Noncovalent Bonds, Spectral and Thermal Properties of Substituted Thiazolo[2,3-b][1,3]thiazinium Triiodides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. X-ray Diffraction Refinement
2.3. Sample Characterization
2.4. Theoretical Calculations
3. Results and Discussion
3.1. Structural Characterization of Compounds 2a–c
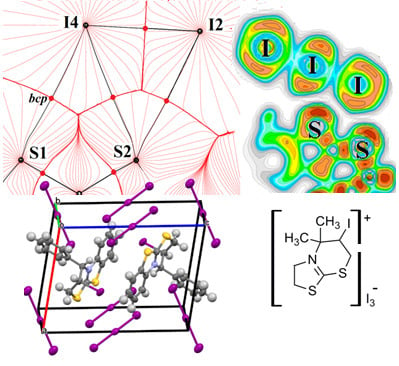
3.2. Noncovalent Bonds Formed by Triiodide Anions: Electron Density Calculations
3.3. Raman Spectroscopy Data
3.4. Thermal Analysis Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. 2019, 58, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen Bonding: An Electrostatically-Driven Highly Directional Noncovalent Interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond. Iupac Recomm. 2019. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and ρ-Holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990; pp. 1–438. [Google Scholar]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Tsirelson, V.G.; Zhou, P.F.; Tang, T.-H.; Bader, R.F.W. Topological definition of crystal structure: Determination of the bonded interactions in solid molecular chlorine. Acta Crystallogr. Sect. A 1995, A51, 143–153. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Non-Covalent Interaction. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Abramov, P.A.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. Halogen Bonding-assisted Assembly of Bromoantimonate (V) and Polybromide-bromoantimonate-based Frameworks. CrystEngComm 2019, 2, 850–856. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Abramov, P.A.; Novikov, A.S.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. Chlorobismuthates Trapping Dibromine: Formation of Two-Dimensional Supramolecular Polyhalide Networks with Br2 Linkers. Eur. J. Inorg. Chem. 2017, 4925–4929. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Abramov, P.A.; Novikov, A.S.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. A Bromo- and polybromoantimonates (V): Structural and theoretical studies of hybrid halogen-rich halometalate frameworks. Chem. Eur. J. 2018, 24, 10165–10170. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, E.; Malinen, H.; Haukka, M.; Konu, J. Reactivity of 4-Aminopyridine with Halogens and Interhalogens: Weak Interactions Supported Networks of 4-Aminopyridine and 4-Aminopyridinium. Cryst. Growth Des. 2019, 19, 2434–2445. [Google Scholar] [CrossRef]
- Gross, M.M.; Manthiram, A. Long-Life Polysulfide-Polyhalide Batteries with a Mediator-Ion Solid Electrolyte. ACS Appl. Energy Mater. 2019, 2, 3445–3451. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Pröhm, P.; Schwarze, N.; Müller, C.; Beckers, H.; Riedel, S. Investigation of Large Polychloride Anions: [Cl11]−, [Cl12]2−, and [Cl13]−. Angew. Chem. Int. Ed. 2018, 57, 9136–9140. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Martínez-Camarena, Á.; Bazzicalupi, C.; Delgado-Pinar, E.; Llinares, J.M.; Mariani, P.; Verdejo, B.; García-España, E.; Bianchi, A. Stabilization of Supramolecular Networks of Polyiodides with Protonated Small Tetra-azacyclophanes. Inorganics 2019, 7, 48. [Google Scholar] [CrossRef]
- Yu, H.-L.; He, Y.-C.; Zhao, F.-H.; Wang, Y.; Wang, A.-N.; Hao, M.-G.; Si, Z.-S.; You, J. Synthesis, Structure and Properties of a New Polyiodide Compound with the 1D→3D Interdigitated Architecture. Polyhedron 2019, 169, 183–186. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Yethiraj, A. Grotthuss Transport of Iodide in EMIM/I3 Ionic Crystal. J. Phys. Chem. B 2018, 122, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Shestimerova, T.A.; Bykov, M.A.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Crystal Structure and Two-level Supramolecular Organization of Glycinium Triiodide. Russ. Chem. Bull. 2019, 68, 1520–1524. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Yelavik, N.A.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Role of I2 Molecules and Weak Interactions in Supramolecular Assembling of Pseudo-Three-Dimensional Hybrid Bismuth Polyiodides: Synthesis, Structure, and Optical Properties of Phenylenediammonium Polyiodobismuthate(III). Cryst. Growth Des. 2018, 18, 2572–2578. [Google Scholar] [CrossRef]
