The Effect of Pressure on Magnetic Properties of Prussian Blue Analogues
Abstract
:1. Introduction
2. Magnetic Prussian Blue Analogues
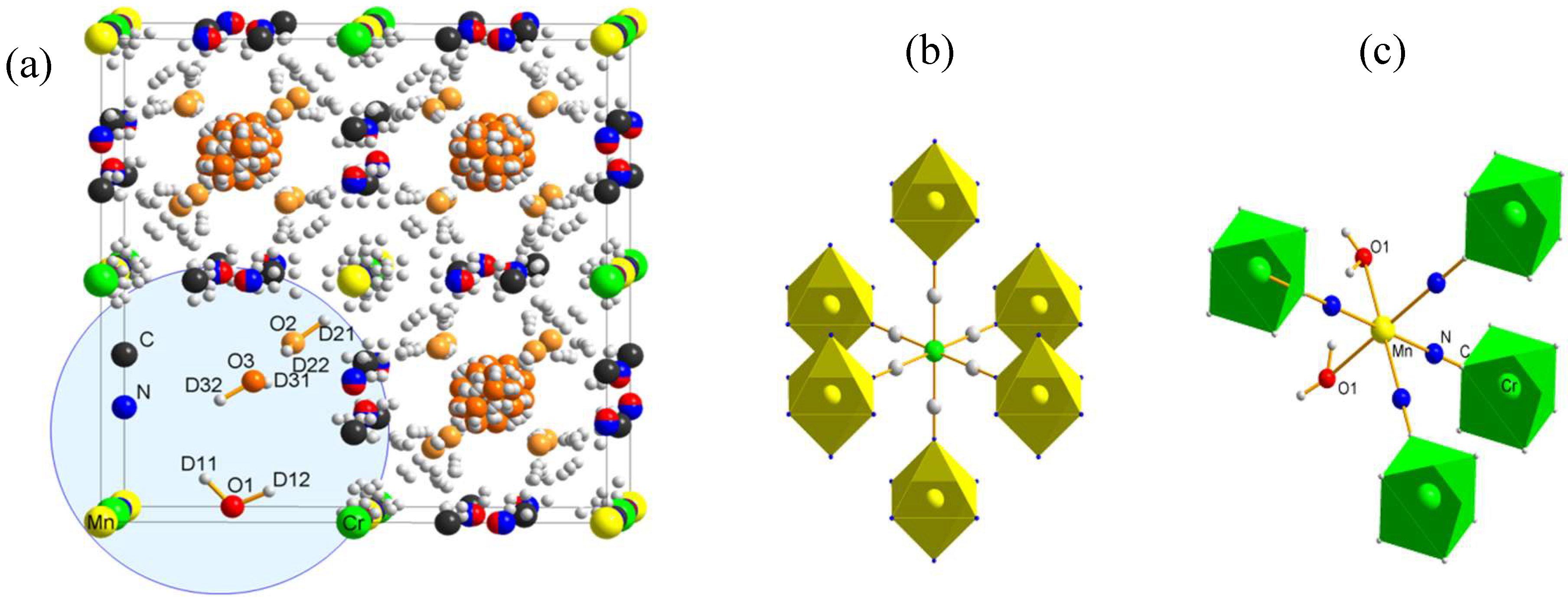
2.1. Crystal Structure of Prussian Blue Analogues
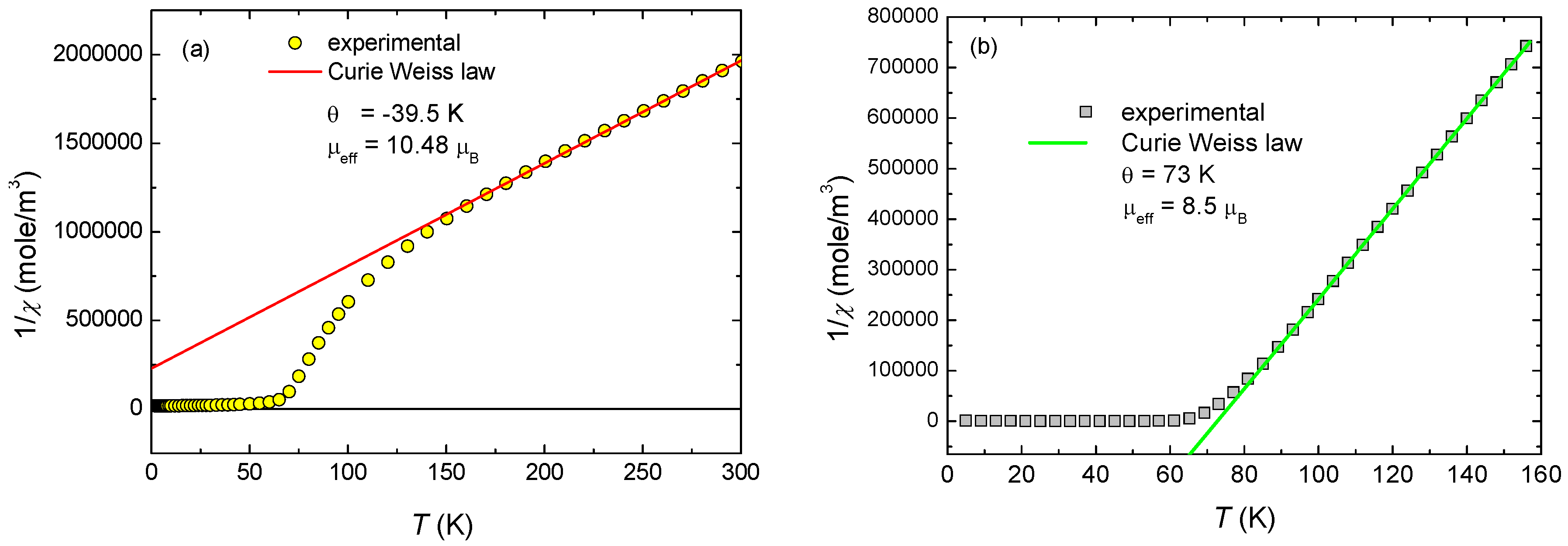
2.2. Magnetic Properties
- [B(CN)6]4− for B = VII, MnII
- [B(CN)6]3− for B = TiIII, CrIII, MnIII, FeIII
- [B(CN)6]2− for B = MnIV
3. Probing of Magnetocrystalline Correlations Using External Pressure
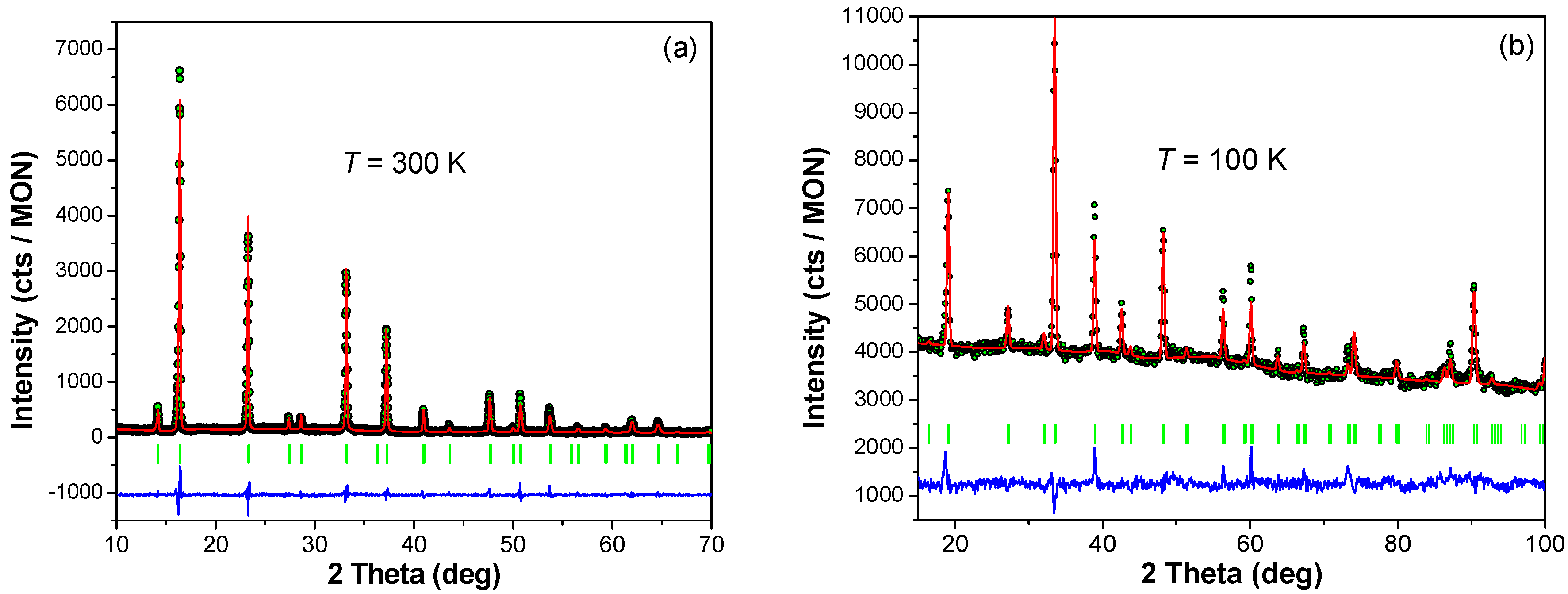
3.1. Crystal Structure of TM2+-CrIII-PBs
3.2. Effect of Pressure on the Crystal Structure of TM2+-CrIII-PBs
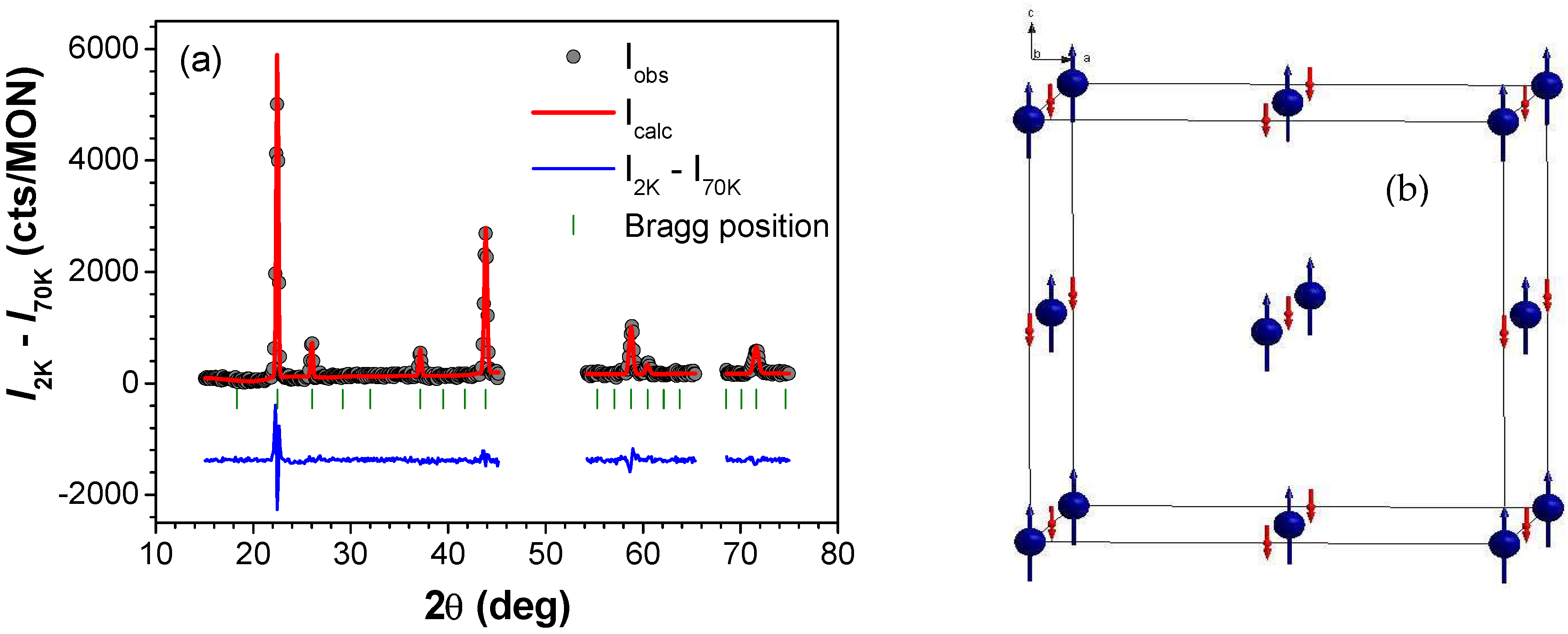
3.3. Magnetic Structure of TM2+-CrIII-PBs
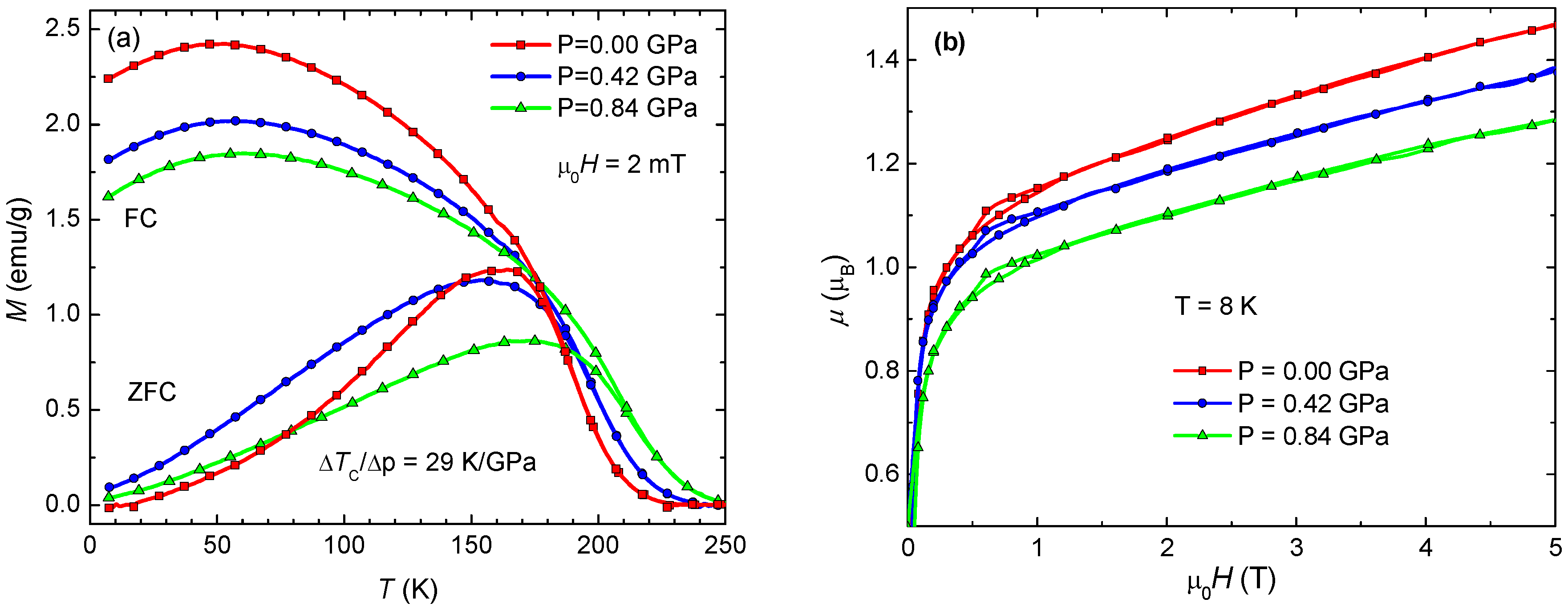
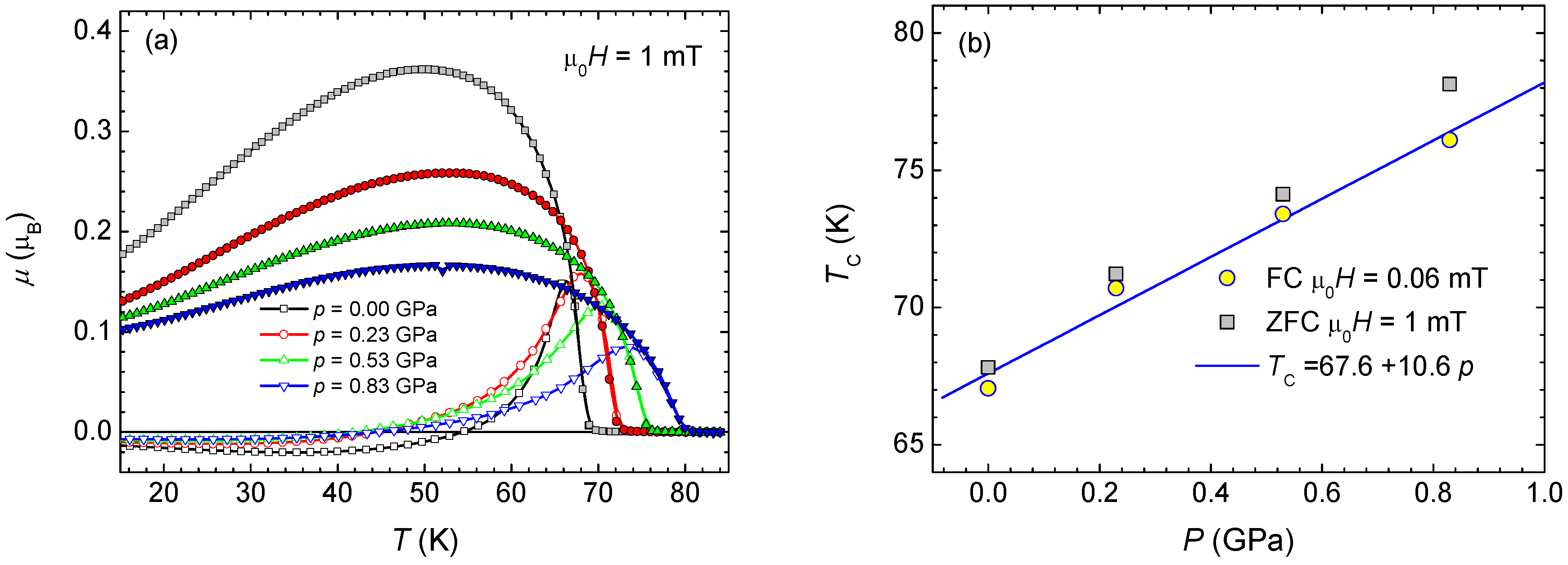
3.4. Pressure Effect on Magnetic Properties of Polycrystaline Samples
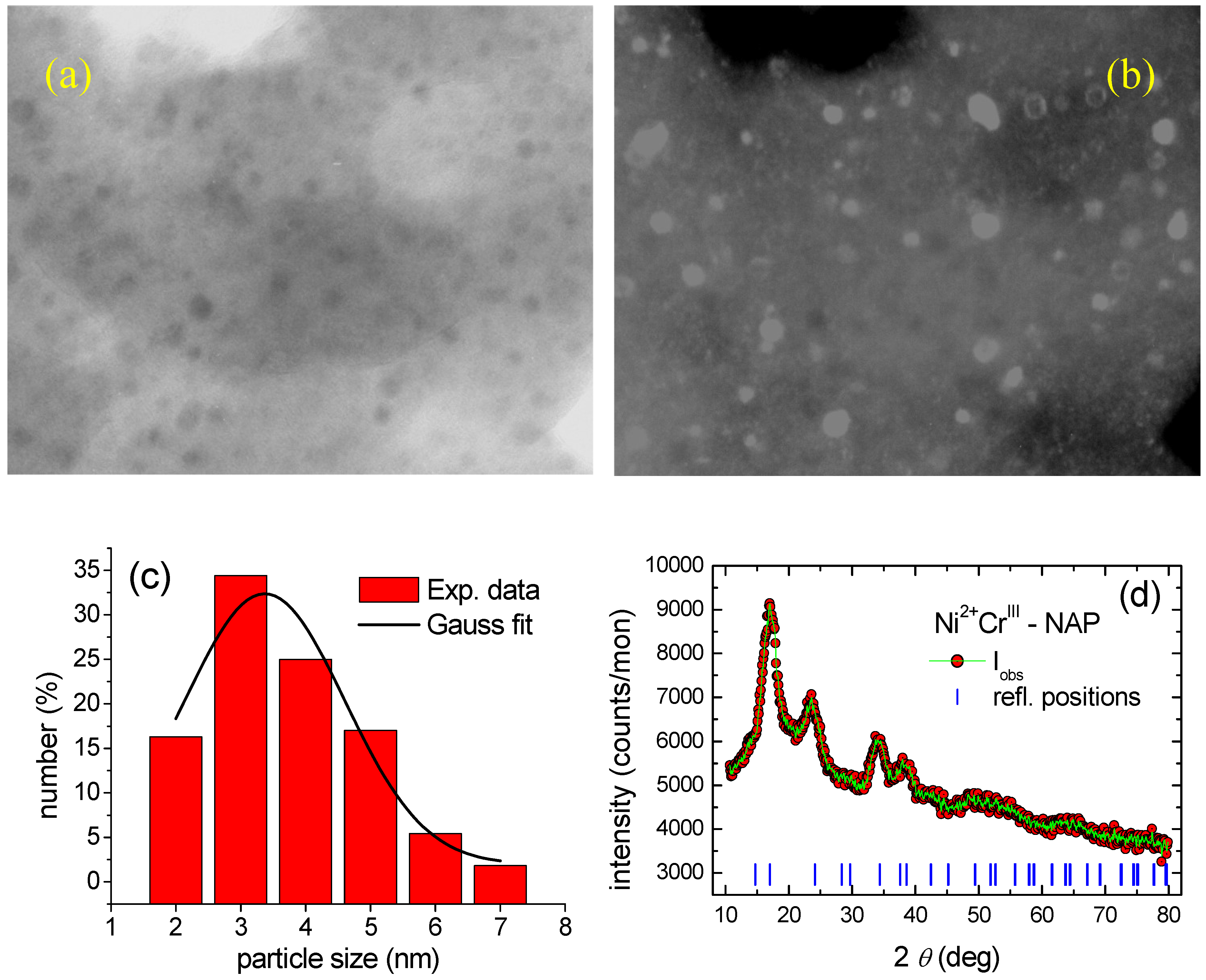
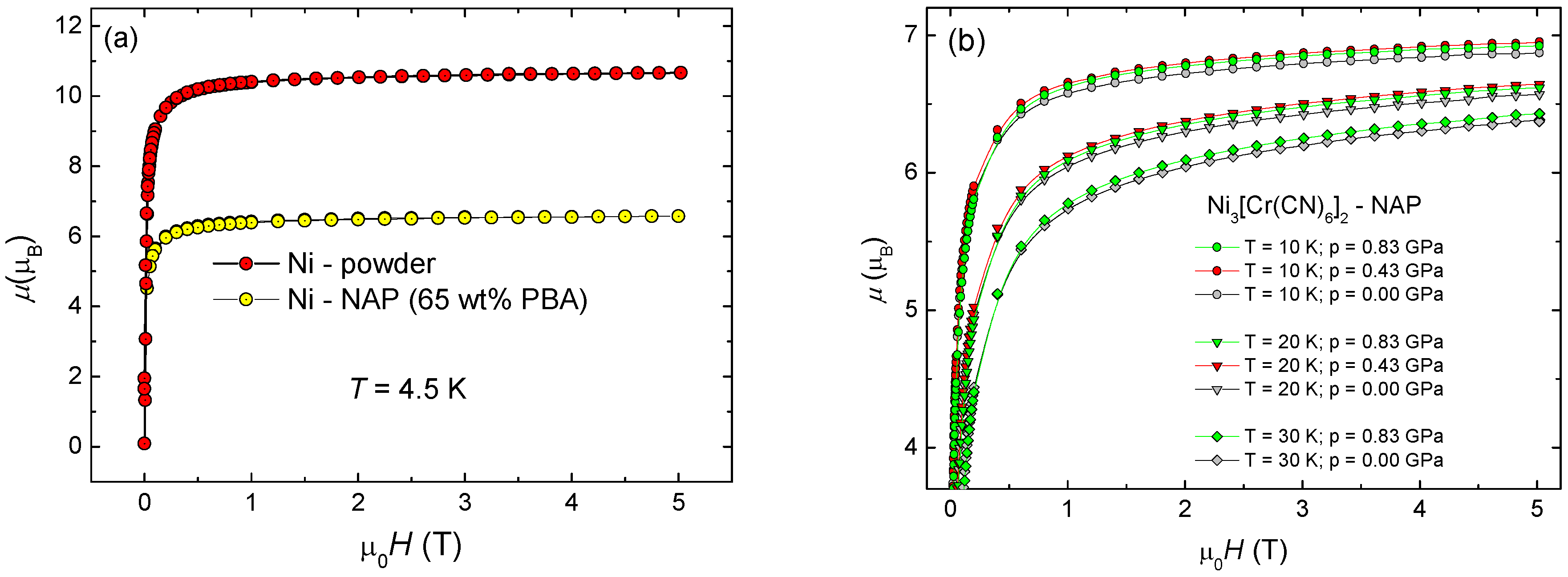
3.5. Magnetic Properties of Magnetic Nanoparticles
3.6. CrIII-PBs Core–Shell Heterostructures under Pressure
4. Conclusions
Funding
Conflicts of Interest
References
- Verdaguer, M.; Girolami, G. Magnetic Prussian Blue Analogs. In Magnetism: Molecules to Materials V; Miller, J.S., Drilon, M., Eds.; Wiley–VCH & KGaA: Weinheim, Germany, 2004; pp. 283–386. ISBN 3-527-30665-X. [Google Scholar]
- Ma, F.; Li, Q.; Wang, T.; Zhang, H.; Wu, G. Energy storage materials derived from Prussian blue analogues. Sci. Bull. 2017, 62, 358–368. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian Blue Analogs for Rechargeable Batteries. Science 2018, 3, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Krap, C.P.; Balmaseda, J.; Zamora, B.; Reguera, E. Hydrogen storage in the iron series of porous Prussian blue analogues. Int. J. Hydrog. Energy 2010, 35, 10381–10386. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. The role of vacancies in the hydrogen storage properties of Prussian blue analogues. Catal. Today 2007, 120, 311–316. [Google Scholar] [CrossRef]
