Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals
Abstract
1. Introduction
2. Zona Pellucida Glycoproteins
2.1. ZP Glycoproteins in the Mouse Model
2.2. ZP Glycoproteins in the Humans
2.3. ZP Glycoproteins in the Pig Model
2.4. ZP Glycoproteins in the Bovine Model
3. Carbohydrate Structure and Glycosylation of ZP Glycoproteins
3.1. Glycosylation in the Mouse Model
3.2. Glycosylation in the Humans
3.3. Glycosylation in the Pig Model
3.4. Glycosylation in the Bovine Model
4. Sperm-Zona Pellucida Interaction Ligands
4.1. ZP Ligands for Sperm Binding in the Mouse Model
4.2. ZP Ligands for Sperm Binding in the Human
4.3. ZP Ligands for Sperm Binding in the Pig Model
4.4. ZP Ligands for Sperm Binding in the Bovine model
5. Sperm Surface Receptors with ZP-Binding Affinity
5.1. Evolutionarily Conserved Mammalian Sperm-ZP Receptors and Other ZP-Binding Proteins
5.1.1. Galactosyltransferase (B4GALT1/GalTase)
5.1.2. Proacrosin/Acrosin (ACR)
5.1.3. Zonadhesin (ZAN)
5.1.4. Arylsulphatase A (ARSA/AS-A)
5.1.5. MFGE8/SED1/p47/Lactadherin
5.1.6. ZP3R (Syn. sp56/AM67)
5.1.7. ZPB1/sp38/IAM38
5.1.8. SPACA2/SP-10/ACV1
5.2. Mouse and Human Sperm-ZP Binding Receptors
5.2.1. α-1-3-Fucosyltransferase (FUT5)
5.2.2. α-D-Mannosidase (MAN2)
5.2.3. Cysteine-Rich Secretory Protein (CRISP1)
5.2.4. Zona Receptor Kinase (ZRK)
5.2.5. Fertilization Antigen-1 (FA-1)
5.2.6. Angiotensin-Converting Enzyme 1 (ACE1)
5.2.7. P34H/Carbonyl Reductase/DCXR
5.2.8. Other Human Sperm-ZP Binding Proteins
5.3. Candidate Boar Sperm-ZP Receptors
5.3.1. Spermadhesins
5.3.2. DQH/BSP1/pB1
5.3.3. Other Boar Sperm-ZP Binding Proteins
5.4. Candidate Bull Sperm-ZP Receptors
6. Lipid Microdomains and Multiprotein Complexes Implicated in Sperm-ZP Interaction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewis, I.A.; Gadella, B.M. Sperm surface proteomics: From protein lists to biological function. Mol. Hum. Reprod. 2010, 16, 68–79. [Google Scholar] [CrossRef]
- Byrne, K.; Leahy, T.; McCulloch, R.; Colgrave, M.L.; Holland, M.K. Comprehensive mapping of the bull sperm surface proteome. Proteomics 2012, 12, 3559–3579. [Google Scholar] [CrossRef]
- Kasvandik, S.; Sillaste, G.; Velthut-Meikas, A.; Mikelsaar, A.V.; Hallap, T.; Padrik, P.; Tenson, T.; Jaakma, U.; Koks, S.; Salumets, A. Bovine sperm plasma membrane proteomics through biotinylation and subcellular enrichment. Proteomics 2015, 15, 1906–1920. [Google Scholar] [CrossRef]
- Rickard, J.P.; de Graaf, S.P. Sperm surface changes and their consequences for sperm transit through the female reproductive tract. Theriogenology 2020. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; Volume 1, pp. 189–317. [Google Scholar]
- Gadella, B.M.; Boerke, A. An update on post-ejaculatory remodeling of the sperm surface before mammalian fertilization. Theriogenology 2016, 85, 113–124. [Google Scholar] [CrossRef]
- Clark, G.F. A role for carbohydrate recognition in mammalian sperm-egg binding. Biochem. Biophys. Res. Commun. 2014, 450, 1195–1203. [Google Scholar] [CrossRef]
- Wassarman, P.M. Contribution of mouse egg zona pellucida glycoproteins to gamete recognition during fertilization. J. Cell. Physiol. 2005, 204, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.; Caille, A.M.; Gallardo-Rios, M.; Munuce, M.J. D-Mannose-binding sites are putative sperm determinants of human oocyte recognition and fertilization. Reprod. Biomed. Online 2007, 15, 182–190. [Google Scholar] [CrossRef]
- Sinowatz, F.; Wessa, E.; Neumüller, C.; Palma, G. On the species specificity of sperm binding and sperm penetration of the zona pellucida. Reprod. Domest. Anim. 2003, 38, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Topfer-Petersen, E.; Ekhlasi-Hundrieser, M.; Tsolova, M. Glycobiology of fertilization in the pig. Int. J. Dev. Biol. 2008, 52, 717–736. [Google Scholar] [CrossRef]
- Takahashi, K.; Kikuchi, K.; Uchida, Y.; Kanai-Kitayama, S.; Suzuki, R.; Sato, R.; Toma, K.; Geshi, M.; Akagi, S.; Nakano, M.; et al. Binding of Sperm to the Zona Pellucida Mediated by Sperm Carbohydrate-Binding Proteins is not Species-Specific in Vitro between Pigs and Cattle. Biomolecules 2013, 3, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M.; Litscher, E.S. The mouse egg’s zona pellucida. In Current Topics in Developmental Biology; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 130, pp. 331–356. [Google Scholar]
