Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review
Abstract
1. Introduction
Overview of the Microwave Frequency Effect
2. Methods of Microwave Energy Generation and Delivery
2.1. Local Heating and “Hot Spots”
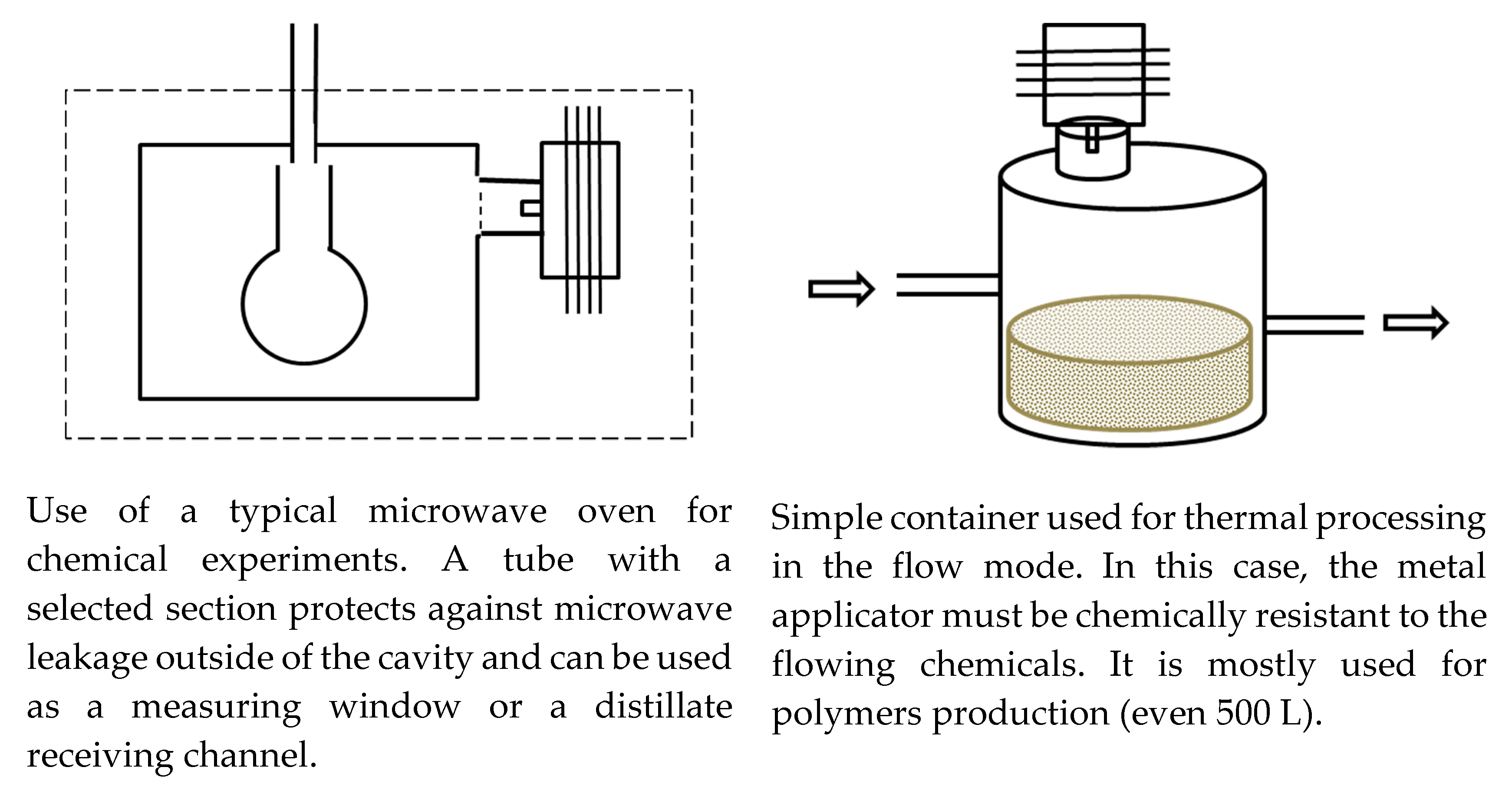
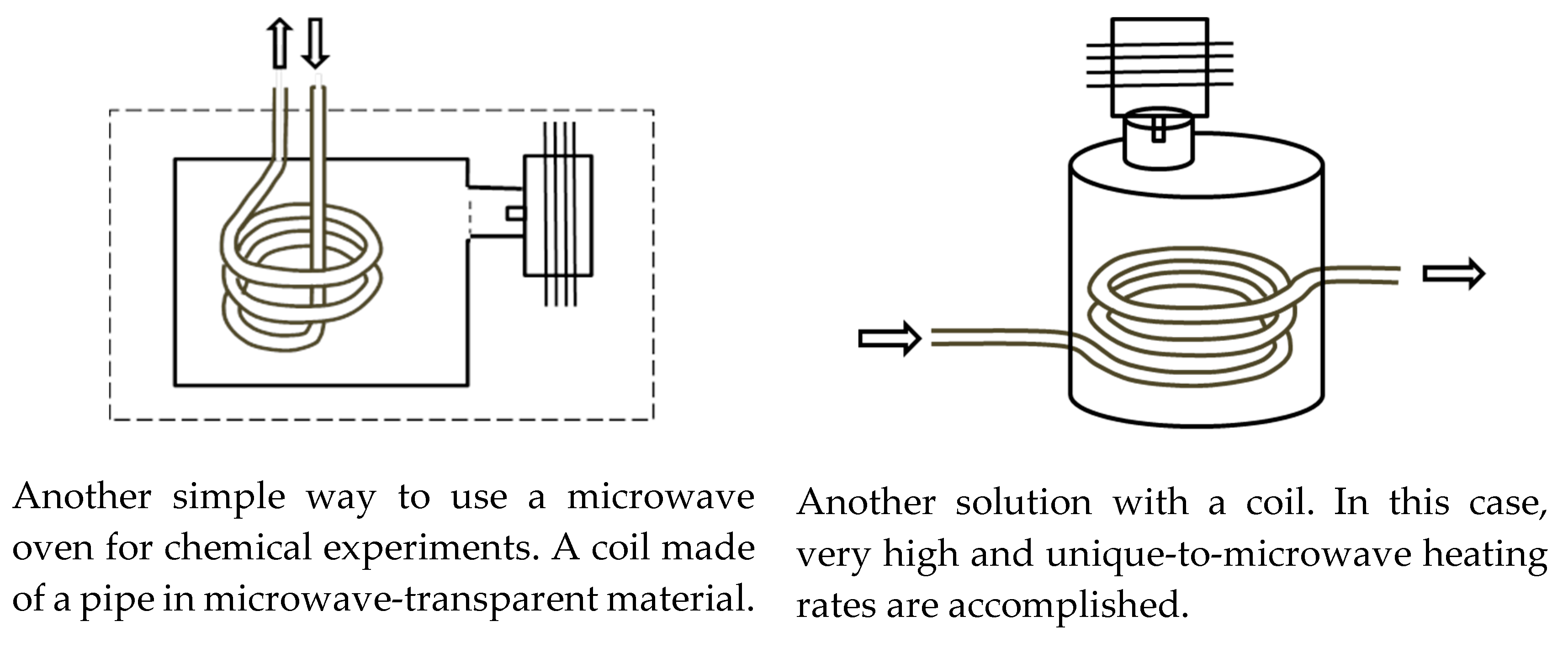
2.2. Typical Designs of Microwave Laboratory Devices
2.3. Process Parameters Control and Enhanced Pressure
Measurements Problems
2.4. Optimal Performance and Configurations
- No power delivery components—the magnetrons are mounted directly to the oven cavity (for big loss processes with known static loads);
- Impedance matching only—the magnetron is mounted to a short section of the waveguide (for high loss and static loads processes);
- No insulator—the typical laboratory set without a “dummy load” (it is a cheaper version than the “typical” one, but it can cause magnetron damage);
- Directional coupler before an insulator—the coupler is not set after the circulator like in typical heating system [34];
- Systems in which the microwave energy is supplied to the reactants by specially formed antennas.
2.5. Materials for the Reactor
- High-quality polytetrafluoroethylene (PTFE), commercialized as Teflon®, ALGOFLON® HOSTAFLON®, FLUON®, CHEMLOY®, and so forth. This family of fluoroplastic resins has exceptional resistance to high temperatures, chemical reaction, corrosion, and stress-cracking. The mechanical toughness, and electrical and low-friction properties of Teflon® make it the preferred plastic for molding many different products and stock shapes, such as rods, tubes, and sheets. Products fabricated from PTFE are rated for continuous service at 260 °C and provide exceptional low-temperature toughness, as well as unique low adhesion and flame resistance.
- Pure quartz glass, fused quartz, or fused silica are transparent materials consisting of silica in an amorphous (non-crystalline) form. They differ from traditional glasses, because no other oxides have been added during the melting process, thus leading to high working and melting temperatures. The optical and thermal properties of fused quartz are superior to those of other types of glass due to its purity; in particular, its most useful qualities are as follows: (i) It has a very low thermal expansion and an excellent thermal shock resistance; and (ii) it is very hard and chemically inert to most elements and compounds, including virtually all acids, regardless of concentration, except hydrofluoric acid, which is very reactive even in fairly low concentrations. Quartz glass possesses a marked refractoriness and can retain its shape, such as in crucibles, trays, and rollers, up to high temperatures (1000–1500 °C). Unfortunately, it is fragile and easy to crack.