- Kumar, A.; Kanagare, A.B.; Banerjee, S.; Kumar, P.; Kumar, M.; Jagannath; Sudarsan, V. Synthesis of cobalt hexacyanoferrate nanoparticles and its hydrogen storage properties. Int. J. Hydrog. Energy 2018, 43, 7998–8006. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classifications. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Martin, M.; O’Neill, R.D.; Gonzales-Mora, J.L. In vivo biosensor based on Prussian blue for brain chemistry monitoring > Methodological Review and biological applications. In In Vivo Neuropharmacology and Neurophysiology, Neuromethods; Springer Science + Business Media: New York, NY, USA, 2017; Volume 121, pp. 155–179. [Google Scholar] [CrossRef]
- Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Photoinduced Magnetization of a Cobalt-Iron Cyanide. Science 1996, 272, 704–705. [Google Scholar] [CrossRef]
- Bleuzen, A.; Cafun, J.D.; Bachschmidt, A.; Verdaguer, M.; Munsch, P.; Baudelet, F.; Itie, J.P. CoFe Prussian blue analogues under variable pressure. Evidence of departure from cubic symmetry: X-ray diffraction and absorbtion study. J. Phys. Chem. C 2008, 112, 17709–17715. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Bistability and Information Storage. In Proceedings of the Twelfth Annual International Conference of the IEEE in Engineering in Medicine and Biology Society, Philadelphia, PA, USA, 1–4 November 1990; pp. 1683–1685. [Google Scholar] [CrossRef]
- Ksenofontov, V.; Levchenko, G.; Reiman, S.; Gutlich, P.; Bleuzen, A.; Escax, V.; Verdaguer, M. Pressure induced electron transfer in ferromagnetic Prussian blue analogues. Phys. Rev. B 2003, 68, 024415. [Google Scholar] [CrossRef]