- Savinkina, E.V.; Golubev, D.V.; Grigoriev, M.S. Synthesis, Characterization, and Crystal Structures of Iodides and Polyiodides of Scandium Complexes with Urea and Acetamide. J. Coord. Chem. 2019, 72, 347–357. [Google Scholar] [CrossRef]
- Ma, L.; Peng, H.; Lu, X.; Liu, L.; Shao, X. Building up 1-D, 2-D, and 3-D Polyiodide Frameworks by Finely Tuning the Size of Aryls on Ar-S-TTF in the Charge-Transfer (CT) Complexes of Ar-S-TTFs and Iodine. Chin. J. Chem. 2018, 36, 845–850. [Google Scholar] [CrossRef]
- Blake, A.J.; Li, W.-S.; Lippolis, V.; Schröder, M.; Devillanova, F.A.; Gould, R.O.; Parsons, S.; Radek, C. Template self-assembly of polyiodide networks. Chem. Soc. Rev. 1998, 27, 195–206. [Google Scholar] [CrossRef]
- Rybkovskiy, D.V.; Impellizzeri, A.; Obraztsova, E.D.; Ewels, C.P. Polyiodide Structures in Thin Single-walled Carbon Nanotubes: A large-scale Density-functional Study. Carbon 2019, 142, 123–130. [Google Scholar] [CrossRef]
- Li, H.-H.; Chen, Z.-R.; Cheng, L.-C.; Liu, J.-B.; Chen, X.-B.; Li, J.-Q. A New Hybrid Optical Semiconductor Based on Polymeric Iodoplumbate Co-Templated by Both Organic Cation and Polyiodide Anion. Cryst. Growth Des. 2008, 8, 4355–4358. [Google Scholar] [CrossRef]
- Wlaźlak, E.; Kalinowska-Tłuścik, E.J.; Nitek, W.; Klejna, S.; Mech, K.; Macyk, W.; Szaciłowski, K. Triiodide Organic Salts: Photoelectrochemistry at the Border between Insulators and Semiconductors. Chemelectrochem 2018, 5, 3486–3497. [Google Scholar] [CrossRef]
- Poręba, T.; Ernst, M.; Zimmer, D.; Macchi, P.; Casati, N. Pressure-Induced Polymerization and Electrical Conductivity of a Polyiodide. Angew. Chem. Int. Ed. 2019, 58, 6625–6629. [Google Scholar] [CrossRef]
- Starkholm, A.; Kloo, L.; Svensson, P.H. Polyiodide hybrid perovskites: A strategy to convert intrinsic 2D systems into 3D photovoltaic materials. ACS Appl. Energy Mater. 2019, 2, 477–485. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Q.-X.; Zeng, M.-H. Iodine Release and Recovery, Influence of Polyiodide Anions on Electrical Conductivity and Nonlinear Optical Activity in an Interdigitated and Interpenetrated Bipillared-Bilayer Metal−Organic Framework. J. Am. Chem. Soc. 2012, 134, 4857–4863. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide−Iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar]
- Corban, G.J.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Tiekink, E.R.T.; Butler, I.S.; Drougas, E.; Kosmas, A.M. Synthesis, Structural Characterization, and Computational Studies of Novel Diiodine Adducts with the Heterocyclic Thioamides N-Methylbenzothiazole-2-thione and Benzimidazole-2-thione: Implications with the Mechanism of Action of Antithyroid Drugs. Inorg. Chem. 2005, 44, 8617–8627. [Google Scholar] [CrossRef]
- Roy, G.; Nethaji, M.; Mugesh, G. Interaction of anti-thyroid drugs with iodine: The isolation of two unusual ionic compounds derived from Se-methimazole. Org. Biomol. Chem. 2006, 4, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Aragoni, M.C.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Mancini, A.; Verani, G. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rabie, U.M.; Abou-El-Wafa, M.H.; Nassar, H. In vitro simulation of the chemical scenario of the action of an anti-thyroid drug: Charge transfer interaction of thiazolidine-2-thione with iodine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; El-Hawary, W.F.; Moussa, M.A. IR, 1H NMR, mass, XRD and TGA/DTA investigations on the ciprofloxacin/iodine charge-transfer complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, H.S.; Salman, H.M.A.; El-Kemary, M. Halogen vs hydrogen bonding in thiazoline-2-thione stabilization with σ- and π-electron acceptors adducts: Theoretical and experimental study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Punyani, S.; Narayana, P.; Singh, H.; Vasudevan, P. Iodine based water disinfection: A review. J. Sci. Ind. Res. (India) 2006, 65, 116–120. [Google Scholar]
- Kulkarni, P.V.; Arora, V.; Rooney, A.S.; White, C.; Bennett, M.; Antich, P.P.; Bonte, F.J. Radiolabeled probes for imaging Alzheimer’s plaques. Nucl. Instrum. Methods Phys. Res. B 2005, B241, 676–680. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, Q.; Chen, Y.; Zhao, Z.; Lv, Y.; Hou, M.; Yang, P.; He, B.; Yu, L. Solid-state dye-sensitized solar cells from poly(ethylene oxide)/polyaniline electrolytes with catalytic and hole-transporting characteristics. J. Mater. Chem. A 2015, 3, 5368–5374. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sumida, R.; Tan, S.E.; Akazome, M. Synthesis of Iodinated Thiazolo[2,3-a]isoquinolinium salts and their Crystal Structures with/without Halogen Bond. Heterocycles 2018, 97, 755–775. [Google Scholar] [CrossRef]
- Kuznetsova, E.A.; Zhuravlev, S.V.; Stepanova, T.N. Synthesis and Properties of Derivatives of 2-Mercaptobenzothiazole. VIII. (Perhydro-1,3-thiazino)[2,3-b]benzothiazolines. Chem. Heterocycl. Comp. 1969, 5, 467–469. [Google Scholar] [CrossRef]
- Gregory, J.; Wei, P.H.L. Thiazolo[2,3-b]benzo(and azobenzo)-thiazole Derivatives, Process for their Preparation and Pharmaceutical Compositions Containing Them. EP Patent 0021773, 1 January 1981. [Google Scholar]
- Beard, C.C. 5(6)-Benzene Ring Substituted Benzimidazole-2-carbamate Derivatives Having Anti-protozoal Activity. U.S. Patent 4031228, 22 June 1977. [Google Scholar]
- Clark, A.D.; Sykes, P. Thiazolinium salts and their reactions with nucleophiles. J. Chem. Soc. 1971, 103–110. [Google Scholar] [CrossRef]
- Deplano, P.; Devillanova, F.; Francesco, A.; Ferraro, J.R.; Mercuri, M.L.; Lippolis, V.; Trogu, E.F. FT-Raman Study on Charge-Transfer Polyiodide Complexes and Comparison with Resonance Raman Results. Appl. Spectrosc. 1994, 48, 1236–1241. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Yushina, I.D.; Stash, A.I.; Tsirelson, V.G. Halogen Bonding and Other Iodine Interactions in Crystals of Dihydrothiazolo(oxazino)quinolinium Oligoiodides from the Electron-Density Viewpoint. Cryst. Growth Des. 2014, 14, 5674–5684. [Google Scholar] [CrossRef]
- Yu, H.; Yan, L.; He, Y.; Meng, H.; Huang, W. An Unusual Photoconductive Property of Polyiodide and Enhancement by Catenating with 3-Thiophenemethylamine Salt. Chem. Commun. 2017, 53, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Mukhitdinova, S.E.; Yushina, I.D.; Tsirelson, V.G. Electronic criterion for categorizing the chalcogen and halogen bonds: Sulfur–iodine interactions in crystals. Acta Crystallogr. Sect. B 2019, B75, 117–126. [Google Scholar] [CrossRef]
- Tarasova, N.M.; Kim, D.G. Synthesis and Halocyclization of Allyl Derivatives of 4,5-Dihydro-1,3-thiazol-2-thione. Bull. South Ural State Univ. Sect. Chem. 2015, 7, 4–10. [Google Scholar]
- Bruker. SMART and SAINT-Plus, Versions 5.0; Data Collection and Processing Software for the SMART System; Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Bruker. SHELXTL/PC, Versions 5.10; An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data; Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Yushina, I.D.; Kolesov, B.A.; Bartashevich, E.V. Raman Spectroscopy Study of New Thia- and Oxazinoquinolinium Triodides. New J. Chem. 2015, 39, 6163–6170. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rerat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wires Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Iodine Basis Set. Available online: http://www.tcm.phy.cam.ac.uk/~mdt26/basis_sets/I_basis.txt (accessed on 27 September 2019).
