] [CrossRef] [PubMed]
- Alsfasser, R.; Janiak, C.; Klapötke, T.M.; Meyer, H.-J. Moderne Anorganische Chemie, 4th ed.; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Pergamon Press: Cambridge, UK, 1998. [Google Scholar]
- Wells, A.F. Structural Inorganic Chemistry, 3rd ed.; Oxford University Press: Oxford, UK, 1962. [Google Scholar]
- Müller, U. Anorganische Strukturchemie, 2nd ed.; Teubner Studienbuecher Chemie: Stuttgart, Germany, 1992. [Google Scholar]
- Holleman, A.F.; Wiberg, E. Lehrbuch der Anorganischen Chemie, 101th ed.; W. de Gruyter & Co: Berlin, Germany, 1985. [Google Scholar]
- Pearson, G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Cox, P.A. Transition Metal Oxides: Introduction to Their Electronic Structure and Properties; Oxford University Press: Oxford, UK, 1995; p. 272. [Google Scholar]
- Shannon Radii—Atomistic Simulation Group. Available online: http://abulafia.mt.ic.ac.uk/shannon/ptable.php. (accessed on 10 December 2016).
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Miller, T.M.; Bederson, B. Atomic and molecular polarizabilities—A review of recent advances. Adv. At. Mol. Phys. 1978, 13, 1–55. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 53rd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Evans, H.T.; Konnert, J.A. Crystal structure refinement of covellite. Am. Mineral. 1976, 61, 996–1000. [Google Scholar]
- Prener, J.S. Nonstoichiometry in Chalcogenide Systems; American Chemical Society: Washington, DC, USA, 1963. [Google Scholar]
- Pedoussaut, N.M.; Lind, C. Facile Synthesis of Troilite. Inorg. Chem. 2008, 47, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Liu, X.; Ma, J.; Du, Q. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal sulfides. Mater. Sci. Eng. B 2007, 145, 17–22. [Google Scholar] [CrossRef]
- Geng, B.; Liu, X.; Du, Q.; Ma, J.; Liu, X. Size-dependent blue luminescent CdS nanocrystals synthesized through a single-source molecular precursor route. Mater. Res. Bull. 2008, 43, 1093–1098. [Google Scholar] [CrossRef]
- Ludi, B.; Olliges-Stadler, I.; Rossell, M.D.; Niederberger, M. Extension of the benzyl alcohol route to metal sulfides: “Nonhydrolytic” thio sol-gel synthesis of ZnS and SnS2. Chem. Commun. 2011, 47, 5280–5282. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Su, Y.; Xie, Y.; Chen, Q.; Chen, Z. Hydrothermal preparation and characterization of nanocrystalline powder of sphalerite. Mater. Res. Bull. 1995, 30, 601–605. [Google Scholar]
- Chen, Q.; Qian, Y.T.; Chen, Z.Y.; Shi, L.; Li, X.G.; Zhou, G.E.; Zhang, Y.H. Preparation of zinc sulfide thin films by the hydrothermal method. Thin Solid Films 1996, 272, 1–3. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Qian, Y.; Zhu, J.; Zhou, G.; Zhang, W.; Zhang, Y. Photoluminescence in ultrafine zinc sulfide thin film. Appl. Phys. Lett. 1996, 68, 3582–3584. [Google Scholar] [CrossRef]
- Li, Y.; Yi, D.; Yue, Z.; Qian, Y. Photophysical properties of ZnS quantum dots. J. Phys. Chem. Solids 1998, 60, 13–15. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, B.; Zhu, L.; Yu, J.; Sun, W.; Xu, L. Cation exchange synthesis of ZnS-Ag2S microspheric composites with enhanced photocatalytic activity. Appl. Surf. Sci. 2013, 270, 133–138. [Google Scholar] [CrossRef]
- Yu, S.-H.; Yang, J.; Wu, Y.-S.; Han, Z.-H.; Lu, J.; Xie, Y.; Qian, Y.-T. A new low temperature one-step route to metal chalcogenide semiconductors: PbE, Bi2E3 (E = S, Se, Te). J. Mater. Chem. 1998, 8, 1949–1951. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Wang, X.; Zhang, R.; Liu, X.; Lin, W.; Qian, Y. Synthesis of novel copper sulfide hollow spheres generated from copper (II)–thiourea complex. J. Cryst. Growth 2004, 263, 570–574. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Hydrothermal Growth of CuS Nanowires from Cu−Dithiooxamide, a Novel Single-Source Precursor. Cryst. Growth Des. 2006, 6, 1921–1926. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, S.; Xie, Q.; Hu, Z.; Yang, Y.; Zhang, S.; Qian, Y. Large-scale synthesis of ultralong Bi2S3 nanoribbons via a solvothermal process. Adv. Mater. 2003, 15, 936–940. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, D.; Liu, Z. Solvothermal synthesis route to one-dimensional nanostructures. Trans. Mater. Res. Soc. Jpn. 2004, 29, 2233–2238. [Google Scholar]
- Qian, Y.T. Solvothermal synthesis of nanocrystalline III-V semiconductors. Adv. Mater. 1999, 11, 1101–1102. [Google Scholar] [CrossRef]
- Li, Y.; Liao, H.; Ding, Y.; Fan, Y.; Zhang, Y.; Qian, Y. Solvothermal Elemental Direct Reaction to CdE (E = S, Se, Te) Semiconductor Nanorod. Inorg. Chem. 1999, 38, 1382–1387. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.-H.; Yu, S.-H.; Yang, L.; Zhou, G.-E.; Qian, Y.-T. Formation Process of CdS Nanorods via Solvothermal Route. Chem. Mater. 2000, 12, 3259–3263. [Google Scholar] [CrossRef]
- Amiri, O.; Salavati-Niasari, M.; Sabet, M.; Ghanbari, D. Sonochemical method for preparation of copper indium sulfide nanoparticles and their application for solar cell. Comb. Chem. High Throughput Screen. 2014, 17, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Baranchikov, A.Y.; Ivanov, V.K.; Tretyakov, Y.D. Sonochemical synthesis of inorganic materials. Russ. Chem. Rev. 2007, 76, 133–151. [Google Scholar] [CrossRef]
- Behboudnia, M.; Khanbabaee, B. Conformational study of CdS nanoparticles prepared by ultrasonic waves. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 229–232. [Google Scholar] [CrossRef]
- Wang, S.F.; Gu, F.; Lü, M.K.; Zhou, G.J.; Zhang, A.Y. Sonochemical synthesis of PbS nanocubes, nanorods and nanotubes. J. Cryst. Growth 2006, 289, 621–625. [Google Scholar] [CrossRef]
- García-Gómez, N.A.; de la Parra-Arcieniega, S.M.; Garza-Tovar, L.L.; Torres-González, L.C.; Sánchez, E.M. Ionic liquid-assisted sonochemical synthesis of SnS nanostructures. J. Alloy. Compd. 2014, 588, 638–643. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.-X.; Chen, G.; Yu, Y.-G.; Zhou, Y.-S.; Han, Z.-H.; Liu, Y. Sonochemistry synthesis of Bi2S3/CdS heterostructure with enhanced performance for photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2014, 39, 14479–14486. [Google Scholar] [CrossRef]
- Hosseini, Z.; Azizian-Kalandaragh, Y.; Khodayari, A.; Nedaee-Shakarab, B. Sonochemically prepared PbS nanostructures and investigation of their optical and structural properties. Optoelectron. Adv. Mater. 2014, 8, 201–203. [Google Scholar]
- Kis-Csitári, J.; Kónya, Z.; Kiricsi, I. Sonochemical Synthesis of Inorganic Nanoparticles. In Functionalized Nanoscale Materials, Devices and Systems. NATO Science for Peace and Security Series B: Physics and Biophysics; Vaseashta, A., Mihailescu, I.N., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 369–372. [Google Scholar]
- Kumar, R.V.; Palchik, O.; Koltypin, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis and characterization of Ag2S/PVA and CuS/PVA nanocomposite. Ultrason. Sonochem. 2002, 9, 65–70. [Google Scholar] [CrossRef]
- Lee, G.-J.; Anandan, S.; Masten, S.J.; Wu, J.J. Sonochemical Synthesis of Hollow Copper Doped Zinc Sulfide Nanostructures: Optical and Catalytic Properties for Visible Light Assisted Photosplitting of Water. Ind. Eng. Chem. Res. 2014, 53, 8766–8772. [Google Scholar] [CrossRef]
- Lopes, P.A.L.; Santos, M.B.; Mascarenhas, A.J.S.; Silva, L.A. Synthesis of CdS nano-spheres by a simple and fast sonochemical method at room temperature. Mater. Lett. 2014, 136, 111–113. [Google Scholar] [CrossRef]
- Murcia, M.J.; Shaw, D.L.; Woodruff, H.; Naumann, C.A.; Young, B.A.; Long, E.C. Facile Sonochemical Synthesis of Highly Luminescent ZnS−Shelled CdSe Quantum Dots. Chem. Mater. 2006, 18, 2219–2225. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P.; Stróz, D.; Szala, J.; Rzychoń, T.; Bober, Ł.; Toroń, B.; Nowrot, A. Sonochemical preparation of SbS(1-x)Se(x)I nanowires. Ultrason. Sonochem. 2010, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, M.; Jung, W.M.; Lee, W.Y.; Kim, H.; Kim, Y.; Hwang, C.; Shim, I.-W. Syntheses of Cu2SnS3 and Cu2ZnSnS4 nanoparticles with tunable Zn/Sn ratios under multibubble sonoluminescence conditions. Dalton Trans. 2013, 42, 10545–10550. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Estevané, P.; Sánchez, E.M. Preparation of Sb2S3 Nanostructures by the Ionic Liquid-Assisted Sonochemical Method. Cryst. Growth Des. 2010, 10, 3917–3924. [Google Scholar] [CrossRef]