- Levchenko, G.G.; Berezhnaya, L.V.; Filimonov, G.G.; Han, W. Charge Transfer, Change the Spin Value and Driving of Magnetic Order by Pressure in Bimetallic Molecular Complexes. J. Phys. Chem. B 2018, 122, 6846–6853. [Google Scholar] [CrossRef]
- Sugimoto, M.; Yamashita, S.; Akutsu, H.; Nakazawa, Y.; DaSilva, J.G.; Kareis, C.M.; Miller, J.S. Increase in the Magnetic Ordering Temperature (TC) as a Function of the Applied Pressure for A2Mn[Mn(CN)6] (A = K, Rb, Cs) Prussian Blue Analogues. Inorg. Chem. 2017, 56, 10452–10457. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gimenez-Lopez, M.C.; Levchenko, G.; Romero, M.F.; Garcia-Baonza, V.; Milner, A.; Paz-Pasternak, M. Pressure tuning of magnetism and linkage isomerism in iron(II) hexacyanochromate. J. Am. Chem. Soc. 2005, 127, 4580–4581. [Google Scholar] [CrossRef] [PubMed]
- Egan, L.; Kamenev, K.; Papanikolau, D.; Takabayashi, Y.; Margadonna, S. Pressure-induced sequential magnetic pole inversion and antiferromagnetic/ferromagnetic crossover in a trimetallic Prussian blue analogue. J. Am. Chem. Soc. 2006, 128, 6034–6035. [Google Scholar] [CrossRef] [PubMed]
- Papnikolau, D.; Kosaka, W.; Margadonna, S.; Kagi, H.; Ohkoshi, S.-I.; Prassides, K. Piezomagnetic behaviour of the spin crossover Prussian blue analogue CsFe[Cr(CN)6]. J. Phys. Chem. C 2007, 111, 8086–8091. [Google Scholar] [CrossRef]
- Levchenko, G.G.; Khristov, A.V.; Varyukhin, V.N. Spin crossover in iron(II)-containing complex compounds under a pressure. Low Temp. Phys. 2014, 40, 571–585. [Google Scholar] [CrossRef]
- Frisch, J.L. Notitia Caerulei Berolinensis Nuper Inventi. Miscellanea Berolinensia ad incrementum Scientiorum 1710, 1, 377–378. [Google Scholar]
- Kraft, A. On the discovery and history of Prussian blue. Bull. Hist. Chem. 2008, 33, 61–67. [Google Scholar]