- Gatti, C.; Saunders, V.R.; Roetti, C. Crystal field effects on the topological properties of the electron density in molecular crystals: The case of urea. J. Chem. Phys. 1994, 101, 10686–10696. [Google Scholar] [CrossRef]
- Gatti, C.; Casassa, S. TOPOND14 User’s Manual; CNR-ISTM of Milano: Milano, Italy, 2013. [Google Scholar]
- Clark, T. σ-Holes. Wires Comput. Mol. Sci. 2013, 3, 13–20. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Yushina, I.D.; Kropotina, K.K.; Muhitdinova, S.E.; Tsirelson, V.G. Testing the Tools for Revealing and Characterizing the Iodine-Iodine Halogen Bond in Crystals. Acta Crystallogr. Sect. B 2017, B73, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bol’shakov, O.I.; Yushina, I.D.; Bartashevich, E.V.; Nelyubina, Y.V.; Aysin, R.R.; Rakitin, O.A. Asymmetric triiodide-diiodine interactions in the crystal of (Z)-4-chloro-5-((2-((4-chloro-5H-1,2,3-dithiazol-5-ylidene) amino)phenyl)amino)-1,2,3-dithiazol-1-ium oligoiodide. Struct. Chem. 2017, 28, 1927–1934. [Google Scholar] [CrossRef]
- Tsirelson, V.G.; Avilov, A.S.; Lepeshov, G.G.; Kulygin, A.K.; Stahn, J.; Pietsch, U.; Spence, J.C.H. Quantitative Analysis of the Electrostatic Potential in Rock-Salt Crystals Using Accurate Electron Diffraction Data. J. Phys. Chem. B 2001, 105, 5068–5074. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Grigoreva, E.A.; Yushina, I.D.; Bulatova, L.M.; Tsirelson, V.G. Modern level for properties prediction of iodine-containing organic compounds: The halogen bonds formed by iodine. Russ. Chem. Bull. 2017, 66, 1345–1356. [Google Scholar] [CrossRef]
- Arca, M.; Aragoni, M.C.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Mancini, A.; Verani, G. Reactions between chalcogen donors and dihalogens/interalogens: Typology of products and their characterization by FT-Raman spectroscopy. Bioinorg. Chem. Appl. 2006, 2006, 58937. [Google Scholar] [CrossRef] [PubMed]
- Yushina, I.D.; Rudakov, B.V.; Krivtsov, I.V.; Bartashevich, E.V. Thermal decomposition of tetraalkylammonium iodides. J. Anal. Calorim. 2014, 118, 425–429. [Google Scholar] [CrossRef]
- Yushina, I.D.; Pikhulya, D.G.; Bartashevich, E.V. The features of iodine loss at high temperatures. The case study of crystalline thiazoloquinolinium polyiodides. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wang, W. Intermolecular and very strong intramolecular C–SeO/N chalcogen bonds in nitrophenyl selenocyanate crystals. Phys. Chem. Chem. Phys. 2018, 20, 5227–5234. [Google Scholar] [CrossRef]







| Structure | 2a | 2b | 2c |
|---|---|---|---|
| Empirical formula | C16H13NS2I4, | C11H11NS2I4, | C8H13NS2I4 |
| Temperature (K) | 293 | 293 | 296.15 |
| Crystal system | triclinic | triclinic | monoclinic |
| Space group | P-1 | P-1 | P21/n |
| a (Å) | 9.775(7) | 7.913(5) | 12.1447(7) |
| b (Å) | 9.868(6) | 7.994(5) | 10.2762(5) |
| c (Å) | 12.890(8) | 14.883(11) | 13.9862(7) |
| α (°) | 96.416(19) | 101.68(3) | 90 |
| β (°) | 98.49(2) | 96.33(3) | 108.053(2) |
| γ (°) | 117.72(4) | 101.22(3) | 90 |