- Polyetheretherketone (PEEK) is a colorless organic thermoplastic polymer in the polyaryletherketone (PAEK) family with excellent mechanical and chemical resistance properties that are retained in high temperatures. Commercial PAEK products are as follows: AROTNE®, KARDEL®, ZYEX®, and PEEK-VICTREX®. The processing conditions used to mold PEEK can influence its mechanical properties (Young’s modulus: 3.6 GPa; tensile strength: 90–100 MPa). PEEK has a useful operating temperature up to 250 °C, and it is highly resistant to attack in both organic and aqueous environments. It can withstand attacks by halogens and strong Brønsted and Lewis acids, as well as some halogenated compounds and aliphatic hydrocarbons, at high temperatures. It is soluble in concentrated sulfuric acid at room temperature with a slow kinetic rate. For these reasons, PEEK is often used with an internal PTFE coating.
2.6. Microwave Reactors Applied to Chemical Processes
2.6.1. Microwave Digestors for Sample Mineralization
2.6.2. Inorganic Synthesis of Nanomaterials
2.6.3. Examples of Applications for Organic Synthesis
3. Overview of Microwave Reactors
3.1. Main Producers of Microwave Devices with Parameter Control
3.2. Reactors Designed for Scale-Up
3.3. Hybrid Reactors
3.4. High-Temperature Microwave Reactors (Sintering Furnaces)
4. Future Developments in Microwave Chemistry
- The development of microwave technology has allowed for its wider use in further areas of research and production. We believe that we can expect particularly intensive progress in the field of new materials, which is useful for increasing the operational parameters of devices and further disseminating of effective methods for the synthesis of valuable products (e.g., pharmaceuticals, nanopowders).
- The development of materials, such as semiconductor generators for replacing magnetrons and TWT for power supplies in microwave reactors, will continue to increase efficiency. Unfortunately, semiconductor supplies are currently still too expensive for use in all devices. In the next few years, the hope is that the price will fall dramatically, and it will be possible to use semiconductors (very small supply) for domestic cooking purposes to obtain shorter heating times than those that are currently available.
- The increased use of Internet/Bluetooth connections will have positive benefits on cooking household and laboratory experiments. This will allow users to avoid having to keep an “eye” on everything, because dices could be started and initialized by using an Internet/Bluetooth connection. The laboratory data can be sent by the cloud, and some calculations can be done for the user while the reaction is running.