- Keggin, J.F.; Miles, F.D. Structures and formulae of the Prussian blues and related compounds. Nature 1936, 137, 577–578. [Google Scholar] [CrossRef]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3.xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Herren, F.; Fischer, P.; Ludi, A.; Hälg, W. Neutron diffraction study of Prussian blue Fe4[Fe(CN)6]3.xH2O. Location of water molecules and long/range magnetic order. Inorg. Chem. 1980, 19, 956–959. [Google Scholar] [CrossRef]
- Bozorth, R.M.; Williams, H.J.; Walsh, D.E. Magnetic Properties of Some Orthoferrites and Cyanides at Low Temperatures. Phys. Rev. 1956, 103, 572–578. [Google Scholar] [CrossRef]
- Babel, D. Magnetism and Structure: Model Studies on Transition Metal Fluorides and Cyanides. Comments Inorg. Chem. 1986, 5, 285–320. [Google Scholar] [CrossRef]
- Goodenough, J.B. Theory of the Role of Covalence in the Perovskite-Type Manganites [La, M(II)]MnO3. Phys. Rev. 1955, 100, 564–573. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87. [Google Scholar] [CrossRef]
- Anderson, P.W. New approach to the theory of superexchange interactions. Phys. Rev. 1959, 115. [Google Scholar] [CrossRef]
- Zentková, M.; Arnold, Z.; Kamarád, J.; Kavečanský, V.; Lukáčová, M.; Maťaš, S.; Mihalik, M.; Mitróoová, Z.; Zentko, A. Effect of pressure on magnetic properties of TM3[Cr(CN)6]2·12H2O. J. Phys.: Condens. Matter 2007, 19, 266217. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, M.; Kavečanský, V.; Maťaš, S.; Zentková, M.; Prokhenko, O.; André, G. Magnetic properties and neutron diffraction study of (NixMn1-x)3[ Cr(CN)6]2 molecule-based magnets. J. Phys.: Conf. Ser. 2010, 200, 022035. [Google Scholar] [CrossRef]
- Zentkova, M.; Mihalik, M.; Kovac, J.; Zentko, A.; Mitroova, Z.; Lukacova, M.; Kavecansky, V.; Kiss, L.F. Magnetic properties of TM3[Cr(CN)6]2·nH2O. Phys. Stat. Sol. (b) 2006, 243, 272–276. [Google Scholar] [CrossRef]
