Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications
Abstract
1. Introduction
2. Gold Nanoparticles
2.1. Physical and Chemical Properties of Gold
2.2. Historical Perspective: From Bulk to Gold Nanoparticles
2.3. Properties of Gold Nanoparticles
3. Synthesis of Gold Nanoparticles
3.1. Toward Green Synthesis
3.2. The Behavior of Polymers in Gold Nanoparticle Synthesis
3.2.1. Polymers as Reducing and Stabilizing Agents
3.2.2. Polymers as Soft Template
3.3. Biosynthesis
4. Characterization of Hybrid Gold/Polymer Nanomaterials
4.1. Surface Characterization Microscopy
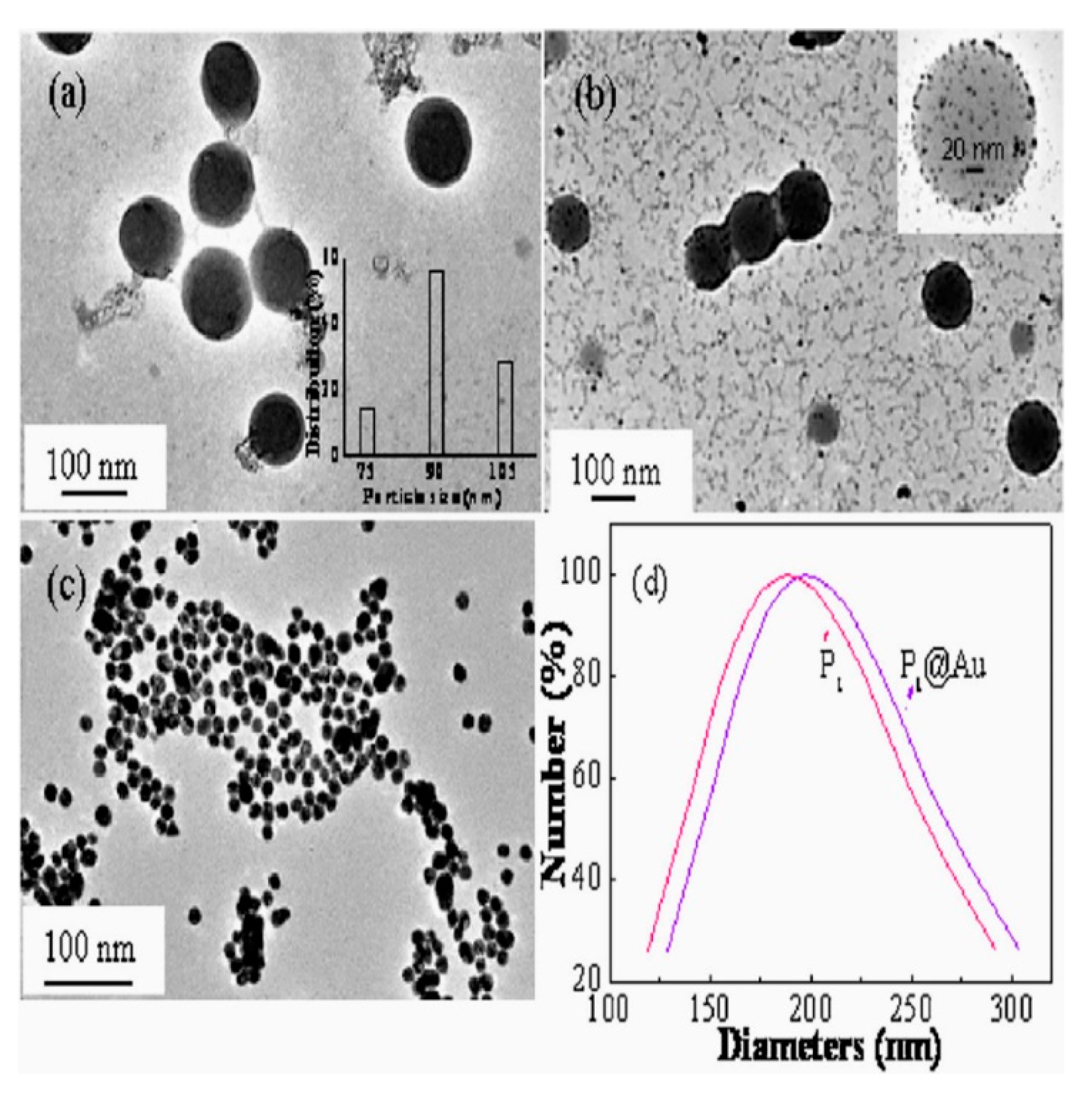
4.1.1. Transmission Electron Microscopy, (TEM)
4.1.2. Atomic Force Microscopy, (AFM)
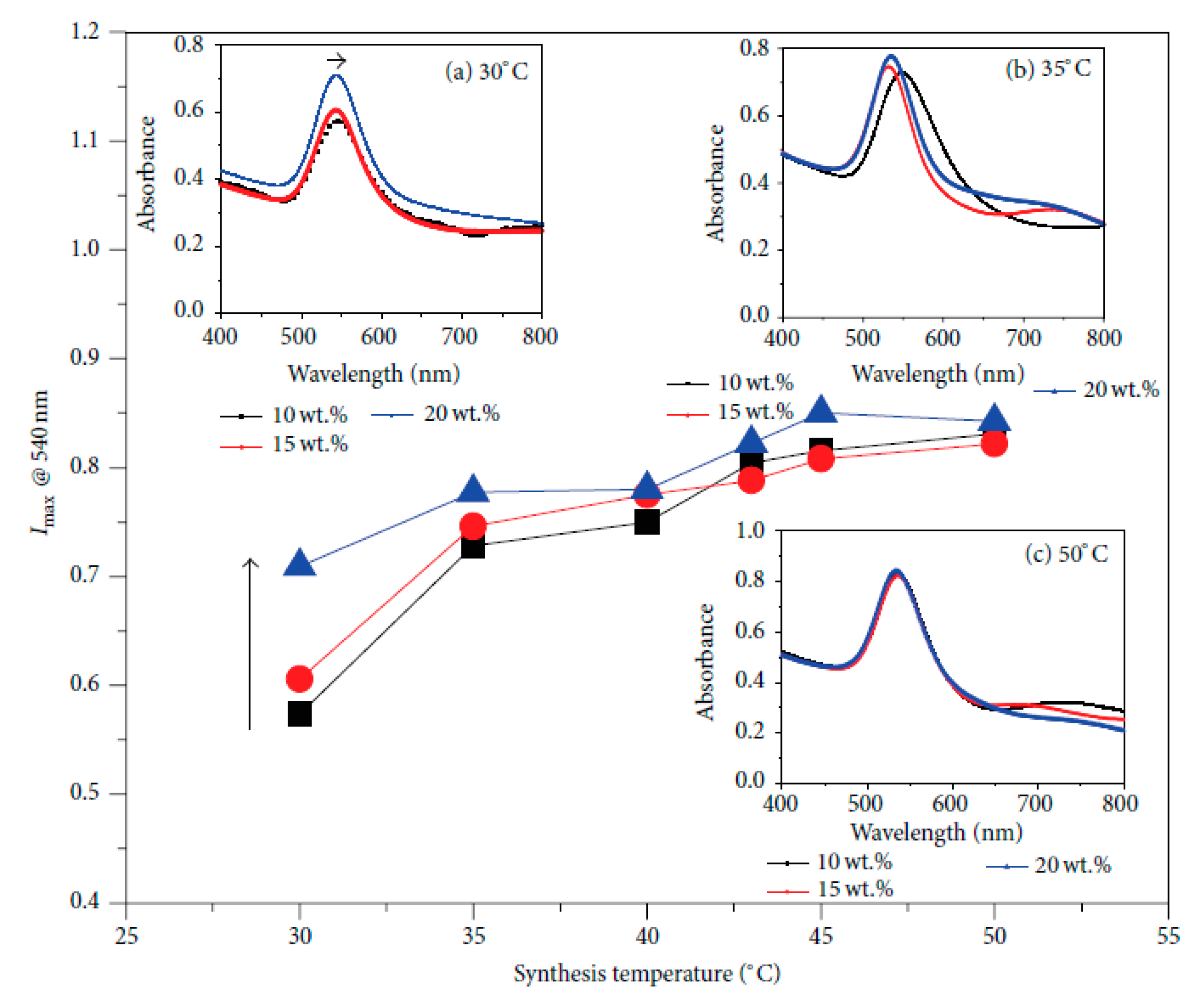
4.2. Spectroscopic Method: UV-Visible Spectroscopy, (UV-Vis)
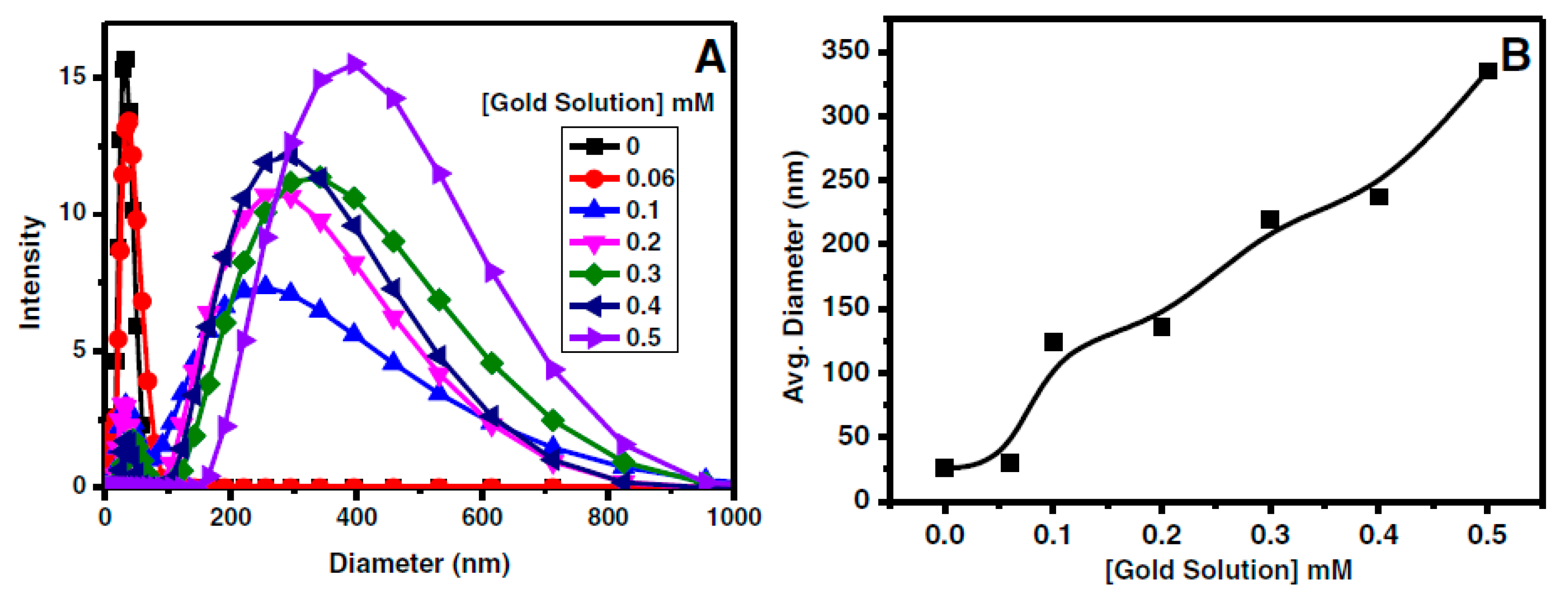
4.3. Light Scattering Method: Dynamic Light Scattering, (DLS)
4.4. X-ray Spectroscopy: X-Ray Diffraction, XRD
4.5. Electron Spectroscopy: X-Ray Photoelectron Spectroscopy, XPS
4.6. Synchrotron X-Ray: Small-Angle X-Ray Scattering, (SAXS)
4.7. Flow Property Characterization: Rheology
5. Technological Applications of Hybrid Gold/Polymer Nanomaterials
5.1. Antibacterial
5.2. Catalysis
5.2.1. Catalysis of Biosynthesis Routes
5.2.2. Heterogeneous Catalysis
5.3. Medical Applications
5.3.1. Drug Nanocarriers
5.3.2. Photodynamic and Photothermal Therapy
5.3.3. Nanotheranostics
5.3.4. Treatment of Other Diseases
5.4. Sensors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry and Biology; World Scientific: Singapore, 2012; ISBN 978-1-84816-806-0. [Google Scholar]
- Mingos, D.M.P. Historical Introduction to Gold Colloids, Clusters and Nanoparticles. In Gold Clusters, Colloids and Nanoparticles I. Structure and Bonding; Springer: Cham, Switzerland, 2014; Volume 161, ISBN 978-3-319-07848-9. [Google Scholar]
- Sakai, T.; Alexandridis, P. Single-Step Synthesis and Stabilization of Metal Nanoparticles in Aqueous Pluronic Block Copolymer Solutions at Ambient Temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Habashi, F. Handbook of extractive metallurgy; Wiley-VCH: Weinheim, Germany, 1997; ISBN 3-527-28792-2. [Google Scholar]
- Schmid, G.; Corain, B. Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities. Eur. J. Inorg. Chem. 2003, 2003, 3081–3098. [Google Scholar] [CrossRef]
- Laguna, A. Modern Supramolecular Gold Chemistry; Wiley: Weinheim, Germany, 2008; ISBN 9783527320295. [Google Scholar]
- Pyykkö, P. Theoretical Chemistry of Gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar]
- Kretsinger, R.H.; Uversky, V.N.; Permyakov, E.A. (Eds.) Encyclopedia of Metalloproteins; Springer New York: New York, NY, USA, 2013; ISBN 978-1-4614-1532-9. [Google Scholar]
- Dai, Y.; Zhang, X. Recent Advances in Amphiphilic Polymers as the Stabilizers of Colloidal Gold Nanoparticles. Macromol. Mater. Eng. 2018, 303, 1800105. [Google Scholar] [CrossRef]
- Johal, M.S.; Johnson, L.E. Understanding Nanomaterials, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780815354383. [Google Scholar]
- Bond, G. The early history of catalysis by gold. Gold Bull. 2008, 41, 235–241. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Catalytic Science Series; Gold Bull, World Scientific: Singapore, 2006; ISBN 978-1-86094-658-5. [Google Scholar]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Heal. Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Schatz, G.C. Electrodynamics of nonspherical noble metal nanoparticles and nanoparticle aggregates. J. Mol. Struct. THEOCHEM 2001, 573, 73–80. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Capek, I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. [Google Scholar] [CrossRef]
- Tantra, R. Nanomaterial Characterization: An Introduction; Tantra, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118753460. [Google Scholar]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R. Nanomaterials for the Life Science; Metallic Nanomaterials; Wiley-VCH: Weinheim, Germany, 2009; Volume 1, ISBN 978-3-527-32151-3. [Google Scholar]
- Lin, L.; Wang, W.; Huang, J.; Li, Q.; Sun, D.; Yang, X.; Wang, H.; He, N.; Wang, Y. Nature factory of silver nanowires: Plant-mediated synthesis using broth of Cassia fistula leaf. Chem. Eng. J. 2010, 162, 852–858. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thünemann, A.F.; Kraehnert, R. Mechanism of Gold Nanoparticle Formation in the Classical Citrate Synthesis Method Derived from Coupled In Situ XANES and SAXS Evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef]
- Köth, A.; Tiersch, B.; Appelhans, D.; Gradzielski, M.; Cölfen, H.; Koetz, J. Synthesis of Core-Shell Gold Nanoparticles with Maltose-Modified Poly(Ethyleneimine). J. Dispers. Sci. Technol. 2012, 33, 52–60. [Google Scholar] [CrossRef]
- Polte, J.; Emmerling, F.; Radtke, M.; Reinholz, U.; Riesemeier, H.; Thünemann, A.F. Real-Time Monitoring of Copolymer Stabilized Growing Gold Nanoparticles. Langmuir 2010, 26, 5889–5894. [Google Scholar] [CrossRef]
- Nalwa, H.S. (Ed.) Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Valencia, CA, USA, 2005; Volume 42, ISBN 1588830012. [Google Scholar]
- Li, C.; Li, D.; Wan, G.; Xu, J.; Hou, W. Facile synthesis of concentrated gold nanoparticles with low size-distribution in water: Temperature and pH controls. Nanoscale Res. Lett. 2011, 6, 440. [Google Scholar] [CrossRef]
- Shan, J.; Tenhu, H. Recent advances in polymer protected gold nanoparticles: Synthesis, properties and applications. Chem. Commun. 2007, 4580. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Saldías, C.; Bonardd, S.; Quezada, C.; Radi´c, D.; Leiva, A. The Role of Polymers in the Synthesis of Noble Metal Nanoparticles: A Review. J. Nanosci. Nanotechnol. 2017, 17, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P. Gold Nanoparticle Synthesis, Morphology Control, and Stabilization Facilitated by Functional Polymers. Chem. Eng. Technol. 2011, 34, 15–28. [Google Scholar] [CrossRef]
- Parveen, R.; Tremiliosi-Filho, G. A step ahead towards the green synthesis of monodisperse gold nanoparticles: The use of crude glycerol as a greener and low-cost reducing agent. RSC Adv. 2016, 6, 95210–95219. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatt, M.; Vyas, G.; Bhatt, S.; Paul, P. Sunlight Induced Preparation of Functionalized Gold Nanoparticles as Recyclable Colorimetric Dual Sensor for Aluminum and Fluoride in Water. ACS Appl. Mater. Interfaces 2017, 9, 17359–17368. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Mechanism of Gold Metal Ion Reduction, Nanoparticle Growth and Size Control in Aqueous Amphiphilic Block Copolymer Solutions at Ambient Conditions. J. Phys. Chem. B 2005, 109, 7766–7777. [Google Scholar] [CrossRef]
- Tepale, N.; Fernández-Escamilla, V.V.A.; Álvarez, C.; Flores-Aquino, E.; González-Coronel, V.J.; Cruz, D.; Sánchez-Cantú, M. Morphological and Rheological Characterization of Gold Nanoparticles Synthesized Using Pluronic P103 as Soft Template. J. Nanomater. 2016, 2016, 45. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Bani Yaseen, A.I.; Kailani, M.H. Synthesis of Monodispersed Gold Nanoparticles with Exceptional Colloidal Stability with Grafted Polyethylene Glycol- g -polyvinyl Alcohol. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Abrica-González, P.; Zamora-Justo, J.A.; Sotelo-López, A.; Vázquez-Martínez, G.R.; Balderas-López, J.A.; Muñoz-Diosdado, A.; Ibáñez-Hernández, M. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res. Lett. 2019, 14, 258. [Google Scholar] [CrossRef]