- Sensors for properly using power and managing the time of cooking/chemical reactions will be very beneficial. In the cooking industry, this can involve the IR scanning of every frozen or non-prepared food having a universal product code, such that a microwave can “read” the information put on the pack. The microwave oven is able to use the time and power instructions that are put on the product by producer. In the laboratory, some UPC codes can be used by the operator to sign some prepared precursor to be heated by the microwave in some clear ways. The device will use an IR sensor to put accurate data in the chemical reactor.
- Greater efficiency of microwave reactions can be obtained by using continuous flow, stop-flow, or large batch (with large power) modes. The type of reactor for microwave synthesis depends on the needs and costs. IHPP PAS developed the MSS2 reactor for hydro- and solvothermal synthesis with stop-flo and also made an added mode—a batch system with a bigger volume of a batch. The bigger volume of batch mode needs to optimize every part of the device and take into consideration the penetration depths of the solvents and materials for each cup/batch/vessel.
- The development of new materials for microwave devices and new types of microwave reactors needs stronger materials with better resistance to high temperature or materials with bigger penetration depth that are not expensive compared with “old used materials” (Section 2.5).
- New devices for the generation plasma in microwave-induced plasma analytical spectrometry (MIC-OES), new types of emission microwave plasma (using semiconductor sources), and induced plasma torches are being developed. More new methods of microwave excitation are being used in the industries, with cheaper methods from known ICP instruments [89].
- Current and future trends in the microwave reactors industry include the following:
- ○
- pharmacy (synthesis, e.g., aspirin);
- ○
- extractions (food stuff);
- ○
- biofuels, biomass;
- ○
- nanopowder productions; and
- ○
- sterilization (in-vitro cells nutrition, dental tools).
5. Summary
- Microwave heating allows for better internal heating than conventional techniques, the reaction can be completed right at a selected time or at a specified heating rate, and microwaves create a narrow size distribution of the NPs;
- Microwaves accelerate the synthesis time relative to the conventional method and can result in obtaining a smaller grain size;
- The possibility of stopping the reaction immediately at any time during the reaction is very important, and the temperature continues to decrease in the seconds after stopping the reaction; thus, microwave technology gives a better reaction in controlling this and the ability to easily repeat the results. The example of this type of control is the MSS2 reactor in the stop-flow mode;
- Microwave hydrothermal synthesis is called “green” chemistry because of the use of water as a solvent, and the fact that it allows for the design of chemical products and processes that can reduce or can eliminate the generation of hazardous and dangerous substances. Using chemically inert materials in reactors, such as PTFE or PEEK, to minimalize pollution and facilitate easy cleaning is another way in which it represents “green” chemistry;
- Observing the reflected power during the reaction helps refine the understanding of the structure of changes in the synthesis, and recording and interpreting such data during microwave heating gives more knowledge about the process of the synthesis;
- Metallic particles may be obtained in a microwave reactor (e.g., Cu, Ag, Au), provided that they do not agglomerate and are only in a small quantity. It should be taken into consideration that metals can strongly absorb MW in reactions and create sparking, that is, “arcing”.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HAP | hydroxyapatite |
| ICP-MS | inductively coupled plasma mass spectrometry |
| ICP-OES | inductively coupled plasma atomic emission spectroscopy |
| IHPP PAS | Institute of High Pressure Physics Polish Academy of Sciences |
| IR | infrared |
| ISM | industrial, scientific and medical |
| ITU-R MIC-OES | International Telecommunication Union Radiocommunications Sector Microwave-induced plasma analytical spectrometry |
| MW | microwave |
| PAEK | polyaryletherketone |
| PEEK | polyetheretherketone |
| PTFE | polytetrafluoroethylene |
| SSA BET | specific surface area by the BET method |
| SSS or S3 | solid-state source |
| TWT | traveling wave tube |
References
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry; DeGruyterTextbook; De Gruyter: Berlin, Germany, 2017; ISBN 978-3-11-047993-5. [Google Scholar]
- Microwave History. Available online: http://ideafinder.com/history/inventions/microwave.htm (accessed on 10 April 2018).
- Leadbeater, N.E.; McGowan, C.B. Laboratory Experiments Using Microwave Heating; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2013, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- General Technical Description of the Magnetron S-Band 750 Kw. Available online: http://njr.com/products/micro/pdf/M1901S.pdf (accessed on 10 April 2018).
- Industrial Magnetron on Market. Available online: https://alibaba.com/showroom/industrial-magnetron.html (accessed on 10 April 2018).
- Hartnett, J.P. Advances in Heat Transfer, 1st ed.; Academic Press: New York, NY, USA, 1999; Volume 33. [Google Scholar]
- Bandura, A.V.; Lvov, S.N. The Ionization Constant of Water over Wide Ranges of Temperature and Density. J. Phys. Chem. Ref. Data 2006, 35. [Google Scholar] [CrossRef]
- Keglevich, G. (Ed.) The spread of the application of the microwave technique in organic synthesis. In Milestones in Microwave Chemistry; Springer: New York, NY, USA, 2016; Chapter 4. [Google Scholar]
- Kappe, C.O. Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols; Wiley-VCH Verlag: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Patil, N.G.; Benaskar, F.; Rebrov, E.V. Scale-up of Microwave Assisted Flow Synthesis by Transient Processing through Monomode Cavities in Series Schouten. Org. Process Res. Dev. 2014, 18, 1400–1407. [Google Scholar] [CrossRef]
- What Is a Gyrotron? Learn about DNP-NMR Spectroscopy. Available online: http://bridge12.com/what-is-a-gyrotron/ (accessed on 10 April 2018).