- Mitróová, Z.; Maťaš, S.; Mihalik, M.; Zentková, M.; Arnold, Z.; Kamarád, J. Effect of Pressure on Magnetic Properties of Hexacyanochromates. Acta Phys. Polonica A 2008, 113, 469–472. [Google Scholar] [CrossRef]
- Lukacova, M.; Kiss, L.F.; Marysko, M.; Mihalik, M.; Mitroova, Z.; Stopka, P.; Zentko, A.; Zentkova, M. New magnetic phenomena in vanadium hexacyanochromates. Phys. Stat. Sol. (a) Appl. Res. 2003, 196, 240–243. [Google Scholar] [CrossRef]
- Zentková, M.; Mihalik, M.; Arnold, Z.; Kamarád, J. Effect of pressure on magnetic properties of mixed ferro—Ferrimagnet (Ni0.38Mn0.62)3[Cr(CN)6]2.zH2O. J. Phys. Conf. Ser. 2010, 200, 022074. [Google Scholar] [CrossRef]
- Bokor, M.; Tompa, K.; Kiss, L.F.; Zentková, M.; Zentko, A.; Mihalik, M.; Maťaš, S.; Mitróová, Z. 1H NMR on (Nix Mn1−x)3 [Cr(CN)6 ]2·nH2 O. Acta Phys. Polonica A 2008, 113, 485–488. [Google Scholar] [CrossRef]
- Vavra, M.; Antoňák, M.; Jagličic, Z.; Mihalik, M.; Mihalik, M., Jr.; Csach, K.; Zentková, M. Magnetic Properties of (CuxMn1−x)3[Cr(CN)6]2·zH2O Complexes. Acta Phys. Polonica A 2010, 118, 998–999. [Google Scholar] [CrossRef]
- Zentko, A.; Kavečanský, V.; Mihalik, M.; Maťaš, S.; Mitróová, Z.; Zentková, M.; Maryško, M.; Jagličić, Z. Magnetic Relaxation and Memory Effect in Nickel-Chromium Cyanide Nanoparticles. Acta Phys. Polonica A 2008, 113, 511–514. [Google Scholar] [CrossRef]
- Zentko, A.; Zentková, M.; Kavečanský, V.; Mihalik, M.; Mitróová, Z.; Arnold, Z.; Kamarád, J.; Cieslar, M.; Zeleňák, V. Effect of Pressure on Magnetic Properties of TM3[Cr(CN)6]2·nH2O Nanoparticles. Acta Phys. Polonica A 2008, 113, 489–492. [Google Scholar] [CrossRef]
- Vavra, M.; Hrabčák, P.; Zentková, M.; Mihalik, M.; Mihalik, M., Jr.; Csach, K. The Effect of Pressure on Magnetic Properties of KMnCr(CN)6. EPJ Web Conf. 2013, 40, 14001. [Google Scholar] [CrossRef]
