Simultaneous Enhancement of Electrical Conductivity and Seebeck Coefficient of [6,6]-Phenyl-C71 Butyric Acid Methyl Ester (PC70BM) by Adding Co-Solvents
Abstract
:1. Introduction
2. Experimental
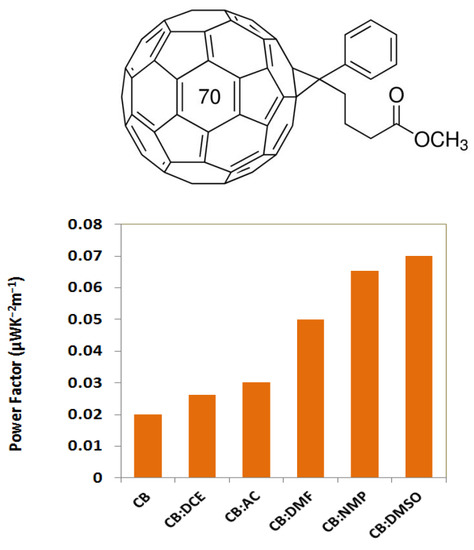
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fan, S.; Xiao, N.; Liu, D.; Tay, Y.Y.; Yu, C.; Sim, D.; Hng, H.H.; Zhang, Q.; Boey, F.; et al. Flexible Carbon Nanotube Papers with Improved Thermoelectric Properties. Energy Environ. Sci. 2012, 5, 5364–5369. [Google Scholar] [CrossRef]
- Culebras, M.; Gómez, C.M.; Cantarero, A. Thermoelectric measurements of PEDOT:PSS/expanded graphite composites. J. Mater. Sci. 2013, 48, 2855–2860. [Google Scholar] [CrossRef]
- Dubey, N.; Leclerc, M. Conducting Polymers: Efficient Thermoelectric Materials. J. Polym. Sci. B Polym. Phys. 2011, 49, 467–475. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.M.; Xu, W.; Zhu, D.B. Thermoelectric energy from flexible P3HT films doped with a ferric salt of triflimide anions. Energy Environ. Sci. 2012, 5, 9639–9644. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Xiong, Y.; Wu, P.; Hu, B.; Xu, L. Fabricate organic thermoelectric modules use modified PCBM and PEDOT:PSS materials. Front. Optoelectron. 2017, 10, 117–123. [Google Scholar] [CrossRef]
- Petsagkourakis, I.; Pavlopoulou, E.; Portale, G.; Kuropatwa, B.A.; Dilhaire, S.; Fleury, G.; Hadziioannou, G. Structurally-driven enhancement of thermoelectric properties within poly(3,4-ethylenedioxythiophene) thin films. Sci. Rep. 2016, 6, 30501. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yeh, M.L.; Jung, B.J.; Zhang, B.; Feser, J.; Majumdar, A.; Katz, H.E. Simultaneous increase in Seebeck coefficient and conductivity in a doped poly(alkylthiophene) blend with defined density of states. Macromolecules 2010, 43, 2897–2903. [Google Scholar] [CrossRef]
- Maiz, J.; Rojo, M.M.; Abad, B.; Wilson, A.A.; Nogales, A.; Borca-Tasciuc, D.A.; Borca-Tasciuc, T.; Martín-González, M. Enhancement of thermoelectric efficiency of doped PCDTBT polymer films. RSC Adv. 2015, 5, 66687–66694. [Google Scholar] [CrossRef]
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Thermoelectric properties of conducting polymers: The case of poly(3-hexylthiophene). Phys. Rev. B 2010, 82, 115454. [Google Scholar] [CrossRef]
- Mateeva, N.; Niculescu, H.; Schlenoff, J.; Testardi, L.R. Correlation of Seebeck coefficient and electric conductivity in polyaniline and polypyrrole. J. Appl. Phys. 1998, 83, 3111–3117. [Google Scholar] [CrossRef]
- Kaiser, A.B. Thermoelectric power and conductivity of heterogeneous conducting polymers. Phys. Rev. B 1989, 40, 2806. [Google Scholar] [CrossRef]
- Toshima, N. Conductive polymers as a new type of thermoelectric material. Macromol. Symp. 2002, 186, 81–86. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Song, H.; Shi, H.; Yao, Y.; Xu, J.; Zhang, G.; Lu, B. Improved thermoelectric performance of PEDOT:PSS films prepared by polar-solvent vapor annealing method. J. Mater. Sci. Mater. Electron. 2013, 24, 4240–4246. [Google Scholar] [CrossRef]
