Synthesis and Simulation of CaF2@Al(OH)3 Core-Shell Coated Solid Lubricant Composite Powder
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Processing
2.2. Synthesis of CaF2@Al(OH)3 Powders
2.3. Sample Characterization
3. Results and Discussion
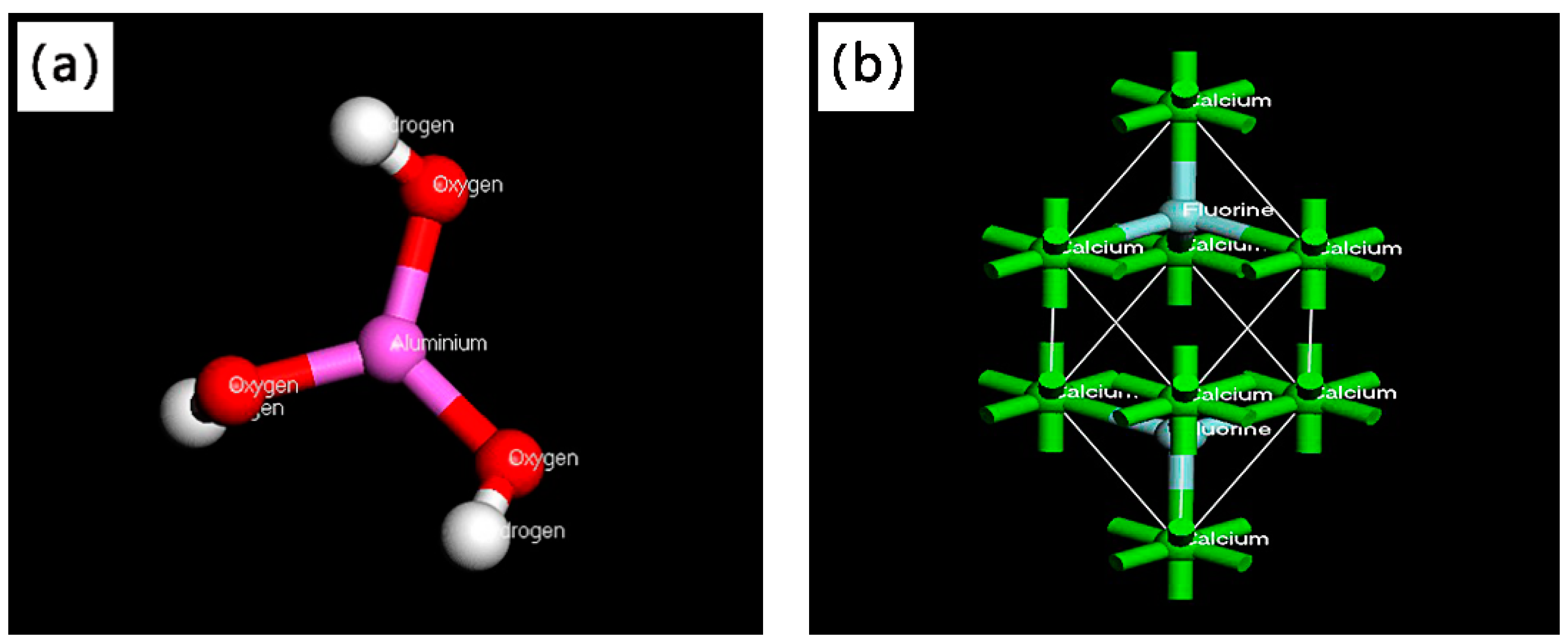
3.1. Simulation of Adsorption of Al(OH)3 Molecules on CaF2 Surface
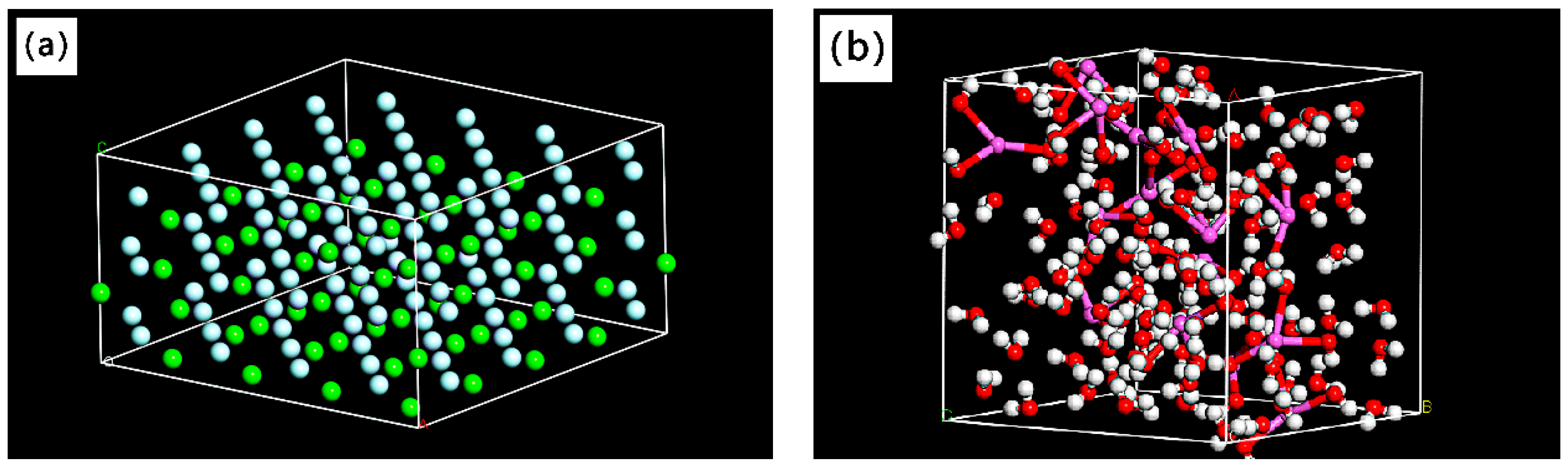
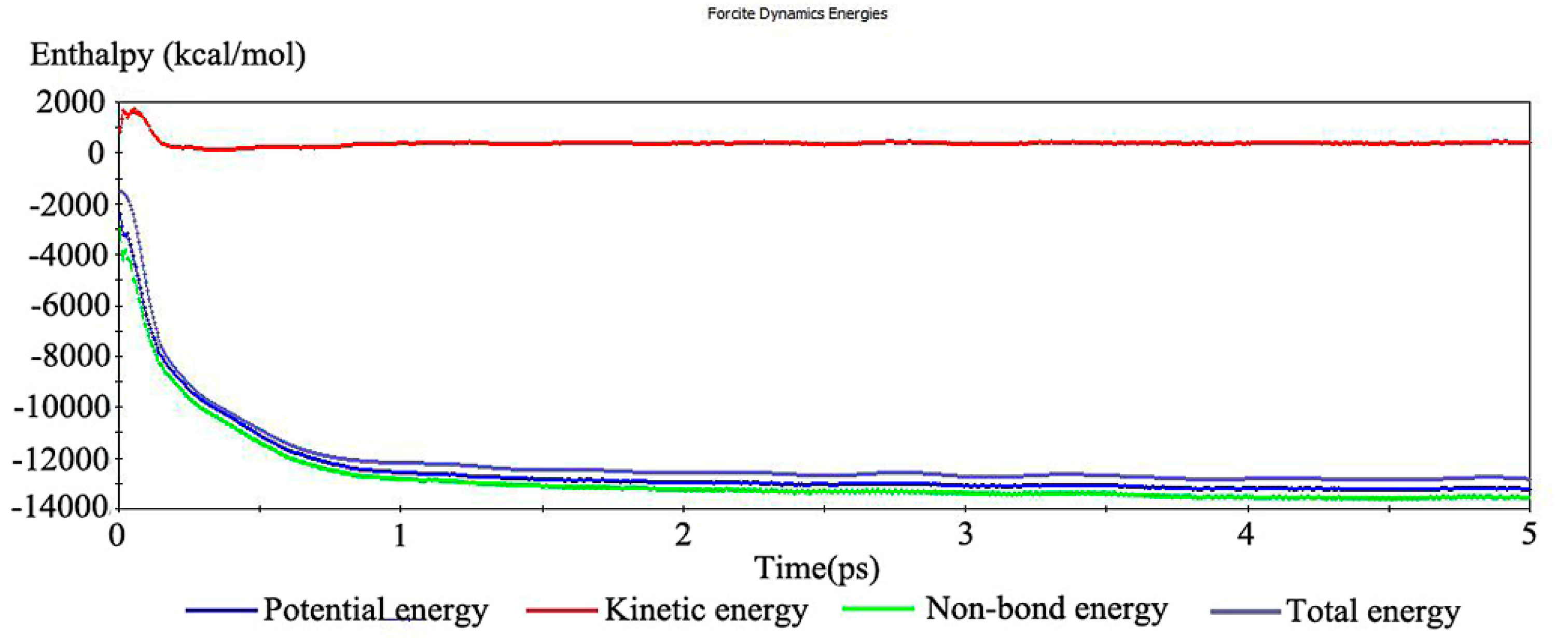
3.2. Simulation of Suspension Adsorption Interface
3.3. Test Analysis
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Gajrani, K.K.; Sankar, M.R.; Dixit, U.S. Environmentally friendly machining with MoS2-filled mechanically microtextured cutting tools. J. Mech. Sci. Technol. 2018, 32, 3797–3805. [Google Scholar] [CrossRef]
- Xing, Y.; Deng, J.; Wu, Z.; Liu, L.; Huang, P.; Jiao, A. Analysis of tool-chip interface characteristics of self-lubricating tools with nanotextures and WS2/Zr coatings in dry cutting. Int. J. Adv. Manuf. Technol. 2018, 97, 1637–1647. [Google Scholar] [CrossRef]
- Deng, J.; Yang, X.; Liu, J. Self-lubricating behaviours of ceramic tools in dry cutting. Int. J. Mach. Mach. Mater. 2006, 1, 213–222. [Google Scholar]
- Xu, C.; Xiao, G.; Zhang, Y.; Fang, B. Finite element design and fabrication of Al2O3/TiC/CaF2 gradient self-lubricating ceramic tool material. Ceram. Int. 2014, 40, 10971–10983. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Heidari, F.; Manshaei, M.; Vashaeef, D.; Tayebi, L. In vivo study of nanostructured diopside (CaMgSi2O6) coating on magnesium alloy as biodegradable orthopedic implants. Appl. Surf. Sci. 2014, 313, 60–66. [Google Scholar] [CrossRef]
- Wu, Q. Applicatin of Modification Technology on Surface Coating to Ceramic Process. Adv. Ceram. 2000, 4. [Google Scholar] [CrossRef]