- Valdez, J.; Gómez, I. One-Step Green Synthesis of Metallic Nanoparticles Using Sodium Alginate. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Chairam, S.; Konkamdee, W.; Parakhun, R. Starch-supported gold nanoparticles and their use in 4-nitrophenol reduction. J. Saudi Chem. Soc. 2017, 21, 656–663. [Google Scholar] [CrossRef]
- Gajendiran, M.; Jo, H.; Kim, K.; Balasubramanian, S. Green synthesis of multifunctional PEG-carboxylate π back-bonded gold nanoconjugates for breast cancer treatment. Int. J. Nanomed. 2019, 14, 819–834. [Google Scholar] [CrossRef]
- Otari, S.V.; Patel, S.K.S.; Jeong, J.-H.; Lee, J.H.; Lee, J.-K. A green chemistry approach for synthesizing thermostable antimicrobial peptide-coated gold nanoparticles immobilized in an alginate biohydrogel. RSC Adv. 2016, 6, 86808–86816. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Lim, I.G.; Oh, J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int. J. Pharm. 2016, 511, 367–379. [Google Scholar] [CrossRef]
- Goy-López, S.; Castro, E.; Taboada, P.; Mosquera, V. Block Copolymer-Mediated Synthesis of Size-Tunable Gold Nanospheres and Nanoplates. Langmuir 2008, 24, 13186–13196. [Google Scholar] [CrossRef]
- Ray, D.; Aswal, V.K.; Srivastava, D. Concentration effect on tuning of block copolymer-mediated synthesis of gold nanoparticles. J. Nanosci. Nanotechnol. 2010. [Google Scholar] [CrossRef]
- Saldías, C.; Leiva, A.; Quezada, C.; Jaque, P.; Gargallo, L.; Radic, D. Structural effects of amphiphilic block copolymers on the gold nanoplates synthesis. Experimental and theoretical study. Eur. Polym. J. 2011, 47, 1866–1876. [Google Scholar] [CrossRef]
- Simon, T.; Boca, S.C.; Astilean, S. Pluronic-Nanogold hybrids: Synthesis and tagging with photosensitizing molecules. Colloids Surfaces B Biointerfaces 2012, 97, 77–83. [Google Scholar] [CrossRef]
- Khullar, P.; Singh, V.; Mahal, A.; Kumar, H.; Kaur, G.; Bakshi, M.S. Block Copolymer Micelles as Nanoreactors for Self-Assembled Morphologies of Gold Nanoparticles. J. Phys. Chem. B 2013, 117, 3028–3039. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Y.; Wang, S. ABC triblock copolymer-stabilized gold nanoparticles for catalytic reduction of 4-nitrophenol. J. Catal. 2015, 329, 425–430. [Google Scholar] [CrossRef]
- Gomes, D.S.B.; Paterno, L.G.; Santos, A.B.S.; Garay, A.V.; Mertz, D.; Freitas, S.M.; Soler, M.A.G. New insights on the formation of gold nanoparticles and Pluronic nanocomposites: Kinetics and thermodynamics parameters. J. Mol. Liq. 2018, 268, 181–189. [Google Scholar] [CrossRef]
- Santos, D.C.; de Souza, V.C.; Vasconcelos, D.A.; Andrade, G.R.S.; Gimenez, I.F.; Teixeira, Z. Triblock copolymer-mediated synthesis of catalytically active gold nanostructures. J. Nanopart. Res. 2018, 20, 105. [Google Scholar] [CrossRef]
- Das, S.; Pandey, A.; Pal, S.; Kolya, H.; Tripathy, T. Green synthesis, characterization and antibacterial activity of gold nanoparticles using hydroxyethyl starch-g-poly (methylacrylate-co-sodium acrylate): A novel biodegradable graft copolymer. J. Mol. Liq. 2015, 212, 259–265. [Google Scholar] [CrossRef]
- Kariuki, V.M.; Hoffmeier, J.C.; Yazgan, I.; Sadik, O.A. Seedless synthesis and SERS characterization of multi-branched gold nanoflowers using water soluble polymers. Nanoscale 2017, 9, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Khullar, P.; Mahal, A.; Singh, V.; Banipal, T.S.; Kaur, G.; Bakshi, M.S. How PEO-PPO-PEO Triblock Polymer Micelles Control the Synthesis of Gold Nanoparticles: Temperature and Hydrophobic Effects. Langmuir 2010, 26, 11363–11371. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.S. Colloidal micelles of block copolymers as nanoreactors, templates for gold nanoparticles, and vehicles for biomedical applications. Adv. Colloid Interface Sci. 2014, 213, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, X.; Wang, J.; An, Y.; Zhang, Z.; Shi, L. Thermosensitive mixed shell polymeric micelles decorated with gold nanoparticles at the outmost surface: Tunable surface plasmon resonance and enhanced catalytic properties with excellent colloidal stability. RSC Adv. 2015, 5, 47458–47465. [Google Scholar] [CrossRef]
- Seo, E.; Kim, J.; Hong, Y.; Kim, Y.S.; Lee, D.; Kim, B.-S. Double Hydrophilic Block Copolymer Templated Au Nanoparticles with Enhanced Catalytic Activity toward Nitroarene Reduction. J. Phys. Chem. C 2013, 117, 11686–11693. [Google Scholar] [CrossRef]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Spatz, J.P.; Mössmer, S.; Hartmann, C.; Möller, M.; Herzog, T.; Krieger, M.; Boyen, H.-G.; Ziemann, P.; Kabius, B. Ordered Deposition of Inorganic Clusters from Micellar Block Copolymer Films. Langmuir 2000, 16, 407–415. [Google Scholar] [CrossRef]
- Enomoto-Rogers, Y.; Kamitakahara, H.; Yoshinaga, A.; Takano, T. Radially oriented cellulose triacetate chains on gold nanoparticles. Cellulose 2010, 17, 923–936. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Kaura, A.; Bhandari, P.; Kaur, G.; Torigoe, K.; Esumi, K. Synthesis of Colloidal Gold Nanoparticles of Different Morphologies in the Presence of Triblock Polymer Micelles. J. Nanosci. Nanotechnol. 2006, 6, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.P.B.; Lee, C.H.; Chen, L.; Ho, K.M.; Lu, Y.; Ballauff, M.; Li, P. Facile synthesis of gold/polymer nanocomposite particles using polymeric amine-based particles as dual reductants and templates. Polymer (Guildf) 2015, 76, 271–279. [Google Scholar] [CrossRef]
- Antonisamy, J.D.; Swain, J.; Dash, S. Study on binding and fluorescence energy transfer efficiency of Rhodamine B with Pluronic F127-gold nanohybrid using optical spectroscopy methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications; Cao, G., Ed.; World Scientific: Singapore, 2004; ISBN 1-86094-415-9. [Google Scholar]
- Gan, P.P.; Li, S.F.Y. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev. Environ. Sci. Bio/Technol. 2012, 11, 169–206. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe vera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surfaces B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, N.; Laskar, R.A.; Sk, I.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids Surfaces B Biointerfaces 2011, 82, 497–504. [Google Scholar] [CrossRef]
- Daisy, P. Saipriya Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 1189. [Google Scholar] [CrossRef]
- Jafarizad, A.; Safaee, K.; Gharibian, S.; Omidi, Y.; Ekinci, D. Biosynthesis and In-vitro Study of Gold Nanoparticles Using Mentha and Pelargonium Extracts. Procedia Mater. Sci. 2015, 11, 224–230. [Google Scholar] [CrossRef]