- Ma, W.; Shi, K.; Wu, Y.; Lu, Z.Y.; Liu, H.Y.; Wang, J.Y.; Pei, J. Enhanced molecular packing of a conjugated polymer with high organic thermoelectric power factor. ACS Appl. Mater. Interfaces 2016, 8, 24737–24743. [Google Scholar] [CrossRef] [PubMed]
- Rastegaralam, M.; Lee, C.; Dettlaff-Weglikowska, U. Solvent-Dependent Thermoelectric Properties of PTB7 and Effect of 1,8-Diiodooctane Additive. Crystals 2017, 7, 292. [Google Scholar] [CrossRef]
- Kim, S.S.; Bae, S.; Jo, W.H. Performance enhancement of planar heterojunction perovskite solar cells by n-doping of the electron transporting layer. Chem. Commun. 2015, 51, 17413–17416. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Oh, J.H.; Dong, G.; Bao, Z. Use of a 1H-Benzoimidazole Derivative as an n-Type Dopant and to Enable Air-Stable Solution-Processed n-Channel Organic Thin-Film Transistors. JACS 2010, 132, 8852–8853. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Uehara, K.; Klug, D.D.; Tse, J.S. Rational design of high-efficiency thermoelectric materials with low band gap conductive polymers. Comput. Mater. Sci. 2006, 36, 49–53. [Google Scholar] [CrossRef]
- Wuesten, J.; Ziegler, C.; Ertl, T. Electron transport in pristine and alkali metal doped perylene-3,4,9,10-tetracarboxylicdianhydride (PTCDA) thin films. Phys. Rev. B 2006, 74, 125205. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 25, 554. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Wang, L.; Chen, L.D.; Nolas, G.S. Enhanced Seebeck coefficient through energy-barrier scattering in PbTe nanocomposites. Phys. Rev. B 2009, 79, 115311. [Google Scholar] [CrossRef]









| Material | Boiling Point (°C) |
|---|---|
| Chlorobenzene | 131 |
| 1-Methyl-2-pyrrolidone | 202 |
| Dimethylsulfoxide | 189 |
| N,N-Dimethylformamide | 153 |
| 1,2-Dichloroethane | 83 |
| Acetonitrile | 82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastegaralam, M.; Lee, C.; Dettlaff-Weglikowska, U. Simultaneous Enhancement of Electrical Conductivity and Seebeck Coefficient of [6,6]-Phenyl-C71 Butyric Acid Methyl Ester (PC70BM) by Adding Co-Solvents. Crystals 2018, 8, 237. https://doi.org/10.3390/cryst8060237
Rastegaralam M, Lee C, Dettlaff-Weglikowska U. Simultaneous Enhancement of Electrical Conductivity and Seebeck Coefficient of [6,6]-Phenyl-C71 Butyric Acid Methyl Ester (PC70BM) by Adding Co-Solvents. Crystals. 2018; 8(6):237. https://doi.org/10.3390/cryst8060237
Chicago/Turabian StyleRastegaralam, Mina, Changhee Lee, and Urszula Dettlaff-Weglikowska. 2018. "Simultaneous Enhancement of Electrical Conductivity and Seebeck Coefficient of [6,6]-Phenyl-C71 Butyric Acid Methyl Ester (PC70BM) by Adding Co-Solvents" Crystals 8, no. 6: 237. https://doi.org/10.3390/cryst8060237
APA StyleRastegaralam, M., Lee, C., & Dettlaff-Weglikowska, U. (2018). Simultaneous Enhancement of Electrical Conductivity and Seebeck Coefficient of [6,6]-Phenyl-C71 Butyric Acid Methyl Ester (PC70BM) by Adding Co-Solvents. Crystals, 8(6), 237. https://doi.org/10.3390/cryst8060237