- Zentková, M.; Vavra, M.; Mihalik, M.; Mihalik, M., Jr.; Lazurová, J.; Arnold, Z.; Kamarád, J.; Kamenev, K.; Míšek, M. Raman spectroscopy and magnetic properties of KMCr(CN)6 under pressure. High Press. Res. 2015, 35, 22–27. [Google Scholar] [CrossRef]
- Maeda, T.; Mito, M.; Deguchi, H.; Takagi, S.; Kaneko, W.; Ohba, M.; Okawa, H. Pressure effects on a dimetallic ferrimagnet [Mn(en)]3[Cr(CN)6]2·4H2O. Polyhedron 2005, 24, 2497–2500. [Google Scholar] [CrossRef]
- Ohba, M.; Kaneko, W.; Kitagawa, S.; Maeda, T.; Mito, M. Pressure Response of Three-Dimensional Cyanide-Bridged Bimetallic Magnets. J. Am. Chem. Soc. 2008, 130, 4475–4484. [Google Scholar] [CrossRef]
- Giriat, G.; Wang, W.; Attfield, J.P.; Huxley, A.D.; Kamenev, K.V. Turnbuckle diamond anvil cell for high-pressure measurements in a superconducting quantum interference device magnetometer. Rev. Sci. Instrum. 2010, 81, 073905–073910. [Google Scholar] [CrossRef]
- Pajerowski, D.M.; Conklin, S.E.; Leao, J.; Harriger, L.W.; Phelan, D. High-pressure neutron scattering of the magnetoelastic Ni-Cr Prussian blue analog. Phys. Rev. B 2015, 91, 094104–094110. [Google Scholar] [CrossRef]
- Coronado, E.; Gimenez-Lopez, M.; Korzeniak, T.; Levchenko, G.; Romero, F.; Segura, A.; Garcia-Baonza, V.; Cezar, J.; de Groot, F.M.F.; Milner, A.; et al. Pressure-Induced Magnetic Switching and Linkage Isomerism in K0.4Fe4[Cr(CN)6]2.8·16H2O: X-ray Absorption and Magnetic Circular Dichroism Studies. J. Am. Chem. Soc. 2007, 130, 15519–15532. [Google Scholar] [CrossRef] [PubMed]
- Awaga, K.; Sekine, T.; Okawa, M.; Fujita, W.; Holmes, S.M.; Girolami, G.S. High-pressure effects on a manganese hexacyanomanganate ferrimagnet with TN = 29K. Chem. Phys. Lett. 1998, 293, 352–356. [Google Scholar] [CrossRef]
- Kavečanský, V.; Mihalik, M.; Lukáčová, M.; Mitróová, Z.; Maťaš, S. Neutron Diffraction Study of Crystal and Magnetic Structure of Dy[Fe(CN)6].4D2O. J. Phys. Suppl. D 2004, 54, D571–D574. [Google Scholar] [CrossRef]
