Quasi-Equilibrium, Multifoil Platelets of Copper- and Titanium-Substituted Bismuth Vanadate, Bi2V0.9(Cu0.1−xTix)O5.5−δ, by Molten Salt Synthesis
Abstract
:1. Introduction
2. Materials and Methods
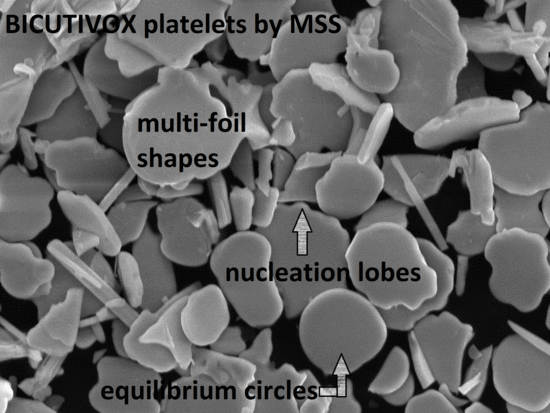
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, K.; Debreuille, M.; Mairesse, G.; Nowogrocki, G. Phase transitions and ionic conductivity in Bi4V2O11 an oxide with a layered structure. Solid State Ion. 1988, 28–30, 529–532. [Google Scholar] [CrossRef]
- Abraham, F.; Boivin, J.; Mairesse, G.; Nowogrocki, G. The BIMEVOX Series: A New Family of High Performance Oxide Ion Conductors. Solid State Ion. 1990, 40–41, 934–937. [Google Scholar] [CrossRef]
- Yaremchenko, A.; Avdeev, M.; Kharton, K.; Kovalevsky, A.; Naumovich, E.; Marques, F. Structure and electronic conductivity of Bi2−xLaxV0.9Cu0.1O5.5−δ. Mater. Chem. Phys. 2002, 77, 552–558. [Google Scholar] [CrossRef]
- Kurek, P.; Dygas, J.; Breiter, M. Impedance measurements on single crystals of the oxygen ion conductor BICUVOX. J. Electroanal. Chem. 1994, 378, 77–83. [Google Scholar] [CrossRef]
- Goodenough, J. Oxide-Ion Electrolytes, a review. Ann. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Kendall, K.; Navas, C.; Thomas, J.; Loye, H. Recent Developments in Oxide Ion Conductors: Aurivillius Phases. Chem. Mater. 1996, 8, 642–649. [Google Scholar] [CrossRef]
- Cho, H.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Preparation of BiMeVOx (Me=Cu, Ti, Zr, Nb, Ta) compounds as solid electrolyte and behavior of their oxygen concentration cells. Sens. Actuators B Chem. 2005, 109, 307–314. [Google Scholar] [CrossRef]
- Kida, T.; Minami, T.; Kishi, S.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Planar-type BiCuVOx solid electrolyte sensor for the detection of volatile organic compounds. Sens. Actuators B Chem. 2009, 137, 147–153. [Google Scholar] [CrossRef]
- Kida, T.; Harano, H.; Minami, T.; Kishi, S.; Morinaga, N.; Yamazoe, N.; Shimanoe, K. Control of electrode reactions in a mixed-potential-type gas sensor based on a BiCuVOx solid electrolyte. J. Phys. Chem. C 2010, 114, 15141–15148. [Google Scholar] [CrossRef]
- Patil, B.; Sharma, S.; Mohanta, H.; Roy, B. BINIVOX catalyst for hydrogen production from ethanol by low temperature steam reforming (LTSR). J. Chem. Sci. 2017, 129, 1741–1746. [Google Scholar] [CrossRef]
- Fuierer, P.; Maier, R.; Roder-Roith, U.; Moos, R. Processing Issues Related to the Bi-dimensional Ionic Conductivity of BIMEVOX Ceramics. J. Mater. Sci. 2011, 46, 5447–5453. [Google Scholar] [CrossRef]
- Sant, C.; Contour, J. Pulsed laser deposition of Bi4Cu2xV2(1−x)O11 thin films. J. Cryst. Growth 1995, 153, 63–67. [Google Scholar] [CrossRef]
