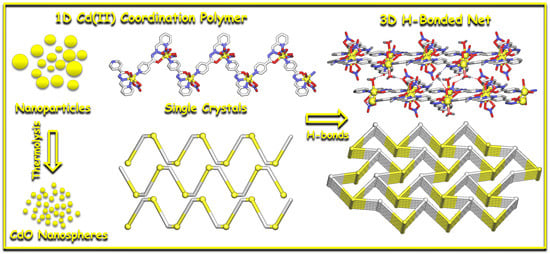
Sonochemical Synthesis of Cadmium(II) Coordination Polymer Nanospheres as Precursor for Cadmium Oxide Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Measurements
2.2. Structure Determination
2.3. Topological Analysis
2.4. Synthesis of {[Cd2(µ-HL)(µ-L)(NO3)3(H2O)]·H2O}n (1)
2.5. Synthesis of {[Cd2(µ-HL)(µ-L)(NO3)3(H2O)]·H2O}n (1)
2.6. Preparation of CdO Nanoparticles
3. Results and Discussion
3.1. Synthesis of 1
3.2. Structural and Topological Description of 1
3.3. FT-IR and TGA
3.4. Characterization of Nanoparticles of 1 and CdO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Visakh, P.M.; Morlanes, M.J.M. (Eds.) Nanomaterials and Nanocomposites: Zero- to Three-Dimensional Materials and Their Composites; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz-Mendez, U. Handbook of Less-Common Nanostructures; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Fan, Z.; Zhang, H. Crystal phase-controlled synthesis, properties and applications of noble metal nanomaterials. Chem. Soc. Rev. 2016, 45, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Shen, X.-F.; Yan, X.P. Facile shape-controlled synthesis of well-aligned nanowire architectures in binary aqueous solution. Angew. Chem. Int. Ed. 2007, 46, 7659–7663. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-S.; Hsu, S.-C.; Ke, W.-H.; Chen, L.-J.; Huang, M.H. Facet-Dependent Electrical Conductivity Properties of Cu2O Crystals. Nano Lett. 2015, 15, 2155–2160. [Google Scholar] [CrossRef]
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131. [Google Scholar] [CrossRef]
- Peng, W.; Qu, S.; Cong, G.; Wang, Z. Synthesis and Structures of Morphology-Controlled ZnO Nano- and Microcrystals. Cryst. Growth Des. 2006, 6, 1518–1522. [Google Scholar] [CrossRef]
- Han, W.; Yi, L.; Zhao, N.; Tang, A.; Gao, M.; Tang, Z. Synthesis and Shape-Tailoring of Copper Sulfide/Indium Sulfide-Based Nanocrystals. J. Am. Chem. Soc. 2008, 130, 13152–13161. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yang, P.; Lian, H.; Lin, J. Hydrothermal Synthesis of Lanthanide Fluorides LnF3 (Ln = La to Lu) Nano-/Microcrystals with Multiform Structures and Morphologies. Chem. Mater. 2008, 20, 4317–4326. [Google Scholar] [CrossRef]
- Mehta, J.P.; Tian, T.; Zeng, Z.; Divitini, G.; Connolly, B.M.; Midgley, P.A.; Tan, J.-C.; Fairen-Jimenez, D.A.; Wheatley, E.H. Sol–Gel Synthesis of Robust Metal–Organic Frameworks for Nanoparticle Encapsulation. Adv. Funct. Mater. 2018, 28, 1705588. [Google Scholar] [CrossRef]
- Blin, J.-L.; Stébé, M.-J.; Lebeau, B. Hybrid/porous materials obtained from nano-emulsions. Curr. Opin. Colloid. Interfaces Sci. 2016, 25, 75–82. [Google Scholar] [CrossRef]
- Chhatre, A.; Duttagupta, S.; Thaokar, R.; Mehra, A. Mechanism of Nanorod Formation by Wormlike Micelle-Assisted Assembly of Nanospheres. Langmuir 2015, 31, 10524–10531. [Google Scholar] [CrossRef]
- Moulik, S.P.; De, G.C.; Panda, A.K.; Bhowmik, B.B.; Das, A.R. Dispersed Molecular Aggregates. 1. Synthesis and Characterization of Nanoparticles of Cu2[Fe(CN)6] in H2O/AOT/n-Heptane Water-in-Oil Microemulsion Media. Langmuir 1999, 15, 8361–8367. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Ameta, S.C.; Ameta, R.; Ameta, G. (Eds.) Sonochemistry: An Emerging Green Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Colmenares, J.C.; Chatel, G. (Eds.) Sonochemistry: From Basic Principles to Innovative Applications; Springer: Berlin, Germany, 2017. [Google Scholar]