- Evans, J.P. Preventing polyspermy in mammalian eggs-Contributions of the membrane block and other mechanisms. Mol. Reprod. Dev. 2020, 87, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkamp, E.; Algarra, B.; Jovine, L. Mammalian egg coat modifications and the block to polyspermy. Mol. Reprod. Dev. 2020, 87, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Hibler, D.W.; Fontenot, G.K.; Hsu, K.T.; Yurewicz, E.C.; Sacco, A.G. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: The ZPA, ZPB and ZPC gene families. DNA Seq. 1994, 4, 361–393. [Google Scholar] [CrossRef]
- Spargo, S.C.; Hope, R.M. Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod. 2003, 68, 358–362. [Google Scholar] [CrossRef]
- Goudet, G.; Mugnier, S.; Callebaut, I.; Monget, P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biol. Reprod. 2008, 78, 796–806. [Google Scholar] [CrossRef]
- Smith, J.; Paton, I.R.; Hughes, D.C.; Burt, D.W. Isolation and mapping the chicken zona pellucida genes: An insight into the evolution of orthologous genes in different species. Mol. Reprod. Dev. 2005, 70, 133–145. [Google Scholar] [CrossRef]
- Gupta, S.K. The human egg’s zona pellucida. In Current Topics in Developmental Biology; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 130, pp. 379–411. [Google Scholar]
- Bokhove, M.; Jovine, L. Structure of Zona Pellucida Module Proteins. Curr. Top. Dev. Biol. 2018, 130, 413–442. [Google Scholar] [CrossRef]
- Conner, S.J.; Lefievre, L.; Hughes, D.C.; Barratt, C.L. Cracking the egg: Increased complexity in the zona pellucida. Hum. Reprod. 2005, 20, 1148–1152. [Google Scholar] [CrossRef]
- Greve, J.M.; Wassarman, P.M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J. Mol. Biol. 1985, 181, 253–264. [Google Scholar] [CrossRef]
- Hughes, D.C.; Barratt, C.L. Identification of the true human orthologue of the mouse Zp1 gene: Evidence for greater complexity in the mammalian zona pellucida? Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1999, 1447, 303–306. [Google Scholar] [CrossRef]
- Wassarman, P.M. Zona pellucida glycoproteins. Annu. Rev. Biochem. 1988, 57, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Wong, B.S.; Lee, C.L.; Pang, R.T.; Lee, K.F.; Sumitro, S.B.; Gupta, S.K.; Yeung, W.S. Native human zona pellucida glycoproteins: Purification and binding properties. Hum. Reprod. 2008, 23, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Bhandari, B.; Shrestha, A.; Biswal, B.K.; Palaniappan, C.; Malhotra, S.S.; Gupta, N. Mammalian zona pellucida glycoproteins: Structure and function during fertilization. Cell Tissue Res. 2012, 349, 665–678. [Google Scholar] [CrossRef]
- Lefièvre, L.; Conner, S.J.; Salpekar, A.; Olufowobi, O.; Ashton, P.; Pavlovic, B.; Lenton, W.; Afnan, M.; Brewis, I.A.; Monk, M.; et al. Four zona pellucida glycoproteins are expressed in the human. Hum. Reprod. 2004, 19, 1580–1586. [Google Scholar] [CrossRef]
- Nishimura, K.; Dioguardi, E.; Nishio, S.; Villa, A.; Han, L.; Matsuda, T.; Jovine, L. Molecular basis of egg coat cross-linking sheds light on ZP1-associated female infertility. Nat. Commun. 2019, 10, 3086. [Google Scholar] [CrossRef]
- Hasegawa, A.; Koyama, K.; Okazaki, Y.; Sugimoto, M.; Isojima, S. Amino acid sequence of a porcine zona pellucida glycoprotein ZP4 determined by peptide mapping and cDNA cloning. J. Reprod. Fertil. 1994, 100, 245–255. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Wardrip, N.J. Isolation of the zona pellucida and purification of its glycoprotein families from pig oocytes. Anal. Biochem. 1986, 157, 63–70. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Wardrip, N.J. On the macromolecular composition of the zona pellucida from porcine oocytes. Dev. Biol. 1987, 121, 478–488. [Google Scholar] [CrossRef]
- Nakano, M.; Hatanaka, Y.; Sawai, T.; Kobayashi, N.; Tobita, T. Fractionation of glycoproteins from porcine zonae pellucidae into three families by high-performance liquid chromatography. Biochem. Int. 1987, 14, 417–423. [Google Scholar]
