Li and Co Ordering in the Nitridocobaltate(I) SrLi2{Li[CoN2]}
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
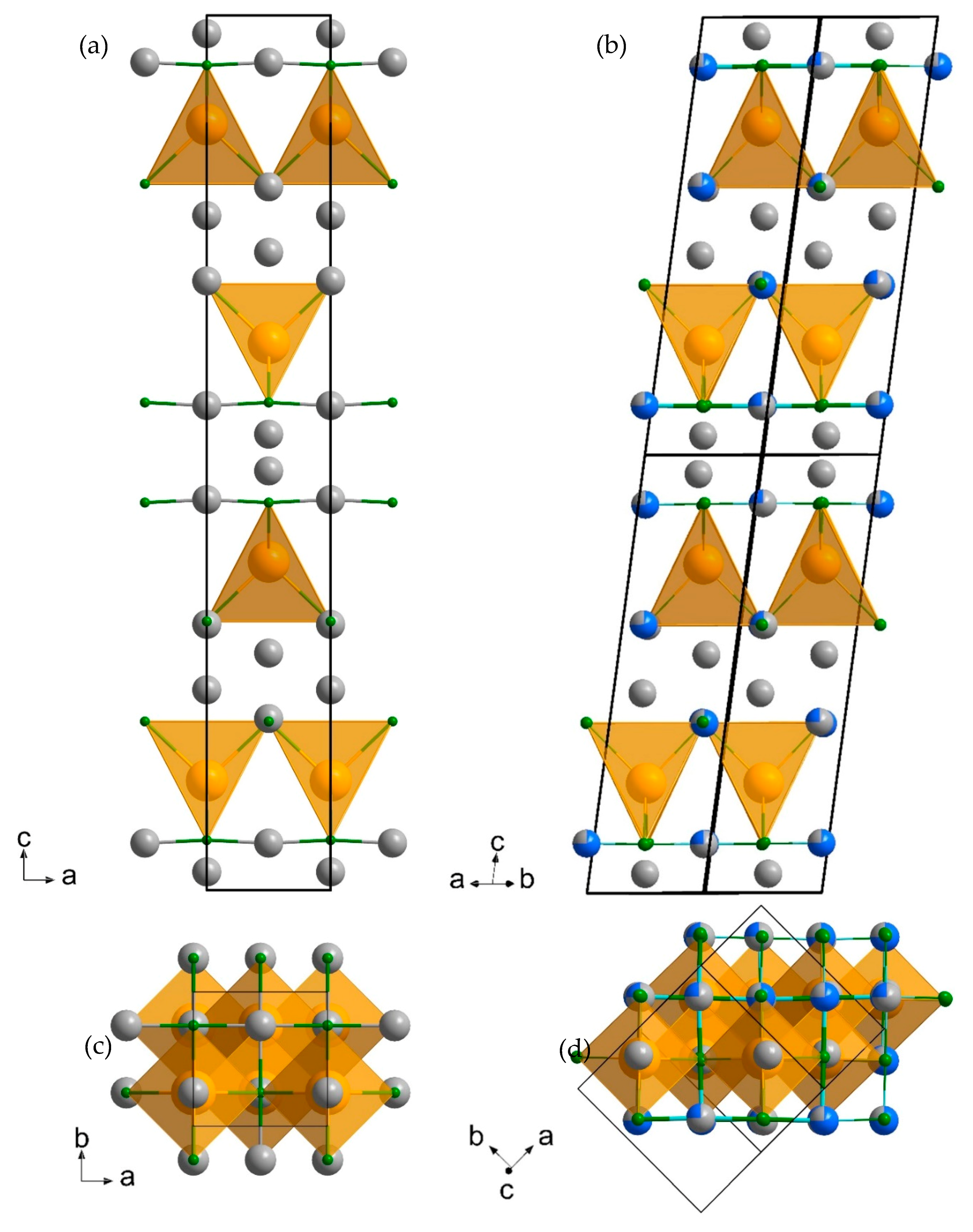
3.1. Diffraction Data and Refinement
3.2. Measurements of the Magnetic Susceptibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregory, D.H. Lithium nitrides as sustainable energy materials. Chem. Rec. 2008, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, A. Lithium nitride and related materials case study of the use of modern solid state research techniques. Solid State Ionics 1982, 6, 277–293. [Google Scholar] [CrossRef]
- Cordier, G.; Gudat, A.; Kniep, R.; Rabenau, A. LiCaN and Li4SrN2, Derivatives of the fluorite and lithium nitride structures. Angew. Chemie Int. Ed. 1989, 28, 1702–1703. [Google Scholar] [CrossRef]
- Klatyk, J.; Kniep, R. Crystal structure of alkaline earth dilithium bis(nitridolithiate/ferrates(I)), Ca{Li2[(Li1−xFex)N]2}, x = 0.30 and Sr{Li2[(Li1−xFexN)]2}, x = 0.46. Z. Anorg. Allg. Chem. 1999, 214, 449–450. [Google Scholar] [CrossRef]
- Gudat, A.; Kniep, R.; Rabenau, A. Ternary and quaternary metal-nitrides in the Li–Sr–Ni–N-system. Thermochim. Acta. 1990, 160, 49–56. [Google Scholar] [CrossRef]
- Jäger, J.; Kniep, R. Nitridocuprate(I): Quaternäre Phasen in den Systemen Li–Ca/Sr–Cu–N. Z. Naturforschung. B. 1993, 47, 1290–1296. [Google Scholar] [CrossRef]
- Höhn, P.; Kniep, R. LiSr2[CoN2]: Ein Nitridocobaltat(I) mit gestreckten Anionen [CoN2]5−/LiSr2[CoN2]: A Nitridocobaltate(I) with Linear Anions [CoN2]5−. Z. Naturforsch. B. 1992, 47, 434–436. [Google Scholar] [CrossRef]
- Gudat, A.; Kniep, R.; Rabenau, A. Li3Sr3Ni4N4: Ein Niedervalentes Nitridoniccolat mit-Ketten. Z. Anorg. Allg. Chem. 1991, 597, 61–67. [Google Scholar] [CrossRef]
- Höhn, P.; Niewa, R. Nitrides of non-maingroup elements. In Handbook of Solid State Chemistry, 1st ed.; Dronskowski, R., Kikkawa, S., Stein, A., Eds.; Wiley-VCH: Weinheim, Germany, 5870; Volume 1, pp. 251–359. ISBN 9783527325870. [Google Scholar]