- Takahashi, N.; Hashimoto, S.; Daiko, Y.; Honda, S.; Iwamoto, Y. High-temperature shrinkage suppression in refractory ceramic fiber board using novel surface coating agent. Ceram. Int. 2018, 44, 16725–16731. [Google Scholar] [CrossRef]
- Weng, B.; Yang, M.Q.; Zhang, N.; Xu, Y.J. Toward the enhanced photoactivity and photostability of ZnO nanospheres via intimate surface coating with reduced graphene oxide. J. Mater. Chem. A 2014, 2, 9380–9389. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B.; Dikici, B. Effect of hydroxyapatite/SiO2, hybride coatings on surface morphology and corrosion resistance of REX-734 alloy. Ceram. Int. 2016, 42, 10151–10158. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Z. Microstructure and Tribology Behaviors of In-situ WC/Fe Carbide Coating Fabricated by Plasma Transferred Arc Metallurgic Reaction. Appl. Surf. Sci. 2017, 423, 13–24. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, K.; Li, Y.; Deng, C.; Zeng, K. High temperature wear performance of HVOF-sprayed Cr3C2-WC-NiCoCrMo and Cr3C2-NiCr hardmetal coatings. Appl. Surf. Sci. 2017, 416, 33–44. [Google Scholar] [CrossRef]
- Sahan, H.; Göktepe, H.; Patat, S. A Novel Method to Improve the Electrochemical Performance of LiMn2O4 Cathode Active Material by CaCO3 Surface Coating. J. Mater. Sci. Technol. 2011, 27, 415–420. [Google Scholar] [CrossRef]
- Liu, Z.; Miyauchi, M.; Uemura, Y.; Cui, Y.; Hara, K.; Zhao, Z.; Sunahara, K.; Furube, A. Enhancing the performance of quantum dots sensitized solar cell by SiO2 surface coating. Appl. Phys. Lett. 2010, 96, 3183. [Google Scholar] [CrossRef]
- Shen-Wei, X.U.; Jiang, L.; Huang, X.Z.; Xu, S.; Jiang, L.; Huang, X.; Du, Z.; Xiao, L. Silicon Carbide Doped Beryllium Foam Ceramic Preparation by the Surface of Polyurethane Coating. Surf. Technol. 2016. [Google Scholar] [CrossRef]