- Abdolalian, P.; Morsali, A.; Bruno, G. Sonochemical synthesis and characterization of microrod to nanoparticle of new mixed-ligand zinc(II) fumarate metal-organic polymer. Ultrason. Sonochem. 2017, 37, 654–659. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Montazerozohori, M.; Masoudiasl, A.; Mahmoudi, G.; White, J.M. Sonication-assisted synthesis of a new cationic zinc nitrate complex with a tetradentate Schiff base ligand: Crystal structure, Hirshfeld surface analysis and investigation of different parameters influence on morphological properties. Ultrason. Sonochem. 2018, 46, 26–35. [Google Scholar] [CrossRef]
- Morsali, A.; Monfared, H.H.; Janiak, C. Ultrasonic irradiation assisted syntheses of one-dimensional di(azido)-dipyridylamine Cu(II) coordination polymer nanoparticles. Ultrason. Sonochem. 2015, 23, 208–211. [Google Scholar] [CrossRef]
- Derakhshandeh, P.G.; Soleimannejad, J.; Janczak, J. Sonochemical synthesis of a new nano-sized cerium(III) coordination polymer and its conversion to nanoceria. Ultrason. Sonochem. 2015, 26, 273–280. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal–organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Fillion, H.; Luche, J.L. Synthetic Organic Sonochemistry; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- MacGillivray, L.R. (Ed.) Metal-Organic Frameworks: Design and Application; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sander, J.R.G.; Bucar, D.K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Pharmaceutical Nano-Cocrystals: Sonochemical Synthesis by Solvent Selection and Use of a Surfactant. Angew. Chem., Int. Ed. 2010, 49, 7284–7288. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.W.; Tejedor, M.I.; Nelson, B.P.; Anderson, M.A. Mesoporous Metal Oxide Semiconductor-Clad Waveguides. J. Phys. Chem. B 1999, 103, 8490–8492. [Google Scholar] [CrossRef]
- Fernández, M.; Martínez, A.; Hanson, J.C.; Rodriguez, A.J. Nanostructured Oxides in Chemistry: Characterization and Properties. Chem. Rev. 2004, 104, 4063–4104. [Google Scholar] [CrossRef]
- Jefferson, P.H.; Hatfield, S.A.; Veal, T.D.; King, P.D.C.; McConville, C.F. Bandgap and effective mass of epitaxial cadmium oxide. Appl. Phys. Lett. 2008, 92, 022101. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.A.; O’Brien, P. Organometallic and Metallo-Organic Precursors for Nanoparticles Precursor Chemistry of Advanced Materials; Fischer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; p. 173. [Google Scholar]
- Leong, W.L.; Vittal, J.J. One-Dimensional Coordination Polymers: Complexity and Diversity in Structures, Properties, and Applications. Chem. Rev. 2010, 111, 688–764. [Google Scholar] [CrossRef]
- Vittal, J.J.; Ng, M.T. Chemistry of Metal Thio- and Selenocarboxylates: Precursors for Metal Sulfide/Selenide Materials, Thin Films, and Nanocrystals. Acc. Chem. Res. 2006, 39, 869–877. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Applications of metal–organic coordination polymers as precursors for preparation of nano-materials. Coord. Chem. Rev. 2012, 256, 2921–2943. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Stilinović, V.; Bauzá, A.; Frontera, A.; Bartyzel, A.; Ruiz-Pérez, C.; Kirillov, A.M. Inorganic–organic hybrid materials based on PbBr2 and pyridine–hydrazone blocks – structural and theoretical study. RSC Adv. 2016, 6, 60385–60393. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Khandar, A.A.; White, J.; Mitoraj, M.P.; Jena, H.S.; Van Der Voort, P.; Qureshi, N.; Kirillov, A.M.; Robeynsi, K.; Safin, D.A. Polar protic solvent-trapping polymorphism of the HgII-hydrazone coordination polymer: experimental and theoretical findings. CrystEngComm 2017, 19, 3017–3025. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, J.; Wu, D.; Xu, J.; Xue, B.; Kirillov, A.M. A Highly Stable Nanotubular MOF Rotator for Selective Adsorption of Benzene and Separation of Xylene Isomers. Inorg. Chem. 2015, 54, 10524–10526. [Google Scholar] [CrossRef]
- Iqbal, K.; Iqbal, A.; Kirillov, A.M.; Liu, W.; Tang, Y. Hybrid Metal–Organic-Framework/Inorganic Nanocatalyst toward Highly Efficient Discoloration of Organic Dyes in Aqueous Medium. Inorg. Chem. 2018, 57, 13270–13278. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Cai, Y.; Wen, M.; Shi, Z.-F.; Kirillov, A.M. A New Series of Cd(II) Metal–Organic Architectures Driven by Soft Ether-Bridged Tricarboxylate Spacers: Synthesis, Structural and Topological Versatility, and Photocatalytic Properties. Dalton Trans. 2018, 47, 14327–14339. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Cui, Y.-H.; Liang, X.-X.; Wu, J.; Lv, D.; Kirillov, A.M. Structurally Distinct Metal-Organic and H-bonded Networks Derived from 5-(6-Carboxypyridin-3-yl)isophthalic Acid: Coordination and Template Effect of 4,4′-Bipyridine. Cryst. Growth Des. 2016, 16, 4658–4670. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Kłak, J.; Kirillova, M.V.; Kirillov, A.M. Copper(II) Coordination Polymers Self-assembled from Aminoalcohols and Pyromellitic Acid: Highly Active Pre-catalysts for the Mild Water-promoted Oxidation of Alkanes. Inorg. Chem. 2016, 55, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-Z.; Wen, M.; Liang, X.; Shi, Z.-F.; Kirillova, M.V.; Kirillov, A.M. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ.; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2016.
- Bruker Saint Plus; Saint Plus 8.34A; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Bruker SADABS, TWINABS, SADABS 2012/1; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B.J. ShelXle: A Qt graphical user interface for SHELXL. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr Comp. Comm. Newslett. 2006, 7, 4–38. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Richardson, D.R.; Becker, E.; Bernhardt, P.V. The biologically active iron chelators 2-pyridylcarboxaldehyde isonicotinoylhydrazone, 2-pyridylcarboxaldehyde benzoylhydrazone monohydrate and 2-furaldehyde isonicotinoylhydrazone. Acta Crystallogr. 1999, C55, 2102–2105. [Google Scholar] [CrossRef]
- Yumnam, S.; Rajkumari, L. Thermodynamics of the Complexation of N-(Pyridin-2-ylmethylene) Isonicotinohydrazide with Lighter Lanthanides. J. Chem. Eng. Data 2009, 54, 28–34. [Google Scholar] [CrossRef]
- Khandar, A.A.; Afkhami, F.A.; Yazdi, S.A.H.; White, J.M.; Kassel, S.; Dougherty, W.G.; Lipkowski, J.; Van Derveer, D.; Giester, G.; Costantino, F. Anion influence in the structural diversity of cadmium coordination polymers constructed from a pyridine based Schiff base ligand. Inorg. Chim. Acta 2015, 427, 87–96. [Google Scholar] [CrossRef]