- Gordon, A.; Smith, R.; Wilson, C.; Stoeva, Z.; Gregory, D.H. Crystal growth, defect structure and magnetism of new Li3N-derived lithium nitridocobaltates. ChemComm 2004, 24, 2812–2813. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Rau, D.; Toffoletti, L.; Müller, C.; Burkhardt, U.; Schnelle, W.; Niewa, R.; Schwarz, U. Ternary metastable nitrides ε-Fe2TMN (TM = Co, Ni): High-pressure, high-temperature synthesis, crystal structure, thermal stability, and magnetic properties. Chem. Mater. 2012, 24, 4600–4606. [Google Scholar] [CrossRef]
- Nishijima, M. Synthesis and electrochemical studies of a new anode material, Li3−xCoxN. Solid State Ionics 1996, 83, 107–111. [Google Scholar] [CrossRef]
- STOE & Cie GmbH. WinXPow: Program for Handling and Refinement of Powder Diffraction Data; Version 3.0.1.13; STOE & Cie GmbH: Darmstadt, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sachsze, W.; Juza, R. Metallamide und Metallnitride, 21. Mitteilung. Ueber Mischkristalle der Zusammensetzung (Li,Co)3N, (Li,Ni)3N und (Li,Cu)3N. Z. Anorg. Allg. Chem. 1949, 259, 278–290. [Google Scholar] [CrossRef]
- Bärnighausen, H. Group-subgroup relations between space groups: A useful tool in crystal chemistry. MATCH Commun. Math. Comput. Chem. 1980, 9, 139–175. [Google Scholar]
- Niewa, R.; Huang, Z.L.; Schnelle, W.; Hu, Z.; Kniep, R. Preparation, crystallographic, spectroscopic and magnetic characterization of low-valency nitridometalates Li2[(Li1−xMx)N] with M = Cu, Ni. Z. Anorg. Allg. Chem. 2003, 629, 1778–1786. [Google Scholar] [CrossRef]
- Jesche, A.; Ke, L.; Jacobs, J.L.; Harmon., B.; Houk, R.S.; Canfield, P.C. Alternating magnetic anisotropy of Li2(Li1−xTx)N (T = Mn, Fe, Co, and Ni). Phys. Rev. B 2015, 91, 1–5. [Google Scholar] [CrossRef]



| Composition | SrLi2{Li0.55(1)Co0.45(1)[Co0.55(1)Li0.45(1)N2]} | SrLi2{Li0.65Co0.35[Co0.65Li0.35N2]} |
|---|---|---|
| Crystal System | Tetragonal | Monoclinic |
| Space Group | I41/amd (Nr. 141) | P21/c (Nr. 14) |
| Z | 4 | 4 |
| a/Å | 3.741(2) | 5.2958(3) |
| b/Å | 3.741(2) | 5.2898(4) |
| c/Å | 27.93(2) | 14.2164(5) |
| β/° | 90 | 100.728(1) |
| ρcalc/gcm-3 | 3.319 | 3.317 |
| Volume V/Å3 | 390.98 | 391.29 |
| Measurement temperature/K | 293(2) | 293(2) |
