Comparative Study on AC Susceptibility of YBa2Cu3O7−δ Added with BaZrO3 Nanoparticles Prepared via Solid-State and Co-Precipitation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid-State (SS) Method
2.2. Co-Precipitation (COP) Method
2.3. Sample Characterization
3. Discussion
3.1. X-ray Diffraction (XRD) Analysis
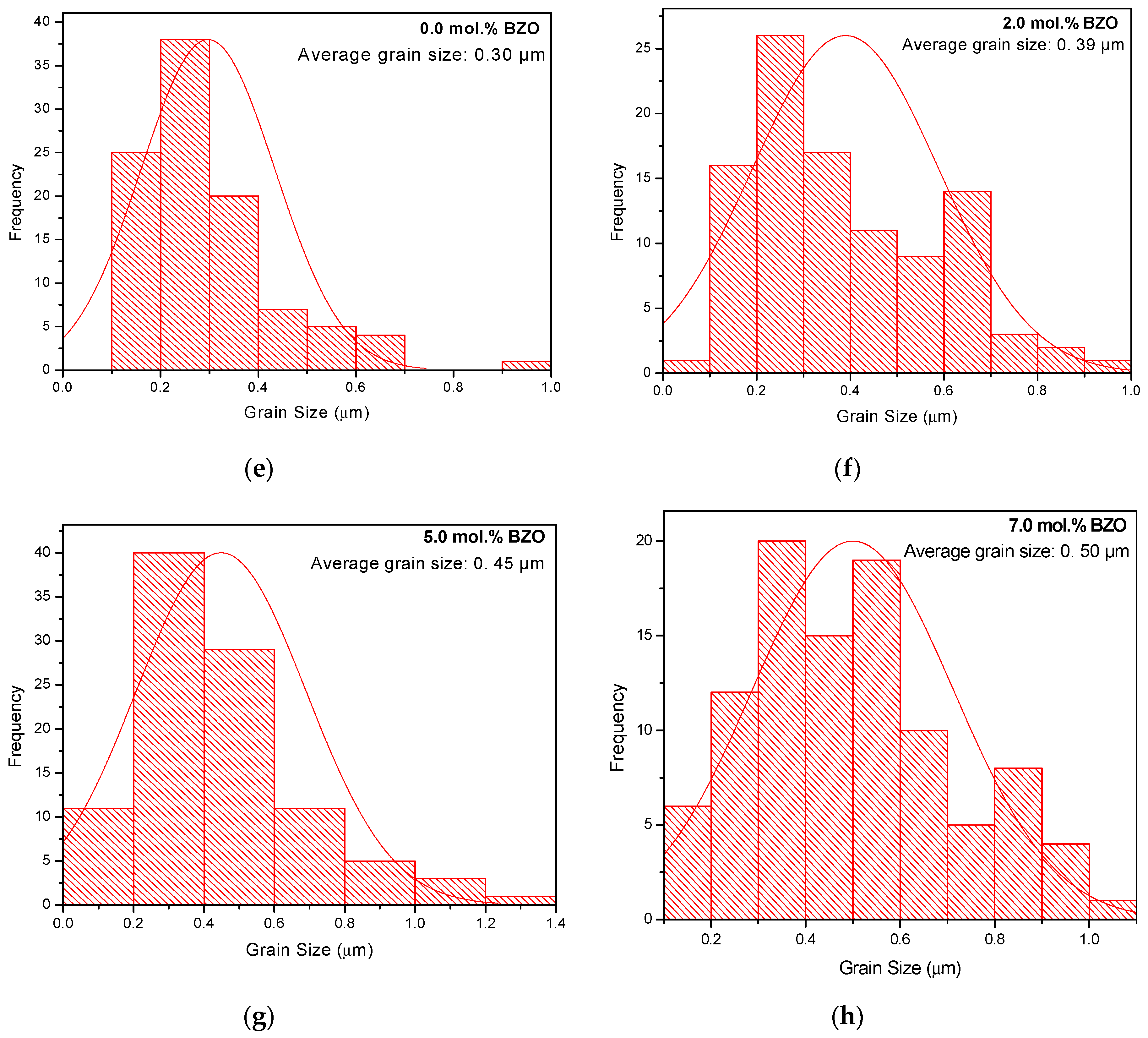
3.2. Microstructure Analysis
3.3. Superconducting Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, P.N.; Haugan, T.J.; Baca, F.J.; Varanasi, C.V.; Wheeler, R.; Meisenkothen, F.; Sathiraju, S. Inducing self-assembly of Y2BaCuO5 nanoparticles via Ca-doping for improved pinning in YBa2Cu3O7−x. Physica C 2009, 469, 2029–2032. [Google Scholar] [CrossRef]
- Horvath, D.; Harnois, C.; Noudem, J.G. Li and Ce doping of melt-textured YBCO: Improved Jc at medium fields. Mater. Sci. Eng. B 2008, 151, 36–39. [Google Scholar] [CrossRef]
- Klie, R.F.; Buban, J.P.; Varela, M.; Franceschetti, A.; Jooss, C.; Zhu, Y.; Browning, N.D.; Pantelides, S.T.; Pennycook, S.J. Enhanced current transport at grain boundaries in high-Tc superconductors. Nature 2005, 435, 475–478. [Google Scholar] [CrossRef]
- Jin, L.H.; Zhang, S.N.; Yu, Z.M.; Li, C.S.; Feng, J.Q.; Sulpice, A.; Wang, Y.; Zhang, P.X. Influences of BaZrO3 particles on the microstructure and flux pinning of YBCO film prepared by using modified TFA-MOD approach. Mater. Chem. Phys. 2015, 149–150, 188–192. [Google Scholar] [CrossRef]
- Awano, M.; Fujishiro, Y.; Moon, J.; Takagi, H.; Rybchenko, S.; Bredikhin, S. Microstructure control of an oxide superconductor on interaction of pinning centers and growing crystal surface. Physica C 2000, 341–348, 2017–2018. [Google Scholar] [CrossRef]
- Paulose, K.V.; Koshy, J.; Damodaran, A.D. Superconductivity in YBa2Cu3O7−δ-ZrO2 systems. Supercond. Sci. Technol. 1991, 4, 96–101. [Google Scholar] [CrossRef]
- Palonen, H.; Huhtinen, H.; Shakhov, M.A.; Paturi, P. Electron mass anisotropy of BaZrO3 doped YBCO thin films in pulsed magnetic fields up to 30 T. Supercond. Sci. Technol. 2013, 26, 045003. [Google Scholar] [CrossRef]
- Bretos, I.; Schneller, T.; Falter, M.; Backer, M.; Hollmann, E.; Wordenweber, R.; Molina-Luna, L.; Tendelooe, G.V.; Eibl, O. Solution-derived YBa2Cu3O7−δ (YBCO) superconducting films with BaZrO3 (BZO) nanodots based on reverse micelle stabilized nanoparticles. J. Mater. Chem. C 2015, 3, 3971–3979. [Google Scholar] [CrossRef]
- Peurla, M.; Paturi, P.; Stepanov, Y.P.; Huhtinen, H.; Tse, Y.Y.; Bodi, A.C.; Raittila, J.; Laiho, R. Optimization of the BaZrO3 concentration in YBCO films prepared by pulsed laser deposition. Supercond. Sci. Technol. 2006, 19, 767–771. [Google Scholar] [CrossRef]