- Lim, S.H.; Ahn, E.-Y.; Park, Y. Green Synthesis and Catalytic Activity of Gold Nanoparticles Synthesized by Artemisia capillaris Water Extract. Nanoscale Res. Lett. 2016, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Kwon, Y.; Baek, K.-H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv. Powder Technol. 2016, 27, 2204–2213. [Google Scholar] [CrossRef]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Chitra, K.; Reena, K.; Manikandan, A.; Antony, S.A. Antibacterial Studies and Effect of Poloxamer on Gold Nanoparticles by Zingiber Officinale Extracted Green Synthesis. J. Nanosci. Nanotechnol. 2015, 15, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Reena, K.; Balashanmugam, P.; Gajendiran, M.; Arul Antony, S. Synthesis of leucas aspera extract loaded gold-PLA-PEG-PLA amphiphilic copolymer nanoconjugates: In vitro cytotoxicity and anti-inflammatory activity studies. J. Nanosci. Nanotechnol. 2016, 16, 4762–4770. [Google Scholar] [CrossRef]
- Suganya, P.; Vaseeharan, B.; Vijayakumar, S.; Balan, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer zein-coated gold nanoparticles: Synthesis, antibacterial potential, toxicity and histopathological effects against the Zika virus vector Aedes aegypti. J. Photochem. Photobiol. B Biol. 2017, 173, 404–411. [Google Scholar] [CrossRef]
- Nazirov, A.; Pestov, A.; Privar, Y.; Ustinov, A.; Modin, E.; Bratskaya, S. One-pot green synthesis of luminescent gold nanoparticles using imidazole derivative of chitosan. Carbohydr. Polym. 2016, 151, 649–655. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Zhang, X.-Y.; Yu, H.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. Preparation, self-assembly and performance modulation of gold nanoparticles decorated ferrocene-containing hybrid block copolymer multifunctional materials. J. Ind. Eng. Chem. 2018, 65, 224–235. [Google Scholar] [CrossRef]
- Yoo, M.; Kim, S.; Lim, J.; Kramer, E.J.; Hawker, C.J.; Kim, B.J.; Bang, J. Facile Synthesis of Thermally Stable Core−Shell Gold Nanoparticles via Photo-Cross-Linkable Polymeric Ligands. Macromolecules 2010, 43, 3570–3575. [Google Scholar] [CrossRef]
- Amgoth, C.; Suman Joshi, D.S.D.; Dharmapuri, G.; Lakavathu, M. Self-assembled block copolymer [(BenzA)-b-(PCL)] micelles to orient randomly distributed AuNPs into hollow core-shell morphology and its role as payload for nanomedicines. Mater. Sci. Eng. C 2018, 92, 790–799. [Google Scholar] [CrossRef]
- Díaz, M.; Barrera, A.; López-Cuenca, S.; Martínez-Salazar, S.Y.; Rabelero, M.; Ceja, I.; Fernández, V.V.A.; Aguilar, J. Size-controlled gold nanoparticles inside polyacrylamide microgels. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Yan, N.; Zhu, Y.; Jiang, W.; Shi, D. Self-assembly of block copolymers into sieve-like particles with arrayed switchable channels and as scaffolds to guide the arrangement of gold nanoparticles. Nanoscale 2017, 9, 15056–15061. [Google Scholar] [CrossRef] [PubMed]
- Aubrit, F.; Testard, F.; Paquirissamy, A.; Gobeaux, F.; Wang, X.; Nallet, F.; Fontaine, P.; Ponsinet, V.; Guenoun, P. Ligand-free synthesis of gold nanoparticles incorporated within cylindrical block copolymer films. J. Mater. Chem. C 2018, 6, 8194–8204. [Google Scholar] [CrossRef]
- Scarabelli, L.; Schumacher, M.; Jimenez de Aberasturi, D.; Merkl, J.; Henriksen-Lacey, M.; Milagres de Oliveira, T.; Janschel, M.; Schmidtke, C.; Bals, S.; Weller, H.; et al. Encapsulation of Noble Metal Nanoparticles through Seeded Emulsion Polymerization as Highly Stable Plasmonic Systems. Adv. Funct. Mater. 2019, 29, 1809071. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, P.; Zhang, J.; Yu, C.; Yan, D.; Mai, Y. Crystallization-Driven Two-Dimensional Self-Assembly of Amphiphilic PCL- b -PEO Coated Gold Nanoparticles in Aqueous Solution. ACS Macro Lett. 2018, 7, 1062–1067. [Google Scholar] [CrossRef]
- Ikai, A. The World of Nano-Biomechanics; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780444527776. [Google Scholar]
- Sabir, T.S.; Rowland, L.K.; Milligan, J.R.; Yan, D.; Aruni, A.W.; Chen, Q.; Boskovic, D.S.; Kurti, R.S.; Perry, C.C. Mechanistic Investigation of Seeded Growth in Triblock Copolymer Stabilized Gold Nanoparticles. Langmuir 2013, 29, 3903–3911. [Google Scholar] [CrossRef][Green Version]
- Jenczyk, J.; Woźniak-Budych, M.; Jancelewicz, M.; Jarek, M.; Jurga, S. Structural and dynamic study of block copolymer – Nanoparticles nanocomposites. Polymer (Guildf) 2019, 167, 130–137. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Astruc, D.; Lin, W.; Gu, H. Highly-branched amphiphilic organometallic dendronized diblock copolymer: ROMP synthesis, self-assembly and long-term Au and Ag nanoparticle stabilizer for high-efficiency catalysis. Polymer (Guildf) 2019, 173, 1–10. [Google Scholar] [CrossRef]
- Zhu, H.; Lussier, F.; Ducrot, C.; Bourque, M.-J.; Spatz, J.P.; Cui, W.; Yu, L.; Peng, W.; Trudeau, L.-É.; Bazuin, C.G.; et al. Block Copolymer Brush Layer-Templated Gold Nanoparticles on Nanofibers for Surface-Enhanced Raman Scattering Optophysiology. ACS Appl. Mater. Interfaces 2019, 11, 4373–4384. [Google Scholar] [CrossRef]
- Perkampus, H.-H. UV-VIS spectroscopy and Its Applications; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 0387554211. [Google Scholar]
- Singh, M.P.; Strouse, G.F. Involvement of the LSPR Spectral Overlap for Energy Transfer between a Dye and Au Nanoparticle. J. Am. Chem. Soc. 2010, 132, 9383–9391. [Google Scholar] [CrossRef]
- Rakshit, S.; Moulik, S.P.; Bhattacharya, S.C. Deciphering the Role of the Length of the Corona in Controlled NSET within Triblock Copolymers. J. Phys. Chem. B 2015, 119, 8457–8467. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Aswal, V.K. Step-addition Method for Enhancing the Yield of Gold Nanoparticles in Block Copolymer Solution. J. Macromol. Sci. Part B 2010, 49, 810–820. [Google Scholar] [CrossRef]
- Xu, J.-P.; Yang, X.; Lv, L.-P.; Wei, Y.; Xu, F.-M.; Ji, J. Gold-Nanoparticle-Stabilized Pluronic Micelles Exhibiting Glutathione Triggered Morphology Evolution Properties. Langmuir 2010, 26, 16841–16847. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-W.; Yang, H.-Y. Tunable Arrangement of Gold Nanoparticles in Epoxidated Poly(styrene-block-butadiene) Diblock Copolymer Matrices. Macromol. Chem. Phys. 2011, 212, 2249–2259. [Google Scholar] [CrossRef]
- Pamies, R.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Temperature effects on the stability of gold nanoparticles in the presence of a cationic thermoresponsive copolymer. J. Nanopart. Res. 2016, 18, 319. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; García-Soria, S.V.; Paraguay-Delgado, F.; Licea-Claveríe, A. Synthesis of gold nanoparticles using poly(ethyleneglycol)-b-poly(N,N-diethylaminoethylmethacrylate) as nanoreactors. Polym. Bull. 2017, 74, 3527–3544. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, Y.; Peng, C.; Xu, W.; Banks, M.K.; Wu, R. Synthesis of a novel graphene-based gold nanocomposite using PVEIM- b -PNIPAM as a stabilizer and its thermosensitivity for the catalytic reduction of 4-nitrophenol. Inorg. Chem. Front. 2019, 6, 903–913. [Google Scholar] [CrossRef]
- Schmitz, K.S.; Phillies, G.D.J. An Introduction to Dynamic Light Scattering by Macromolecules; American Institute of Physics: Melville, NY, USA, 1991; Volume 44. [Google Scholar]
- Shi, Y.; Selin, V.; Wang, Y.; Sukhishvili, S.A. Multiresponsive Block Copolymer-Modified “Hairy” Gold Nanoparticles for Remote Control of Interfaces. Part. Part. Syst. Charact. 2013, 30, 950–957. [Google Scholar] [CrossRef]