- Sebben, J.; Canevese, V.A.; Alessandretti, R.; Pereira, G.K.; Sarkis-Onofre, R.; Bacchi, A.; Spazzin, A.O. Effect of Surface Coating on Bond Strength between Etched Feldspar Ceramic and Resin-Based Luting Agents. BioMed Res. Int. 2018, 2018, 3039251. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; He, M.; Wangyang, P.; Lu, Y.; Huang, H.; Xu, L. Microstructural characteristic, outward-inward growth behavior and formation mechanism of MAO ceramic coating on the surface of ADC12 Al alloy with micro-groove. Ceram. Int. 2018, 44, 7656–7662. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Vereschaka, A.A.; Vereschaka, A.S.; Kutin, A.A. Cutting Tools Made of Layered Composite Ceramics with Nano-Scale Multilayered Coatings. Procedia CIRP 2012, 1, 301–306. [Google Scholar] [CrossRef]
- He, G.; Deng, X.; Cen, Y.K.; Li, X.Y.; Luo, E.; Nie, R.R.; Zhao, Y.; Liang, Z.H.; Chen, Z.Q. Development and Characterization of Nano-TiO2/HA Composite Bioceramic Coating on Titanium Surface. Key Eng. Mater. 2007, 336–338, 1802–1805. [Google Scholar] [CrossRef]
- Nazemosadat, S.M.; Foroozmehr, E.; Badrossamay, M. Preparation of Alumina/Polystyrene Core-Shell Composite Powder via Phase Inversion process for Indirect Selective Laser Sintering Applications. Ceram. Int. 2018, 44, 596–604. [Google Scholar] [CrossRef]
- Lin, J.F.; Cao, S.H.; Huang, Q.F. Preparation of Co/Fe Core-shell composite powder by chemical plating. Mater. Sci. Eng. Powder Metall. 2010, 15, 265–270. [Google Scholar]