- Matas, S.; Kavecansky, V.; Lukacova, M.; Mihalik, M.; Mitroova, Z.; Zentkova, M. The symmetry analysis and magnetic model of Dy[Fe(CN)6]·4D2O. J. Alloy. Compd. 2008, 459, 526–530. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Magnetic properties of mixed ferro-ferrimagnets composed of Prussian blue analogs. Phys. Rev. B 1997, 56, 11642–11652. [Google Scholar] [CrossRef]
- Kahn, O. The magnetic turnabout. Nature 1999, 399, 21–23. [Google Scholar] [CrossRef]
- Néel, M.L. Propriétés magnétiques des ferrites; ferrimagnétisme et antiferromagnétisme. Ann. Phys. 1948, 12, 137–198. [Google Scholar] [CrossRef]
- Middlemiss, D.S.; Lawton, L.M.; Wilson, C.C. A solid-state hybrid density functional theory study of Prussian blue analogues and related chlorides at pressure. J. Phys. Condens. Matter 2008, 20, 335231. [Google Scholar] [CrossRef]
- Thomas, L.; Lionti, F.; Ballou, R.; Gatteschi, D.; Sessoli, R.; Barbara, B. Macroscopic quantum tunnelling of magnetization in a single crystal of nanomagnets. Nature 1996, 383, 145–147. [Google Scholar] [CrossRef]
- Lerunberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larionova, J.; Gross, M.; Pilkington, M.; Andres, H.; Stoeckli-Evans, H.; Güdel, H.U.; Decurtins, S. High-Spin Molecules: A Novel Cyano-Bridged MnMo Molecular Cluster with a S = 51/2 Ground State and Ferromagnetic Intercluster Ordering at Low Temperatures. Angew. Chem. Int. Ed 2000, 39, 1605–1609. [Google Scholar] [CrossRef]
- Pileni, M.P. Nanocrystal Self-Assemblies: Fabrication and Collective Properties. J. Phys. Chem. B 2001, 105, 3358–3371. [Google Scholar] [CrossRef]
- Vaucher, S.; Li, M.; Mann, S. Synthesis of Prussian Blue Nanoparticles and Nanocrystal Superlattices in Reverse Microemulsions. Angew. Chem. Int. Ed. 2000, 39, 1793–1796. [Google Scholar] [CrossRef]