- Malmivirta, M.; Rijckaert, H.; Paasonen, V.; Huhtinen, H.; Hynninen, T.; Jha, R.; Awana, V.S.; Driessche, I.V.; Paturi, P. Enhanced flux pinning in YBCO multilayer films with BCO nanodots and segmented BZO nanorods. Sci. Rep. 2017, 7, 14682. [Google Scholar] [CrossRef]
- Wang, F.; Tian, H. BaZrO3 (BZO) nanoparticles as effective pinning centers for -YBa2Cu3O7−δ (YBCO) superconducting thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 4137–4143. [Google Scholar] [CrossRef]
- Jha, A.K.; Khare, N. Investigation of flux pinning properties of YBCO: BaZrO3 composite superconductor from temperature dependent magnetization studies. J. Magn. Magn. Mater. 2010, 322, 2653–2657. [Google Scholar] [CrossRef]
- Arlina, A.; Halim, S.A.; Awang Kechik, M.M.; Chen, S.K. Superconductivity in Bi-Pb-Sr-Ca-Cu-O ceramics with YBCO as additive. J. Alloys Compd. 2015, 645, 269–273. [Google Scholar] [CrossRef]
- Paz-Pujalt, G.R.; Mehrotra, A.K.; Ferranti, S.A.; Agostinelli, J.A. Solid state reactions in the formation of YBa2Cu3O7−δ high Tc superconductor powders. Solid State Ionics 1989, 32–33, 1179–1182. [Google Scholar] [CrossRef]
- Fujihara, S.; Kozuka, H.; Yoko, T.; Sakka, S. Mechanism of formation of YBa2Cu4O8 superconductor in the sol-gel synthesis. J. Sol-Gel Sci. Technol. 1994, 1, 133–140. [Google Scholar] [CrossRef]
- Ramli, A.; Halim, S.A.; Chen, S.K.; Awang Kechik, M.M. The effect of Gd2O3 nanoparticles addition on microstructural and electrical properties of YBCO superconductor. ARPN J. Eng. Appl. Sci. 2016, 11, 13708–13715. [Google Scholar]
- Hamadneh, I.; Rosli, A.M.; Abd-Shukor, R.; Suib, N.R.M.; Yahya, S.Y. Superconductivity of REBa2Cu3O7−δ (RE = Y, Dy, Er) ceramic synthesized via coprecipitation method. J. Phys. Conf. Ser. 2008, 97, 012063. [Google Scholar] [CrossRef]
- Bhargava, A.; Mackinnon, I.D.R.; Yamashita, T.; Page, D. Bulk manufacture of YBCO powders by coprecipitation. Physica C 1995, 241, 53–62. [Google Scholar] [CrossRef]
- Mousa Dihom, M.; Shaari, A.H.; Baqiah, H.; Al-Hada, N.M.; Talib, Z.A.; Chen, S.K.; Aziz, R.S.; Awang Kechik, M.M.; Lim, K.P.; Shukor, R.A. Structural and superconducting properties of Y(Ba1-xKx)2Cu3O7−δ ceramics. Ceram. Int. 2017, 43, 11339–11344. [Google Scholar] [CrossRef]
- Wahid, M.H.; Zainal, Z.; Hamadneh, I.; Tan, K.B.; Halim, S.A.; Rosli, A.M.; Alaghbari, E.S.; Nazarudin, M.F.; Kadri, E.F. Phase formation of REBa2Cu3O7−δ (RE: Y0.5Gd0.5, Y0.5Nd0.5, Nd0.5Gd0.5) superconductors from nanopowders synthesised via co-precipitation. Ceram. Int. 2012, 38, 1187–1193. [Google Scholar] [CrossRef]