- Gerling, J.F. Recent Developments in Solid-State Microwave Heating. AMPERE Newslett. 2016, 89, 8–10. [Google Scholar]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing, 1st ed.; Springer: Singapore, 2018; ISBN 978-981-10-6465-4. [Google Scholar]
- Jerby, E. Editorial of the Special Issue on Solid-State Microwave Heating. AMPERE Newslett. 2016, 89, 2–3. [Google Scholar]
- Neupane, U.; Rai, R.N. Solid state synthesis of novel charge transfer complex and studies of its crystal structure and optical properties. J. Solid State Chem. 2018, 268, 67–74. [Google Scholar] [CrossRef]
- Kiruthigaa, G.; Manoharan, C.; Raju, C.; Jayabharathi, J.; Dhanapandian, S. Solid state synthesis and spectral investigations of nanostructure SnS2. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Veronesi, P. Chapter 2: Microwave Reactors for Chemical Synthesis and Biofuels Preparation. In Production of Biofuels and Chemicals with Microwave, Biofuels and Biorefineries; Fang, Z., Smith, R.L., Jr., Qi, X., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 17–40. [Google Scholar]
- Chudoba, T. SHS SiC Synthesis Initiated by Microwaves. Ph.D. Thesis, Akademia Górniczo-Hutnicza im Stanisława Staszica Wydział Inżynierii Materiałowej, Kraków, Poland, 2005. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. Engl. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G. (Ed.) Interpretation of the Effects of Microwaves. In Milestones in Microwave Chemistry; Springer: New York, NY, USA, 2016; Chapter 1. [Google Scholar]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Microwave Frequency Effects in Organic Synthesis in Microwaves in Organic Synthesis; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Cao, W. (Ed.) Chapter 7: Microwave Apparatus for Kinetic Studies and in-situ Observations in Hydrothermal of High-Pressure. In The Development and Application of Microwave Heating; IntechOpen: London, UK, 2012; ISBN 978-953-51-0835-1. [Google Scholar]
- Smith, F.E.; Arsenault, E.A. Microwave-assisted sample preparation in analytical chemistry. Talanta 1996, 43. [Google Scholar] [CrossRef]
- Thompson, K.; Booske, J.H.; Cooper, R.F.; Gianchandani, Y.B. Temperature Measurement in Microwave-Heated Silicon Wafers. In Microwaves: Theory and Application in Materials Processing V (Ceramic Transactions); The American Ceramic Society: Westerville, OH, USA, 2000; Volume 111, p. 391. [Google Scholar]
- Pert, E.; Carmel, Y.; Birnboim, A.; Olorunyolemi, T.; Gershon, D.; Calame, J.; Lloyd, I.K.; Wilson, O.C., Jr. Temperature Measurements during Microwave Processing: The Significance of Termocouple Effects. J. Am. Ceram. Soc. 2001, 84, 1981–1986. [Google Scholar] [CrossRef]
- Whittaker, G.; Mingos, D.M. Arcing and Other Microwave Characteristics of Metal Powders in Liquid Systems. J. Chem. Soc. Dalton Trans. 2000, 1521–1526. [Google Scholar] [CrossRef]
- Leonelli, C.; Lojkowski, W. Main development directions in the application of microwave irradiation to the synthesis of nanopowders. Chem. Today 2007, 25, 34–38. [Google Scholar]
- Hoz, A.; Díaz-Ortiz, A.; Moreno, A. Review on non-thermal effects of microwave irradiation in organic synthesis. J. Microw. Power Electromagn. Energy 2007, 41, 45–66. [Google Scholar] [CrossRef]
- Milestone. Available online: http://milestonesci.com/ (accessed on 10 April 2018).
- Fang, Z. Production of Biofuels and Chemicals with Microwave; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef] [PubMed]
- Gerling, J.F. Wavegiude Components and Configurations for Optimal Performance. Available online: https://mueggegerling.de/fileadmin/user_upload/muegge.de/Documents/GAE/Technical-References/Waveguide-Components-and-Configurations.pdf/ (accessed on 10 April 2018).
- Bogdal, D. Microwave-Assisted Organic Synthesis: One Hundred Reaction Procedures, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780080446219. [Google Scholar]
- AR-GLAS Schott. Datasheet. Available online: http://us.schott.com/tubing/media/selector/datasheets/english/schott-tubing_datasheet_ar-glas_english.pdf/ (accessed on 10 April 2018).
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Sibera, D.; Strachowski, T.; Lojkowski, W.; Narkiewicz, U.; Chudoba, T.; Jedrzejewski, R.; Majcher, A.; Presz, A. Nano-ZnAl2O4—Hydrothermal mw assisted synthesis in a stop-flow reactor and characterization. Probl. Eksploat. 2010, 91–102. [Google Scholar]
- Sibera, D.; Jedrzejewski, R.; Mizerack, J.; Presz, A.; Narkiewicz, U.; Lojkowski, W. Synthesis and Characterization of ZnO Doped with Fe2O3—Hydrothermal Synthesis and Calcination Process. Acta Phys. Pol. Ser. A 2009, 116. [Google Scholar] [CrossRef]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and Complex ZnO Nanostructures by Microwave-Assisted Synthesis: Morphologies, Growth Mechanism and Classification. Crit. Rev. Solid State Mater. Sci. 2018. [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Gong, J.M.; Zhang, L.Z.; Yu, J.C. Continuous Size Tuning of Monodisperse ZnO Colloidal Nanocrystal clusters by a Microwave-Polyol Process and Their Application for Humidity Sensing. Adv. Mater. 2008, 20, 4845–4850. [Google Scholar] [CrossRef]