- Simon, T.; Boca, S.; Biro, D.; Baldeck, P.; Astilean, S. Gold-Pluronic core-shell nanoparticles: Synthesis, characterization and biological evaluation. J. Nanopart. Res. 2013. [Google Scholar] [CrossRef]
- Fernandez, V.V.A.; Soltero, J.F.A.; Puig, J.E.; Rharbi, Y. Temporal Evolution of the Size Distribution during Exchange Kinetics of Pluronic P103 at Low Temperatures. J. Phys. Chem. B 2009, 113, 3015–3023. [Google Scholar] [CrossRef]
- Seeck, O.H.; Murphy, B.M. X-Ray Diffraction: Modern Experimental Techniques; Taylor & Francis Group: Boca Raton, FL, USA, 2014; ISBN 9780429071898. [Google Scholar]
- Feng, C.; Shen, Z.; Li, Y.; Gu, L.; Zhang, Y.; Lu, G.; Huang, X. PNIPAM- b -(PEA- g -PDMAEA) double-hydrophilic graft copolymer: Synthesis and its application for preparation of gold nanoparticles in aqueous media. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1811–1824. [Google Scholar] [CrossRef]
- Sanches, E.A.; Soares, J.C.; Iost, R.M.; Marangoni, V.S.; Trovati, G.; Batista, T.; Mafud, A.C.; Zucolotto, V.; Mascarenhas, Y.P. Structural Characterization of Emeraldine-Salt Polyaniline/Gold Nanoparticles Complexes. J. Nanomater. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, H.; He, T. Large-Area 2D Gold Nanorod Arrays Assembled on Block Copolymer Templates. Small 2013, 9, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, C.; Wang, Y. Nanoporous block copolymer membranes immobilized with gold nanoparticles for continuous flow catalysis. Polym. Chem. 2019, 10, 1642–1649. [Google Scholar] [CrossRef]
- Pearson, A.C.; Pound, E.; Woolley, A.T.; Linford, M.R.; Harb, J.N.; Davis, R.C. Chemical Alignment of DNA Origami to Block Copolymer Patterned Arrays of 5 nm Gold Nanoparticles. Nano Lett. 2011, 11, 1981–1987. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, Y. In Situ Synchrotron X-Ray Techniques for Real-Time Probing of Colloidal Nanoparticle Synthesis. Part. Part. Syst. Charact. 2013, 30, 399–419. [Google Scholar] [CrossRef]
- Chen, X.; Schröder, J.; Hauschild, S.; Rosenfeldt, S.; Dulle, M.; Förster, S. Simultaneous SAXS/WAXS/UV–Vis Study of the Nucleation and Growth of Nanoparticles: A Test of Classical Nucleation Theory. Langmuir 2015, 31, 11678–11691. [Google Scholar] [CrossRef]
- Abécassis, B.; Testard, F.; Kong, Q.; Francois, B.; Spalla, O. Influence of Monomer Feeding on a Fast Gold Nanoparticles Synthesis: Time-Resolved XANES and SAXS Experiments. Langmuir 2010, 26, 13847–13854. [Google Scholar] [CrossRef]
- Kikhney, A.G.; Svergun, D.I. A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins. FEBS Lett. 2015, 589, 2570–2577. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.; Zavaleta, C.; Massoud, T.F.; Gambhir, S.S. Gold Nanoparticles: A Revival in Precious Metal Administration to Patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Gracheva, T.A.; Kuz’micheva, T.A.; Perevezentsev, V.N.; Smirnova, L.A.; Mochalova, A.E.; Salomatina, E.B. Kinetics and mechanisms of the UV-radiation-assisted formation of gold nanoparticles in HAuCl4-doped chitosan solutions. Tech. Phys. 2017, 62, 1228–1232. [Google Scholar] [CrossRef]
- Yakimovich, N.O.; Smirnova, L.A.; Gracheva, T.A.; Klychkov, K.S.; Bityurin, N.M.; Aleksandrov, A.P. Synthesis of chitosan-stabilized Au nanoparticles with controllable sizes. Polym. Sci. Ser. B 2008, 50, 238–242. [Google Scholar] [CrossRef]
- Dunlop, I.E.; Ryan, M.P.; Goode, A.E.; Schuster, C.; Terrill, N.J.; Weaver, J.V.M. Direct synthesis of PEG-encapsulated gold nanoparticles using branched copolymer nanoreactors. RSC Adv. 2014, 4, 27702–27707. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley VCH: New York, NY, USA, 1994; ISBN 9780471185758. [Google Scholar]
- Fernández, V.V.A.; Tepale, N.; Álvarez, J.G.; Pérez-López, J.H.; Macías, E.R.; Bautista, F.; Pignon, F.; Rharbi, Y.; Gámez-Corrales, R.; Manero, O.; et al. Rheology of the Pluronic P103/water system in a semidilute regime: Evidence of nonequilibrium critical behavior. J. Colloid Interface Sci. 2009, 336, 842–849. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Rheological and dielectric properties of different gold nanoparticle sizes. Lipids Health Dis. 2011. [Google Scholar] [CrossRef]
- Mendoza, C.; Gindy, N.; Gutmann, J.S.; Frömsdorf, A.; Förster, S.; Fahmi, A. In Situ Synthesis and Alignment of Au Nanoparticles within Hexagonally Packed Cylindrical Domains of Diblock Copolymers in Bulk. Langmuir 2009, 25, 9571–9578. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Pietsch, T.; Gutmann, J.S.; Jehnichen, D.; Gindy, N.; Fahmi, A. Block Copolymers with Gold Nanoparticles: Correlation between Structural Characteristics and Mechanical Properties. Macromolecules 2009, 42, 1203–1211. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Mehta, R.V.; Upadhyay, R.V. Highly bacterial resistant silver nanoparticles: Synthesis and antibacterial activities. J. Nanopart. Res. 2010, 12, 1677–1685. [Google Scholar] [CrossRef]
- Marta, B.; Jakab, E.; Potara, M.; Simon, T.; Imre-Lucaci, F.; Barbu-Tudoran, L.; Popescu, O.; Astilean, S. Pluronic-coated silver nanoprisms: Synthesis, characterization and their antibacterial activity. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 441, 77–83. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. Open Access 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, Q.; Wang, X.; Li, L. Preparation of Hybrid Gold/Polymer Nanocomposites and Their Application in a Controlled Antibacterial Assay. ACS Appl. Mater. Interfaces 2016, 8, 29101–29109. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Prosenc, F.; Franko, M. Facile synthesis, structure, biocompatibility and antimicrobial property of gold nanoparticle composites from cellulose and keratin. J. Colloid Interface Sci. 2018, 510, 237–245. [Google Scholar] [CrossRef]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef]
- Khan, S.; Bakht, J.; Syed, F. Green synthesis of gold nanoparticles using Acer pentapomicum leaves extract its characterization, antibacterial, antifungal and antioxidant bioassay. Dig. J. Nanomater. Biostruct. 2018, 579–589. [Google Scholar]
- Borah, D.; Hazarika, M.; Tailor, P.; Silva, A.R.; Chetia, B.; Singaravelu, G.; Das, P. Starch-templated bio-synthesis of gold nanoflowers for in vitro antimicrobial and anticancer activities. Appl. Nanosci. 2018, 8, 241–253. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Liu, W.; Ding, Z.; Ma, H.; Seeram, N.P.; Xu, L.; Mu, Y.; Huang, X.; Li, L. New Sesquiterpenoids from Eugenia jambolana Seeds and Their Anti-microbial Activities. J. Agric. Food Chem. 2017, 65, 10214–10222. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of Size and Surface Charge of Gold Nanoparticles on their Skin Permeability: A Molecular Dynamics Study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef]
- Fernandes, R.; Smyth, N.R.; Muskens, O.L.; Nitti, S.; Heuer-Jungemann, A.; Ardern-Jones, M.R.; Kanaras, A.G. Interactions of Skin with Gold Nanoparticles of Different Surface Charge, Shape, and Functionality. Small 2015, 11, 713–721. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alhusban, A.A.; Ali, J.I.; Al-Bakri, A.G.; Hamed, R.; Khalil, E.A. Preferential Accumulation of Phospholipid-PEG and Cholesterol-PEG Decorated Gold Nanorods into Human Skin Layers and Their Photothermal-Based Antibacterial Activity. Sci. Rep. 2019, 9, 5796. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Shiju, N.R.; Guliants, V.V. Recent developments in catalysis using nanostructured materials. Appl. Catal. A Gen. 2009, 356, 1–17. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, H.P.; Sharma, R.K. Single-pot synthesis: Plant mediated gold nanoparticles catalyzed reduction of methylene blue in presence of stannous chloride. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 367, 102–107. [Google Scholar] [CrossRef]