| Index range (±hmax,±kmax, ±lmax) | 4, 4, 36 | 6, 6, 18 |
| Max. 2θ /deg | 54.85 | 54.98 |
| F(000) | 360.8 | 352.0 |
| µ/mm-1 | 18.03 | 17.64 |
| Measured reflections | 4373 | 7168 |
| Observed reflections | 148 | 878 |
| Rint/Rσ | 0.2109/0.0515 | 0.0985/0.0473 |
| R1/wR2 | 0.0636/0.1611 | 0.0640/0.1470 |
| GooF | 1.136 | 1.133 |
| Remaining electron density (max/min) | 1.55/−3.63 | 1.60/−1.20 |
| BASF | - | 0.49(1) |
| Twin matrix | - | −1 0 0 0 −1 0 1 0 1 |
| Wyckoff Position | x/a | y/b | z/c | Occ. | Ueq | |
|---|---|---|---|---|---|---|
| Sr(1) | 4e | 0.7499(3) | 0.1269(4) | 0.25006(6) | 1 | 0.0221(5) |
| Li(1)/Co(1) | 4e | 0.177(3) | 0.120(1) | 0.1108(3) | 0.65/0.35 | 0.041(1) |
| Li(2)/Co(2) | 4e | 0.686(1) | 0.6307(5) | 0.1130(1) | 0.35/0.65 | 0.0145(5) |
| Li(3) | 4e | 0.164(8) | 0.605(5) | 0.044(1) | 1 | 0.032(5)1 |
| Li(4) | 4e | 0.634(9) | 0.132(7) | 0.044(2) | 1 | 0.037(5)1 |
| N(1) | 4e | 0.926(4) | 0.372(3) | 0.1111(6) | 1 | 0.023(2) |
| N(2) | 4e | 0.436(4) | 0.864(2) | 0.1113(6) | 1 | 0.020(2) |
| Sr(1) | Li(1)/Co(1) | Li(2)/Co(2) | Li(3) | Li(4) | |
|---|---|---|---|---|---|
| M–N(1) | 2.67(1) | 1.89(2) | 1.87(2) | 2.11(4) | 2.09(4) |
| M–N(2) | 2.71(1) | 1.92(2) | 1.81(2) | 2.09(4) | 2.09(4) |
| N(1)–M–N(1) | 121.1(3) | - | - | 117(1) | - |
| N(1)–M–N(2) | 87.8(3) | 179.4(9) | 175.8(7) | 126(2) | 115(1) |
| N(2)–M–N(2) | 121.1(3) | - | - | - | 117(1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, W.P.; Niewa, R. Li and Co Ordering in the Nitridocobaltate(I) SrLi2{Li[CoN2]}. Crystals 2018, 8, 268. https://doi.org/10.3390/cryst8070268
Clark WP, Niewa R. Li and Co Ordering in the Nitridocobaltate(I) SrLi2{Li[CoN2]}. Crystals. 2018; 8(7):268. https://doi.org/10.3390/cryst8070268
Chicago/Turabian StyleClark, William P., and Rainer Niewa. 2018. "Li and Co Ordering in the Nitridocobaltate(I) SrLi2{Li[CoN2]}" Crystals 8, no. 7: 268. https://doi.org/10.3390/cryst8070268
APA StyleClark, W. P., & Niewa, R. (2018). Li and Co Ordering in the Nitridocobaltate(I) SrLi2{Li[CoN2]}. Crystals, 8(7), 268. https://doi.org/10.3390/cryst8070268