- Majcher, A.; Wiejak, J.; Przybylski, J.; Chudoba, T.; Wojnarowicz, J. A Novel Reactor for Microwave Hydrothermal Scale-up Nanopowder Synthesis. Int. J. Chem. React. Eng. 2013, 11, 361–368. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Smoleń, D.; Łojkowski, W.; Majcher, A.; Mazurkiewicz, A. Examples of the Nanoparticles Produced by Microwave Solvothermal Synthesis (MSS) Route. Glass Ceram. 2014, 6, 8–11. [Google Scholar]
- Wojnarowicz, J.; Kusnieruk, S.; Chudoba, T.; Gierlotka, S.; Lojkowski, W.; Knoff, W.; Lukasiewicz, M.I.; Witkowski, B.S.; Wolska, A.; Klepka, M.T.; et al. Paramagnetism of cobalt-doped ZnO nanoparticles obtained by microwave solvothermal synthesis. Beilstein J. Nanotechnol. 2015, 6, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Mukhovskyi, R.; Pietrzykowska, E.; Kusnieruk, S.; Mizeracki, J.; Lojkowski, W. Microwave solvothermal synthesis and characterization of manganese-doped ZnO nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Sobczak, K.; Lojkowski, W. Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals 2018, 8, 179. [Google Scholar] [CrossRef]
- Lojkowski, W.; Gedanken, A.; Grzanka, E.; Opalinska, A.; Strachowski, T.; Pielaszek, R.; Tomaszewska-Grzeda, A.; Yatsunenko, S.; Godlewski, M.; Matysiak, H.; et al. Solvothermal synthesis of nanocrystalline zinc oxide doped with Mn2+, Ni2+, Co2+ and Cr3+ ions. J. Nanopart. Res. 2009, 11, 1991–2002. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Kuśnieruk, S.; Chudoba, T.; Mizeracki, J.; Łojkowski, W. Microwave solvothermal synthesis of Co-doped ZnO nanoparticles. Glass Ceram. 2015, 3, 8–13. [Google Scholar]
- Fidelus, J.; Piticescu, R.; Piticescu, R.; Lojkowski, W.; Giurgiu, L. Solvothermal Synthesis of Co-doped ZnO Nanopowders. Zeitschrift fur Naturforschung B 2008, 63, 725–729. [Google Scholar] [CrossRef]
- Branka, H.; Romcevic, N.; Maja, R.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Narkiewicz, U.; Sibera, D. Raman study of surface optical phonons in ZnO(Co) nanoparticles prepared by hydrothermal method. Hem. Ind. 2013, 67, 695–701. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of Microwave Radiation Power on the Size of Aggregates of ZnO NPs Prepared Using Microwave Solvothermal Synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Corradi, A.B.; Bondioli, F.; Ferrari, A.M.; Focher, B.; Leonelli, C. Synthesis of silica nanoparticles in a continuous-flow microwave reactor. Powder Technol. 2006, 167, 45–48. [Google Scholar] [CrossRef]
- Cabello, G.; Davoglio, R.A.; Pereira, E.C. Microwave-assisted synthesis of anatase-TiO2 nanoparticles with catalytic activity in oxygen reduction. J. Electroanal. Chem. 2017, 794, 36–42. [Google Scholar] [CrossRef]
- Ebadzadeh, T.; Asadian, K. Microwave-assisted synthesis of nanosized α-Al2O3. Powder Technol. 2009, 192, 242–244. [Google Scholar] [CrossRef]
- Shi, S.; Deng, T.; Zhang, M.; Yang, G. Fast facile synthesis of SnO2/Graphene composite assisted by microwave as anode material for lithium-ion batteries. Electrochim. Acta 2017, 246, 1104–1111. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, L.; Chen, Z.-F.; He, H.; Ye, F.; Cheng, G.; Zhang, Q. Microwave-Assisted Synthesis of Fe3O4 Nanocrystals with Predominantly Exposed Facets and Their Heterogeneous UVA/Fenton Catalytic Activity. ACS Appl. Mater. Interfaces 2017, 9, 29203–29212. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH Verlag: Weinheim, Germany, 2013; ISBN 9783527331970. [Google Scholar] [CrossRef]
- Malka, I.; Danelska, A.; Kimmel, G. The Influence of Al2O3 Content on ZrO2-Al2O3 Nanocomposite Formation—The Comparison between Sol-Gel and Microwave Hydrothermal Methods. Mater. Today Proc. 2016, 3, 2713–2724. [Google Scholar] [CrossRef]
- Koltsov, I.; Smalc-Kozirowska, J.; Prześniak-Welenc, M.; Małysa, M.; Kimmel, G.; McGlynn, J.; Ganin, A.; Stelmakh, S. Mechanism of reduced sintering temperature of Al2O3-ZrO2 nanocomposites obtained by microwave hydrothermal synthesis. Materials 2018, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Koltsov, I.; Prześniak-Welenc, M.; Wojnarowicz, J.; Rogowska, A.; Mizeracki, J.; Malysa, M.; Kimmel, G. Thermal and physical properties of ZrO2-AlO(OH) nanopowders synthesised by microwave hydrothermal method. J. Therm. Anal. Calorim. 2018, 131, 2273–2284. [Google Scholar] [CrossRef]
- Smoleń, D.; Chudoba, T.; Gierlotka, S.; Kedzierska, A.; Łojkowski, W.; Sobczak, K.; Święszkowski, W.; Kurzydłowski, K.J. Hydroxyapatite Nanopowder Synthesis with a Programmed Resorption Rate. J. Nanomater. 2012, 2012, 841971. [Google Scholar] [CrossRef]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Opalinska, A.; Malka, I.; Dzwolak, W.; Chudoba, T.; Presz, A.; Lojkowski, W. Size-dependent density of zirconia nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lojkowski, W.; Leonelii, C.; Chudoba, T.; Wojnarowicz, J.; Majcher, A.; Mazurkiewicz, A. High-Energy-Low-Temperature Technologies for the Synthesis of Nanoparticles: Microwaves and High Pressure. Inorganics 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Opalinska, A.; Leonelli, C.; Lojkowski, W.; Pielaszek, R.; Grzanka, E.; Chudoba, T.; Matysiak, H.; Wejrzanowski, T.; Kurzydlowski, K.J. Effect of pressure on synthesis of Pr-doped Zirconia powders produced by microwave-driven hydrothermal reaction. J. Nanomater. 2006, 2006, 98769. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Varma, R.S. Green Chemistry with Microwave Energy. In Innovations in Green Chemistry and Green Engineering; Anastas, P., Zimmerman, J., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ravichandran, S.; Karthikeyan, E. Microwave Synthesis-A Potential Tool for Green Chemistry. Int. J. Chem. Tech. Res. 2011, 3, 466–470. [Google Scholar]