- Mitsudome, T.; Kaneda, K. Gold nanoparticle catalysts for selective hydrogenations. Green Chem. 2013, 15, 2636–2654. [Google Scholar] [CrossRef]
- Haruta, M. When gold is not noble: Catalysis by nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. J. Am. Chem. Soc. 2005. [Google Scholar] [CrossRef]
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S. Aerobic Oxidation of Alcohols at Room Temperature and Atmospheric Conditions Catalyzed by Reusable Gold Nanoclusters Stabilized by the Benzene Rings of Polystyrene Derivatives. Angew. Chem. Int. Ed. 2007, 46, 4151–4154. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Guo, R. Reactive Template Method to Synthesize Gold Nanoparticles with Controllable Size and Morphology Supported on Shells of Polymer Hollow Microspheres and Their Application for Aerobic Alcohol Oxidation in Water. Adv. Funct. Mater. 2009, 19, 1112–1117. [Google Scholar] [CrossRef]
- Dai, Y.; Ren, T.; Wang, Y.; Zhang, X. The synergistic effect of nitrogen atoms and triblock structure on stabilizing gold nanoparticles for catalytic reduction of 4-nitrophenol. Gold Bull. 2017, 50, 123–129. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green synthesis of Ag-Au bimetallic nanocomposites using a biodegradable synthetic graft copolymer; hydroxyethyl starch-g-poly (acrylamide- co -acrylic acid) and evaluation of their catalytic activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Yuan, P.; Sun, Q.; Jia, Y.; Yan, W.; Chen, Z.; Xu, Q. Au nanoparticle decorated N-containing polymer spheres: Additive-free synthesis and remarkable catalytic behavior for reduction of 4-nitrophenol. J. Mater. Sci. 2015, 50, 1323–1332. [Google Scholar] [CrossRef]
- Yan, W.; Chen, C.; Wang, L.; Zhang, D.; Li, A.-J.; Yao, Z.; Shi, L.-Y. Facile and green synthesis of cellulose nanocrystal-supported gold nanoparticles with superior catalytic activity. Carbohydr. Polym. 2016, 140, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kratošová, G.; Holišová, V.; Konvičková, Z.; Ingle, A.P.; Gaikwad, S.; Škrlová, K.; Prokop, A.; Rai, M.; Plachá, D. From biotechnology principles to functional and low-cost metallic bionanocatalysts. Biotechnol. Adv. 2019, 37, 154–176. [Google Scholar] [CrossRef]
- Aiken, J.D.; Finke, R.G. A review of modern transition-metal nanoclusters: Their synthesis, characterization, and applications in catalysis. J. Mol. Catal. A Chem. 1999, 145, 1–44. [Google Scholar] [CrossRef]
- Munir, A.; Joya, K.S.; Ul Haq, T.; Babar, N.; Hussain, S.Z.; Qurashi, A.; Ullah, N.; Hussain, I. Metal Nanoclusters: New Paradigm in Catalysis for Water Splitting, Solar and Chemical Energy Conversion. ChemSusChem 2019, 12, 1517–1548. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic hydrogen production over flame spray pyrolysis-synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Li, W.; Yao, L.; Zhang, Z.; Geng, H.; Li, C.; Yu, Y.; Sheng, P.; Li, S. Tiny Au nanoparticles mediation strategy for preparation of NIR CuInS2 QDs based 1D TiO2 hybrid photoelectrode with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Mondal, C.; Pal, J.; Ganguly, M.; Sinha, A.K.; Jana, J.; Pal, T. A one pot synthesis of Au–ZnO nanocomposites for plasmon-enhanced sunlight driven photocatalytic activity. New J. Chem. 2014, 38, 2999–3005. [Google Scholar] [CrossRef]
- Padikkaparambil, S.; Narayanan, B.; Yaakob, Z.; Viswanathan, S.; Tasirin, S.M. Au/TiO2 Reusable Photocatalysts for Dye Degradation. Int. J. Photoenergy 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Lakshminarayana, B.; Satyanarayana, G.; Subrahmanyam, C. Bimetallic Pd–Au/TiO2 Nanoparticles: An Efficient and Sustainable Heterogeneous Catalyst for Rapid Catalytic Hydrogen Transfer Reduction of Nitroarenes. ACS Omega 2018, 3, 13065–13072. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, X.; Xu, Z.; Zhang, J.; Rendón, U.F.; Razzari, L.; Chaker, M.; Ma, D. Plasmonic Au-Loaded Hierarchical Hollow Porous TiO2 Spheres: Synergistic Catalysts for Nitroaromatic Reduction. J. Phys. Chem. Lett. 2018, 9, 5317–5326. [Google Scholar] [CrossRef]
- Pradhan, S.; Ghosh, D.; Chen, S. Janus Nanostructures Based on Au−TiO2 Heterodimers and Their Photocatalytic Activity in the Oxidation of Methanol. ACS Appl. Mater. Interfaces 2009, 1, 2060–2065. [Google Scholar] [CrossRef]
- Martínez, L.; Benito, M.; Mata, I.; Soler, L.; Molins, E.; Llorca, J. Preparation and photocatalytic activity of Au/TiO2 lyogels for hydrogen production. Sustain. Energy Fuels 2018, 2, 2284–2295. [Google Scholar] [CrossRef]
- Khore, S.K.; Kadam, S.R.; Naik, S.D.; Kale, B.B.; Sonawane, R.S. Solar light active plasmonic Au@TiO2 nanocomposite with superior photocatalytic performance for H2 production and pollutant degradation. New J. Chem. 2018, 42, 10958–10968. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Frenkel, A.I.; Morris, J.R. Catalysis and Photocatalysis by Nanoscale Au/TiO2: Perspectives for Renewable Energy. ACS Energy Lett. 2017, 2, 1223–1231. [Google Scholar] [CrossRef]
- Ratliff, J.S.; Tenney, S.A.; Hu, X.; Conner, S.F.; Ma, S.; Chen, D.A. Decomposition of Dimethyl Methylphosphonate on Pt, Au, and Au−Pt Clusters Supported on TiO2 (110). Langmuir 2009, 25, 216–225. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017. [Google Scholar]
- Sun, Y.; Sun, Y.; Zhang, T.; Chen, G.; Zhang, F.; Liu, D.; Cai, W.; Li, Y.; Yang, X.; Li, C. Complete Au@ZnO core–shell nanoparticles with enhanced plasmonic absorption enabling significantly improved photocatalysis. Nanoscale 2016, 8, 10774–10782. [Google Scholar] [CrossRef]
- Pougin, A.; Dodekatos, G.; Dilla, M.; Tüysüz, H.; Strunk, J. Au@TiO2 Core-Shell Composites for the Photocatalytic Reduction of CO2. Chem. A Eur. J. 2018, 24, 12416–12425. [Google Scholar] [CrossRef]
- Gavade, N.L.; Babar, S.B.; Kadam, A.N.; Gophane, A.D.; Garadkar, K.M. Fabrication of M@CuxO/ZnO (M = Ag, Au) Heterostructured Nanocomposite with Enhanced Photocatalytic Performance under Sunlight. Ind. Eng. Chem. Res. 2017, 56, 14489–14501. [Google Scholar] [CrossRef]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef]
- Sasidharan, A.; Monteiro-Riviere, N.A. Biomedical applications of gold nanomaterials: Opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 779–796. [Google Scholar]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 2. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 612–618. [Google Scholar] [CrossRef]
- Dai, Z. (Ed.) Advances in Nanotheranostics II; Springer Series in Biomaterials Science and Engineering; Springer Singapore: Singapore, 2016; Volume 7, ISBN 978-981-10-0061-4. [Google Scholar]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum Arabic as a Phytochemical Construct for the Stabilization of Gold Nanoparticles: In Vivo Pharmacokinetics and X-ray-Contrast-Imaging Studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Harrison, E.; Coulter, J.A.; Dixon, D. Gold nanoparticle surface functionalization: Mixed monolayer versus hetero bifunctional peg linker. Nanomedicine 2016, 11, 851–865. [Google Scholar] [CrossRef]