- Khandar, A.A.; Ghosh, B.K.; Lampropoulos, C.; Gargari, M.S.; Yilmaz, V.T.; Bhar, K.; Yazdi, S.A.H.; Cain, J.M.; Mahmoudi, G. Coordination complexes and polymers from the initial application of phenyl-2-pyridyl ketone azine in mercury chemistry. Polyhedron 2015, 85, 467–475. [Google Scholar] [CrossRef]
- Huang, J.-H.; Hou, G.-F.; Ma, D.-S.; Yu, Y.-H.; Jiang, W.-H.; Huang, Q.; Gao, J.-S. Two pairs of Zn(II) coordination polymer enantiomers based on chiral aromatic polycarboxylate ligands: synthesis, crystal structures and properties. RSC Adv. 2017, 7, 18650–18657. [Google Scholar] [CrossRef]
- An, Y.-Y.; Lu, L.-P.; Zhu, M.-L. A three-dimensional twofold inter penetrated cobalt(II) MOF containing a flexible carboxylate-based ligand: synthesis, structure and magnetic properties. Acta Cryst. 2018, C74, 418–423. [Google Scholar]









| Formula | C24H23Cd2N11O13 |
|---|---|
| Fw | 898.33 |
| Crystal system | Monoclinic |
| Space group | P21 |
| a (Å) | 7.8082(6) |
| b (Å) | 14.9024(9) |
| c (Å) | 13.4584(10) |
| α (o) | 90.00 |
| β (o) | 98.903(2) |
| γ (o) | 90.00 |
| V (Å3) | 1547.16(19) |
| Temp (K) | 100(2) |
| Z | 2 |
| Dc(g.cm−3) | 1.928 |
| µ(mm−1) | 1.459 |
| Index ranges | −9 < h < 9 |
| −19 < h < 19 | |
| −17 < h < 17 | |
| F(000) | 888 |
| Rint | 0.0290 |
| R1 (I > 2σ(I)) | 0.0388 |
| wR (all data) | 0.0708 |
| GOF | 0.985 |
| Bond Lengths (Å) | Bond Angles (°) | ||
|---|---|---|---|
| Cd1−O3 | 2.528(4) | O4−Cd1−O3 | 52.24(13) |
| Cd1−O4 | 2.381(4) | O7−Cd1−O6 | 52.61(11) |
| Cd1−O6 | 2.526(3) | N3−Cd1−N4 | 67.46(12) |
| Cd1−O7 | 2.351(4) | N3−Cd1−O1 | 64.53(10) |
| Cd1−O1 | 2.516(3) | N5i−Cd1−N4 | 143.21(13) |
| Cd1−N3 | 2.373(3) | N5i−Cd1−N3 | 145.68(12) |
| Cd1−N4 | 2.420(4) | N5i−Cd1−O1 | 84.39(12) |
| Cd1−N5i | 2.317(4) | O10−Cd2−O9 | 51.38(11) |
| Cd2−O9 | 2.585(3) | N7−Cd2−O2 | 67.33(11) |
| Cd2−O10 | 2.384(4) | N7−Cd2−N8 | 68.76(12) |
| Cd2−O2 | 2.360(3) | N1−Cd2−O12 | 84.23(13) |
| Cd2−O12 | 2.341(3) | N1−Cd2−N8 | 140.20(13) |
| Cd2−N1 | 2.341(4) | N1−Cd2−O2 | 83.81(13) |
| Cd2−N7 | 2.308(3) | N1−Cd2−N7 | 148.30(12) |
| Cd2−N8 | 2.467(4) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afkhami, F.A.; Khandar, A.A.; Mahmoudi, G.; Abdollahi, R.; Gurbanov, A.V.; Kirillov, A.M. Sonochemical Synthesis of Cadmium(II) Coordination Polymer Nanospheres as Precursor for Cadmium Oxide Nanoparticles. Crystals 2019, 9, 199. https://doi.org/10.3390/cryst9040199
Afkhami FA, Khandar AA, Mahmoudi G, Abdollahi R, Gurbanov AV, Kirillov AM. Sonochemical Synthesis of Cadmium(II) Coordination Polymer Nanospheres as Precursor for Cadmium Oxide Nanoparticles. Crystals. 2019; 9(4):199. https://doi.org/10.3390/cryst9040199
Chicago/Turabian StyleAfkhami, Farhad Akbari, Ali Akbar Khandar, Ghodrat Mahmoudi, Reza Abdollahi, Atash V. Gurbanov, and Alexander M. Kirillov. 2019. "Sonochemical Synthesis of Cadmium(II) Coordination Polymer Nanospheres as Precursor for Cadmium Oxide Nanoparticles" Crystals 9, no. 4: 199. https://doi.org/10.3390/cryst9040199
APA StyleAfkhami, F. A., Khandar, A. A., Mahmoudi, G., Abdollahi, R., Gurbanov, A. V., & Kirillov, A. M. (2019). Sonochemical Synthesis of Cadmium(II) Coordination Polymer Nanospheres as Precursor for Cadmium Oxide Nanoparticles. Crystals, 9(4), 199. https://doi.org/10.3390/cryst9040199