- Wang, G.Z.; Geng, B.Y.; Huang, X.M.; Wang, Y.W.; Li, G.H.; Zhang, L.D. A convenient ultrasonic irradiation technique for in situ synthesis of zinc sulfide nanocrystallites at room temperature. Appl. Phys. A 2003, 77, 933–936. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.-R.; Zhao, X.-N.; Xu, S.; Zhu, J.-J. Preparation of copper monosulfide and nickel monosulfide nanoparticles by sonochemical method. Mater. Lett. 2002, 55, 253–258. [Google Scholar] [CrossRef]
- Wu, G.S.; Yuan, X.Y.; Xie, T.; Xu, G.C.; Zhang, L.D.; Zhuang, Y.L. A simple synthesis route to CdS nanomaterials with different morphologies by sonochemical reduction. Mater. Lett. 2004, 58, 794–797. [Google Scholar] [CrossRef]
- Yadav, R.S.; Mishra, P.; Mishra, R.; Kumar, M.; Pandey, A.C. Growth mechanism and optical property of CdS nanoparticles synthesized using amino-acid histidine as chelating agent under sonochemical process. Ultrason. Sonochem. 2010, 17, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-M.; Zhang, X.-H.; Meng, X.-M.; Fan, X.; Lee, S.-T.; Wu, S.-K. Sonochemical synthesis of mass single-crystal PbS nanobelts. J. Solid State Chem. 2005, 178, 399–403. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Palchik, O.; Koltypin, Y.; Gedanken, A. A Novel Sonochemical Method for the Preparation of Nanophasic Sulfides: Synthesis of HgS and PbS Nanoparticles. J. Solid State Chem. 2000, 153, 342–348. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, J.; Ma, T.-Y.; Liu, Y.-P.; Du, G.; Yuan, Z.-Y. Sonochemistry-assisted synthesis and optical properties of mesoporous ZnS nanomaterials. J. Mater. Chem. A 2014, 2, 1093. [Google Scholar] [CrossRef]
- Kharazmi, A.; Saion, E.; Faraji, N.; Hussin, R.M.; Yunus, W.M.M. Structural, optical and thermal properties of PVA/CdS nanocomposites synthesized by radiolytic method. Radiat. Phys. Chem. 2014, 97, 212–216. [Google Scholar] [CrossRef]
- Mostafavi, M.; Liu, Y.; Pernot, P.; Belloni, J. Dose rate effect on size of CdS clusters induced by irradiation. Radiat. Phys. Chem. 2000, 59, 49–59. [Google Scholar] [CrossRef]
- Souici, A.H.; Keghouche, N.; Delaire, J.A.; Remita, H.; Mostafavi, M. Radiolytic synthesis and optical properties of ultra-small stabilized ZnS nanoparticles. Chem. Phys. Lett. 2006, 422, 25–29. [Google Scholar] [CrossRef]
- Evans, J.E.; Jungjohann, K.L.; Browning, N.D.; Arslan, I. Controlled growth of nanoparticles from solution with in situ liquid transmission electron microscopy. Nano Lett. 2011, 11, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Micic, O.I.; Nenadovic, M.T.; Swayambunathan, V.; Meisel, D. Radiolytic production and properties of ultrasmall cadmium sulfide particles. J. Phys. Chem. 1989, 93, 4603–4608. [Google Scholar] [CrossRef]
- Swayambunathan, V.; Hayes, D.; Schmidt, K.H.; Liao, Y.X.; Meisel, D. Thiol surface complexation on growing cadmium sulfide clusters. J. Am. Chem. Soc. 1990, 112, 3831–3837. [Google Scholar] [CrossRef]
- Ni, Y.; Ge, X.; Liu, H.; Xu, X.; Zhang, Z. γ-Irradiation preparation of CdS nano-particles and their formation mechanism in non-water system. Radiat. Phys. Chem. 2001, 61, 61–64. [Google Scholar] [CrossRef]
- Ullrich, B.; Ezumi, H.; Keitoku, S.; Kobayashi, T. Luminescence properties of p-type thin CdS films prepared by laser ablation. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 1995, 35, 117–119. [Google Scholar] [CrossRef]
- Zhang, D.S.; Goekce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.X.; Bosman, M.; Thamankar, R.; Zulkifli, M.F.B.; Yen, S.K.; Hariharan, A.; Sudhaharan, T.; Selvan, S.T. Highly Luminescent Heterostructured Copper-Doped Zinc Sulfide Nanocrystals for Application in Cancer Cell Labeling. Chemphyschem 2016, 17, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Chander, H. Development of nanophosphors—A review. Mater. Sci. Eng. R Rep. 2005, 49, 113–155. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, E.; Lian, S.; Kang, Z.; Lan, Y.; Wu, D. Microemulsion-directed synthesis of different CuS nanocrystals. Solid State Commun. 2004, 130, 309–312. [Google Scholar] [CrossRef]
- Selvan, S.T.; Tan, T.T.Y.; Yi, D.K.; Jana, N.R. Functional and multifunctional nanoparticles for bioimaging and biosensing. Langmuir ACS J. Surf. Colloids 2010, 26, 11631–11641. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Chua, S.J.; Liu, B.; Gan, L.M.; Chew, C.H.; Xu, G.Q. Luminescence characteristics of impurities-activated ZnS nanocrystals prepared in microemulsion with hydrothermal treatment. Appl. Phys. Lett. 1998, 73, 478–480. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, L. Copper sulfide flakes and nanodisks. J. Mater. Chem. 2003, 13, 2007–2010. [Google Scholar] [CrossRef]
- Agostiano, A.; Catalano, M.; Curri, M.L.; Della Monica, M.; Manna, L.; Vasanelli, L. Synthesis and structural characterisation of CdS nanoparticles prepared in a four-components “water-in-oil” microemulsion. Micron 2000, 31, 253–258. [Google Scholar] [CrossRef]
- Curri, M.L.; Agostiano, A.; Manna, L.; Della Monica, M.; Catalano, M.; Chiavarone, L.; Spagnolo, V.; Lugara, M. Synthesis and characterization of CdS nanoclusters in a quarternary microemulsion: The role of the cosurfactant. J. Phys. Chem. B 2000, 104, 8391–8397. [Google Scholar] [CrossRef]
- Fini, P.; Curri, M.L.; Castagnolo, M.; Ciampi, F.; Agostiano, A. Calorimetric study of US nanoparticle formation in w/o microemulsions. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2003, 23, 1077–1081. [Google Scholar] [CrossRef]
- Bechthold, N.; Tiarks, F.; Willert, M.; Landfester, K.; Antonietti, M. Miniemulsion polymerization: Applications and new materials. Macromol. Symp. 2000, 151, 549–555. [Google Scholar] [CrossRef]
- Dolcet, P.; Maurizio, C.; Casarin, M.; Pandolfo, L.; Gialanella, S.; Badocco, D.; Pastore, P.; Speghini, A.; Gross, S. An Effective Two-Emulsion Approach to the Synthesis of Doped ZnS Crystalline Nanostructures. Eur. J. Inorg. Chem. 2015, 2015, 706–714. [Google Scholar] [CrossRef]
- Landfester, K. Synthesis of colloidal particles in miniemulsions. Annu. Rev. Mater. Res. 2006, 36, 231–279. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsions for nanoparticle synthesis. Top. Curr. Chem. 2003, 227, 75–123. [Google Scholar]
- Landfester, K. Recent developments in miniemulsions—Formation and stability mechanisms. Macromol. Symp. 2000, 150, 171–178. [Google Scholar] [CrossRef]
- Landfester, K. The Generation of Nanoparticles in Miniemulsions. Adv. Mater. 2001, 13, 765–768. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsions for Nanoparticle Synthesis. In Colloid Chemistry II; Antonietti, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 227, pp. 75–123. [Google Scholar]
- Muñoz-Espí, R.; Weiss, C.K.; Landfester, K. Inorganic nanoparticles prepared in miniemulsion. Curr. Opin. Colloid Interface Sci. 2012, 17, 212–224. [Google Scholar] [CrossRef]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Armelao, L.; Camozzo, D.; Gross, S.; Tondello, E. Synthesis of copper sulfide nanoparticles in carboxylic acids as solvent. J. Nanosci. Nanotechnol. 2006, 6, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Liz-Marzan, L.M. The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev. 2014, 43, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Hyeon, T. Colloidal Chemical Synthesis and Formation Kinetics of Uniformly Sized Nanocrystals of Metals, Oxides, and Chalcogenides. Acc. Chem. Res. 2008, 41, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Leon-Velazquez, M.S.; Irizarry, R.; Castro-Rosario, M.E. Nucleation and Growth of Silver Sulfide Nanoparticles. J. Phys. Chem. C 2010, 114, 5839–5849. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Mudring, A.-V.; Alammar, T.; Baecker, T.; Richter, K. Nanoparticle synthesis in ionic liquids. ACS Symp. Ser. 2009, 1030, 177–188. [Google Scholar]
- Muñoz-Espì, R.; Mastai, Y.; Gross, S.; Landfester, K. Colloidal systems for crystallization processes from liquid phase. CrystEngComm 2013, 15, 2175–2191. [Google Scholar] [CrossRef]
- Najmaei, S.; Lou, J. Synthesis, Characterization and Engineering of Two-Dimensional Transition Metal Dichalcogenides. In Proceedings of the 2014 IEEE 14th International Conference on Nanotechnology (IEEE-Nano), Toronto, ON, Canada, 18–21 August 2014; pp. 616–619. [Google Scholar]
- Palberg, T. Crystallization kinetics of colloidal model suspensions: Recent achievements and new perspectives. J. Phys. Condens. Matter 2014, 26, 333101. [Google Scholar] [CrossRef] [PubMed]