- Miao, Z.; Gao, Z.; Chen, R.; Yu, X.; Su, Z.; Wei, G. Surface-bioengineered Gold Nanoparticles for Biomedical Applications. Curr. Med. Chem. 2018, 25, 1920–1944. [Google Scholar] [CrossRef]
- Chen, H.; Zou, H.; Paholak, H.J.; Ito, M.; Qian, W.; Che, Y.; Sun, D. Thiol-reactive amphiphilic block copolymer for coating gold nanoparticles with neutral and functionable surfaces. Polym. Chem. 2014, 5, 2768–2773. [Google Scholar] [CrossRef]
- Locatelli, E.; Comes Franchini, M. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanopart. Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Anniebell, S.; Gopinath, S.C.B. Polymer Conjugated Gold Nanoparticles in Biomedical Applications. Curr. Med. Chem. 2018, 25, 1433–1445. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Simpson, C.A.; Salleng, K.J.; Cliffel, D.E.; Feldheim, D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Singh, R.K.; Kim, T.H.; Seo, J.W.; Shin, U.S.; Chrzanowski, W.; Kim, H.W. Triple Hit with Drug Carriers: pH- and Temperature-Responsive Theranostics for Multimodal Chemo- and Photothermal Therapy and Diagnostic Applications. ACS Appl. Mater. Interfaces 2016, 8, 8967–8979. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, S.V.; Kulkarni, M.G.; Devarajan, P.V. Polyaspartic acid functionalized gold nanoparticles for tumor targeted doxorubicin delivery. J. Biomed. Nanotechnol. 2014, 10, 143–153. [Google Scholar] [CrossRef]
- Dey, S.; Sherly, M.C.; Rekha, M.R.; Sreenivasan, K. Alginate stabilized gold nanoparticle as multidrug carrier: Evaluation of cellular interactions and hemolytic potential. Carbohydr. Polym. 2016, 136, 71–80. [Google Scholar] [CrossRef]
- Salem, D.S.; Sliem, M.A.; El-Sesy, M.; Shouman, S.A.; Badr, Y. Improved chemo-photothermal therapy of hepatocellular carcinoma using chitosan-coated gold nanoparticles. J Photochem Photobiol B 2018, 182, 92–99. [Google Scholar] [CrossRef]
- Cai, H.; Yao, P. In situ preparation of gold nanoparticle-loaded lysozyme–dextran nanogels and applications for cell imaging and drug delivery. Nanoscale 2013, 5, 2892–2900. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef]
- Laksee, S.; Puthong, S.; Teerawatananond, T.; Palaga, T.; Muangsin, N. Highly efficient and facile fabrication of monodispersed Au nanoparticles using pullulan and their application as anticancer drug carriers. Carbohydr. Polym. 2017, 173, 178–191. [Google Scholar] [CrossRef]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Ponrasu, T.; Raja, M.D.; Subamekala, M.K.; Suguna, L. Green synthesis of pullulan stabilized gold nanoparticles for cancer targeted drug delivery. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Hamishehkar, H. Decoration of gold nanoparticles with thiolated pH-responsive polymeric (PEG-b-p(2-dimethylamio ethyl methacrylate-co-itaconic acid) shell: A novel platform for targeting of anticancer agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Jabri, T.; Imran, M.; Shafiullah; Rao, K.; Ali, I.; Arfan, M.; Shah, M.R. Fabrication of lecithin-gum tragacanth muco-adhesive hybrid nano-carrier system for in-vivo performance of Amphotericin B. Carbohydr. Polym. 2018, 194, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Qiu, Y.; Li, W.; Guo, X.; Li, Q.; Zhang, H.; Zhou, J.; Du, Y.; Yuan, H.; et al. Specific photothermal therapy to the tumors with high EphB4 receptor expression. Biomaterials 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Yao, X.; Zheng, X.; Wang, J.; Jiang, X. Near-IR-triggered photothermal/photodynamic dual-modality therapy system via chitosan hybrid nanospheres. Biomaterials 2013, 34, 8314–8322. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 2018, 157, 107–124. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; Abo-Zeid, M.A.M.; Saleh, S.; Khoobchandani, M.; Katti, K.; Shukla, R.; Katti, K.V. Gum Arabic-encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed. Pharmacother. 2017, 89, 1045–1054. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; El-Hussieny, E.A.; Saleh, S.; Khoobchandani, M.; Bacon, K.L.; Gupta, S.; Katti, K.; Shukla, R.; et al. Photothermal therapy mediated by gum Arabic-conjugated gold nanoparticles suppresses liver preneoplastic lesions in mice. J. Photochem. Photobiol. B 2016, 163, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Rijo, P.; Molpeceres, J.; Ascensão, L.; Roberto, A.; Fernandes, A.S.; Gomes, R.; Pinto Coelho, J.M.; Gabriel, A.; Vieira, P.; et al. Bioproduction of gold nanoparticles for photothermal therapy. Ther. Deliv. 2016, 7, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.; Chen, X. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Shanavas, A.; Rengan, A.K.; Chauhan, D.; George, L.; Vats, M.; Kaur, N.; Yadav, P.; Mathur, P.; Chakraborty, S.; Tejaswini, A.; et al. Glycol chitosan assisted in situ reduction of gold on polymeric template for anti-cancer theranostics. Int. J. Biol. Macromol. 2018, 110, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Hao, Y.; Niu, M.; Hu, Y.; Zhao, H.; Chang, J.; Zhang, Z.; Zhang, Y. Gold nanorod-based poly(lactic-co-glycolic acid) with manganese dioxide core-shell structured multifunctional nanoplatform for cancer theranostic applications. Int. J. Nanomed. 2017, 12, 3059–3075. [Google Scholar] [CrossRef]
- Deng, X.; Li, K.; Cai, X.; Liu, B.; Wei, Y.; Deng, K.; Xie, Z.; Wu, Z.; Ma, P.; Hou, Z.; et al. A Hollow-Structured CuS@Cu2 S@Au Nanohybrid: Synergistically Enhanced Photothermal Efficiency and Photoswitchable Targeting Effect for Cancer Theranostics. Adv. Mater. 2017, 29, 1701266. [Google Scholar] [CrossRef]
- Simon, T.; Potara, M.; Gabudean, A.M.; Licarete, E.; Banciu, M.; Astilean, S. Designing Theranostic Agents Based on Pluronic Stabilized Gold Nanoaggregates Loaded with Methylene Blue for Multimodal Cell Imaging and Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 16191–16201. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, X.; Peng, S.; Gu, H.; Zhang, L. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloids Surf B Biointerfaces 2018, 163, 29–40. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, D.; Wu, M.; Liu, Y.; Zhang, X.; Li, L.; Li, Z.; Han, X.; Wei, X.; Liu, X. Lipid-AuNPs@PDA nanohybrid for MRI/CT imaging and photothermal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 14266–14277. [Google Scholar] [CrossRef]
- Zhao, L.; Kim, T.H.; Kim, H.W.; Ahn, J.C.; Kim, S.Y. Enhanced cellular uptake and phototoxicity of Verteporfin-conjugated gold nanoparticles as theranostic nanocarriers for targeted photodynamic therapy and imaging of cancers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 611–622. [Google Scholar] [CrossRef]
- Zhou, G.; Xiao, H.; Li, X.; Huang, Y.; Song, W.; Song, L.; Chen, M.; Cheng, D.; Shuai, X. Gold nanocage decorated pH-sensitive micelle for highly effective photothermo-chemotherapy and photoacoustic imaging. Acta Biomater. 2017, 64, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green Synthesis of Anisotropic Gold Nanoparticles for Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Chen, W.-J.; Chen, Y.-C. Using Dextran-encapsulated gold nanoparticles as insulin carriers to prolong insulin activity. Nanomedicine 2017, 12, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef]