- Zovinka, E.P.; Stock, A.E. Microwave Instruments: Green Machines for Green Chemistry? J. Chem. Educ. 2010, 87, 350–352. [Google Scholar] [CrossRef]
- Morschhäuser, R.; Krull, M.; Kayser, C.; Boberski, C.; Bierbaum, R.; Püschner, P.A.; Glasnov, T.N.; Kappe, C.O. Microwave-assisted continuous flow synthesis on industrial scale. Green Process. Synth. 2012, 1, 281–290. [Google Scholar] [CrossRef]
- Tierney, J.; Lidstrom, P. Microwave Assisted Organic Synthesis; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2005; ISBN 1-4051-1560-2. [Google Scholar] [CrossRef]
- Galema, S.A. Microwave Chemistry. Chem. Soc. Rev. 1997, 26, 233–238. [Google Scholar] [CrossRef]
- Bogdal, D.; Lukasiewicz, M.; Bednarz, S. Microwave induced thermal gradients in solventless reaction systems. Tetrahedron 2006, 62, 9440–9445. [Google Scholar] [CrossRef]
- Bogdal, D.; Prociak, A. Microwave-Enhanced Polymer Chemistry and Technology; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Dworakowska, S.; Bogdał, D.; Prociak, A. Microwave-Assisted Synthesis of Polyols from Rapeseed Oil and Properties of Flexible Polyurethane Foams. Polymers 2012, 4, 1462–1477. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2012; Volume 52. [Google Scholar]
- Chemat, F.; Cravotto, G. (Eds.) Microwave–Assisted Extraction for Bioactive Compounds Theory and Practice; Springer Science + Business Media: New York, NY, USA, 2013; ISBN 978-1-4614-4829-7. [Google Scholar] [CrossRef]
- Wathey, B.; Tierney, J.; Lidstrom, P.; Westman, J. The Impact of Microwave-Assisted Organic Chemistry on Drug Discovery. Drug Discov. Today 2002, 7, 373–380. [Google Scholar] [CrossRef]
- Fang, Z.; Smith, R.L., Jr.; Qi, X. (Eds.) Production of Biofuels and Chemicals with Microwave Tom 3 Biofuels and Biorefineries; Springer: New York, NY, USA, 2014; ISBN 9789401796125. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The Combined Use of Microwaves and Ultrasound: Improved Tools in Process Chemistry and Organic Synthesis. Chemistry 2007, 13, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Klána, P.; Hájek, M.; Cı́rkva, V. The electrodeless discharge lamp: A prospective tool for photochemistry: Part 3. The microwave photochemistry reactor. J. Photochem. Photobiol. A Chem. 2001, 140, 185–189. [Google Scholar] [CrossRef]
- Rizzuti, A.; Leonelli, C. Crystallization of aragonite particles from solution under microwave irradiation. Powder Technol. 2008, 186, 255–262 101016/jpowtec200712012. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Denti, L.; Gatto, A.; Iuliano, L. Microwave assisted sintering of green metal parts. J. Mater. Process. Technol. 2008, 205, 489–496. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Reszke, E. Microwave Induced Plasma Analytical Spectrometry; RSC Publishing: London, UK, 2011. [Google Scholar]



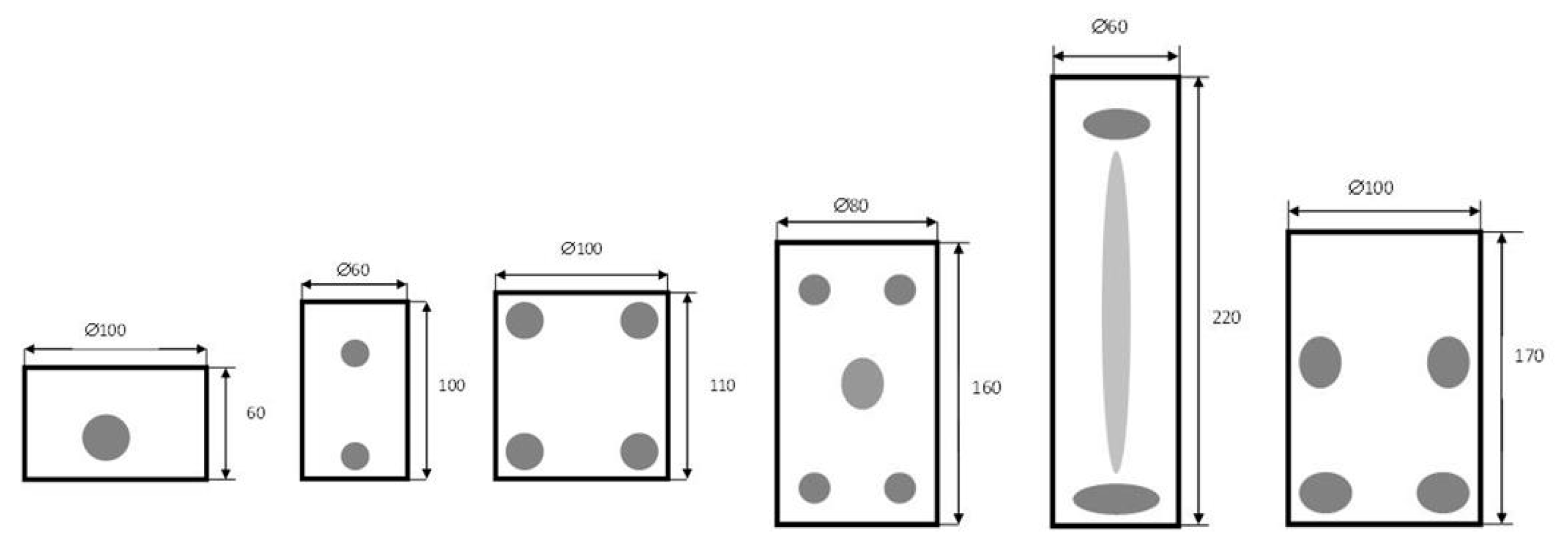





| Material | Temperature in °C | Penetration Depth in cm |
|---|---|---|
| Methanol | 25 | <1 |
| Water (distilled) | 45 | 1.4 |
| Water (distilled) | 95 | 5.7 |
| Acetone | 25 | 7 |
| Toulene | 25 | >40 |
| Polytetrafluoroethylene (PTFE) | 25 | 9200 |
| Quartz glass | 25 | 16,000 |
| MICROWAVE DEVICES AT THE LABORATORY SCALE | |
|---|---|
| EXAMPLE PICTURE | DESCRIPTION |
| www.berghof-instruments.com | Berghof Speedwave Xpert, with a toploader design, is easy to handle. The digestion vessels are inserted into a rotor from above and taken out again. The power output of the magnetron is 2000 W. |
| www.berghof-instruments.com | Berghof Speedwave Entry is optimized for the digestion of sample materials. It is easy and quick to start. The software offers a range of applications to the routine analysis. The magnetron power output is 1000 W. |
 www.ertec.pl [IHPP PAS] | ERTEC Magnum II has a focused microwave system with a detector of reflected power and with a safety system to ensure the proper closing of the shield of the reactor. It can handle a reaction in a liquid phase in a tight Teflon chamber with a usable volume of 70 mL, a temperature of 260 °C, and a pressure of 100 bar. |
| www.nade17.com | Nade XT-9912 Intelligent Microwave Digestion/Extraction System has a microwave output power and frequency of 0–1300 W, with stepless adjustment. The multi-vessel pressure monitoring has a range of 1–50 bar. It has multiple non-contact pressure sensors, real-time monitoring, and 12 digestion vessels. |
 www.cem.com | CEM/MARS 6 is a multimode reactor that can run 40 vessels in parallel. It has a simple all-in-one MW reactor for almost every application. The delivered power is about 1600 W, and there is magnetic stirring with a rotor speed of 8.5 rpm. The temperature is measured by an outside dual IR remote sensor. |