- Ochsenkuhn-Petropoulou, M.; Argyropoulou, R.; Tarantilis, P.; Kokkinos, E.; Ochsenkuhn, K.M.; Parissakis, G. Comparison of the oxalate co-precipitation and the solid state reaction methods for the production of high temperature superconducting powders and coating. J. Mater. Process. Technol. 2002, 127, 122–128. [Google Scholar] [CrossRef]
- Mohd Hapipi, N.; Chen, S.K.; Shaari, A.H.; Awang Kechik, M.M.; Tan, K.B.; Lim, K.P. Superconductivity of Y2O3 and BaZrO3 nanoparticles co-added YBa2Cu3O7−δ bulks prepared using co-precipitation method. J. Mater. Sci. Mater. Electron. 2018, 29, 18684–18692. [Google Scholar] [CrossRef]
- Mohd Hapipi, N.; Chen, S.K.; Shaari, A.H.; Awang Kechik, M.M.; Tan, K.B.; Lim, K.P.; Lee, O.J. AC susceptibility of BaZrO3 nanoparticles added YBa2Cu3O7−δ superconductor prepared via coprecipitation method. J. Supercond. Novel Magn. 2018, 32, 1191–1198. [Google Scholar] [CrossRef]
- Patnaik, P. Dean’s Analytical Chemistry Handbook, 2nd ed.; McGraw Hill Professional: New York, NY, USA, 2004. [Google Scholar]
- Zhang, C.J.; Oyanagi, H. The synthesis condition and its influence on Tc in Mn doped La1.85Sr0.15CuO4. Physica C 2008, 468, 1155–1158. [Google Scholar] [CrossRef]
- Pomar, A.; Vlad, V.R.; Llordes, A.; Palau, A.; Gutiérrez, J.; Ricart, S.; Puig, T.; Obradors, X.; Usoskin, A. Enhanced vortex pinning in YBCO coated conductors with BZO nanoparticles from chemical solution deposition. IEEE Trans. Appl. Supercond. 2009, 19, 3258–3261. [Google Scholar] [CrossRef]
- Cracolice, M.S.; Peters, E.I. Introductory Chemistry: An Active Learning Approach; Brooks Cole: Belmont, CA, USA, 2012. [Google Scholar]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Mazaheri, M.; Aminzare, M.; Sadrnezhaad, S.K. Reverse precipitation synthesis and characterization of CeO2 nanopowder. J. Alloys Compd. 2010, 491, 499–502. [Google Scholar] [CrossRef]
- Mondal, O.; Pal, M.; Singh, R.; Sen, D.; Mazumder, S.; Pal, M. Influence of doping on crystal growth, structure and optical properties of nanocrystalline CaTiO3: A case study using small-angle neutron scattering. J. Appl. Crystallogr. 2015, 48, 836–843. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Wu, Y.C.; Xiong, X.M.; Li, Q.Y.; Gawalek, W.; He, Z.H. Effects of precursors with fine BaZrO3 inclusions on the growth and microstructure of textured YBCO. J. Supercond. 2000, 13, 575–581. [Google Scholar] [CrossRef]
- Nikolo, M. Superconductivity: A guide to alternating current susceptibility measurements and alternating current susceptometer design. Am. J. Phys. 1995, 63, 57–65. [Google Scholar] [CrossRef]