- Rempel, J.Y.; Bawendi, M.G.; Jensen, K.F. Insights into the Kinetics of Semiconductor Nanocrystal Nucleation and Growth. J. Am. Chem. Soc. 2009, 131, 4479–4489. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.R.; Habas, S.; Yang, P.D. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Warad, H.C.; Ghosh, C.K.; Hemtanon, B.; Thanachayanont, C.; Dutta, J. Luminescent nanoparticles of Mn doped ZnS passivated with sodium hexametaphosphate. Sci. Technol. Adv. Mater. 2005, 6, 296. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Dharsana, U.S.; Varsha, M.; Behlol, A.A.K.; Veerappan, A.; Thiagarajan, R. Sulfidation modulates the toxicity of biogenic copper nanoparticles. RSC Adv. 2015, 5, 30248–30259. [Google Scholar] [CrossRef]
- Gonzalez-Estrella, J.; Puyol, D.; Sierra-Alvarez, R.; Field, J.A. Role of biogenic sulfide in attenuating zinc oxide and copper nanoparticle toxicity to acetoclastic methanogenesis. J. Hazard. Mater. 2015, 283, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kennedy, D.W.; Dohnalkova, A.; Moore, D.A.; Nachimuthu, P.; Reed, S.B.; Fredrickson, J.K. Manganese sulfide formation via concomitant microbial manganese oxide and thiosulfate reduction. Environ. Microbiol. 2011, 13, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Duran, N. Biogenic nanoparticles: Copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K.; Doktycz, M.J.; Wang, W.; Moon, J.W.; Gu, B.H.; Meyer, H.M.; Hensley, D.K.; Allison, D.P.; Phelps, T.J.; Pelletier, D.A. Monodispersed biocompatible silver sulfide nanoparticles: Facile extracellular biosynthesis using the gamma-proteobacterium, Shewanella oneidensis. Acta Biomater. 2011, 7, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; Lens, P.N.L.; Balakrishnan, R.M. Microbial synthesis of chalcogenide semiconductor nanoparticles: A review. Microb. Biotechnol. 2016, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Solution Processing of Inorganic Materials; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Diodati, S.; Dolcet, P.; Casarin, M.; Gross, S. Pursuing the Crystallization of Mono- and Polymetallic Nanosized Crystalline Inorganic Compounds by Low-Temperature Wet-Chemistry and Colloidal Routes. Chem. Rev. 2015, 115, 11449–11502. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Solution-processed inorganic semiconductors. J. Mater. Chem. 2004, 14, 2355–2365. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles—Formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [PubMed]
- Balantseva, E.; Berlier, G.; Camino, B.; Lessio, M.; Ferrari, A.M. Surface Properties of ZnS Nanoparticles: A Combined DFT and Experimental Study. J. Phys. Chem. C 2014, 118, 23853–23862. [Google Scholar] [CrossRef]
- Busca, G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys. Chem. Chem. Phys. 1999, 1, 723–736. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Lorenzelli, V. Low-temperature CO2 adsorption on metal oxides: Spectroscopic characterization of some weakly adsorbed species. Mater. Chem. Phys. 1991, 29, 425–435. [Google Scholar] [CrossRef]
- Dinter, N.; Rusanen, M.; Raybaud, P.; Kasztelan, S.; da Silva, P.; Toulhoat, H. Temperature-programed reduction of unpromoted MoS2-based hydrodesulfurization catalysts: Experiments and kinetic modeling from first principles. J. Catal. 2009, 267, 67–77. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Becker, U.; Wright, K. Sulphide mineral surfaces: Theory and experiment. Int. J. Miner. Process. 1997, 51, 1–14. [Google Scholar] [CrossRef]
- Emin, S.; Lisjak, D.; Pitcher, M.; Valant, M. Structural and morphological transformations of textural porous zinc sulfide microspheres. Microporous Mesoporous Mater. 2013, 165, 185–192. [Google Scholar] [CrossRef]
- Hamad, S.; Cristol, S.; Catlow, C.R.A. Surface structures and crystal morphology of ZnS: Computational study. J. Phys. Chem. B 2002, 106, 11002–11008. [Google Scholar] [CrossRef]
- Balantseva, E.; Camino, B.; Ferrari, A.M.; Berlier, G. Effect of Post-Synthesis Treatments on the Properties of ZnS Nanoparticles: An Experimental and Computational Study. Oil Gas Sci. Technol. Revue d’IFP Energies Nouvelles 2015, 70, 817–829. [Google Scholar] [CrossRef]
- Tasker, P.W. The stability of ionic crystal surfaces. J. Phys. C 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Wells, S.; Alfe, D.; Blanchard, L.; Brodholt, J.; Calleja, M.; Catloe, R.; Price, D.; Tyler, R.; Wright, K. Ab-initio simulations of magnetic iron sulphides. Mol. Simul. 2005, 31, 379–384. [Google Scholar] [CrossRef]
- Spirko, J.A.; Neiman, M.L.; Oelker, A.M.; Klier, K. Electronic structure and reactivity of defect MoS2I. Relative stabilities of clusters and edges, and electronic surface states. Surf. Sci. 2003, 542, 192–204. [Google Scholar] [CrossRef]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2 (111) and (210) surfaces. Surf. Sci. 2002, 520, 111–119. [Google Scholar] [CrossRef]
- Wen, X.D.; Ren, J.; Li, Y.W.; Wang, J.G.; Jiao, H.J. NO adsorption on triangular Mo28S60 cluster. Chem. Phys. Lett. 2007, 436, 209–212. [Google Scholar] [CrossRef]
- Wen, X.D.; Zeng, T.; Teng, B.T.; Zhang, F.Q.; Li, Y.W.; Wang, H.G.; Jiao, H.J. Hydrogen adsorption on a Mo(27)S(54)cluster: A density functional theory study. J. Mol. Catal. A Chem. 2006, 249, 191–200. [Google Scholar] [CrossRef]
- Temel, B.; Tuxen, A.K.; Kibsgaard, J.; Topsoe, N.Y.; Hinnemann, B.; Knudsen, K.G.; Topsoe, H.; Lauritsen, J.V.; Besenbacher, F. Atomic-scale insight into the origin of pyridine inhibition of MoS2-based hydrotreating catalysts. J. Catal. 2010, 271, 280–289. [Google Scholar] [CrossRef]
- Tuxen, A.; Gobel, H.; Hinnemann, B.; Li, Z.S.; Knudsen, K.G.; Topsoe, H.; Lauritsen, J.V.; Besenbacher, F. An atomic-scale investigation of carbon in MoS2 hydrotreating catalysts sulfided by organosulfur compounds. J. Catal. 2011, 281, 345–351. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Tuxen, A.; Hinnemann, B.; Lauritsen, J.V.; Knudsen, K.G.; Besenbacher, F.; Topsoe, H. Spectroscopy, microscopy and theoretical study of NO adsorption on MoS2 and Co-Mo-S hydrotreating catalysts. J. Catal. 2011, 279, 337–351. [Google Scholar] [CrossRef]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [PubMed]
- Moses, P.G.; Grabow, L.C.; Fernandez, E.M.; Hinnemann, B.; Topsoe, H.; Knudsen, K.G.; Norskov, J.K. Trends in Hydrodesulfurization Catalysis Based on Realistic Surface Models. Catal. Lett. 2014, 144, 1425–1432. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.H.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Tsyganenko, A.A.; Can, F.; Travert, A.; Mauge, F. FTIR study of unsupported molybdenum sulfide—In situ synthesis and surface properties characterization. Appl. Catal. A Gen. 2004, 268, 189–197. [Google Scholar] [CrossRef]
- Mauge, F.; Lamotte, J.; Nesterenko, N.S.; Manoilova, O.; Tsyganenko, A.A. FT-IR study of surface properties of unsupported MoS2. Catal. Today 2001, 70, 271–284. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Woodruff, S. In Situ Fourier Transform Infrared Characterization of Sulfur Species Resulting from the Reaction of Water Vapor and Oxygen with Zinc Sulfide. Ind. Eng. Chem. Res. 1997, 36, 5277–5281. [Google Scholar] [CrossRef]
- Kendelewicz, T.; Doyle, C.S.; Bostick, B.C.; Brown, G.E. Initial oxidation of fractured surfaces of FeS2(100) by molecular oxygen, water vapor, and air. Surf. Sci. 2004, 558, 80–88. [Google Scholar] [CrossRef]
- Guevremont, J.M.; Bebie, J.; Elsetinow, A.R.; Strongin, D.R.; Schoonen, M.A.A. Reactivity of the (100) plane of pyrite in oxidizing gaseous and aqueous environments: Effects of surface imperfections. Environ. Sci. Technol. 1998, 32, 3743–3748. [Google Scholar] [CrossRef]
- Guevremont, J.M.; Elsetinow, A.R.; Strongin, D.R.; Bebie, J.; Schoonen, M.A.A. Structure sensitivity of pyrite oxidation: Comparison of the (100) and (111) planes. Am. Mineral. 1998, 83, 1353–1356. [Google Scholar] [CrossRef]
- Guevremont, J.M.; Strongin, D.R.; Schoonen, M.A.A. Thermal chemistry of H2S and H2O on the (100) plane of pyrite: Unique reactivity of defect sites. Am. Mineral. 1998, 83, 1246–1255. [Google Scholar] [CrossRef]
- Blanchard, M.; Wright, K.; Gale, J.D.; Catlow, C.R.A. Adsorption of As(OH)(3) on the (001) surface of FeS2 pyrite: A quantum-mechanical DFT study. J. Phys. Chem. C 2007, 111, 11390–11396. [Google Scholar] [CrossRef]
- Zezza, F.; Comparelli, R.; Striccoli, M.; Curri, M.L.; Tommasi, R.; Agostiano, A.; Della Monica, M. High quality CdS nanocrystals: Surface effects. Synth. Met. 2003, 139, 597–600. [Google Scholar] [CrossRef]