| Volume (Å3) | 1065.24 | 893.29 | 1659.57(15) |
| Z | 2 | 2 | 4 |
| Density (g/cm3) | 2.466 | 2.710 | 2.781 |
| µ, (mm−1) | 6.045 | 7.195 | 7.74 |
| F(000) | 720.0 | 653.4 | 1242.9 |
| Crystal size (mm) | 0.25 × 0.14 × 0.11 | 0.77 × 0.27 × 0.22 | 0.47 × 0.29 × 0.17 |
| 2θ range of data collection (deg) | 5.9 to 59.22 | 5.66 to 66.48 | 6.12 to 79.2 |
| Range of refraction indices | –13 ≤ h ≤ 13, –13 ≤ k ≤ 13, –17 ≤ l ≤ 17 | –12 ≤ h ≤ 12, –12 ≤ k ≤ 12, –22 ≤ l ≤ 22 | –21 ≤ h ≤ 21, –18 ≤ k ≤ 18, –24 ≤ l ≤ 25 |
| Measured reflections | 47057 | 52396 | 81480 |
| Independent reflections | 5968 (Rint = 0.0346, Rsigma = 0.0186 | 6839 (Rint = 0.0357, Rsigma = 0.0208) | 9984 (Rint = 0.0432, Rsigma = 0.0315) |
| Refinement variables | 211 | 165 | 142 |
| Goodness-of-fit on F2 | 1.039 | 1.049 | 1.104 |
| R factors for F2 > 2σ(F2) | R1 = 0.0283, wR2 = 0.0623 | R1 = 0.0450, wR2 = 0.1007 | R1 = 0.0543, wR2 = 0.117 |
| R factors for all reflections | R1 = 0.0431 wR2 = 0.0683 | R1 = 0.0611 wR2 = 0.1098 | R1 = 0.1107 wR2 = 0.1171 |
| Residual electron density (min/max) (e/Å3) | 0.61/−1.29 | 1.98/−2.24 | 3.13/−3.71 |
| Distance S…I, Å | C–S…I Angle, ° | S…I–I Angle, ° | I–I in I3−, Å | |
|---|---|---|---|---|
| 2a | S2…I2: 3.707 | C1–S2…I2: 142.27 | S2…I2–I4: 110.54 | 2.917; 2.917 |
| 2b | S2…I2: 3.775 | C1–S2…I2: 161.54 | S2…I2–I1: 110.53; | I3– (1): 2.912; 2.912 |
| S2b…I2: 3.778 | C10–S2b…I2: 153.69 | S2b…I2–I1: 32.93 | I3– (2): 2.931; 2.931 | |
| 2c | S4…I3: 3.910 | C9–S4…I3: 165.72 | S4…I3–I2: 96.39 | I3– (1): 2.904; 2.936 |
| S3…I8: 3.699 | C9–S3…I8: 166.14 | S3…I8–I7: 96.41 | ||
| S2…I1: 3.902 | C1–S2…I1: 172.07 | S2…I1–I2: 77.78 | I3– (2): 2.931; 2.933 | |
| S1…I6: 3.734 | C1–S1…I6: 165.23 | S1…I6–I7: 96.74 |
| 2a | 2b | 2c | |
|---|---|---|---|
| Melting Point (°C) | 141 | 127 | 122.5 |
| Decomposition start (°C) | 162*smooth | 222 | 160 |
| DTG peak 1 (°C) | 180–210 | 249 | 215 |
| DTG peak 2 (°C) | 277 | 310 | 252 |
| Mass loss, peak 1 and 2 (%) | 83.4 | 89.9 | 90.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushina, I.; Tarasova, N.; Kim, D.; Sharutin, V.; Bartashevich, E. Noncovalent Bonds, Spectral and Thermal Properties of Substituted Thiazolo[2,3-b][1,3]thiazinium Triiodides. Crystals 2019, 9, 506. https://doi.org/10.3390/cryst9100506
Yushina I, Tarasova N, Kim D, Sharutin V, Bartashevich E. Noncovalent Bonds, Spectral and Thermal Properties of Substituted Thiazolo[2,3-b][1,3]thiazinium Triiodides. Crystals. 2019; 9(10):506. https://doi.org/10.3390/cryst9100506
Chicago/Turabian StyleYushina, Irina, Natalya Tarasova, Dmitry Kim, Vladimir Sharutin, and Ekaterina Bartashevich. 2019. "Noncovalent Bonds, Spectral and Thermal Properties of Substituted Thiazolo[2,3-b][1,3]thiazinium Triiodides" Crystals 9, no. 10: 506. https://doi.org/10.3390/cryst9100506
APA StyleYushina, I., Tarasova, N., Kim, D., Sharutin, V., & Bartashevich, E. (2019). Noncovalent Bonds, Spectral and Thermal Properties of Substituted Thiazolo[2,3-b][1,3]thiazinium Triiodides. Crystals, 9(10), 506. https://doi.org/10.3390/cryst9100506