- Luo, X.T.; Li, C.X.; Shang, F.L.; Yang, G.J.; Wang, Y.Y.; Li, C.J. WC-Co Composite Coating Deposited by Cold Spraying of a Core-Shell-Structured WC-Co Powder. J. Therm. Spray Technol. 2015, 24, 100–107. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, C.; Xiao, G.; Yi, M.; Chen, Z.; Zhang, J. Electrostatic Self-assembly Preparation of Reduced Graphene Oxide-Encapsulated Alumina Nanoparticles with Enhanced Mechanical Properties of Alumina Nanocomposites. J. Eur. Ceram. Soc. 2018, 38, 5122–5133. [Google Scholar] [CrossRef]
- Wu, G.; Xu, C.; Xiao, G.; Yi, M.; Chen, Z.; Chen, H. An advanced self-lubricating ceramic composite with the addition of core-shell structured h-BN@Ni powders. Int. J. Refract. Met. Hard Mater. 2018, 72, 276–285. [Google Scholar] [CrossRef]
- Chen, H.; Xu, C.; Xiao, G.; Chen, Z.; Ma, J.; Wu, G. Synthesis of (h-BN)/SiO2 core–shell powder for improved self-lubricating ceramic composites. Ceram. Int. 2016, 42, 5504–5511. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Guo, N.; Xu, C.; Ji, L.; Guo, R.; Wang, B. Synthesis and Simulation of CaF2@Al(OH)3 Core-Shell Coated Solid Lubricant Composite Powder. Crystals 2019, 9, 578. https://doi.org/10.3390/cryst9110578
Chen Z, Guo N, Xu C, Ji L, Guo R, Wang B. Synthesis and Simulation of CaF2@Al(OH)3 Core-Shell Coated Solid Lubricant Composite Powder. Crystals. 2019; 9(11):578. https://doi.org/10.3390/cryst9110578
Chicago/Turabian StyleChen, Zhaoqiang, Niansheng Guo, Chonghai Xu, Lianggang Ji, Runxin Guo, and Benyuan Wang. 2019. "Synthesis and Simulation of CaF2@Al(OH)3 Core-Shell Coated Solid Lubricant Composite Powder" Crystals 9, no. 11: 578. https://doi.org/10.3390/cryst9110578
APA StyleChen, Z., Guo, N., Xu, C., Ji, L., Guo, R., & Wang, B. (2019). Synthesis and Simulation of CaF2@Al(OH)3 Core-Shell Coated Solid Lubricant Composite Powder. Crystals, 9(11), 578. https://doi.org/10.3390/cryst9110578