- Fini, P.; Depalo, N.; Comparelli, R.; Curri, M.L.; Striccoli, M.; Castagnolo, M.; Agostiano, A. Interactions between surfactant capped CdS nanocrystals and organic solvent. J. Therm. Anal. Calorim. 2008, 92, 271–277. [Google Scholar] [CrossRef]
- Ingrosso, C.; Panniello, A.; Comparelli, R.; Curri, M.L.; Striccoli, M. Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication. Materials 2010, 3, 1316–1352. [Google Scholar] [CrossRef]
- Curri, M.L.; Comparelli, R.; Striccoli, M.; Agostiano, A. Emerging methods for fabricating functional structures by patterning and assembling engineered nanocrystals. Phys. Chem. Chem. Phys. 2010, 12, 11197–11207. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Fanizza, E.; Corricelli, M.; Comparelli, R.; Striccoli, M.; Curri, M.L. Patterned assembly of luminescent nanocrystals: Role of the molecular chemistry at the interface. J. Nanoparticle Res. 2014, 16, 2468. [Google Scholar] [CrossRef]
- Corricelli, M.; Comparelli, R.; Depalo, N.; Fanizza, E.; Sadhu, V.B.; Huskens, J.; Agostiano, A.; Striccoli, M.; Curri, M.L. Surface Functionalized Luminescent Nanocrystals Electrostatically Assembled onto a Patterned Substrate. Curr. Nanosci. 2016, 12, 386–395. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Curri, M.L.; Innocenti, C.; Sangregorio, C.; Achterhold, K.; Parak, F.G.; Agostiano, A.; et al. Seeded growth of asymmetric binary nanocrystals made of a semiconductor TiO2 rodlike section and a magnetic gamma-Fe2O3 spherical domain. J. Am. Chem. Soc. 2006, 128, 16953–16970. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D. Advanced Wet-Chemical Synthetic Approaches to Inorganic Nanostructures; Transworld Research Network: Kerala, India, 2008; p. 453. [Google Scholar]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Colloidal synthesis of organic-capped ZnO nanocrystals via a sequential reduction-oxidation reaction. J. Phys. Chem. B 2005, 109, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Manna, L. Synthetic strategies to size and shape controlled nanocrystals and nanocrystal heterostructures. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; Volume 620, pp. 1–17. [Google Scholar]
- Cozzoli, P.D.; Manna, L.; Curri, M.L.; Kudera, S.; Giannini, C.; Striccoli, M.; Agostiano, A. Shape and phase control of colloidal ZnSe nanocrystals. Chem. Mater. 2005, 17, 1296–1306. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Snoeck, E.; Garcia, M.A.; Giannini, C.; Guagliardi, A.; Cervellino, A.; Gozzo, F.; Hernando, A.; Achterhold, K.; Ciobanu, N.; et al. Colloidal synthesis and characterization of tetrapod-shaped magnetic nanocrystals. Nano Lett. 2006, 6, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P.; Cozzoli, P.D.; Pinna, N. Self-assembled supracrystals and hetero-structures made from colloidal nanocrystals. CrystEngComm 2014, 16, 9365–9367. [Google Scholar] [CrossRef]
- Kolny-Olesiak, J.; Weller, H. Synthesis and Application of Colloidal CuInS2 Semiconductor Nanocrystals. ACS Appl. Mater. Interfaces 2013, 5, 12221–12237. [Google Scholar] [CrossRef] [PubMed]
- Weller, H. Synthesis and self-assembly of colloidal nanoparticles. Philos. Trans. R. Soc. Lond. Ser. A 2003, 361, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Weller, H. Quantum size colloids: From size-dependent properties of discrete particles to self-organized superstructures. Curr. Opin. Colloid Interface Sci. 1998, 3, 194–199. [Google Scholar] [CrossRef]
- Weller, H. Colloidal semiconductor Q-particles: Chemistry in the transition region between solid and molecular states. Angew. Chem. Int. Ed. 1993, 32, 41–53. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand Exchange and the Stoichiometry of Metal Chalcogenide Nanocrystals: Spectroscopic Observation of Facile Metal-Carboxylate Displacement and Binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Niederberger, M.; Smarsly, B. Self-assembly in inorganic and hybrid systems: Beyond the molecular scale. Dalton Trans. 2008, 18–24. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A Versatile Bottom-up Assembly Approach to Colloidal Spheres from Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef] [PubMed]
- Bealing, C.R.; Baumgardner, W.J.; Choi, J.J.; Hanrath, T.; Hennig, R.G. Predicting Nanocrystal Shape through Consideration of Surface-Ligand Interactions. ACS Nano 2012, 6, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M., Jr. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Brus, L. Noble Metal Nanocrystals: Plasmon Electron Transfer Photochemistry and Single-Molecule Raman Spectroscopy. Acc. Chem. Res. 2008, 41, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, L.; Kitaev, V. On the nature and importance of the transition between molecules and nanocrystals: Towards a chemistry of “nanoscale perfection”. Nanoscale 2011, 3, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal strategies for preparing oxide-based hybrid nanocrystals. Eur. J. Inorg. Chem. 2008, 2008, 837–854. [Google Scholar] [CrossRef]
- Comin, A.; Manna, L. New materials for tunable plasmonic colloidal nanocrystals. Chem. Soc. Rev. 2014, 43, 3957–3975. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; De La Fuente, J.M. Revisiting 30 years of Biofunctionalization and Surface Chemistry of Inorganic Nanoparticles for Nanomedicine. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Courty, A.; Fermon, C.; Pileni, M.P. “Supra crystals” made of nanocrystals. Adv. Mater. 2001, 13, 254–258. [Google Scholar] [CrossRef]
- De Roo, J.; Baquero, E.A.; Coppel, Y.; De Keukeleere, K.; Van Driessche, I.; Nayral, C.; Hens, Z.; Delpech, F. Insights into the Ligand Shell, Coordination Mode, and Reactivity of Carboxylic Acid Capped Metal Oxide Nanocrystals. Chempluschem 2016, 81, 1216–1223. [Google Scholar] [CrossRef]
- Fu, A.; Gu, W.; Larabell, C.; Alivisatos, A.P. Semiconductor nanocrystals for biological imaging. Curr. Opin. Neurobiol. 2005, 15, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Gaponik, N.; Eychmueller, A. Colloidal nanocrystals. On the way from synthesis to applications. Proc. SPIE Int. Soc. Opt. Eng. 2007, 6785, M7850. [Google Scholar]
- Gomez, D.E.; Califano, M.; Mulvaney, P. Optical properties of single semiconductor nanocrystals. Phys. Chem. Chem. Phys. 2006, 8, 4989–5011. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.R. Nanocrystals assembled from the bottom up. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 6, pp. 537–554. [Google Scholar]
- Motte, L.; Courty, A.; Anh-Tu, N.; Lisiecki, I.; Pileni, M.-P. Self-Organization of Inorganic Nanocrystals. Nanocryst. Form. Mesoscopic Struct. 2006, 1–47. [Google Scholar] [CrossRef]
- Niu, W.X.; Xu, G.B. Crystallographic control of noble metal nanocrystals. Nano Today 2011, 6, 265–285. [Google Scholar] [CrossRef]
- Parak, W.J.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, S.C.; Boudreau, R.; Le, G.M.A.; Larabell, C.A.; Alivisatos, A.P. Biological applications of colloidal nanocrystals. Nanotechnology 2003, 14, R15–R27. [Google Scholar] [CrossRef]
- Pileni, M.P. Supracrystals of Inorganic Nanocrystals: An Open Challenge for New Physical Properties. Acc. Chem. Res. 2008, 41, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Self-organization of inorganic nanocrystals. J. Phys. Condens. Matter 2006, 18, S67–S84. [Google Scholar] [CrossRef]
- Pileni, M.P. Nanocrystals: Fabrication, organization and collective properties. Comptes Rendus Chim. 2003, 6, 965–978. [Google Scholar] [CrossRef]
- Pileni, M.P. Nanocrystals: Size and Shape Control; IOS Press: Amsterdam, The Netherlands, 2003; pp. 25–31. [Google Scholar]
- Pileni, M.P. Semiconductor Nanocrystals. In Nanoscale Materials in Chemistry; Klabunde, K.J., Ed.; Wiley VCH: Weinheim, Germany, 2001; pp. 61–84. [Google Scholar]
- Pileni, M.P. Nanocrystals. In The Chemistry of Nanostructured Material; Yang, P., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2003; pp. 127–146. [Google Scholar]
- Pileni, M.P. Self-assembly of inorganic nanocrystals: Fabrication and collective intrinsic properties. Acc. Chem. Res. 2007, 40, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Self assembly of inorganic nanocrystals in 3D supra crystals: Intrinsic properties. Surf. Sci. 2009, 603, 1498–1505. [Google Scholar] [CrossRef]