- Nakano, M.; Yonezawa, N.; Hatanaka, Y.; Noguchi, S. Structure and function of the N-linked carbohydrate chains of pig zona pellucida glycoproteins. J. Reprod. Fertil. Suppl. 1996, 50, 25–34. [Google Scholar] [PubMed]
- Topfer-Petersen, E.; Mann, K.; Calvete, J.J. Identification of porcine oocyte 55 kDa alpha and beta proteins within the zona pellucida glycoprotein families indicates that oocyte sperm receptor activity is associated with different zone pellucida proteins in different mammalian species. Biol. Chem. 1993, 374, 411–417. [Google Scholar]
- Wardrip, N.J.; Hedrick, J.L. Pig zona pellucida 25K and 65K glycoproteins are derived from Hydrolysis and reduction of the 90K family. J. Cell Biol. 1985, 101, 378a. [Google Scholar]
- Yurewicz, E.C.; Pack, B.A.; Sacco, A.G. Isolation, composition, and biological activity of sugar chains of porcine oocyte zona pellucida 55K glycoproteins. Mol. Reprod. Dev. 1991, 30, 126–134. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Sacco, A.G.; Subramanian, M.G. Structural characterization of the Mr = 55,000 antigen (ZP3) of porcine oocyte zona pellucida. Purification and characterization of alpha- and beta-glycoproteins following digestion of lactosaminoglycan with endo-beta-galactosidase. J. Biol. Chem. 1987, 262, 564–571. [Google Scholar] [CrossRef]
- Noguchi, S.; Yonezawa, N.; Katsumata, T.; Hashizume, K.; Kuwayama, M.; Hamano, S.; Watanabe, S.; Nakano, M. Characterization of the zona pellucida glycoproteins from bovine ovarian and fertilized eggs. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1994, 1201, 7–14. [Google Scholar] [CrossRef]
- Yonezawa, N.; Fukui, N.; Kuno, M.; Shinoda, M.; Goko, S.; Mitsui, S.; Nakano, M. Molecular cloning of bovine zona pellucida glycoproteins ZPA and ZPB and analysis for sperm-binding component of the zona. Eur. J. Biochem. 2001, 268, 3587–3594. [Google Scholar] [CrossRef]
- Yonezawa, N.; Kanai, S.; Nakano, M. Structural significance of N-glycans of the zona pellucida on species-selective recognition of spermatozoa between pig and cattle. Soc. Reprod. Fertil. Suppl. 2007, 63, 217–228. [Google Scholar]
- Abou-Haila, A.; Bendahmane, M.; Tulsiani, D.R. Significance of egg’s zona pellucida glycoproteins in sperm-egg interaction and fertilization. Minerva Ginecol. 2014, 66, 409–419. [Google Scholar]
- Yonezawa, N. Posttranslational modifications of zona pellucida proteins. Adv. Exp. Med. Biol. 2014, 759, 111–140. [Google Scholar] [CrossRef]
- Yonezawa, N. Involvement of Carbohydrate Residues of the Zona Pellucida in In Vitro Sperm Recognition in Pigs and Cattle. In Sexual Reproduction in Animals and Plants; Sawada, H., Inoue, N., Iwano, M., Eds.; Springer: Tokyo, Japan, 2014; pp. 409–415. [Google Scholar]
- Topfer-Petersen, E. Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum. Reprod. Update 1999, 5, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M.; Litscher, E.S. Towards the molecular basis of sperm and egg interaction during mammalian fertilization. Cells Tissues Organs 2001, 168, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hoodbhoy, T.; Dean, J. Insights into the molecular basis of sperm-egg recognition in mammals. Reproduction 2004, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Shalgi, R.; Maymon, R.; Bar-Shira, B.; Amihai, D.; Skutelsky, E. Distribution of lectin receptors sites in the zona pellucida of follicular and ovulated rat oocytes. Mol. Reprod. Dev. 1991, 29, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Maymon, B.B.; Maymon, R.; Ben-Nun, I.; Ghetler, Y.; Shalgi, R.; Skutelsky, E. Distribution of carbohydrates in the zona pellucida of human oocytes. J. Reprod. Fertil. 1994, 102, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Parillo, F.; Stradaioli, G.; Dall’Aglio, C.; Verini-Supplizi, A. Characterization of the complex carbohydrates in the zona pellucida of mammalian oocytes using lectin histochemistry. Vet. Res. Commun. 1996, 20, 225–236. [Google Scholar] [CrossRef]
- Katsumata, T.; Noguchi, S.; Yonezawa, N.; Tanokura, M.; Nakano, M. Structural characterization of the N-linked carbohydrate chains of the zona pellucida glycoproteins from bovine ovarian and fertilized eggs. Eur. J. Biochem. 1996, 240, 448–453. [Google Scholar] [CrossRef]
- