Impact of Crystal Habit on Solubility of Ticagrelor
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Crystallization Experiments
2.3. Solubility Studies
2.4. X-Ray Powder Diffraction (PXRD)
2.5. Single-Crystal X-Ray Diffraction
2.6. Optical Microscopy
2.7. X-Ray Photoelectron Spectroscopy (XPS)
2.8. Specific Surface Area
2.9. Molecular Modeling
2.10. Face Indexation
3. Results
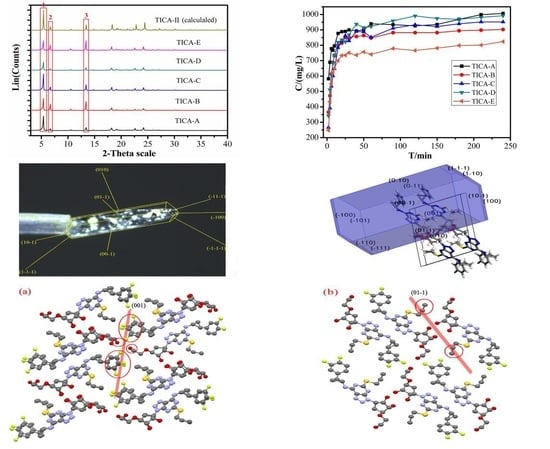
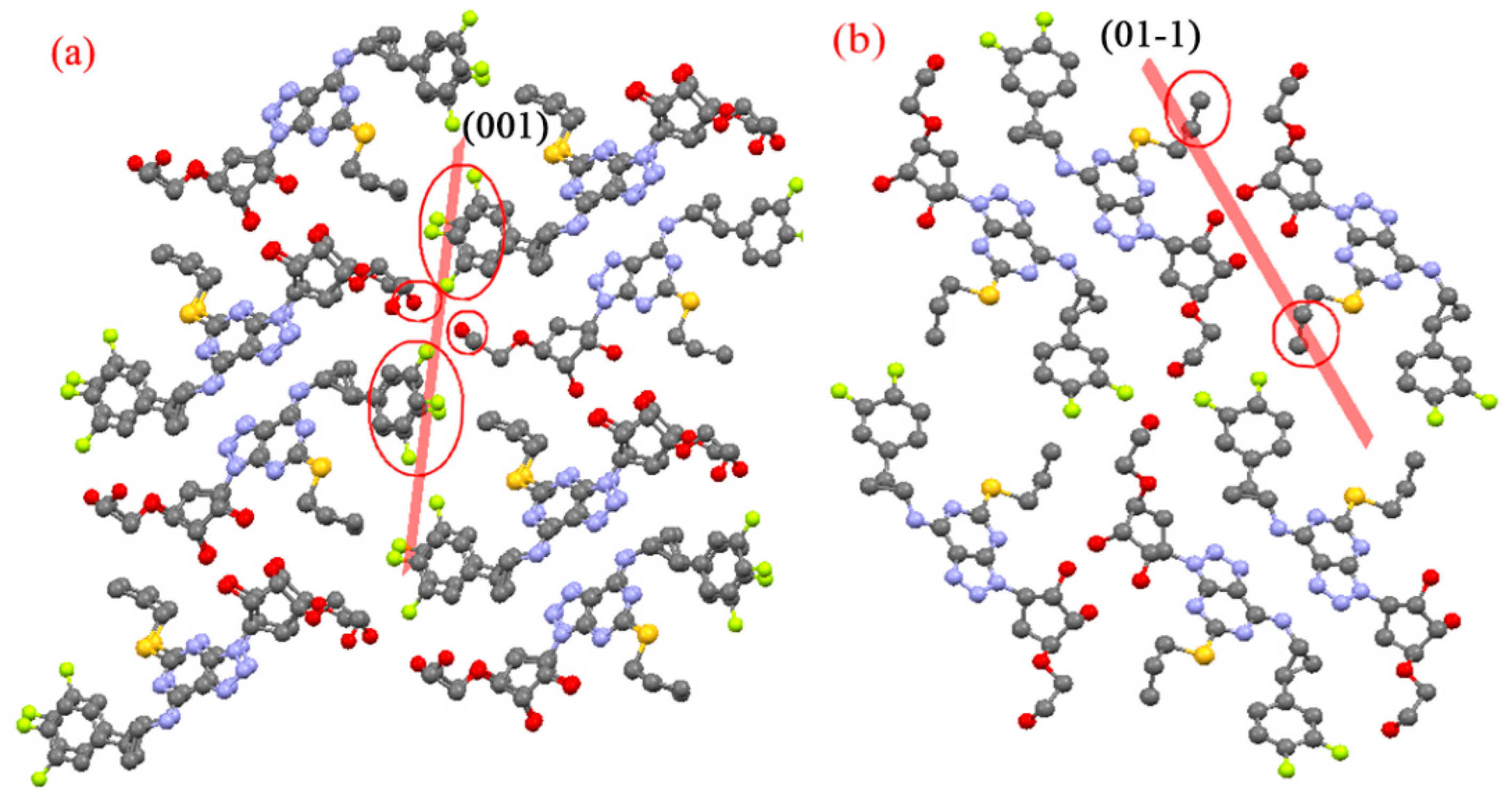
3.1. Single-Crystal X-Ray Diffraction
3.2. Predicted Morphology of the TICA Crystal
3.3. Powder X-Ray Diffraction
3.4. Optical and Polarized Light Microscopy
3.5. XPS
3.6. Solubility Study
3.7. Specific Surface Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marzio, H.D.; Navarro, V.J. Chapter 29–Hepatotoxicity of Cardiovascular and Antidiabetic Drugs. In Drug-Induced Liver Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 519–540. [Google Scholar]
- Cave, B.; Rawal, A.; Ardeshna, D.; Ibebuogu, U.N.; Sai-Sudhakar, C.B.; Khouzam, R.N. Targeting ticagrelor: A novel therapy for emergency reversal. Ann. Transl. Med. 2019, 7, 115–119. [Google Scholar] [CrossRef]
- Kubisa, M.J.; Jezewski, M.P.; Gasecka, A.; Siller-Matula, J.M.; Postula, M. Ticagrelor—toward more efficient platelet inhibition and beyond. Ther. Clin. Risk Manag. 2018, 14, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Van Giezen, J.J.J.; Berntsson, P.; Zachrisson, H.; Bjorkman, J.A. Comparison of ticagrelor and thienopyridine P2Y(12) binding characteristics and antithrombotic and bleeding effects in rat and dog models of thrombosis/hemostasis. Thromb. Res. 2009, 124, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; Emanuelsson, H.; Heptinstall, S.; Sandset, P.M.; Wickens, M.; Peters, G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y(12) antagonist AZD6140 with aspirin in patients with atherosclerosis: A double-blind comparison to clopidogrel with aspirin. Eur. Heart J. 2006, 27, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Husted, S.; Harrington, R.A.; Scirica, B.M.; Emanuelsson, H.; Storey, R.F.; Invest, D. Safety, tolerability, and initial efficacy of AZD6140, the first reversivle oral adenosine diphosphate receptor antagonist, compared with clopidigrel, in patients with non-ST-segment elevation acute coronary syndrome—Primary results of the DISPERSE-2 trial. J. Am. Coll. Cardiol. 2007, 50, 1844–1851. [Google Scholar] [CrossRef]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J.; Drug Induced Liver Injury Network. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Mohammad, R.A.; Goldberg, T.; Dorsch, M.P.; Cheng, J.W.M. Antiplatelet therapy after placement of a drug-eluting stent: A review of efficacy and safety studies. Clin. Ther. 2010, 32, 2265–2281. [Google Scholar] [CrossRef]
- Reuben, A.; Koch, D.G.; Lee, W.M.; Acute Liver Failure Study Group. Drug-induced acute liver failure: Results of a U.S. multicenter, prospective study. Hepatology 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [Green Version]
- Martin, B. New Cristalline and Amorphous form of a Triazolo(4,5-D) pyridinine Compound. U.S. Patent WO 01/92262 A1, 6 December 2001. [Google Scholar]
- Agarwal, V.K. Crystalline Form of Ticagrelor. U.S. Patent Application No. 16/066,425, 3 January 2017. [Google Scholar]
- Bojarska, J.; Remko, M.; Fruzinski, A.; Maniukiewicz, W. The experimental and theoretical landscape of a new antiplatelet drug ticagrelor: Insight into supramolecular architecture directed by C-H … F, π …π and C-H… π interactions. J. Mol. Struct. 2018, 1154, 290–300. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, X.-R.; Yang, S.-P.; Liu, J.-J.; Chen, C.-J. Improved solubility of vortioxetine using C2-C4 straight-chain dicarboxylic acid salt hydrates. Crystals 2018, 8, 352. [Google Scholar] [CrossRef]
- Miletic, T.; Kyriakos, K.; Graovac, A.; Ibric, S. Spray-dried voriconazole-cyclodextrin complexes: Solubility, dissolution rate and chemical stability. Carbohydr. Polym. 2013, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, N.; Muller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Grant, D.J.W. Influence of crystal shape on the tableting performance of L-lysine monohydrochloride dihydrate. J. Pharm. Sci. 2001, 90, 569–579. [Google Scholar] [CrossRef]
- Banga, S.; Chawla, G.; Varandani, D.; Mehta, B.R.; Bansal, A.K. Modification of the crystal habit of celecoxib for improved processability. J. Pharm. Pharmacol. 2007, 59, 29–39. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Tiwary, A.K. Modification of crystal habit and its role in dosage form performance. Drug Dev. Ind. Pharm. 2001, 27, 699–709. [Google Scholar] [CrossRef]
- Mittal, A.; Malhotra, D.; Jain, P.; Kalia, A.; Shunmugaperumal, T. Studies on Aspirin Crystals Generated by a Modified Vapor Diffusion Method. Aaps Pharmscitech 2016, 17, 988–994. [Google Scholar] [CrossRef]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Mishnev, A.; Stepanovs, D. Crystal structure explains crystal habit for the antiviral drug rimantadine hydrochloride. Z. Fur Nat. Sect. B A J. Chem. Sci. 2014, 69, 823–828. [Google Scholar] [CrossRef]
- Maghsoodi, M. Role of solvents in improvement of dissolution rate of drugs: Crystal habit and crystal agglomeration. Adv. Pharm. Bull. 2015, 5, 13–18. [Google Scholar] [CrossRef]
- Modi, S.R.; Dantuluri, A.K.R.; Puri, V.; Pawar, Y.B.; Nandekar, P.; Sangamwar, A.T.; Perumalla, S.R.; Sun, C.C.; Bansal, A.K. Impact of crystal habit on biopharmaceutical performance of celecoxib. Cryst. Growth Des. 2013, 13, 2824–2832. [Google Scholar] [CrossRef]
- Di Martino, P.; Censi, R.; Malaj, L.; Capsoni, D.; Massarotti, V.; Martelli, S. Influence of solvent and crystallization method on the crystal habit of metronidazole. Cryst. Res. Technol. 2007, 42, 800–806. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Bolourtchian, N.; Farid, D. Effects of hydrophilic excipients and compression pressure on physical properties and release behavior of aspirin-tableted microcapsules. Drug Dev. Ind. Pharm. 1999, 25, 711–716. [Google Scholar] [CrossRef]
- Ishikawa, K.; Eanes, E.D.; Tung, M.S. The effect of supersaturation on apatite crystal formation in aqueous solutions at physiologic pH and temperature. J. Dent. Res. 1994, 73, 1462–1469. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Bolourtchian, N.; Dinarvand, R. Crystal modification of phenytoin using different solvents and crystallization conditions. Int. J. Pharm. 2003, 250, 85–97. [Google Scholar] [CrossRef]
- Shariare, M.H.; Blagden, N.; de Matas, M.; Leusen, F.J.J.; York, P. Influence of solvent on the morphology and subsequent comminution of ibuprofen crystals by air jet milling. J. Pharm. Sci. 2012, 101, 1108–1119. [Google Scholar] [CrossRef]
- Stoica, C.; Verwer, P.; Meekes, H.; van Hoof, P.; Kaspersen, F.M.; Vlieg, E. Understanding the effect of a solvent on the crystal habit. Cryst. Growth Des. 2004, 4, 765–768. [Google Scholar] [CrossRef]
- Umprayn, K.; Luengtummuen, A.; Kitiyadisai, C.; Pornpiputsakul, T. Modification of crystal habit of ibuprofen using the phase partition technique: Effect of Aerosil and Tween80 in binding solvent. Drug Dev. Ind. Pharm. 2001, 27, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.M.; Roberts, K.J.; Maginn, S.J. A molecular dynamics study of solvent and impurity interaction on the crystal habit surfaces of epsilon-caprolactam. Langmuir 1998, 14, 5620–5630. [Google Scholar] [CrossRef]
- Prywer, J. Explanation of some peculiarities of crystal morphology deduced from the BFDH law. J. Cryst. Growth 2004, 270, 699–710. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, P.; Dang, L. The Morphology Prediction of Lysozyme Crystals Deduced from the BFDH Law and Attachment Energy Model Based on the Intermolecular Interaction. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar]
- Bruker AXS announces novel APEX(TM) DUO, the most versatile system for small molecule X-ray crystallography. Anti-Corros. Methods Mater. 2007, 54, 375.
- Sheldrick, G.M. SADABS; Version 2.10.; University of Gottingen: Gottingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguezmonge, L.; Taylor, R.; van de Streek, D.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2010, 41, 466–470. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Li, W.D.; Zhang, M.; Li, Y.; Liu, G.X.; Li, Z.J. Effect of heat preservation time on the micro morphology and field emission properties of La-doped SiC nanowires. Crystengcomm 2019, 21, 3993–4000. [Google Scholar] [CrossRef]
- Abourahma, H.; Cocuzza, D.S.; Melendez, J.; Urban, J.M. Pyrazinamide cocrystals and the search for polymorphs. Crystengcomm 2011, 13, 6442–6450. [Google Scholar] [CrossRef]
- Etter, M.C. ChemInform abstract: Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Laville, G. Elementary study on refraction in a prisma which is out of the main section. Bravais law. J. De Phys. Et Le Radium 1921, 2, 62–64. [Google Scholar] [CrossRef]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the law of bravais. Am. Mineral. 1937, 22, 446–467. [Google Scholar]
- Hadjittofis, E.; Isbell, M.A.; Karde, V.; Varghese, S.; Ghoroi, C.; Heng, J.Y.Y. Influences of Crystal Anisotropy in Pharmaceutical Process Development. Pharm. Res. 2018, 35, 100. [Google Scholar] [CrossRef]
- Bukovec, P.; Benkic, P.; Smrkolj, M.; Vrecer, F. Effect of crystal habit on the dissolution behaviour of simvastatin crystals and its relationship to crystallization solvent properties. Pharmazie 2016, 71, 263–268. [Google Scholar] [CrossRef]









| TICA-Ⅱ | |
|---|---|
| Formula | C23H28F2N6O4S |
| Mr | 522.57 |
| Temperature/K | 170(2) |
| Crystal system | triclinic |
| Space group | P1 |
| a/Å | 9.8863(5) |
| b/Å | 15.7349(7) |
| c/Å | 17.6069(9) |
| α/° | 105.538(2) |
| β/° | 100.841(2) |
| γ/° | 103.091(2) |
| Volume/Å3 | 2478.3(2) |
| Z | 4 |
| D/g·cm−3 | 1.401 |
| μ/mm−1 | 0.188 |
| F(000) | 1096.0 |
| Crystal size/mm3 | 0.248 × 0.18 × 0.067 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 4.434 to 53.486 |
| Index ranges | −12 ≤ h ≤ 12, −19 ≤ k ≤ 19, −22 ≤ l ≤ 22 |
| Reflections collected | 70727 |
| Independent reflections | 20802 [Rint = 0.0541, Rsigma = 0.0563] |
| Data/restraints/parameters | 20802/6/1319 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0756, wR2 = 0.1948 |
| Final R indexes [all data] | R1 = 0.0886, wR2 = 0.2089 |
| Largest diff. peak/hole / e Å−3 | 1.58/−0.33 |
| Flack parameter | 0.06(3) |
| Diffractometer | Bruker APEX-II CCD |
| Absorption correction CCDC No. | multi-scan 1953772 |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| O2-H2…O1B | 0.84 | 1.89 | 2.724(5) | 171.8 |
| O4-H4…O4B | 0.84 | 1.92 | 2.734(6) | 162.1 |
| N1-H1A…N4B | 0.88 | 2.19 | 3.057(6) | 169.9 |
| O2A-H2AA…O1C | 0.84 | 1.90 | 2.734(5) | 170.0 |
| O4A-H4A…O4 | 0.84 | 2.01 | 2.849(6) | 174.8 |
| N1A-H1AB…N4C | 0.88 | 2.18 | 3.045(6) | 167.4 |
| O1B-H1BA…N3B | 0.84 | 1.86 | 2.661(5) | 158.6 |
| O2B-H2B…O1 | 0.84 | 2.05 | 2.854(5) | 160.2 |
| O4B-H4B…O3B | 0.84 | 2.52 | 2.856(6) | 105.4 |
| O4B-H4B…O4C | 0.84 | 1.96 | 2.743(7) | 155.5 |
| N1B-H1BB…N4 | 0.88 | 2.16 | 3.019(6) | 166.3 |
| O1C-H1C…N3C | 0.84 | 1.87 | 2.665(6) | 157.7 |
| O2C-H2C…O1A | 0.84 | 2.04 | 2.861(5) | 164.5 |
| N1C-H1CA…N4A | 0.88 | 2.16 | 3.025(6) | 165.7 |
| O1-H1…O2C | 0.84(3) | 2.33(8) | 3.082(5) | 150(13) |
| O1A-H1AA…O2B | 0.84(3) | 2.24(5) | 3.065(5) | 165(14) |
| O4C-H4C…O3C | 0.86(3) | 2.34(11) | 2.764(7) | 111(9) |
| Number | 2θ (°) | Crystal Face | TICA-A | TICA-B | TICA-C | TICA-D | TICA-E | TICA-Ⅱ |
|---|---|---|---|---|---|---|---|---|
| Relative Intensity (I/I0)/% | ||||||||
| 1 | 5.4 | 001 | 100 | 100 | 100 | 100 | 97.1 | 100 |
| 2 | 6.7 | 01-1 | 12.3 | 53.5 | 20.9 | 20.9 | 49.9 | 19.0 |
| 3 | 13.4 | 02-2 | 21.2 | 76.0 | 35.3 | 33.1 | 100 | 27.0 |
| Elemental Composition (%) | (O + N + S)/(C + F) | |||||
|---|---|---|---|---|---|---|
| O 1s | N 1s | S 2p | F 1s | C 1s | ||
| TICA-A | 12.19 | 13.74 | 2.32 | 6.48 | 65.27 | 0.394 |
| TICA-B | 12.38 | 12.63 | 2.14 | 5.52 | 67.33 | 0.372 |
| TICA-C | 11.04 | 14.48 | 2.27 | 6.65 | 65.56 | 0.387 |
| TICA-D | 11.46 | 14.36 | 2.32 | 6.40 | 65.46 | 0.392 |
| TICA-E | 11.23 | 13.85 | 2.21 | 5.87 | 66.85 | 0.375 |
| Sample Name | Specific Surface Area (m2/g) |
|---|---|
| TICA-A | 1.56 |
| TICA-B | 1.60 |
| TICA-C | 1.53 |
| TICA-D | 1.51 |
| TICA-E | 1.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Shen, J.; Yu, K.; Phan, C.U.; Chen, G.; Liu, J.; Hu, X.; Feng, J. Impact of Crystal Habit on Solubility of Ticagrelor. Crystals 2019, 9, 556. https://doi.org/10.3390/cryst9110556
Ren Y, Shen J, Yu K, Phan CU, Chen G, Liu J, Hu X, Feng J. Impact of Crystal Habit on Solubility of Ticagrelor. Crystals. 2019; 9(11):556. https://doi.org/10.3390/cryst9110556
Chicago/Turabian StyleRen, Yan, Jie Shen, Kaxi Yu, Chi Uyen Phan, Guanxi Chen, Jiyong Liu, Xiurong Hu, and Jianyue Feng. 2019. "Impact of Crystal Habit on Solubility of Ticagrelor" Crystals 9, no. 11: 556. https://doi.org/10.3390/cryst9110556
APA StyleRen, Y., Shen, J., Yu, K., Phan, C. U., Chen, G., Liu, J., Hu, X., & Feng, J. (2019). Impact of Crystal Habit on Solubility of Ticagrelor. Crystals, 9(11), 556. https://doi.org/10.3390/cryst9110556