- Pileni, M.P.; Lalatonne, Y.; Ingert, D.; Lisiecki, I.; Courty, A. Self assemblies of nanocrystals: Preparation, collective properties and uses. Faraday Discuss. 2004, 125, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Polarz, S. Shape Matters: Anisotropy of the Morphology of Inorganic Colloidal Particles—Synthesis and Function. Adv. Funct. Mater. 2011, 21, 3214–3230. [Google Scholar] [CrossRef]
- Pradhan, N.; Reifsnyder, D.; Xie, R.G.; Aldana, J.; Peng, X.G. Surface ligand dynamics in growth of nanocrystals. J. Am. Chem. Soc. 2007, 129, 9500–9509. [Google Scholar] [CrossRef] [PubMed]
- Rogach, A.L.; Talapin, D.V.; Weller, H. Semiconductor Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 52–95. [Google Scholar]
- Scher, E.C.; Manna, L.; Alivisatos, A.P. Shape control and applications of nanocrystals. Philos. Trans. R. Soc. Lond. Ser. A 2003, 361, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Shavel, A.; Gaponik, N.; Eychmueller, A. The assembling of semiconductor nanocrystals. Eur. J. Inorg. Chem. 2005, 3613–3623. [Google Scholar] [CrossRef]
- Van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The Heat-Up Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, X. Aqueous-based route toward noble metal nanocrystals: Morphology-controlled synthesis and their applications. Nanoscale 2010, 2, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Weller, H. Quantized semiconductor particles: A novel state of matter for materials science. Adv. Mater. 1993, 5, 88–95. [Google Scholar] [CrossRef]
- Weller, H. Colloidal semiconductor Q-particles: Chemistry in the transition between solid-state and molecules. Angew. Chem.-Int. Ed. Engl. 1993, 32, 41–53. [Google Scholar] [CrossRef]
- De Roo, J.; De Keukeleere, K.; Hens, Z.; Van Driessche, I. From ligands to binding motifs and beyond; the enhanced versatility of nanocrystal surfaces. Dalton Trans. 2016, 45, 13277–13283. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Van den Broeck, F.; De Keukeleere, K.; Martins, J.C.; Van Driessche, I.; Hens, Z. Unravelling the Surface Chemistry of Metal Oxide Nanocrystals, the Role of Acids and Bases. J. Am. Chem. Soc. 2014, 136, 9650–9657. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Debellis, D.; Suranna, G.P.; Gigli, G.; Giansante, C. The Dynamic Organic/Inorganic Interface of Colloidal PbS Quantum Dots. Angew. Chem.-Int. Ed. 2016, 55, 6626–6632. [Google Scholar]
- Hens, Z.; Martins, J.C. A Solution NMR Toolbox for Characterizing the Surface Chemistry of Colloidal Nanocrystals. Chem. Mater. 2013, 25, 1211–1221. [Google Scholar] [CrossRef]
- Hens, Z.; Van den Broeck, F.; De Roo, J.; Dierick, R.; Van Driessche, I.; Martins, J.C. Surface Chemistry of Colloidal Nanocrystals—From Semiconductors to Metal Oxides. In Abstracts of Papers of the American Chemical Society. VOL. 248. 1155 16TH ST, NW; Amer. Chemical Soc.: Washington, DC, USA, 2014. [Google Scholar]
- Mews, A. Surface chemistry of semiconductor nanocrystals. Z. Phys. Chem. 2007, 221, 295–306. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, N. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 2013, 8, 168–197. [Google Scholar] [CrossRef]
- Beaulac, R.; Ochsenbein, S.T.; Gamelin, D.R. Colloidal transition-metal-doped quantum dots. In Nanocrystal Quantum Dots; Klimov, V.I., Ed.; Taylor & Francis: New York, NY, USA, 2010; p. 397. [Google Scholar]
- Clift, M.J.D.; Stone, V. Quantum Dots: An Insight and Perspective of Their Biological Interaction and How This Relates to Their Relevance for Clinical Use. Theranostics 2012, 2, 668–680. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Van Driessche, I.; Martins, J.C.; Hens, Z. Colloidal metal oxide nanocrystal catalysis by sustained chemically driven ligand displacement. Nat. Mater. 2016, 15, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Maillard, M.; Motte, L.; Pileni, M.P. Rings and hexagons made of nanocrystals. Adv. Mater. 2001, 13, 200–204. [Google Scholar] [CrossRef]
- Capetti, E.; Ferretti, A.M.; Dal Santo, V.; Ponti, A. Surfactant-controlled composition and crystal structure of manganese(II) sulfide nanocrystals prepared by solvothermal synthesis. Beilstein J. Nanotechnol. 2015, 6, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, A.A.A.; Martins, M.; Soares, D.A.W.; França, É.J. Modeling of ZnS quantum dots synthesis by DFT techniques. J. Mol. Struct. 2008, 873, 121–129. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, R.; Hendricks, M.P.; Cossairt, B.M.; Liu, H.T.; Owen, J.S. Conversion Reactions of Cadmium Chalcogenide Nanocrystal Precursors. Chem. Mater. 2013, 25, 1233–1249. [Google Scholar] [CrossRef]
- Kolny-Olesiak, J. Synthesis of copper sulphide-based hybrid nanostructures and their application in shape control of colloidal semiconductor nanocrystals. Crystengcomm 2014, 16, 9381–9390. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial Biosynthesis of Cadmium Sulfide Nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Bertagnolli, H.; Gross, S.; Krishnan, V.; Lavrencic-Stangar, U.; Mueller, K.; Orel, B.; Srinivasan, G.; Tondello, E.; Zattin, A. Zr and Hf oxoclusters as building blocks for the preparation of nanostructured hybrid materials and binary oxides MO2-SiO2 (M = Hf, Zr). J. Mater. Chem. 2005, 15, 1954–1965. [Google Scholar] [CrossRef]
- Patel, J.D.; Mighri, F.; Ajji, A. Generalized chemical route to develop fatty acid capped highly dispersed semiconducting metal sulphide nanocrystals. Mater. Res. Bull. 2012, 47, 2016–2021. [Google Scholar] [CrossRef]
- Bae, S.; Mannan, M.B.; Lee, W. Adsorption of cationic cetylpyridinium chloride on pyrite surface. J. Ind. Eng. Chem. 2012, 18, 1482–1488. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal silver nanoparticles: Photochemical preparation and interaction with O-2, CCl4, and some metal ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Henglein, A.; Fojtik, A.; Weller, H. Reactions on colloidal semiconductor particles. Ber. Bunsen-Ges. Phys. Chem. 1987, 91, 441–446. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Hao, W.; Sun, Z.-X. Surface stoichiometry of zinc sulfide and its effect on the adsorption behaviors of xanthate. Chem. Cent. J. 2011, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, J.; Feng, Z.; Li, M.; Li, C. Visible emission characteristics from different defects of ZnS nanocrystals. Phys. Chem. Chem. Phys. PCCP 2011, 13, 4715–4723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, F.; Gilbert, B.; Banfield, J.F. Molecular Dynamics Simulations, Thermodynamic Analysis, and Experimental Study of Phase Stability of Zinc Sulfide Nanoparticles. J. Phys. Chem. B 2003, 107, 13051–13060. [Google Scholar] [CrossRef]
- Moreels, I.; Justo, Y.; De Geyter, B.; Haustraete, K.; Martins, J.C.; Hens, Z. Size-Tunable, Bright, and Stable PbS Quantum Dots: A Surface Chemistry Study. ACS Nano 2011, 5, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Justo, Y.; De Keukeleere, K.; Van den Broeck, F.; Martins, J.C.; Van Driessche, I.; Hens, Z. Carboxylic-Acid-Passivated Metal Oxide Nanocrystals: Ligand Exchange Characteristics of a New Binding Motif. Angew. Chem. Int. Ed. 2015, 54, 6488–6491. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Ying, J.Y. Functionalization of Inorganic Nanoparticles for Bioimaging Applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.S.; Hackett, M.J.; Hyeon, T. Surface ligands in synthesis, modification, assembly and biomedical applications of nanoparticles. Nano Today 2014, 9, 457–477. [Google Scholar] [CrossRef]
- Lü, C.; Gao, J.; Fu, Y.; Du, Y.; Shi, Y.; Su, Z. A Ligand Exchange Route to Highly Luminescent Surface-Functionalized ZnS Nanoparticles and Their Transparent Polymer Nanocomposites. Adv. Funct. Mater. 2008, 18, 3070–3079. [Google Scholar] [CrossRef]
- Nag, A.; Zhang, H.; Janke, E.; Talapin, D.V. Inorganic Surface Ligands for Colloidal Nanomaterials. Z. Phys. Chem. 2015, 229, 85–107. [Google Scholar] [CrossRef]
- Smolensky, E.D.; Park, H.Y.E.; Berquó, T.S.; Pierre, V.C. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications—Effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Tilley, R.D.; Watkins, J.J. Simple Ligand Exchange Reactions Enabling Excellent Dispersibility and Stability of Magnetic Nanoparticles in Polar Organic, Aromatic, and Protic Solvents. Langmuir 2014, 30, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Yang, P.; Matras-Postolek, K.; Wang, J.P.; Che, Q.D.; Cao, Y.Q.; Ma, Q. Low toxic and highly luminescent CdSe/CdxZn1-xS quantum dots with thin organic SiO2 coating for application in cell imaging. J. Nanopart. Res. 2016, 18, 37. [Google Scholar] [CrossRef]
- Morgese, G.; Causin, V.; Maggini, M.; Corra, S.; Gross, S.; Benetti, E.M. Ultrastable Suspensions of Polyoxazoline-Functionalized ZnO Single Nanocrystals. Chem. Mater. 2015, 27, 2957–2964. [Google Scholar] [CrossRef]
- Amiens, C.; Ciuculescu-Pradines, D.; Philippot, K. Controlled metal nanostructures: Fertile ground for coordination chemists. Coord. Chem. Rev. 2016, 308, 409–432. [Google Scholar] [CrossRef]
- Ma, M.-G.; Cölfen, H. Mesocrystals—Applications and potential. Curr. Opin. Colloid Interface Sci. 2014, 19, 56–65. [Google Scholar] [CrossRef]
- Choi, J.J.; Bealing, C.R.; Bian, K.F.; Hughes, K.J.; Zhang, W.Y.; Smilgies, D.M.; Hennig, R.G.; Engstrom, J.R.; Hanrath, T. Controlling Nanocrystal Superlattice Symmetry and Shape-Anisotropic Interactions through Variable Ligand Surface Coverage. J. Am. Chem. Soc. 2011, 133, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.H. A new approach to the formal classification of covalent compounds of the elements. J. Organomet. Chem. 1995, 500, 127–148. [Google Scholar] [CrossRef]
- Douglas, R.N.; Williamson, C.B.; Hanrath, T.; Robinson, R.D. Surface chemistry of cadmium sulfide magic-sized clusters: A window into ligand-nanoparticle interactions. Chem. Commun. 2017. [Google Scholar] [CrossRef]
- Cölfen, H. Double-hydrophilic block copolymers: Synthesis and application as novel surfactants and crystal growth modifiers. Macromol. Rapid Commun. 2001, 22, 219–252. [Google Scholar] [CrossRef]
- Cölfen, H. Polymer-Mediated Growth of Crystals and Mesocrystals. In Research Methods in Biomineralization Science; Yoreo, J.J.D., Ed.; Elsevier Science Publishing Co., Inc.: New York, NY, USA, 2013; Volume 532, pp. 277–304. [Google Scholar]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Edit. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Garnweitner, G.; Krumeich, F.; Nesper, R.; Cölfen, H.; Antonietti, M. Tailoring the Surface and Solubility Properties of Nanocrystalline Titania by a Nonaqueous In Situ Functionalization Process. Chem. Mater. 2004, 16, 1202–1208. [Google Scholar] [CrossRef]
- Boncher, W.; Dalafu, H.; Rosa, N.; Stoll, S. Europium chalcogenide magnetic semiconductor nanostructures. Coord. Chem. Rev. 2015, 289, 279–288. [Google Scholar] [CrossRef]
- Abbad, A.; Bentata, S.; Bentounes, H.A.; Benstaali, W.; Bouadjemi, B. Study of electronic and magnetic properties of binary zinc sulfide and ternary manganese- and iron-substituted alloys. Mater. Sci. Semicond. Process. 2013, 16, 576–581. [Google Scholar] [CrossRef]
- Owens, F.J.; Gladczuk, L.; Szymczak, R.; Dluzewski, P.; Wisniewski, A.; Szymczak, H.; Golnik, A.; Bernhard, C.; Niedermayer, C. High temperature magnetic order in zinc sulfide doped with copper. J. Phys. Chem. Solids 2011, 72, 648–652. [Google Scholar] [CrossRef]
- Cardinali, M.; Valentini, L.; Fabbri, P.; Kenny, J.M. Radiofrequency plasma assisted exfoliation and reduction of large-area graphene oxide platelets produced by a mechanical transfer process. Chem. Phys. Lett. 2011, 508, 285–288. [Google Scholar] [CrossRef]
- Baruah, S.; Ortinero, C.; Shipin, O.V.; Dutta, J. Manganese Doped Zinc Sulfide Quantum Dots for Detection of Escherichia coli. J. Fluoresc. 2012, 22, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Sluydts, M.; De Nolf, K.; Van Speybroeck, V.; Cottenier, S.; Hens, Z. Ligand Addition Energies and the Stoichiometry of Colloidal Nanocrystals. ACS Nano 2016, 10, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.; Johny, S.; Thomas, D.; Setua, S.; Menon, D.; Nair, S. Bio-conjugated luminescent quantum dots of doped ZnS: A cyto-friendly system for targeted cancer imaging. Nanotechnology 2009, 20, 065102. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate conjugated carboxymethyl chitosan—Manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]
- Armelao, L.; Camozzo, D.; Gross, S.; Tondello, E. Embedding of electroluminescent ZnS:Cu phosphors in PMMA matrix by polymerization of particle suspension in MMA monomer. J. Non-Cryst. Solids 2004, 345–346, 402–406. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W. Synthesis and properties of transition metals and rare-earth metals doped ZnS nanoparticles. Opt. Mater. 2006, 28, 536–550. [Google Scholar] [CrossRef]
- Da Silva, A.R.; Aucelio, R.Q.; Rodriguez-Cotto, R.I.; Ortiz-Martinez, M.G.; Rivera-Ramirez, E.; Frias, D.P.; Macchione, M.; Jimenez-Velez, B.; Gioda, A. Physicochemical properties and toxicological assessment of modified CdS nanoparticles. J. Nanopart. Res. 2014, 16, 2655. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Hossain, S.T.; Das, P.; Mukherjee, S.K. Toxicity of cadmium nanoparticles to Bacillus subtilis. Toxicol. Environ. Chem. 2013, 95, 1748–1756. [Google Scholar] [CrossRef]
- Li, H.; Li, M.Y.; Shih, W.Y.; Lelkes, P.I.; Shih, W.H. Cytotoxicity Tests of Water Soluble ZnS and CdS Quantum Dots. J. Nanosci. Nanotechnol. 2011, 11, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Cai, W.; Chen, X.Y. Semiconductor quantum dots for in vivo imaging. J. Nanosci. Nanotechnol. 2007, 7, 2567–2581. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Su, Y.; Peng, F.; Jiang, Z.; Zhong, Y.; Lu, Y.; Jiang, X.; Huang, Q.; Fan, C.; Lee, S.-T.; He, Y. In vivo distribution, pharmacokinetics, and toxicity of aqueous synthesized cadmium-containing quantum dots. Biomaterials 2011, 32, 5855–5862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.W.; Baumer, W.; Monteiro-Riviere, N.A. Cellular uptake mechanisms and toxicity of quantum dots in dendritic cells. Nanomedicine 2011, 6, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Nie, W.; Cheng, Y.H.; Zhou, X.J.; Chen, L.; Qiu, K.X.; Chen, Z.G.; Zhu, M.F.; He, C.L. In vitro and in vivo toxicity studies of copper sulfide nanoplates for potential photothermal applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.X.; Pang, X.J.; Lei, M.Z.; Ma, M.; Guo, F.; Wang, J.P.; Yu, M.; Tan, F.P.; Li, N. An efficient dual-loaded multifunctional nanocarrier for combined photothermal and photodynamic therapy based on copper sulfide and chlorin e6. Int. J. Pharm. 2016, 503, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, I.; Najdoski, M. Optical and Electrical Properties of Copper Sulfide Films of Variable Composition. J. Solid State Chem. 1995, 114, 469–475. [Google Scholar] [CrossRef]
- Öztaş, M.; Bedir, M.; Necmeddin Yazici, A.; Vural Kafadar, E.; Toktamış, H. Characterization of copper-doped sprayed ZnS thin films. Phys. B 2006, 381, 40–46. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Ther. Innov. Regul. Sci. 2013, 47, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S. Functionalization of magnetic nanoparticles for biomedical applications. Korean J. Chem. Eng. 2014, 31, 1289–1305. [Google Scholar]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing Nanoparticles with Biological Molecules: Developing Chemistries that Facilitate Nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Feldgitscher, C.; Peterlik, H.; Puchberger, M.; Kickelbick, G. Structural Investigations on Hybrid Polymers Suitable as a Nanoparticle Precipitation Environment. Chem. Mater. 2009, 21, 695–705. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2012, 64, 246–255. [Google Scholar] [CrossRef]
- Thiry, M.; Boldt, K.; Nikolic, M.S.; Schulz, F.; Ijeh, M.; Panicker, A.; Vossmeyer, T.; Weller, H. Fluorescence Properties of Hydrophilic Semiconductor Nanoparticles with Tridentate Polyethylene Oxide Ligands. ACS Nano 2011, 5, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. Bioconjugation Using Thiols: Old Chemistry Rediscovered to Connect Polymers with Nature’s Building Blocks. ACS Macro Lett. 2013, 2, 14–18. [Google Scholar] [CrossRef]
- Ulman, A.; Kang, J.F.; Shnidman, Y.; Liao, S.; Jordan, R.; Choi, G.Y.; Zaccaro, J.; Myerson, A.S.; Rafailovich, M.; Sokolov, J.; et al. Self-assembled monolayers of rigid thiols. J. Biotechnol. 2000, 74, 175–188. [Google Scholar] [CrossRef]
- Guarise, C.; Pasquato, L.; De Filippis, V.; Scrimin, P. Gold nanoparticles-based protease assay. Proc. Natl. Acad. Sci. USA 2006, 103, 3978–3982. [Google Scholar] [CrossRef] [PubMed]
- Guarise, C.; Pasquato, L.; Scrimin, P. Reversible aggregation/deaggregation of gold nanoparticles induced by a cleavable dithiol linker. Langmuir 2005, 21, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Mancin, F.; Prins, L.J.; Scrimin, P. Catalysis on gold-nanoparticle-passivating monolayers. Curr. Opin. Colloid Interface Sci. 2013, 18, 61–69. [Google Scholar] [CrossRef]