- Ciontea, L.; Celentano, G.; Augieri, A.; Ristoiu, T.; Suciu, R.; Gabor, M.S.; Rufoloni, A.; Vannozzi, A.; Galluzi, V.; Petrisor, T. Chemically processed BaZrO3 nanopowders as artificial pinning centres. J. Phys. Conf. Ser. 2008, 97, 012289. [Google Scholar] [CrossRef]
- MacManus-Driscoll, J.L.; Foltyn, S.R.; Jia, Q.X.; Wang, H.; Serquis, A.; Civale, L.; Maiorov, B.; Hawley, M.E.; Maley, M.P.; Peterson, D.E. Strongly enhanced current densities in superconducting coated conductors of YBa2Cu3O7−x + BaZrO3. Nat. Mater. 2004, 3, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Kameli, P.; Salamati, H.; Eslami, M. The effect of sintering temperature on the intergranular properties of Bi2223 superconductors. Solid State Commun. 2006, 137, 30–35. [Google Scholar] [CrossRef]
- Nedkov, I.; Veneva, A. Grain boundaries contribution to the complex susceptibility of KCu-doped YBCO high-temperature superconductors. J. Low Temp. Phys. 1997, 107, 497–502. [Google Scholar] [CrossRef]
- Deac, I.G.; Burzo, E.; Pop, A.V.; Pop, V.; Tetean, R.; Kovacs, D.; Borodi, G. Intergranular properties of (Y1−x−yZrxCay)Ba2Cu3O7−δ compounds. Int. J. Mod. Phys. B 1999, 13, 1645–1654. [Google Scholar] [CrossRef]
- Sbarciog, C.; Redac, R.T.; Deac, I.G.; Pop, I. Intergranular properties of Zr-substituted Y123 compounds. Mod. Phys. Lett. B 2006, 20, 1191–1198. [Google Scholar] [CrossRef]
- Rani, P.; Jha, R.; Awana, V.P.S. AC susceptibility study of superconducting YBa2Cu3O7: Agx bulk composites (x = 0.0–0.20): The role of intra and intergranular coupling. J. Supercond. Novel Magn. 2013, 26, 2347–2352. [Google Scholar] [CrossRef]
- Nur-Akasyah, J.; Nur-Shamimie, N.H.; Abd-Shukor, R. Effect of CdTe addition on the electrical properties and AC susceptibility of YBa2Cu3O7−δ superconductor. J. Supercond. Novel Magn. 2017, 30, 3361–3365. [Google Scholar] [CrossRef]
- Clem, J.R. Granular and superconducting-glass properties of the high-temperature superconductors. Physica C 1988, 153–155, 50–55. [Google Scholar] [CrossRef]
- Emmen, J.H.P.M.; Brabers, V.A.M.; De Jonge, W.J.M.; Steen, C.V.D.; Dalderop, J.H.J.; Geppaart, P.M.A.; Kopinga, K. Microstructure and properties of Bi-Ca-Sr-Cu-O superconductors. J. Less-Common Met. 1989, 151, 63–69. [Google Scholar] [CrossRef]
- Narlikar, A.V. Field Penetration and Magnetization of High Temperature Superconductors; Nova Science Publisher, Inc.: New York, NY, USA, 1995. [Google Scholar]




| BZO x (mol%) | Weight Percentage of Phases (%) | |||||
|---|---|---|---|---|---|---|
| SS Method | COP Method | |||||
| Y-123 | Y-211 | BZO | Y-123 | Y-211 | BZO | |