- Qu, L.; Biant, C.; Sun, J.; Ren, Z.; Han, J.; Xia, S. Electrochemical synthesis of gold nanoparticles in polypyrrole for antibody immobilization. In Proceedings of the 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2009, Shenzhen, China, 5–8 January 2009. [Google Scholar]
- Qu, L.; Xia, S.; Bian, C.; Sun, J.; Han, J. A micro-potentiometric hemoglobin immunosensor based on electropolymerized polypyrrole–gold nanoparticles composite. Biosens. Bioelectron. 2009, 24, 3419–3424. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Functionalized gold nanoparticle-polypyrrole nanobiocomposite with high effective surface area for electrochemical/pH dual stimuli-responsive smart release of insulin. Colloids Surfaces B Biointerfaces 2015, 126, 502–509. [Google Scholar] [CrossRef]
- Chan, C.K.W.; Zhang, L.; Cheng, C.K.; Yang, H.; Huang, Y.; Tian, X.Y.; Choi, C.H.J. Recent Advances in Managing Atherosclerosis via Nanomedicine. Small 2018, 14, 1702793. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, K.; da Silva, M.N.; Sicchieri, L.B.; de Oliveira Silva, F.R.; de Matos, R.A.; Courrol, L.C. Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 2015, 140, 1974–1980. [Google Scholar] [CrossRef]
- Nascimento da Silva, M.; Sicchieri, L.B.; Rodrigues de Oliveira Silva, F.; Andrade, M.F.; Courrol, L.C. Liquid biopsy of atherosclerosis using protoporphyrin IX as a biomarker. Analyst 2014, 139, 1383. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B Biol. 2011, 102, 26–31. [Google Scholar] [CrossRef]
- Qin, J.; Peng, C.; Zhao, B.; Ye, K.; Yuan, F.; Peng, Z.; Yang, X.; Huang, L.; Jiang, M.; Tang, G.; et al. Noninvasive detection of macrophages in atherosclerotic lesions by computed tomography enhanced with PEGylated gold nanoparticles. Int. J. Nanomed. 2014, 5575–5590. [Google Scholar]
- De Oliveira Gonçalves, K.; Vieira, D.P.; Courrol, L.C. Synthesis and characterization of aminolevulinic acid gold nanoparticles: Photo and sonosensitizer agent for atherosclerosis. J. Lumin. 2018, 197, 317–323. [Google Scholar] [CrossRef]
- Tammam, S.N.; Khalil, M.A.F.; Abdul Gawad, E.; Althani, A.; Zaghloul, H.; Azzazy, H.M.E. Chitosan gold nanoparticles for detection of amplified nucleic acids isolated from sputum. Carbohydr. Polym. 2017, 164, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, Y. Polypyrrole-coated hollow gold nanoshell exerts anti-obesity effects via photothermal lipolysis. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 570, 414–419. [Google Scholar] [CrossRef]
- Kandimalla, R.; Dash, S.; Bhowal, A.C.; Kalita, S.; Talukdar, N.C.; Kundu, S.; Kotoky, J. Glycogen-gold nanohybrid escalates the potency of silymarin. Int. J. Nanomed. 2017, 12, 7025–7038. [Google Scholar] [CrossRef]
- Vinodhini, A.; Govindaraju, K.; Singaravelu, G.; Sadiq, A.M.; Kumar, V.G. Cardioprotective potential of biobased gold nanoparticles. Colloids Surf B Biointerfaces 2014, 117, 480–486. [Google Scholar] [CrossRef]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR Sensor Chip for Detection of Small Molecules Using Molecularly Imprinted Polymer with Embedded Gold Nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef]
- Tian, K.; Siegel, G.; Tiwari, A. A simple and selective colorimetric mercury (II) sensing system based on chitosan stabilized gold nanoparticles and 2,6-pyridinedicarboxylic acid. Mater. Sci. Eng. C 2017, 71, 195–199. [Google Scholar] [CrossRef]
- Xue, L.; Xie, W.; Driessen, L.; Domke, K.F.; Wang, Y.; Schlücker, S.; Gorb, S.N.; Steinhart, M. Advanced SERS Sensor Based on Capillarity-Assisted Preconcentration through Gold Nanoparticle-Decorated Porous Nanorods. Small 2017, 13, 1603947. [Google Scholar] [CrossRef]
- Qiu, Z.; Tang, D.; Shu, J.; Chen, G.; Tang, D. Enzyme-triggered formation of enzyme-tyramine concatamers on nanogold-functionalized dendrimer for impedimetric detection of Hg(II) with sensitivity enhancement. Biosens. Bioelectron. 2016, 75, 108–115. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.Y.; Zhang, X.; Rabin, O.; Briber, R.M. Hexagonally ordered nanoparticles templated using a block copolymer film through Coulombic interactions. Nanotechnology 2013, 24, 45305. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Campione, S.; Caldwell, J.D.; Bezares, F.J.; Culbertson, J.C.; Capolino, F.; Ragan, R. Non-lithographic SERS substrates: Tailoring surface chemistry for Au nanoparticle cluster assembly. Small 2012, 8, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Hammock, M.L.; Sokolov, A.N.; Stoltenberg, R.M.; Naab, B.D.; Bao, Z. Organic transistors with ordered nanoparticle arrays as a tailorable platform for selective, in situ detection. ACS Nano 2012, 6, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-Templated Gold Nanoparticles on Optical Fibers for Enhanced-Sensitivity Localized Surface Plasmon Resonance Biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef] [PubMed]


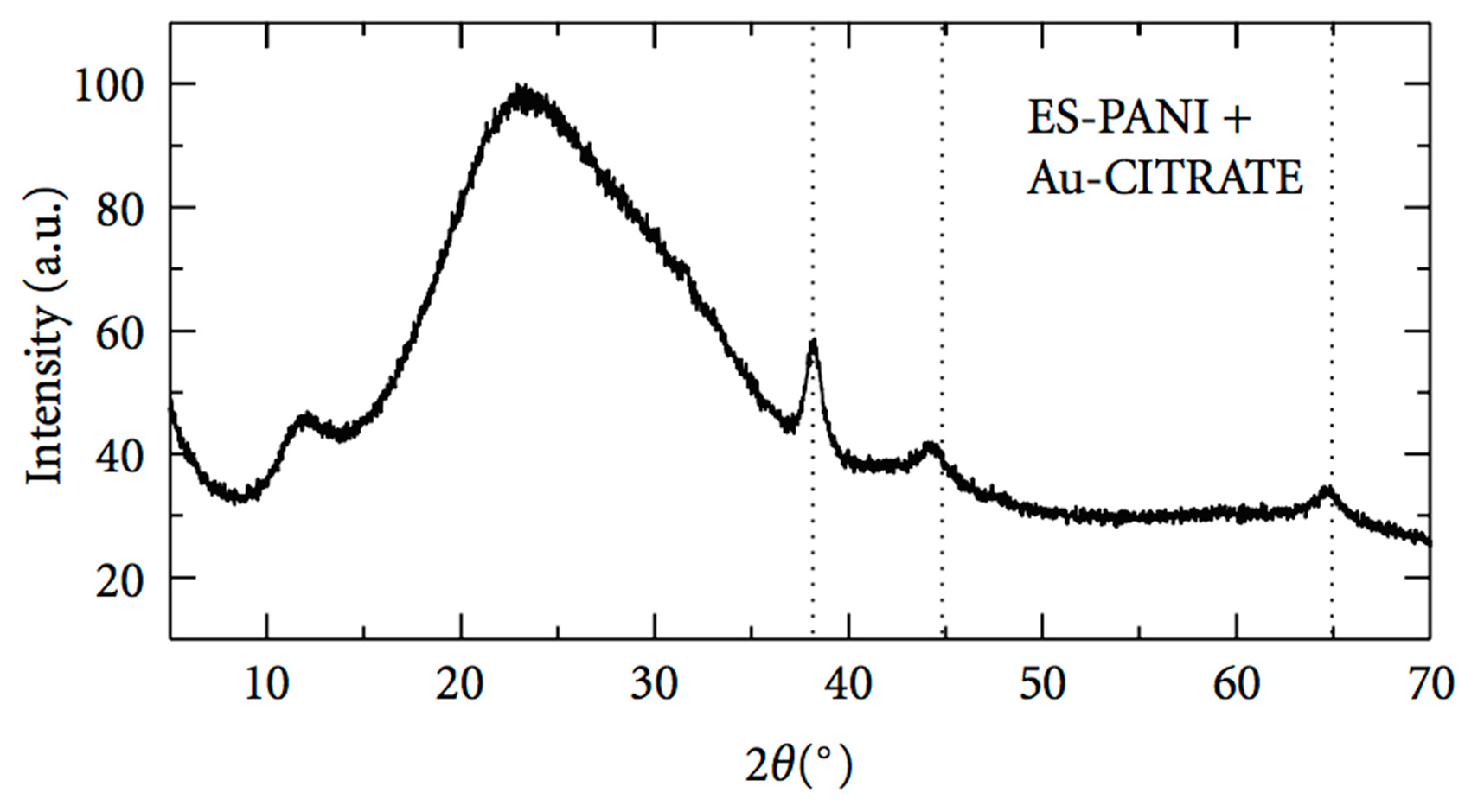
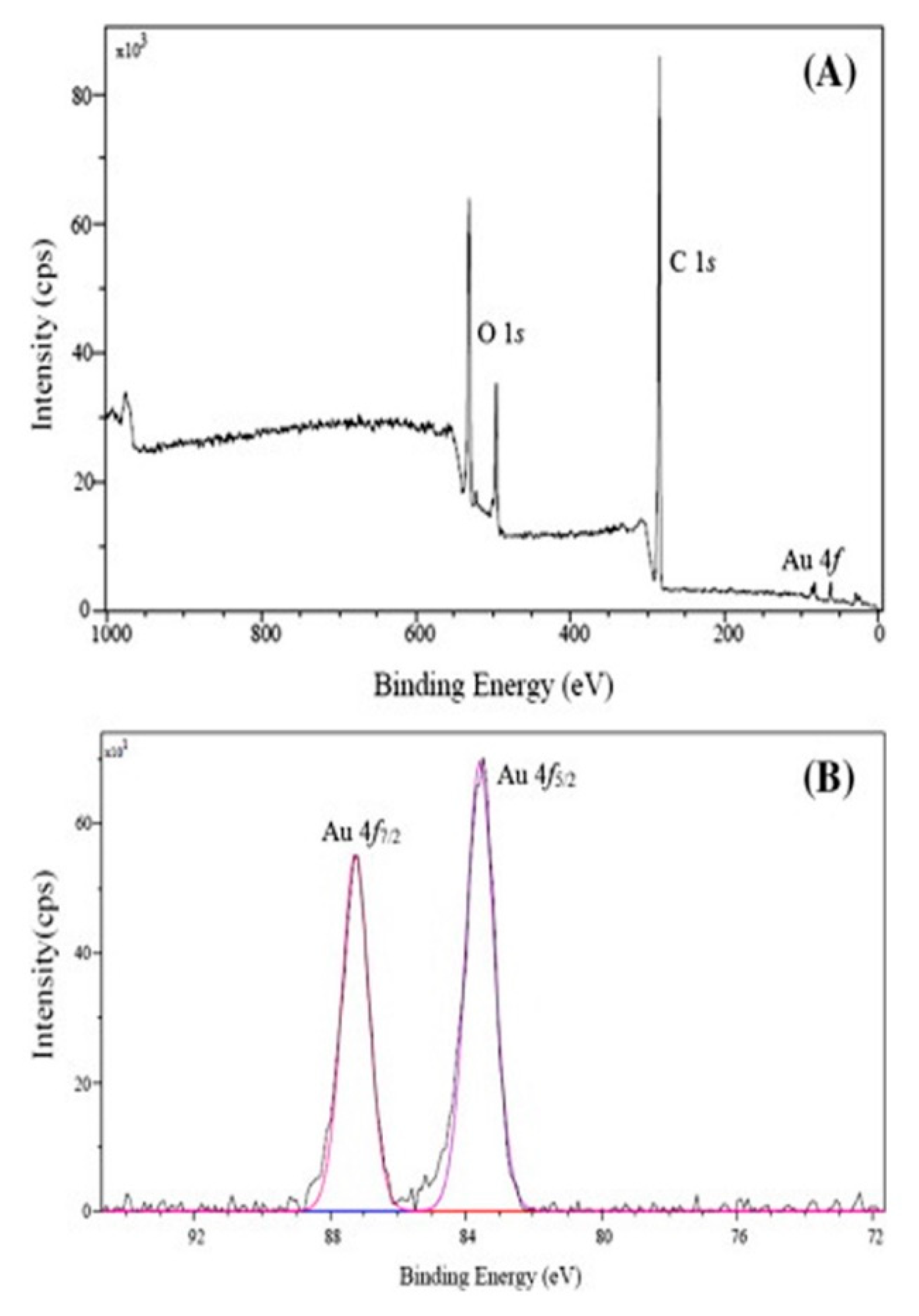
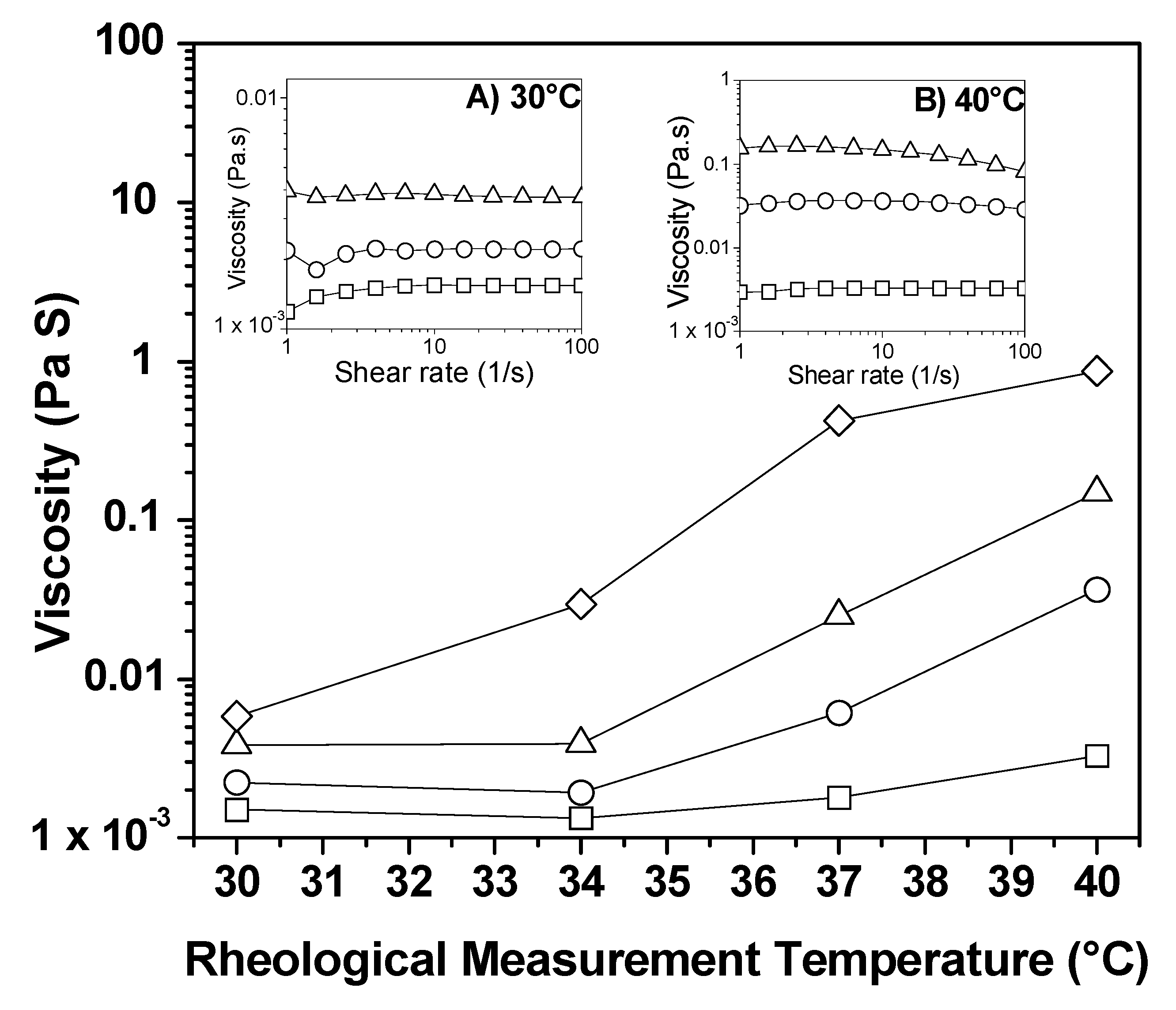
| Polymers | Function | Size [nm] * | Morphology * | Reference |
|---|---|---|---|---|
| Synthetic Polymer Poly(N-vinyl-2-pyrrolidone) (PVP) and Polydiallyldimethylammonium chloride (PDAC) | Stabilizer | ~8 | Spherical | [33] |
| Poly (acrylic acid) (PAA) | Reducer and stabilizer | ~7 | _ | [34] |
| di- or tri-carboxylate- polyethylene glycol (PEG): Citrate-PEG (CPEG); malate-PEG (MAP); tartrate-PEG (TAP) | Reducer and stabilizer | 9–14 | Spherical | [41] |
| Natural Polymer | ||||
| Alginate polymer | Stabilizer | 10–15 | spherical | [42] |
| Sodium alginate (Natural polyhydroxylated polymer) | Reducer and stabilizer | < 100 | Spherical and agglomerates | [39] |
| Mung bean starch (MBS) (Natural polysaccharides) | Reducer and stabilizer | ~10 | Spherical | [40] |
| Chitosan, acylated chitosan (hydrophobically modified chitosan with acyl groups) and chitosan oligosaccharide (short chain chitosan) | Reducer and stabilizer | 3–16 | Spherical | [38] |
| Chitosan oligosaccharide (COS) | Reducer and stabilizer | ~61 | Spherical | [43] |
| Synthetic/Natural Block Copolymer | Size [nm] * | Morphology * | Reference |
|---|---|---|---|
| Pluronic L64, P65, P84, P103, P104, P105, P123, and F127 | ~10 | Spherical | [3] |
| Pluronic L43, L44, L62, L64, P65, F68, P84, P85, F88, P103, P104, P105, F108, P123, and F127 | 7–20 | Spherical | [35] |
| Polyethylene oxide-polystyrene oxide block copolymers | - | Spherical, quiasispherical, and anisotropic shapes (nanoplates) | [44] |
| Pluronic P85 | - | Spherical and quiasispherical | [45] |
| Block copolymers containing a poly (e-caprolactone) central block and poly(N-vinyl 2-pyrrolidone) arms PVP–PCL–PVP | 80–200 | Spherical, triangular, hexagonal, and multi-armed shapes | [46] |
| Pluronic F127 | ~20 | Spherical and quiasispherical | [47] |
| Pluronic L121 | ~100 | Platelike, triangular and hexagonal shapes | [48] |
| Poly(ethylene glycol)-block-linear polyethylenimine-block-poly(e-caprolactone) PEG–PEI–PCL | ~8 | Spherical | [49] |