- Pasquato, L.; Pengo, P.; Scrimin, P. Nanozymes: Functional nanoparticle-based catalysts. Supramol. Chem. 2005, 17, 163–171. [Google Scholar] [CrossRef]
- Di Pietro, P.; Strano, G.; Zuccarello, L.; Satriano, C. Gold and Silver Nanoparticles for Applications in Theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultrastable iron oxide nanoparticle colloidal suspensions using dispersants with catechol-derived anchor groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.; Khalil, F.; Weidmann, C.; Schröder, M.; Rohnke, M.; Janek, J.; Smarsly, B.M.; Maison, W. A biomimetic principle for the chemical modification of metal surfaces: Synthesis of tripodal catecholates as analogues of siderophores and mussel adhesion proteins. Chem. Eur. J. 2011, 17, 8596–8603. [Google Scholar] [CrossRef] [PubMed]
- Maison, W.; Khalil, F.; Franzmann, E. New tripodal catechol derivatives having adamantyl skeleton, useful in a method for the functionalization of surfaces. 2012. [Google Scholar]
- Huang, X.H.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P. Gold Nanoparticle Synthesis, Morphology Control, and Stabilization Facilitated by Functional Polymers. Chem. Eng. Technol. 2011, 34, 15–28. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Lohse, S.E.; Murphy, C.J. The Gold Standard: Gold Nanoparticle Libraries to Understand the Nano-Bio Interface. Acc. Chem. Res. 2013, 46, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.S.; Young, N.P.; Kirkland, A.I.; Van Huis, M.A.; Xu, H. Nanogold: A quantitative phase map. ACS Nano 2009, 3, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Cao-Milan, R.; Liz-Marzan, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Del. 2014, 11, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Gross, S. Colloidal Dispersions of Gold Nanoparticles (Invited Chapter). In Materials Syntheses; Springer Verlag: Wien, Austria, 2008; Volume 1. [Google Scholar]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pasquato, L.; Pengo, P.; Scrimin, P. Functional gold nanoparticles for recognition and catalysis. J. Mater. Chem. 2004, 14, 3481–3487. [Google Scholar] [CrossRef]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for bioimaging. Adv. Colloid Interface Sci. 2006, 123–126, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, O.; Munoz-Rojas, D.; Oro-Sole, J.; Gomez-Romero, P. From silver nanoparticles to nanostructures through matrix chemistry. J. Nanoparticle Res. 2010, 12, 337–345. [Google Scholar] [CrossRef]
- Colomban, P. The Use of Metal Nanoparticles to Produce Yellow, Red and Iridescent Colour, from Bronze Age to Present Times in Lustre Pottery and Glass: Solid State Chemistry, Spectroscopy and Nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R.; Khajeh, M.; Rakhshanipour, M.; Shameli, K. Green synthesis of silver nanoparticles using plant extracts. Korean J. Chem. Eng. 2014, 31, 548–557. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Rybicki, E.; Tarbuk, A.; Pavlovic, G.; Botteri, L. Nanoparticles of silver in antimicrobial treatment of textiles. Tekstil 2011, 60, 629–639. [Google Scholar]
- Henglein, A. Chemisorption effects on colloidal lead nanoparticles. J. Phys. Chem. B 1999, 103, 9302–9305. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Padmos, J.D.; Zhang, P. Surface Structure of Organosulfur Stabilized Silver Nanoparticles Studied with X-ray Absorption Spectroscopy. J. Phys. Chem. C 2012, 116, 23094–23101. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzan, L.M. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzan, L.M. Colloidal silver nanoplates. State of the art and future challenges. J. Mater. Chem. 2008, 18, 1724–1737. [Google Scholar] [CrossRef]
- Pileni, M.P. Fabrication and physical properties of self-organized silver nanocrystals. Pure Appl. Chem. 2000, 72, 53–65. [Google Scholar] [CrossRef]
- Pileni, M.P. Colloidal self-assemblies used as templates to control size, shape and self-organization of nanoparticles. Supramol. Sci. 1998, 5, 321–329. [Google Scholar] [CrossRef]
- Pileni, M.P.; Taleb, A.; Petit, C. Silver metal nanosized particles: Control of particle size, self assemblies in 2D and 3D superlattices and optical properties. J Dispers. Sci. Technol. 1998, 19, 185–206. [Google Scholar] [CrossRef]
- Quester, K.; Avalos-Borja, M.; Castro-Longoria, E. Biosynthesis and microscopic study of metallic nanoparticles. Micron 2013, 54–55, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kapoor, S.; Mukherjee, T. Synthesis and characterisation of silver nanoparticles in viscous solvents and its transfer into non-polar solvents. Res. Chem. Intermed. 2010, 36, 411–421. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C. Silver as an Example of the Applications of Photochemistry to the Synthesis and Uses of Nanomaterials. Photochem. Photobiol. 2012, 88, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Chesser, A.; Singleton, I. Review: Metal-Based Nanoparticles; Size, Function, and Areas for Advancement in Applied Microbiology. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Elsevier Science Publishing Co., Inc.: New York, NY, USA, 2012; Volume 80, pp. 113–142. [Google Scholar]
- Zhang, C.Q.; Hu, Z.Q.; Deng, B.L. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.H.; Susumu, K.; Mei, B.C.; Medintz, I.L.; Delehanty, J.B.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Multidentate poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J. Am. Chem. Soc. 2010, 132, 9804–9813. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yan, X.-P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, M.; Duan, Y.X. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tong, L.; Flores, M.; Lin, S.; Cheng, J.X.; Yan, H.; Liu, Y. High-quality manganese-doped zinc sulfide quantum rods with tunable dual-color and multiphoton emissions. J. Am. Chem. Soc. 2011, 133, 5389–5396. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef] [PubMed]
- Kilina, S.V.; Tamukong, P.K.; Kilin, D.S. Surface Chemistry of Semiconducting Quantum Dots: Theoretical Perspectives. Acc. Chem. Res. 2016, 49, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Kloepfer, J.; Mielke, R. Quantum dots as strain-and metabolism-specific microbiological labels. Appl. Environ. Environ. Microbiol. 2003, 69, 4205–4213. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Xie, B.; Hu, R.; Luo, X.B. Quantum Dots-Converted Light-Emitting Diodes Packaging for Lighting and Display: Status and Perspectives. J. Electron. Packag. 2016, 138, 020803. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 291, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.N.; Xu, Z.Y.; Zhang, Y.C.; Zhang, M. Solvothermal Synthesis of Mn-doped CdS Nanorods Using Single-source Molecular Precursors. In Materials and Design, Pts 1–3; Sang, X.M., Wang, P.C., Ai, L., Li, Y.G., Bu, J.L., Eds.; Elsevier Publishing: New York, NY, USA, 2011; Volume 284–286, pp. 667–670. [Google Scholar]
- Joswig, J.O.; Springborg, M.; Seifert, G. Structural and electronic properties of cadmium sulfide clusters. J. Phys. Chem. B 2000, 104, 2617–2622. [Google Scholar] [CrossRef]
- Kozhevnikova, N.S.; Vorokh, A.S.; Uritskaya, A.A. Cadmium sulfide nanoparticles prepared by chemical bath deposition. Russ. Chem. Rev. 2015, 84, 225–250. [Google Scholar] [CrossRef]
- Ni, T.; Nagesha, D.K.; Robles, J.; Materer, N.F.; Mussig, S.; Kotov, N.A. CdS nanoparticles modified to chalcogen sites: New supramolecular complexes, butterfly bridging, and related optical effects. J. Am. Chem. Soc. 2002, 124, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Optical studies of CdS:Mn nanoparticles. Luminescence 2012, 27, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Garg, S.; Chahal, J.; Raheja, K.; Singh, D.; Singla, M.L. Luminescent behavior of cadmium sulfide quantum dots for gallic acid estimation. Nanotechnology 2013, 24, 115602. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Xia, J.R.; Hu, B.H. Controlled growth of CdS nanoparticles in polyurushiol matrices. Prog. Org. Coat. 2009, 65, 25–29. [Google Scholar] [CrossRef]
- Klein, C.A.; Donadio, R.N. Infrared-active phonons in cubic zinc sulfide. J. Appl. Phys. 1980, 51, 797–800. [Google Scholar] [CrossRef]
- Gross, S.; Camozzo, D.; Di Noto, V.; Armelao, L.; Tondello, E. PMMA: A key macromolecular component for dielectric low-kappa hybrid inorganic-organic polymer films (Invited review). Eur. Polym. J. 2007, 43, 673–696. [Google Scholar] [CrossRef]
- Sharma, R.C.; Chang, Y.A. The S-Zn (Sulfur-Zinc) System. Phase Diagr. Eval. Sect. II 1996, 17, 261–266. [Google Scholar] [CrossRef]
- Desgreniers, S.; Beaulieu, L.; Lepage, I. Pressure-induced structural changes in ZnS. Phys. Rev. B 2000, 61, 8726–8733. [Google Scholar] [CrossRef]
- Gilbert, B.; Huang, F.; Zhang, H.; Waychunas, G.A.; Banfield, J.F. Nanoparticles: Strained and Stiff. Science 2004, 305, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Schrage, C.; Althues, H.; Klausch, A.; Adam, D.; Kaskel, S. ZnS:Cu Polymer Nanocomposites for Thin Film Electroluminescent Devices. J. Nanosci. Nanotechnol. 2010, 10, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, S.; Chen, L. Controllable synthesis of ZnS/PMMA nanocomposite hybrids generated from functionalized ZnS quantum dots nanocrystals. Colloid Polym. Sci. 2007, 285, 1593–1600. [Google Scholar] [CrossRef]
- Song, H.; Lee, S. Photoluminescent (CdSe) ZnS quantum dot-polymethylmethacrylate polymer composite thin films in the visible spectral range. Nanotechnology 2007, 18, 055402. [Google Scholar] [CrossRef]
- Yuan, Y.; Krueger, M. Polymer-nanocrystal hybrid materials for light conversion applications. Polymers 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Anand, K.V.; Chinnu, M.K.; Kumar, R.M.; Mohan, R.; Jayavel, R. Thermal stability and optical properties of HMTA capped zinc sulfide nanoparticles. J. Alloy. Compd. 2010, 496, 665–668. [Google Scholar] [CrossRef]
- Taherian, M.; Sabbagh Alvani, A.A.; Shokrgozar, M.A.; Salimi, R.; Moosakhani, S.; Sameie, H.; Tabatabaee, F. Surface-treated biocompatible ZnS quantum dots: Synthesis, photo-physical and microstructural properties. Electron. Mater. Lett. 2014, 10, 393–400. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, X.; Yu, B.; Pan, Y.; Liu, Z. Synthesis and characterization of ZnS:Mn/ZnS core/shell nanoparticles for tumor targeting and imaging in vivo. J. Biomater. Appl. 2013, 28, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Parfitt, G.D.; Patsis, A.V. Organic Coatings: Science and Technology; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Aswathy, J.; Jahnavi, S.; Krishna, R.; Manzoor, K.; Nair, S.; Menon, D. Targeted Labeling of Cancer Cells Using Biotin Tagged Avidin Functionalized Biocompatible Fluorescent Nanocrystals. J. Nanosci. Nanotechnol. 2011, 11, 7611–7620. [Google Scholar] [CrossRef] [PubMed]
- Koneswaran, M.; Narayanaswamy, R. L-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Q.; Wang, X.; Li, Y. Synthesis and Characterization of Monodisperse ZnS Nanospheres. Chem. Lett. 2004, 33, 1320–1321. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E.J. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Li, Y.B.; Lu, W.; Huang, Q.A.; Huang, M.A.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Pradhan, D. Controlled Synthesis and Catalytic Activity of Copper Sulfide Nanostructured Assemblies with Different Morphologies. ACS Appl. Mater. Interfaces 2014, 6, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Ohmasa, M.; Suzuki, M.; Takeuchi, Y. A refinement of the crystal structure of covellite, CuS. Miner. J. 1977, 8, 311–319. [Google Scholar] [CrossRef]
- Thongtem, T.; Phuruangrat, A.; Thongtem, S. Synthesis and analysis of CuS with different morphologies using cyclic microwave irradiation. J. Mater. Sci. 2007, 42, 9316–9323. [Google Scholar] [CrossRef]
- Chaki, S.H.; Tailor, J.P.; Deshpande, M.P. Synthesis and Characterizations of Undoped and Mn Doped CuS Nanoparticles. Adv. Sci. Lett. 2014, 20, 959–965. [Google Scholar] [CrossRef]
- Biswas, S.; Hait, S.K.; Bhattacharya, S.C.; Moulik, S.P. Synthesis of Nanoparticles of CuI, CuCrO4, and CuS in Water/AOT/Cyclohexanone and Water/TX-100 + i-Propanol/Cyclohexanone Reverse Microemulsions. J. Dispers. Sci. Technol. 2005, 25, 801–816. [Google Scholar] [CrossRef]
- Sugimoto, T.; Chen, S.; Muramatsu, A. Synthesis of uniform particles of CdS, ZnS, PbS and CuS from concentrated solutions of the metal chelates. Colloid Surf. A 1998, 135, 207–226. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Stroyuk, A.L.; Kuchmii, S.Y.; Kryukov, A.I. Catalytic activity of CuS nanoparticles in hydrosulfide ions air oxidation. J. Mol. Catal. A Chem. 2004, 212, 259–265. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Yang, Y.; Jing, L.; Yue, X.; Chen, X.; Dai, Z. Chitosan Functionalized CuS Nanoparticles Boots Gene Transfection via Photothermal Effect. Curr. Drug. Deliv. 2016, 13, 1. [Google Scholar] [CrossRef]
- Liu, R.F.; Jing, L.J.; Peng, D.; Li, Y.; Tian, J.; Dai, Z.F. Manganese (II) Chelate Functionalized Copper Sulfide Nanoparticles for Efficient Magnetic Resonance/Photoacoustic Dual-Modal Imaging Guided Photothermal Therapy. Theranostics 2015, 5, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Liu, Y.Y.; Xiong, Z.; Kaback, D.; Zhao, D.Y. Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 2012, 23, 294007. [Google Scholar] [CrossRef] [PubMed]
- Van Koetsem, F.; Van Havere, L.; Du Laing, G. Impact of carboxymethyl cellulose coating on iron sulphide nanoparticles stability, transport, and mobilization potential of trace metals present in soils and sediment. J. Environ. Manag. 2016, 168, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; He, F.; Zhao, D.Y.; Barnett, M.O. Immobilization of mercury in sediment using stabilized iron sulfide nanoparticles. Water Res. 2009, 43, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.G.; Pauling, L. The crystal structure of molybdenite. J. Am. Chem. Soc. 1923, 45, 1466–1471. [Google Scholar] [CrossRef]
- Bakunin, V.N.; Suslov, A.Y.; Kuzmina, G.N.; Parenago, O.P. Synthesis and application of inorganic nanoparticles as lubricant components—A review. J. Nanoparticle Res. 2004, 6, 273–284. [Google Scholar] [CrossRef]
- Kumar, N.; Seminario, J.M. Computational Chemistry Analysis of Hydrodesulfurization Reactions Catalyzed by Molybdenum Disulfide Nanoparticles. J. Phys. Chem. C 2015, 119, 29157–29170. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Xu, Z.C.; Wang, X. Recent Development of Molybdenum Sulfides as Advanced Electrocatalysts for Hydrogen Evolution Reaction. ACS Catal. 2014, 4, 1693–1705. [Google Scholar] [CrossRef]
- Sun, C.; Berg, J.C. Effect of moisture on the surface free energy and acid–base properties of mineral oxides. J. Chromatogr. A 2002, 969, 59–72. [Google Scholar] [CrossRef]
- Ferretto, L.; Glisenti, A. Study of the surface acidity of an hematite powder. J. Mol. Catal. A Chem. 2002, 187, 119–128. [Google Scholar] [CrossRef]
- Godocikova, E.; Balaz, P.; Bastl, Z.; Brabec, L. Spectroscopic study of the surface oxidation of mechanically activated sulphides. Appl. Surf. Sci. 2002, 200, 36–47. [Google Scholar] [CrossRef]
- Mitchell, M.B. Fundamentals and Applications of Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy; American Chemical Society: Washington, DC, USA, 1993; Volume 236, pp. 351–375. [Google Scholar]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. PCCP 2008, 10, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
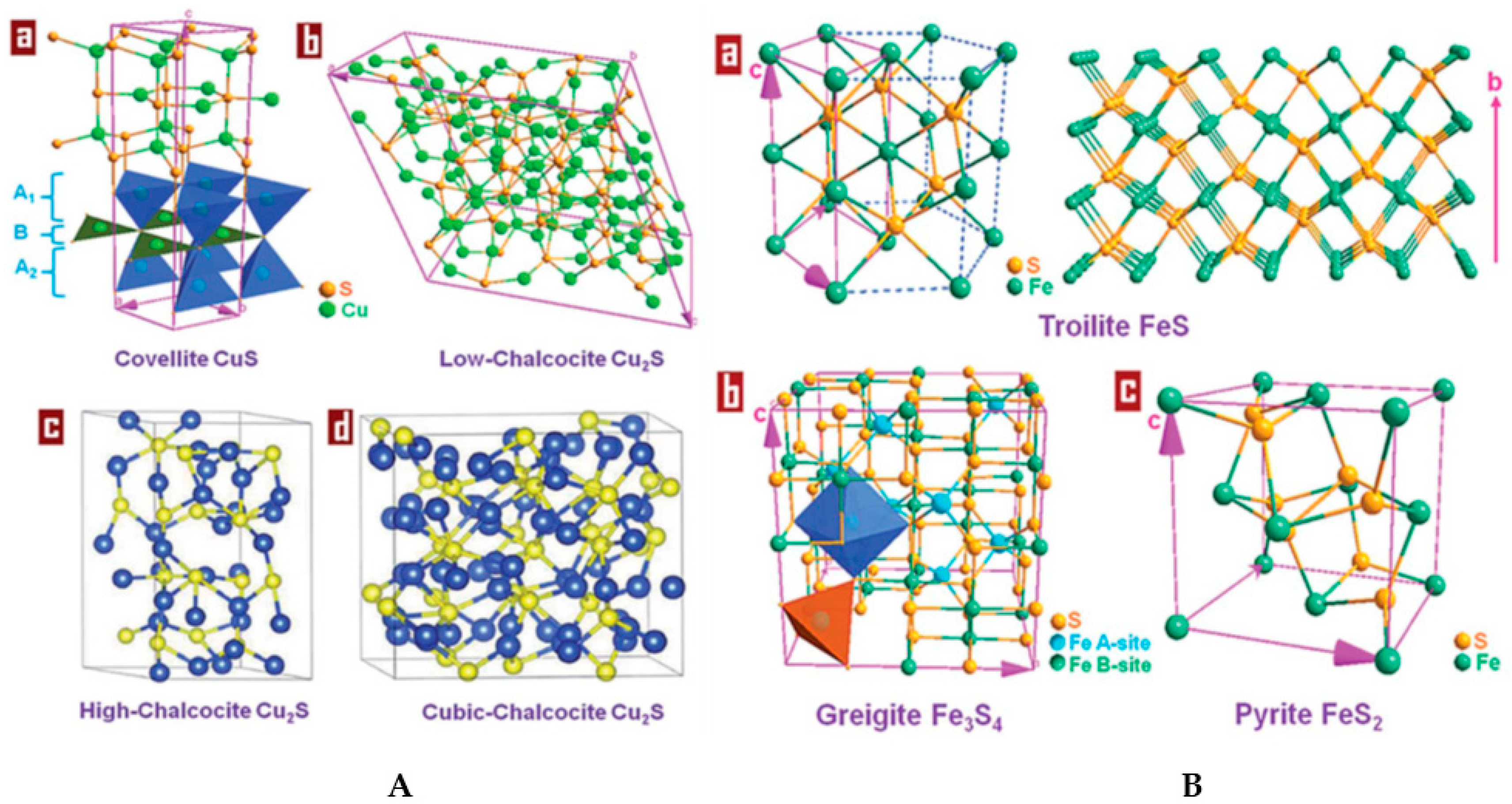
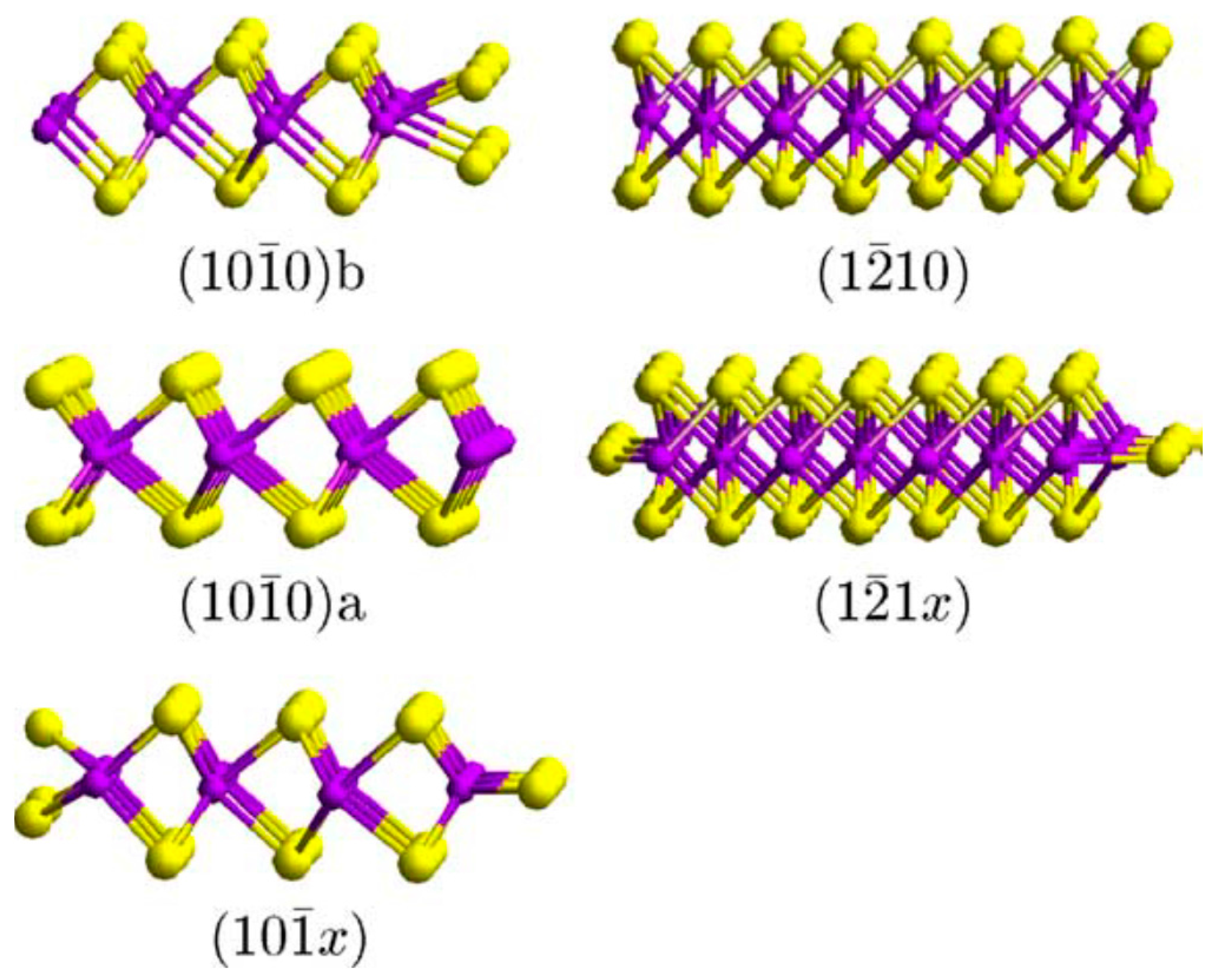
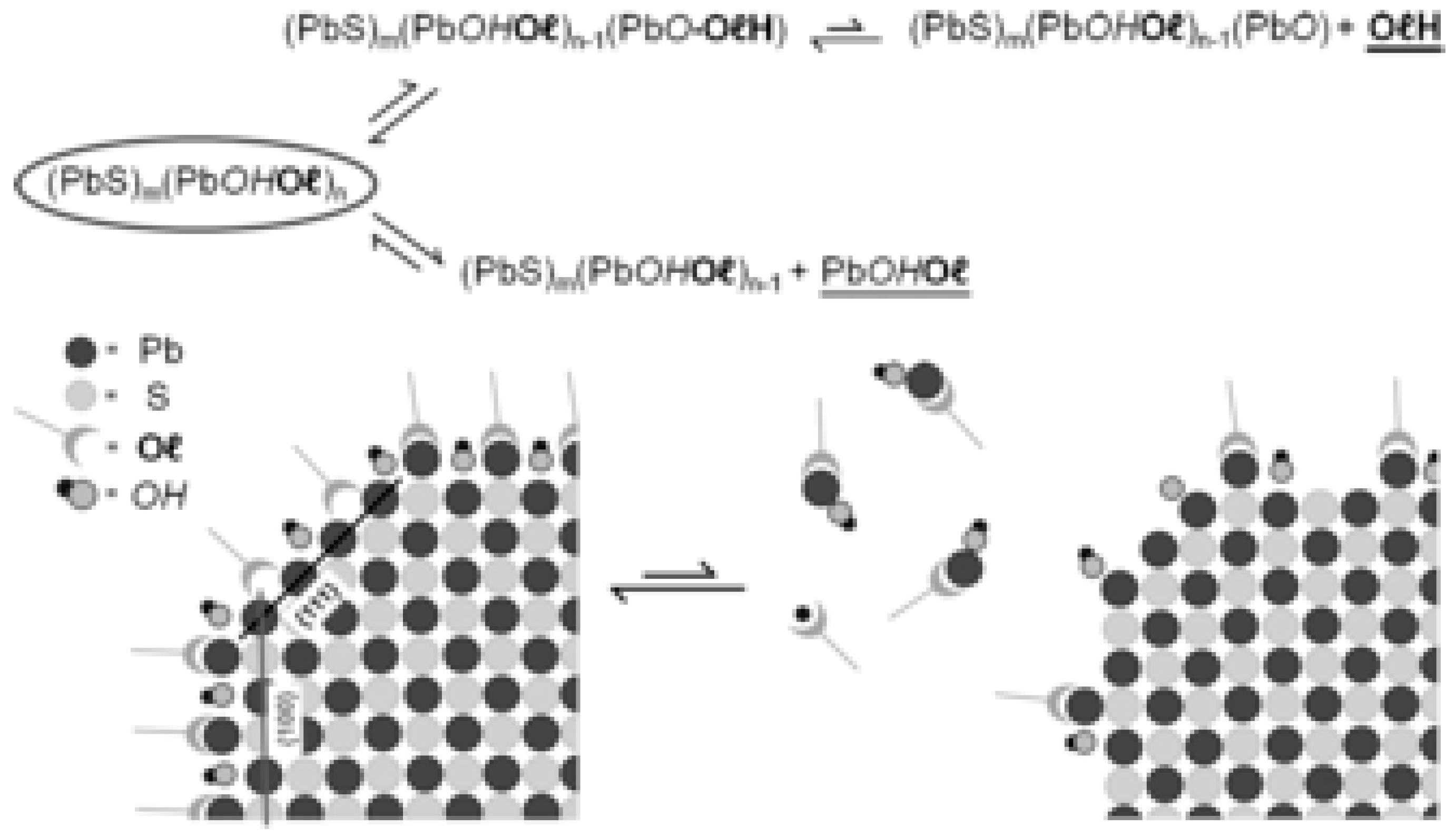
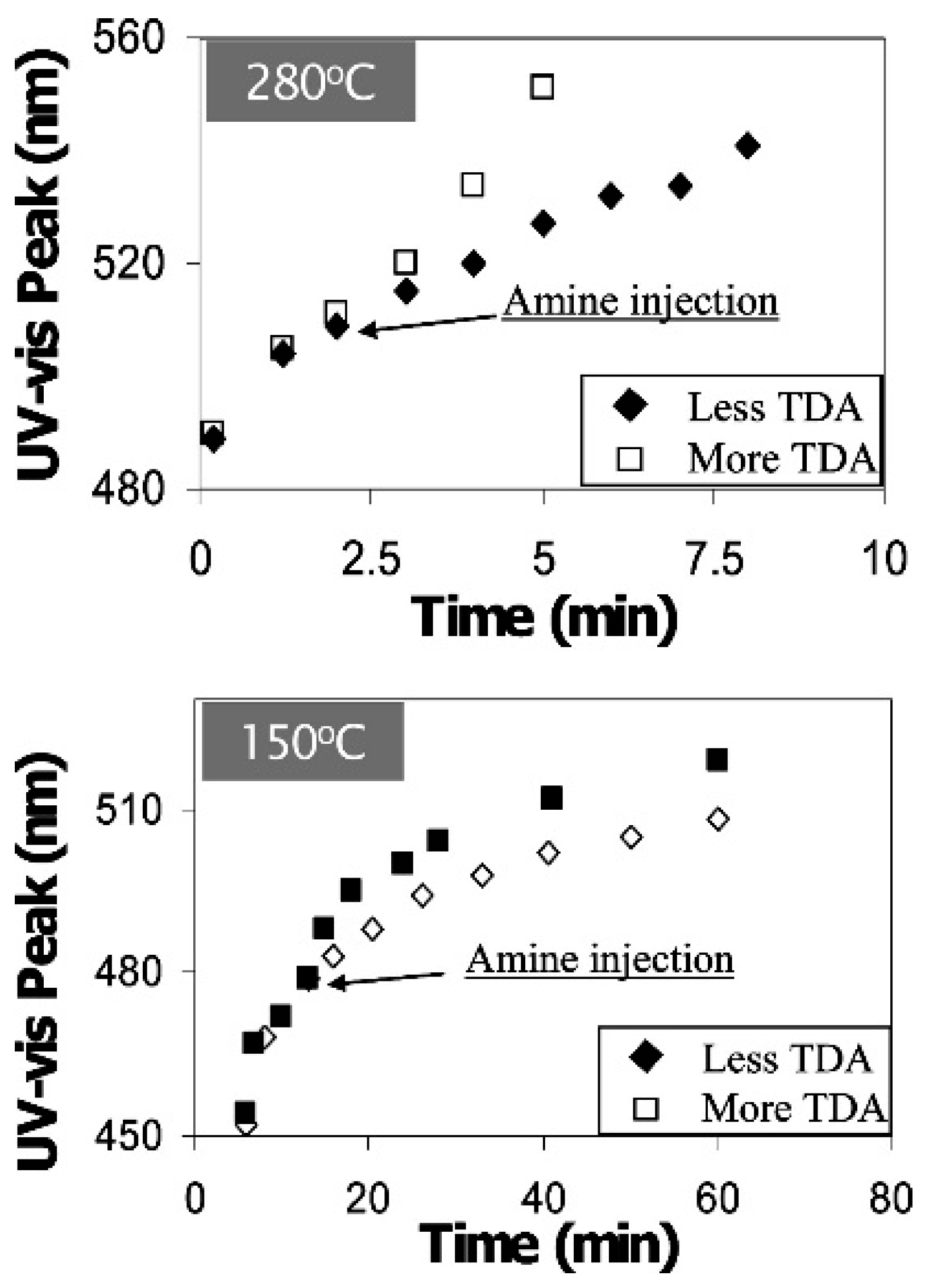
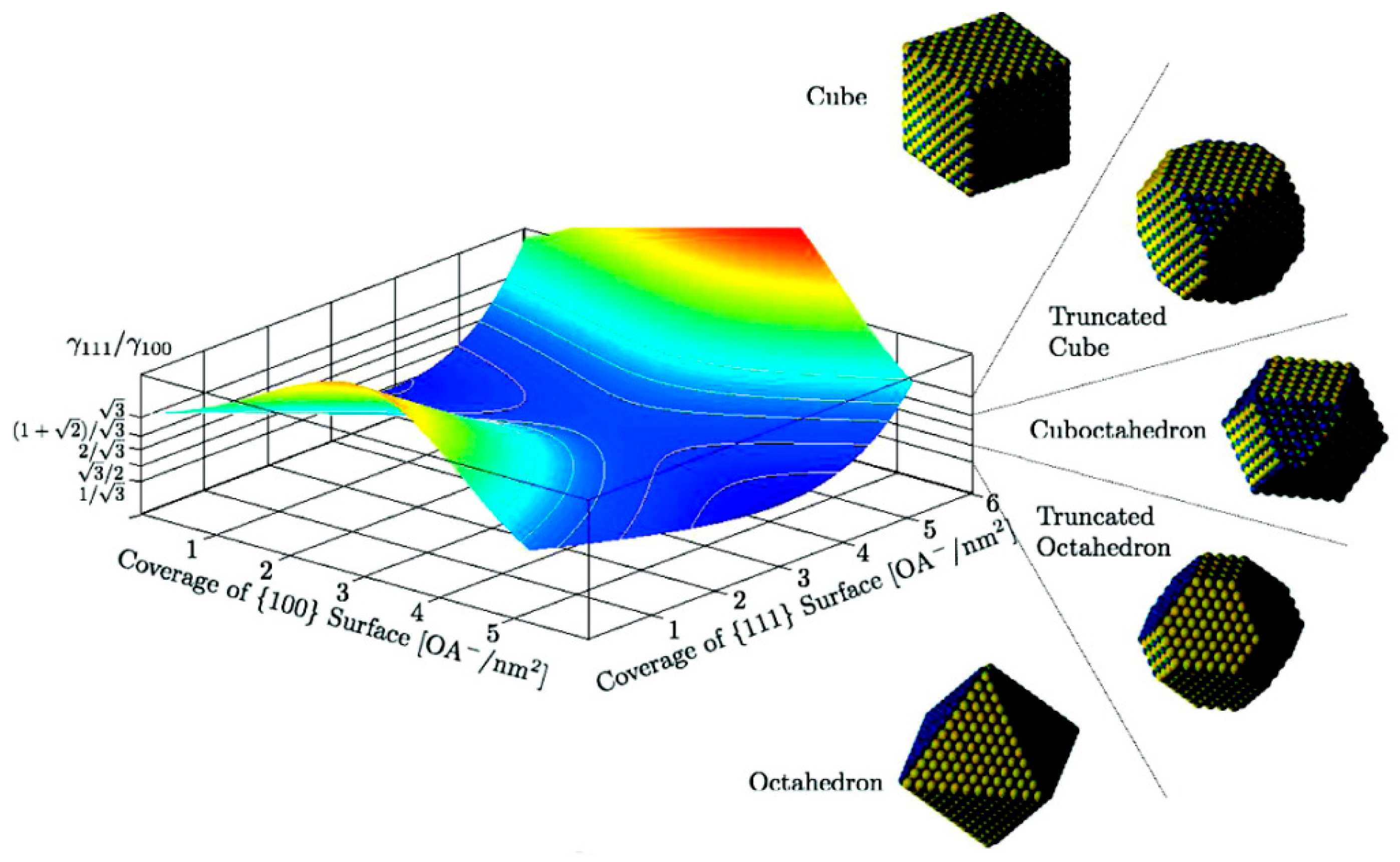
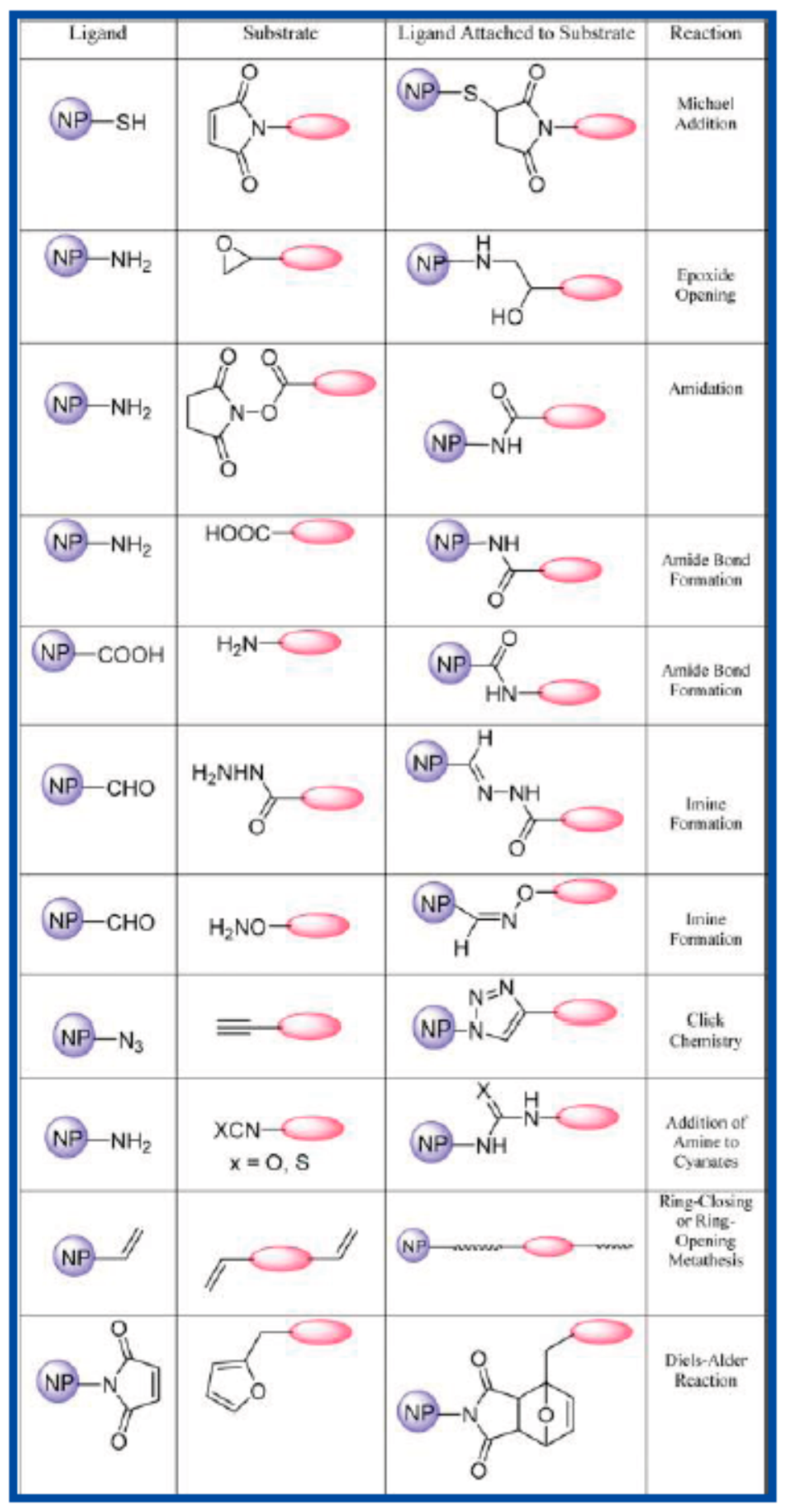
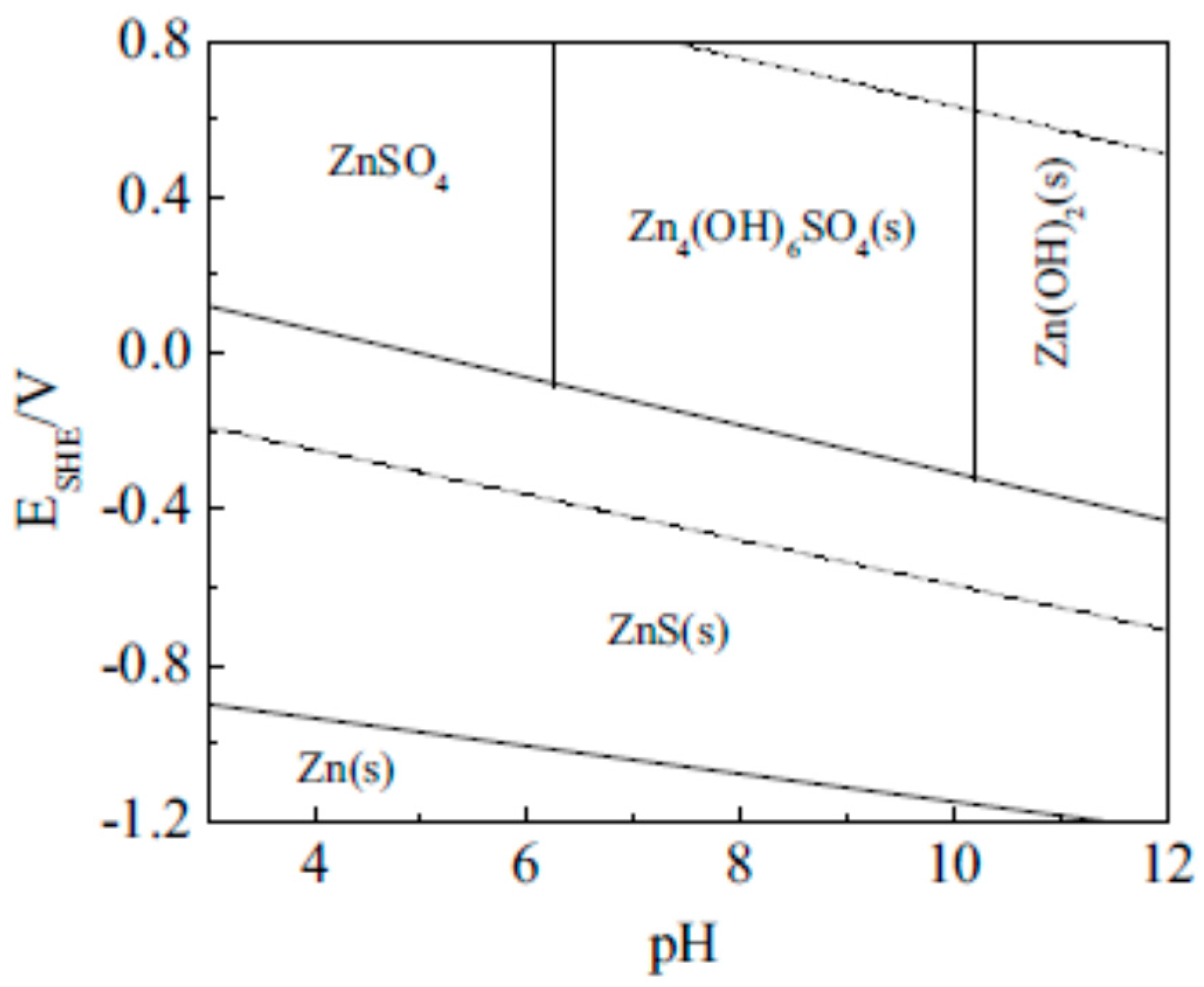

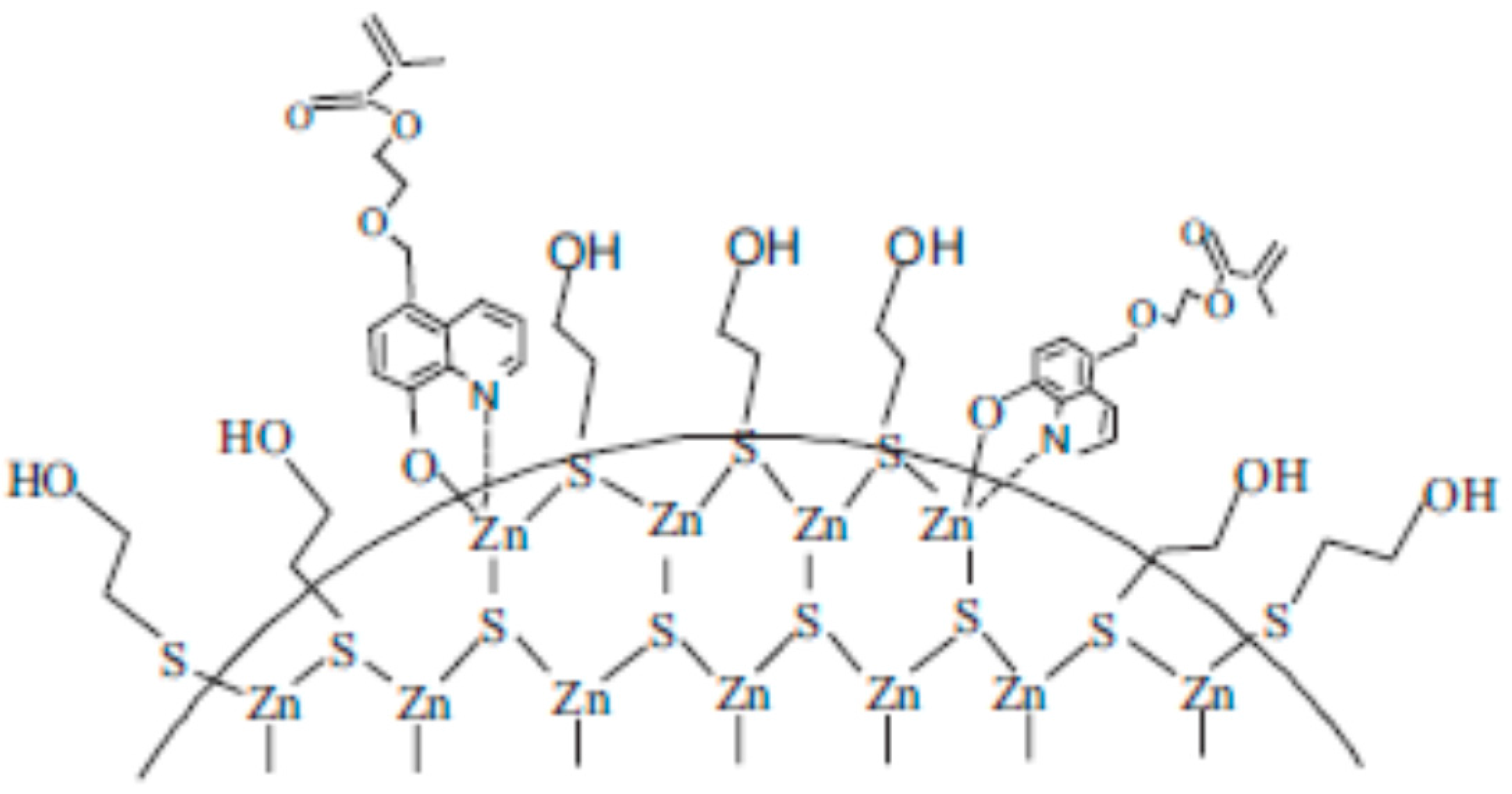
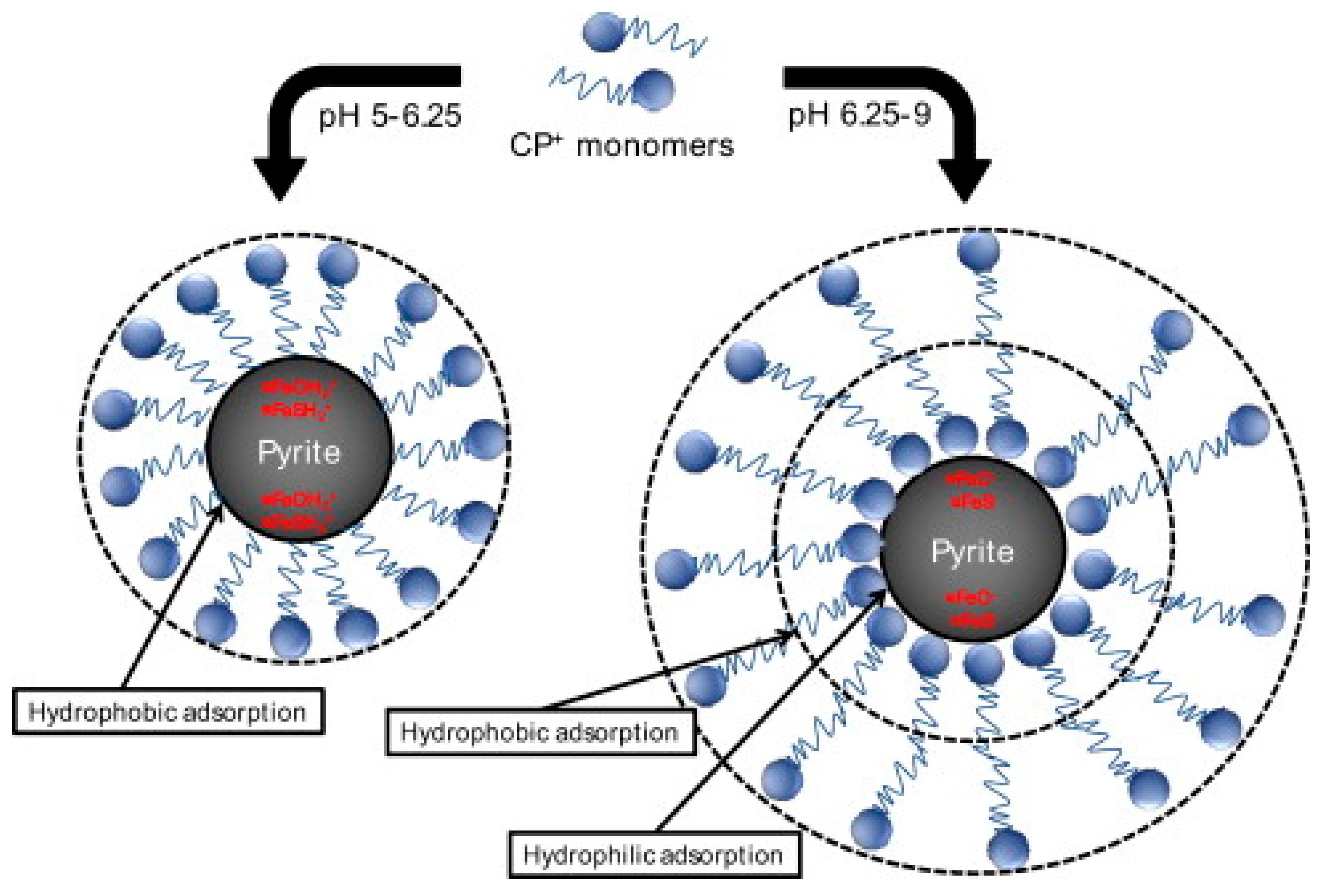
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, S.; Vittadini, A.; Dengo, N. Functionalisation of Colloidal Transition Metal Sulphides Nanocrystals: A Fascinating and Challenging Playground for the Chemist. Crystals 2017, 7, 110. https://doi.org/10.3390/cryst7040110
Gross S, Vittadini A, Dengo N. Functionalisation of Colloidal Transition Metal Sulphides Nanocrystals: A Fascinating and Challenging Playground for the Chemist. Crystals. 2017; 7(4):110. https://doi.org/10.3390/cryst7040110
Chicago/Turabian StyleGross, Silvia, Andrea Vittadini, and Nicola Dengo. 2017. "Functionalisation of Colloidal Transition Metal Sulphides Nanocrystals: A Fascinating and Challenging Playground for the Chemist" Crystals 7, no. 4: 110. https://doi.org/10.3390/cryst7040110
APA StyleGross, S., Vittadini, A., & Dengo, N. (2017). Functionalisation of Colloidal Transition Metal Sulphides Nanocrystals: A Fascinating and Challenging Playground for the Chemist. Crystals, 7(4), 110. https://doi.org/10.3390/cryst7040110