 www.cem.com | CEM/DISCOVER SP-D - Closed Vessel Microwave Digestion is able to perform virtually any type of chemical transformation from organic to inorganic. It has a wide variety of scales of production and temperatures. The manual discover reactor covers a variety of conditions in an open-vessel with up to 125 mL of filling volume and a closed-vessel withup to 50 mL of filling volume. The output power is 300 W. |
 www.cem.com | CEM/VOYAGER SYSTEMS has reaction limits of 250 °C and 18 bar, and the system is also applicable for heterogenous mixtures, slurries, and solid-phase reactions. It is designed to allow the scale up of reactions; it is designed for the stop-flow technique, as well as for the continuous flow system. The stop-flow mode can operate with a special 80 mL vessel to pump the reaction mixture by peristaltic pumps. |
 www.sineomicrowave.com | SINEO/Microwave Digestion and Extraction Workstation has various choices of vessel configurations, with potential throughputs of 16, 18, 40, and 100 vessels, and potential volume capacities of 15, 30, 50, 70, 100, 200, and 500 mL. There is a free lifetime warranty for the core components, including the magnetron of the microwave digestion system. Each digestion vessel may receive great support from the vessel frame on its top and bottom, and it may not be deformed or leaked under pressures of ≤40 bar and temperatures of ≤250 °C. |
 www.sineomicrowave.com | SINEO MDS-15 High-throughput Microwave Sample Preparation Workstation has a 500 mL ultra-large digestion vessel that meets the special requirements for the digestion of samples of up to 10 g. The drying rotor aides in the drying process of samples. Sineo’s patented safety bolt design eliminates the need for explosion-proof membranes and other costly consumables. |
 www.milestonesci.com | Milestone/UltraWAVE/SynthWAVE can achieve a scale up for chemical reactions from grams to the kilogram range. It takes single and multiple reactions at temperatures close to 300 °C and pressures up to 199 bar. Reactions can be carried out directly in the 1 L PTFE vessel or in multiple vials. Its vials are available in glass, quartz, or PTFE, fitted with loose PTFE caps to ensure pressure equalization. Its available rack configurations include 4, 5, 15, and 22 positions. |
 www.anton-paar.com | Anton Paar Masterwave BTR, with productivity increased up to kilogram amounts per day, it has 1700 W installed microwave power. It has an integrated mechanical stirrer with software-guided stirring and can accommodate reaction conditions up to 250 °C and 30 bar. It has constantly circulating microwave-transparent cooling liquid. It offers industry-validated productivity. |
 www.anton-paar.com | Anton Paar Monowave 400 and Monowave 200 are high performance MW reactors designed for small-scale synthesis applications in research and development laboratories. The autosampler option allows for unattended sequential processing of 24 experiments. |
 www.sairem.com | Sairem the MiniFlow 200SS is an easy-to-use microwave-assisted reactor, specifically designed for chemistry and pharmaceutical applications that provides the option to work with small quantities of sample and equally, to be used as a teaching device that allows the user to learn and understand the principles of microwaves. The system can be easily configured to perform different applications, including reactions in the liquid phase, solid phase, and gas phase in homogeneous and heterogeneous mixtures. |
 www.chemspeed.com | Chemspeed SWAVE is a medium microwave synthesizer station from Chemspeed that incorporates a Biotage Initiador with the features necessary to enable a fully automated synthesis workflow form. It allows for the addition of reagents under an inert atmosphere, solid weighing, direct dispensing, and liquid handling. |
| www.enbiogroup.pl | Autoclaws ENBIOJET is a popular, simple sterilizer that uses traditional transformer generators, magnetrons, and waveguides. The specially designed shape of the applicator chamber allows for convenient use of typical laboratory vessels of up to 500 mL capacity. As much as 0.5 L of water in a glass laboratory flask with a diameter of up to 120 mm can be effectively heated to 135 °C in 1.5 min using two microwave sources with a pressure enhanced to 3.5 bar. |
| SCALE UP REACTORS | |
|---|---|
| EXAMPLE PICTURE | DESCRIPTION |
| www.cambrex.com | The CaMWave™ KiloLAB reactor can process more than 100 L in 24 h to produce in excess of 20 kg of product. Larger CaMWave™ reactors are able to manufacture more than 200 kg in 24 h and Good Manufacture Practise equipment is in development. The skid-mounted modular design utilizes current manufacturing plants, keeping capital expenditure low and flexibility high.The the maximum pressure is 20 bar. |
 www.labnano.pl | MSS1 IHPP PAS Warsaw [68] has a power of 2 generators of 1500 W, with continuous-adjustment hydrothermal and solvothermal reactions tested. It supports pressures <40 bar and temperatures up to 300 °C, with a chamber made of Teflon, an automatic working system, and heating up to 5 °C/s. Its productivity is 300 g of powder per day and 30 L of solution per day. The MSS reactors are made strictly for nanopowder synthesis under a variety of pressures and with a variety of powers from generators. |