- Catala, L.; Gacoin, T.; Boilot, J.-P.; Riviere, E.; Paulsen, C.; Lhotel, E.; Mallah, T. Cyanide-Bridged CrIII–NiII Superparamagnetic Nanoparticles. Adv. Mater. 2003, 15, 826–829. [Google Scholar] [CrossRef]
- Catala, L.; Gloter, A.; Stephan, O.; Rogez, G.; Mallah, T. Superparamagnetic bimetallic cyanide-bridged coordination nanoparticles with TB = 9 K. Chem. Commun. 2006, 1018–1020. [Google Scholar] [CrossRef]
- Peprah, M.K.; Li, C.H.; Talham, D.R.; Meisel, M.W. Effect of pressure on the magnetic properties of LiCuFe and LiCuFe@LiNiCr Prussian blue analogues. Polyhedron 2013, 66, 264–267. [Google Scholar] [CrossRef]
- Knowles, E.S.; Li, C.H.; Dumont, M.F.; Peprah, M.K.; Andrus, M.J.; Talham, D.R.; Meisel, M.W. Photoinduced perturbations of the magnetic superexchange in core@shell Prussian blue analogues. Polyhedron 2013, 66, 153–156. [Google Scholar] [CrossRef]
- Peprah, M.K.; VanGennep, D.; Quintero, P.A.; Risset, O.N.; Brinzari, T.V.; Li, C.H.; Dumont, M.F.; Xia, J.-S.; Hamlin, J.J.; Talham, D.R.; et al. Pressure-tuning of the photomagnetic response of heterostructured CoFe@CrCr-PBA core@shell nanoparticles. Polyhedron 2017, 123, 323–327. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zentkova, M.; Mihalik, M. The Effect of Pressure on Magnetic Properties of Prussian Blue Analogues. Crystals 2019, 9, 112. https://doi.org/10.3390/cryst9020112
Zentkova M, Mihalik M. The Effect of Pressure on Magnetic Properties of Prussian Blue Analogues. Crystals. 2019; 9(2):112. https://doi.org/10.3390/cryst9020112
Chicago/Turabian StyleZentkova, Maria, and Marian Mihalik. 2019. "The Effect of Pressure on Magnetic Properties of Prussian Blue Analogues" Crystals 9, no. 2: 112. https://doi.org/10.3390/cryst9020112
APA StyleZentkova, M., & Mihalik, M. (2019). The Effect of Pressure on Magnetic Properties of Prussian Blue Analogues. Crystals, 9(2), 112. https://doi.org/10.3390/cryst9020112