| 0.0 | 93.2 | 6.8 | 0.0 | 97.5 | 2.5 | 0.0 |
| 2.0 | 92.9 | 6.6 | 0.5 | 96.6 | 3.1 | 0.3 |
| 5.0 | 92.8 | 5.7 | 1.5 | 94.8 | 4.1 | 2.7 |
| 7.0 | 92.8 | 3.8 | 3.4 | 94.3 | 3.0 | 2.7 |
| BZO x (mol%) | SS Method | COP Method | ||||
|---|---|---|---|---|---|---|
| FWHM of (103) Peak (º) | Crystallite Size (nm) | Average Grain Size, D (μm) | FWHM of (103) Peak (º) | Crystallite Size (nm) | Average Grain Size, D (μm) | |
| 0.0 | 0.1315 | 101.61 | 1.24 ± 0.14 | 0.1801 | 63.66 | 0.30 ± 0.01 |
| 2.0 | 0.1424 | 89.62 | 1.77 ± 0.06 | 0.1737 | 66.94 | 0.39 ± 0.02 |
| 5.0 | 0.1515 | 81.56 | 2.29 ± 0.01 | 0.1793 | 64.03 | 0.45 ± 0.02 |
| 7.0 | 0.1436 | 88.44 | 2.71 ± 0.11 | 0.1729 | 67.36 | 0.02 |
| BZO x (mol%) | SS Method | COP Method | ||||||
|---|---|---|---|---|---|---|---|---|
| Tp (K) | Tc-onset (K) | Tcj (K) | Io (μA) | Tp (K) | Tc-onset (K) | Tcj (K) | Io (μA) | |
| 0.0 | 87.3 | 90.3 | 87.8 | 51.21 | 84.8 | 90.6 | 86.6 | 31.69 |
| 2.0 | 85.8 | 88.9 | 86.6 | 53.95 | 84.5 | 90.5 | 86.5 | 32.08 |
| 5.0 | 84.1 | 89.9 | 85.4 | 28.20 | 82.9 | 89.8 | 85.6 | 30.56 |
| 7.0 | 84.5 | 88.9 | 85.5 | 36.49 | 83.6 | 89.9 | 85.8 | 30.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hapipi, N.; Lim, J.K.; Chen, S.K.; Lee, O.J.; Shaari, A.H.; Awang Kechik, M.M.; Lim, K.P.; Tan, K.B.; Murakami, M.; Miryala, M. Comparative Study on AC Susceptibility of YBa2Cu3O7−δ Added with BaZrO3 Nanoparticles Prepared via Solid-State and Co-Precipitation Method. Crystals 2019, 9, 655. https://doi.org/10.3390/cryst9120655
Mohd Hapipi N, Lim JK, Chen SK, Lee OJ, Shaari AH, Awang Kechik MM, Lim KP, Tan KB, Murakami M, Miryala M. Comparative Study on AC Susceptibility of YBa2Cu3O7−δ Added with BaZrO3 Nanoparticles Prepared via Solid-State and Co-Precipitation Method. Crystals. 2019; 9(12):655. https://doi.org/10.3390/cryst9120655
Chicago/Turabian StyleMohd Hapipi, Nurhidayah, Jee Khan Lim, Soo Kien Chen, Oon Jew Lee, Abdul Halim Shaari, Mohd Mustafa Awang Kechik, Kean Pah Lim, Kar Ban Tan, Masato Murakami, and Muralidhar Miryala. 2019. "Comparative Study on AC Susceptibility of YBa2Cu3O7−δ Added with BaZrO3 Nanoparticles Prepared via Solid-State and Co-Precipitation Method" Crystals 9, no. 12: 655. https://doi.org/10.3390/cryst9120655
APA StyleMohd Hapipi, N., Lim, J. K., Chen, S. K., Lee, O. J., Shaari, A. H., Awang Kechik, M. M., Lim, K. P., Tan, K. B., Murakami, M., & Miryala, M. (2019). Comparative Study on AC Susceptibility of YBa2Cu3O7−δ Added with BaZrO3 Nanoparticles Prepared via Solid-State and Co-Precipitation Method. Crystals, 9(12), 655. https://doi.org/10.3390/cryst9120655