- Muller, C.; Chateigner, D.; Anne, M.; Bacmann, M.; Fouletier, J.; Rango, P. Pressure and magnetic field effects on the crystallographic texture and electrical conductivity of the compound. J. Phys. D Appl. Phys. 1996, 29, 3106–3111. [Google Scholar] [CrossRef]
- Fuierer, P.; Nichtawitz, A. Electric Field Assisted Hot Forging of Bismuth Titanate. In Proceedings of the IEEE International Symposium on Applications of Ferroelectrics, University Park, PA, USA, 7–10 August 1994; pp. 126–129. [Google Scholar]
- Fuierer, P.; Newnham, R. Newnham, La2Ti2O7 Ceramics. J. Am. Ceram. Soc. 1991, 74, 2876–2881. [Google Scholar] [CrossRef]
- Fuierer, P.; Maier, M.; Exner, J.; Moos, R. Anisotropy and thermal stability of hot-forged BICUTIVOX oxygen ion conducting ceramics. J. Eur. Ceram. Soc. 2014, 34, 943–951. [Google Scholar] [CrossRef]
- Shantha, K.; Varma, K. Fabrication and characterization of grain-oriented bismuth vanadate ceramics. Mater. Res. Bull. 1997, 32, 1581–1591. [Google Scholar] [CrossRef]
- Seth, V.; Schulze, W. Grain-Oriented Fabrication of Bismuth Titanate Ceramics and Its Electrical Properties. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kimura, T.; Yamaguchi, T. Particle Orientation during Tape Casting in the Fabrication of Grain-Oriented Bismuth Titanate. J. Am. Ceram. Soc. 1989, 72, 289–293. [Google Scholar] [CrossRef]
- Horn, J.; Zhang, S.; Selvaraj, U.; Messing, G.; Trolier, S. Templated Grain Growth of Textured Bismuth Titanate. J. Am. Ceram. Soc. 1999, 82, 921–926. [Google Scholar] [CrossRef]
- Yilmaz, H.; Messing, G.; Trolier, S. (Reactive) Templated Grain Growth of Textured Sodium Bismuth Titanate (Na0.5Bi0.5TiO3-BaTiO3) Ceramics-I Processing. J. Electroceram. 2003, 11, 207–215. [Google Scholar] [CrossRef]
- West, D.; Payne, D. Reactive-Templated Grain Growth of Bi0.5(Na,K)0.5TiO3: Effects of Formulation on Texture Development. J. Am. Ceram. Soc. 2003, 86, 1132–1137. [Google Scholar] [CrossRef]
- Kimura, T.; Yoshida, Y. Origin of Texture Development in Barium Bismuth Titanate Prepared by the Templated Grain Growth Method. J. Am. Ceram. Soc. 2006, 89, 869–874. [Google Scholar] [CrossRef]
- Arendt, R.; Rosolowski, J.; Szymaszek, J. Lead zirconate titanate ceramics from molten salt solvent synthesized powders. Mater. Res. Bull. 1979, 15, 703–709. [Google Scholar] [CrossRef]
- Kimura, T.; Yamaguchi, T. Fused Salt Synthesis of Bi4Ti3O12. Ceram. Int. 1983, 9, 13–17. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Hou, J.; Zhou, K. Molten salt synthesis of anisometric Sr3Ti2O7 particles. J. Cryst. Growth 2007, 305, 265–270. [Google Scholar] [CrossRef]
- Kimura, T. Chapter 4. Molten Salt Synthesis of Ceramic Powders. In Advances in Ceramics—Synthesis and Characterization, Processing and Specific Applications; Sikalidis, C., Ed.; INTECH: Rijeka, Croatia, 2011; pp. 75–100. ISBN 978-953-307-505-1. [Google Scholar]