| Polyethylene glycol-g-polyvinyl alcohol PEG-g-PVA | ~23, 44, 62, 79 | Spherical | [37] |
| Pluronic F127 | ~18.5 | Polygonal | [50] |
| Pluronic F127 | ~5 | Quasi-spherical | [51] |
| 10–70 | Mixture of morphologies | ||
| [hydroxyethyl starch-g-poly (methylacrylate-co-sodium acrylate)] (PHES) | 16–20 | Spherical | [52] |
| Pyromellitic dianhydride-p-phenylene diamine-PPDDs | 11–170 | Spherical and nanoflowers (multi branched) | [53] |
| Block Copolymer | Size [nm] * | Reference |
|---|---|---|
| Pluronics P103, P84, P123, and F127 | 2–3 | [61] |
| Pluronics F68 and P103 | 2–3 | [54] |
| Pluronic L121 | 3–6 | [48] |
| Poly (N-isopropyl acrylamide)/polyethyleneimine PNIPAm/PEI | <10 | [62] |
| Pluronic P103 | 2–6 | [36] |
| Pluronic F127 | - | [63] |
| Pluronic F127 | <5 | [51] |
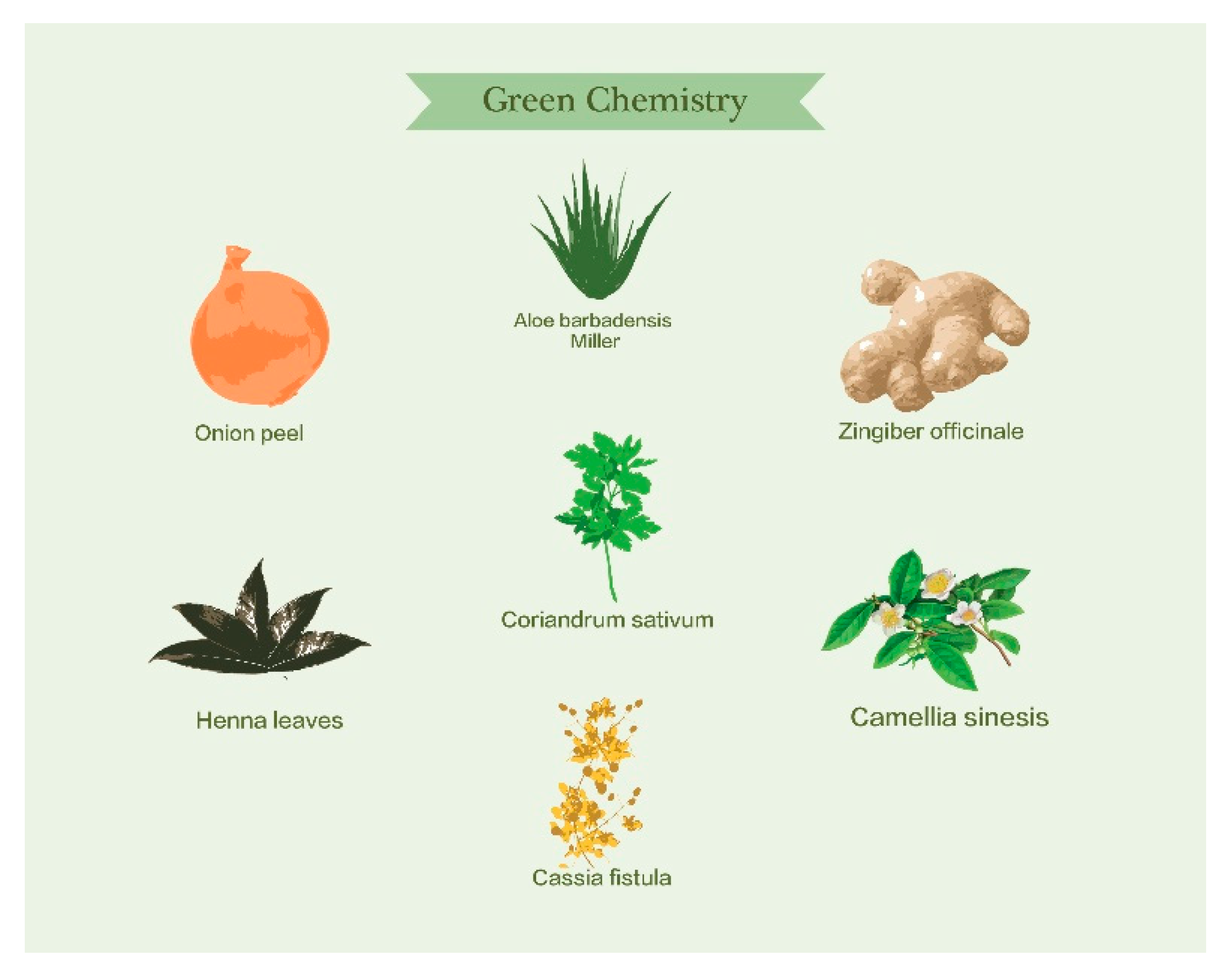
| Plant Origin | Size [nm] * | Morphology * | Applications | Reference |
|---|---|---|---|---|
| Aloe vera | 50–350 | Triangular | Cancer hyperthermia, optical coatings | [66] |
| Coriandrum sativum (coriander leaves) | 6.7–57.9 | Spherical, triangular, truncated triangular, decahedral | Non-linear optics, biomedical and biotechnological applications | [67] |
| Apiin (henna leaves) | 39 | Spherical, triangular, andQuasispheroidal | Hyperthermia of cancer cells and IR-absorbing optical coatings | [68] |
| Camellia sinensis (black tea leaf) | 20 | Spherical, prism | Catalysts, sensors | [69] |
| Cassia fistula (stem bark) | 55–98 | Rectangular and triangular | Diabetes mellitus | [70] |
| Mentha and Pelargonium | 34–33 | Spherical, triangular and polygonal shapes | Medical applications | [71] |
| Artemisia capillaris | 20–30 | Spherical, triangular and rod | Catalytic activity | [72] |
| Onion peel | 45 | Spherical, and triangular shapes | Antibacterial, anticandidal, antioxidant and proteasome inhibitory | [73] |
| Plumeria alba flower | 15–28 | Spherical | Antibacterial activity and catalytic activity | [74] |
| Plant Origin | Size [nm] * | Morphology * | Applications | Reference |
|---|---|---|---|---|
| Zingiber Officinale and Poloxamer 188 | 10–35 | Spherical | Anti-bacterial activity | [75] |
| Leucas Aspera and poly lactic acid-co-poly ethylene glycol-co-poly lactic acid (PLA-PEG-PLA) copolymer | 25 | Spherical | Anti-inflammatory activity | [76] |
| Nigella sativa and zein biopolymer | 50–80 | Nanospherical and nanoplates (hexagons and triangles) | Antimicrobial activity | [77] |
| Block Copolymer and Homopolymer | Template Size [nm] | AuNPs Size [nm] | Additional Techniques Analyzed in Particle Size | Reference |
|---|---|---|---|---|
| a PMAEFc-b-PNIPAM | 90 ± 15 | 5.5–14.0 | DLS | [79] |
| b P(S-b-S-N3) | 2.5 ± 1.5 | - | - | [80] |
| c P4VP-b-PS-b- P4VP | 440 ± 80 | 7.61–11.6 | DLS | [83] |
| d BenzA-b-PCL | 36 ± 9 | ~18.0 | - | [81] |
| e PS-b-PVP | 29 ± 6 | 2.0–6.0 | AFM | [84] |
| f PI-b-PEO | 34 ± 3 | - | DLS | [85] |
| g PCL-b-PEO | 40.2 * | 15.3 ± 1.4 | DLS | [86] |
| h HES-g-PMA | - | 16–20 | - | [52] |
| i COS-PTX | - | 42–76 | DLS | [43] |
| j PAAm | 102 ± 11 | 17–19 | - | [82] |
| Template | Template Size [nm] | AuNPs Size Range [nm] | Additional Techniques Analyzed in Particle Size | Reference |
|---|---|---|---|---|
| a PS-b-PVP | 29 ± 6 | 2–10 | TEM | [84] |
| b L31-F68 | 9 ± 2 59 ± 8 | 2–5 | FESEM, STEM, TEM, DLS, SLS | [88] |
| c PS-b-PEO | ~11 | 5 | - | [89] |
| d TEG-b-Fc | 110 ± 30 (DMSO) 150 ± 35 (THF) | - | SEM, DLS | [90] |
| e,* PS-b-P4VP | - | 46–52 ** | - | [91] |
| Copolymer Template | Plasmon Band Location of AuNPs [nm] | Reference |
|---|---|---|
| a P123 | ~535 | [94] |
| b F127 | ~530 | |
| c P85 | ~540 | [95] |
| F127 | ~508 | [96] |
| d PS-b-PBO | ~521 | [97] |
| e MPEG-b-PNIPAAM-b-PN(+) | ~550 | [98] |
| f PEG-b-PDEAEM | ~534 | [99] |
| g PVEIM-b-PNIPAM | ~525 | [100] |
| Template | Hybrid Particle Size Rh † [nm] | AuNPs Size Rh [nm] | Hybrid Particle Size DH †† [nm] | AuNPs Size DH [nm] | Reference |
|---|---|---|---|---|---|
| a PI-b-PEO (1:2.5) * | - | - | 112 ± 45 | 19 ± 2.0 | [85] |
| b TEG-b-Fc | - | - | 288 (THF) 219 (DMSO) | - | [90] |
| c AuNPs- F127 (Core-shell) | - | - | 34.75 ± 1.56 | 13.48 ± 0.24 | [103] |
| F127- AuNPs | - | - | 24 | - | [63] |
| d MPEG-b-PNIPAAM-b-PN (+) | 200 ± 100.0 | 22 ± 0.0 | - | - | [98] |
| e PEG-b-PDEAEM | - | - | 45 (ethanol) 74 (isopropanol) | 27 ± 3.0 | [99] |
| f PNIPAM-b-PSEMA | - | - | 208 (20 °C) 182 (40 °C) ** | 14.1 ± 1.3 | [102] |
| g COS-PTX | - | - | - | 61.86 ± 3.01 | [43] |
| h CG | - | - | - | 7 ± 2 | [33] |
| PVP | 6.1 | ||||
| PDAC | 8 ± 2 |
| Template | Au (4f) [eV] | N (1s) [eV] | O (1s) [eV] | C (1s) [eV] | Carbon in the Methoxy Group [eV] | Carbon in the Ester Group [eV] | Aromatic Rings [eV] | Reference |
|---|---|---|---|---|---|---|---|---|
| a PVEIM-b-PNIPAM | ~80 | ~400 | ~540 | ~290 | - | - | - | [100] |
| b PS-b-PMMA | - | - | - | 284.6 | 286.8 | 289.0 | 291.7 | [109] |
| c PDMAEMA-b-PS | - | ~400 | - | - | - | - | - | [110] |
| d PS-b-P2VP | 84.5 | - | - | - | - | - | - | [111] |
| e MBS | 83.7 | - | - | - | - | - | - | [40] |
| f TAP-PEG | 83.9 | 288.1 | [41] |
| Hybrid AuNPs | Copolymer | Elastic Modulus G’ [Pa] | Temperature [°C] | Viscosity [Pa·s] | Reference |
|---|---|---|---|---|---|
| a AuNPs-P103 | - | 30.0 | 0.003832 | [36] | |
| (20 wt.%) * | 40.0 | 0.1497 | |||
| c P103 | - | 30.0 | 0.005809 | ||
| (20 wt.%) * | 40.0 | 0.8743 | |||
| b PS-b-(P4VP/HAuCl4) | 3 × 105 | 200 | - | [124] | |
| d PS-b-P4VP | 6 × 105 | 200 | - | ||
| PS-b-(P4VP/HAuCl4) | 105 | 130.0 | - | [123] |
| Synthetic/Natural Polymer or Block Copolymer | Size [nm] | Morphology | Application | Reference |
|---|---|---|---|---|
| Chitosan | 23 | Spherical | Detection of Hg2+ | [226] |
| Polystyrene-block-poly(2-vinyl pyridine) | 16–20 | Spherical | Detection of 5,5′dithiobis(2-nitrobenzoic acid) | [227] |
| Poly(amidoamine) PAMAM | 55 | Spherical | Detection of Hg2+ | [228] |
| Poly(4-vinylpyridine) and polystyrene-b-poly(4-vinylpyridine) | - | Hexagonal | No is specify | [229] |
| Polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA) | 20 | Spherical | Benzenethiol | [230] |
| Poly(4-vinylphenol) (PVP) | - | Variable | Detection of Hg2+ | [231] |
| Poly(styrene-b-4-vinylpyridine) (PS-b-P4VP) | 10–92 | Nanodisk | Human gamma globulin (IgG) and affinipure goat anti-human IgG(H+L) | [232] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612. https://doi.org/10.3390/cryst9120612
Tepale N, Fernández-Escamilla VVA, Carreon-Alvarez C, González-Coronel VJ, Luna-Flores A, Carreon-Alvarez A, Aguilar J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals. 2019; 9(12):612. https://doi.org/10.3390/cryst9120612
Chicago/Turabian StyleTepale, Nancy, Víctor V. A. Fernández-Escamilla, Clara Carreon-Alvarez, Valeria J. González-Coronel, Adan Luna-Flores, Alejandra Carreon-Alvarez, and Jacobo Aguilar. 2019. "Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications" Crystals 9, no. 12: 612. https://doi.org/10.3390/cryst9120612
APA StyleTepale, N., Fernández-Escamilla, V. V. A., Carreon-Alvarez, C., González-Coronel, V. J., Luna-Flores, A., Carreon-Alvarez, A., & Aguilar, J. (2019). Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals, 9(12), 612. https://doi.org/10.3390/cryst9120612