 www.labnano.pl | MSS2 IHPP PAS Warsaw [44,68] is a device, in which all the elements that are in contact with the substrate are made of chemically clean and resistant material, such as PTFE and Al2O3 ceramics, so the maximum operation temperature is 270 °C because of the PTFE. It works with a generator with a 3000 W magnetron by ERTEC Poland. Its productivity is up to 500 g of powder per day and 50 L of solution. Its control system has the following: - regulation of pressure and time of synthesis; - monitoring of processes and emergency maintance; and Its work modes include the stop-flow (automatic, half-automatic and manual) mode and batch mode (manual operation mode). |
 www.sairem.com | The LABOTRON improves considerably the performance of microwave-assisted chemistry due to the following: adjustable power from a few watts to many kilowatts; optimized geometry of the INTLI to achieve high-power densities inside the reactor up to 10 kW/L; direct reading of the forward and reflected power values to enable the correct calculation of the energy absorbed by the sample; and efficient mechanical stirring with adjustable speeds. The reactors are adapted for homogeneous and heterogeneous chemistry in liquid and solid phases. It has quick connections for increased flexibility and rapid cleaning and maintenance. |
| HYBRID REACTORS | |
|---|---|
| EXAMPLE PICTURE | DESCRIPTION |
 www.sineomicrowave.com | UWave-2000 Multifunctional Microwave Chemistry Reaction Workstation integrates the atmospheric pressure and pressurized reaction, microwave, ultrasonic wave and ultraviolet irradiation, and other functions, giving full flexiblility to the user. The microwave automatic frequency conversion control, dual temperature control technology, and piezoelectric crystal pressure can ensure the accurate record and representation of each reaction. The microwave source is 1000 W and the adjustable scope of the ultrasound power is 0–800 W. |
 www.sineomicrowave.com | UWave-1000Sineomicrowave has the following features: automatic variation range of microwave power: 0–1000 W; ultrasonic working frequency: 26–28 KHz; power regulation range: 0–800 W; ultraviolet irradiation light source wavelength: 365 nm; power: 300 W; temperature control: from room temperature to 300 °C.; and reaction container volume: 50–1000 mL (10 mL and 20 mL are optional). The reaction container material is quartz glass, and the range of the operating time is from 1 s to 6000 min. |
 www.milestonesci.com | Milestone ATC-FO 300 MultiSYNTH Single and Multi-Mode Microwave Synthesis System is a so-called hybrid device. It has an ability to merge into a single-mode reactor, as well as a multimode reactor. A single magnetron with a homogenous microwave distribution in the cavity has a 800 W output power for the multimode configuration. For the single mode, the output power is only 400 W. It has an immersed fiber-optic probe and indirect pressure control with an outside IR remote sensor. For the single-mode format, 2.5 and 10 mL glass virals are provided with a temperature limit of 250 °C and a pressure limit of 20 bar. |
| HIGH-TEMPERATURE MICROWAVE REACTORS | |
|---|---|
| EXAMPLE PICTURE | DESCRIPTION |
 www.cem.com | CEM Phoenix™ Microwave Muffle Furnace has a speed that is up to 10 times faster than the conventional muffle furnaces and a capacity of up to 15 samples at one time. The high-temperature furnaces reach 1200 °C and can process up to eight 25 mL crucibles. For laboratories needing greater throughput, the high-capacity furnaces reach up to 1000 °C and hold up to fifteen 25 mL crucibles. It can process any crucible that can be used in a conventional muffle furnace (including platinum). |
 www.milestonesci.com | FlexiWAVE is a high-temperature muffle reactor with an extremely fast heating rate (5 min to 800 °C and 10 min to 1000 °C). Nowadays, it is a first MW reactor for chemical synthesis able to work at 1200 °C. |
 www.weisstechnik.be | Vötsch Industrietechnik was introduced at JEC Europe 2013. As a very energy efficient method for curing fiber composite components in microwave technology, the modular microwave system can accomodate large components. The patented hexagonal cavity shape for a uniform field distribution is useful for making high-quality products. Compared with convectional heating methods, this device saves up to 70% energy and has a working chamber volume between 750 L and 7000 L. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. https://doi.org/10.3390/cryst8100379
Dąbrowska S, Chudoba T, Wojnarowicz J, Łojkowski W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals. 2018; 8(10):379. https://doi.org/10.3390/cryst8100379
Chicago/Turabian StyleDąbrowska, Sylwia, Tadeusz Chudoba, Jacek Wojnarowicz, and Witold Łojkowski. 2018. "Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review" Crystals 8, no. 10: 379. https://doi.org/10.3390/cryst8100379
APA StyleDąbrowska, S., Chudoba, T., Wojnarowicz, J., & Łojkowski, W. (2018). Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals, 8(10), 379. https://doi.org/10.3390/cryst8100379