- Liu, X.; Fechler, N.; Antonietti, M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev. 2013, 42, 8237–8265. [Google Scholar] [CrossRef] [PubMed]
- Preethi, G.; Ninan, A.; Kumar, K.; Balan, R.; Nagaswarupa, H. Molten Salt Synthesis of Nanocrystalline ZnFe2O4 and Its Photocatalytic Dye Degradation Studies. Mater. Today Proc. 2017, 4, 11816–11819. [Google Scholar] [CrossRef]
- Kang, M.; Kim, D.; Hwang, N. Ostwald ripening kinetics of angular grains dispersed in a liquid phase by two dimensional nucleation and abnormal grain growth. J. Eur. Ceram. Soc. 2002, 22, 603–612. [Google Scholar] [CrossRef]
- Roy, B.; Fuierer, P. Molten Salt Synthesis of Bi4(V0.85Co0.15)2O11−δ (BICOVOX) Ceramic Powders. J. Am. Ceram. Soc. 2009, 92, 520–523. [Google Scholar] [CrossRef]
- Sangster, J.; Pelton, A. Phase Diagrams and Thermodynamic Properties of the 70 Binary Alkali Halide Systems Having Common Ions. J. Phys. Chem. Ref. Data 1987, 16, 509. [Google Scholar] [CrossRef]
- International Center for Diffraction Data. Powder Diffraction File #01-070-9191, Bismuth Vanadium Copper Oxide; International Center for Diffraction Data: Newtown Square, PA, USA.
- Inoue, M.; Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4·2H2O. J. Cryst. Growth 2013, 380, 169–175. [Google Scholar] [CrossRef]
- Roy, B.; Scott, P.; Ahrenkiel, Y.; Fuierer, P. Controlling the Size and Morphology of TiO2 Powder by Molten and Solid Salt Synthesis. J. Am. Ceram. Soc. 2008, 91, 2455–2463. [Google Scholar] [CrossRef]
- Betz, W.; Webb, H. Plane Geometry, Book, V. Regular Polygons and Circles; Ginn & Co.: Cambridge, UK, 1921; p. 321. [Google Scholar]
- Lotgering, F. Topotactical Reaction with Ferrimagnetic Oxides Having Hexagonal Structures. J. Inorg. Nucl. Chem. 1959, 9, 113–123. [Google Scholar] [CrossRef]
- Yoon, K.; Yong, S.; Kang, D. Review: Molten Salt synthesis of lead-based relaxors. J. Mater. Sci. 1998, 33, 2977–2984. [Google Scholar] [CrossRef]
- Kapadia, L. The Mechanism of Grain Growth in Ceramics; NASA Report; NASA: Washington, DC, USA, 1973; pp. 6–34.
- Gu, Y.; Huang, J.; Li, L.; Zhang, K.; Wang, X.; Li, Q.; Tan, X.; Xu, H. Molten salt synthesis of anisotropy BaBi4Ti4O15 powders in K2SO4-Na2SO4 flux. Mater. Sci. Forum 2011, 687, 333–338. [Google Scholar] [CrossRef]
- Wulff, G. Zur Frage der Geschwindigkeit des Wachsthums und der Auflösung der Krystallflächen. Z. Kristallogr. 1901, 34, 449–530. [Google Scholar] [CrossRef]
- Herring, C. Some Theorems on the Free Energies of Crystal Surfaces. Phys Rev. 1951, 82, 87–93. [Google Scholar] [CrossRef]
- Kang, S.C. 15 Grain Shape and Grain Growth in a Liquid Matrix. In Sintering; Elsevier: London, UK, 2005; p. 218. [Google Scholar]
- Kimura, T.; Tani, T. Chapt 15, Processing and Properties of Textured Bismuth Layer-Structured Ferroelectrics. In Lead Free Piezoelectrics; Priya, S., Nahm, S., Eds.; Springer: New York, NY, USA, 2012; p. 465. ISBN 978-1-4419-9598-8. [Google Scholar]
- Hollenberg, G.; Gordon, R. Origin of Anomalously High Activation Energies in Sintering and Creep of Impure Refractory. J. Am. Ceram. Soc. 1973, 56, 109–110. [Google Scholar] [CrossRef]
- Afanasiev, P. Preparation of Mixed Phosphates in Molten Alkali Metal Nitrates. Chem. Mater. 1999, 11, 1999–2007. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Kan, Y.M.; Wang, P.L.; Li, Y.G.; Zhang, G.J. Nd/V Co-Doped Bi4Ti3O12 Powder Prepared by Molten Salt Synthesis. J. Am. Ceram. Soc. 2007, 90, 3353–3356. [Google Scholar] [CrossRef]
- Chiang, Y.; Birnie, D.; Kingery, W. Chapter 5, Microstructure. In Physical Ceramics; J. Wiley & Sons: New York, NY, USA, 1997; p. 354. ISBN 0-471-59873-9. [Google Scholar]










| Phase | Treatment | F(001) | Dave ± DStDev | Dmax | AR ± ARStDev |
|---|---|---|---|---|---|
| T(°C)/t(h) | (µm) | (µm) | |||
| BICUVOX | 610/8 | 0.14 | 7.2 ± 1.5 | 11.7 | 8 ± 3 |
| 650/2 | 24.5 ± 2.0 | 36.3 | 13 ± 3 | ||
| 650/4 | 27.0 ± 4.5 | 47.5 | 13 ± 3 | ||
| 650/6 | 0.33 | 26.9 ± 7.2 | 35.1 | 12 ± 3 | |
| 650/8 | 0.43 | 33.5 ±10.3 | 49.4 | 15 ± 8 | |
| 650/10 | 29.1 ± 5.5 | 44.9 | 9 ± 2 | ||
| 675/8 | 0.23 | 37.3 ± 21.1 | 65.5 | 15 ± 9 | |
| BICUTIVOX | 650/2 | 9.6 ± 0.6 | 12.9 | 8 ± 3 | |
| 650/4 | 11.0 ± 3.2 | 21.7 | 11 ± 3 | ||
| 650/6 | 13.6 ± 0.5 | 18.3 | 9 ± 2 | ||
| 650/8 | 0.6 | 16.1 ± 2.7 | 29.2 | 10 ± 2 | |
| 650/10 | 16.3 ± 3.0 | 24.3 | 7 ± 1 | ||
| 675/8 | 0.47 | 17.9 ± 2.8 | 25.0 | 11 ± 3 | |
| 700/8 | 0.22 | 21.3 ± 4.2 | 32.1 | 10 ± 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ring, K.; Fuierer, P. Quasi-Equilibrium, Multifoil Platelets of Copper- and Titanium-Substituted Bismuth Vanadate, Bi2V0.9(Cu0.1−xTix)O5.5−δ, by Molten Salt Synthesis. Crystals 2018, 8, 170. https://doi.org/10.3390/cryst8040170
Ring K, Fuierer P. Quasi-Equilibrium, Multifoil Platelets of Copper- and Titanium-Substituted Bismuth Vanadate, Bi2V0.9(Cu0.1−xTix)O5.5−δ, by Molten Salt Synthesis. Crystals. 2018; 8(4):170. https://doi.org/10.3390/cryst8040170
Chicago/Turabian StyleRing, Kevin, and Paul Fuierer. 2018. "Quasi-Equilibrium, Multifoil Platelets of Copper- and Titanium-Substituted Bismuth Vanadate, Bi2V0.9(Cu0.1−xTix)O5.5−δ, by Molten Salt Synthesis" Crystals 8, no. 4: 170. https://doi.org/10.3390/cryst8040170
APA StyleRing, K., & Fuierer, P. (2018). Quasi-Equilibrium, Multifoil Platelets of Copper- and Titanium-Substituted Bismuth Vanadate, Bi2V0.9(Cu0.1−xTix)O5.5−δ, by Molten Salt Synthesis. Crystals, 8(4), 170. https://doi.org/10.3390/cryst8040170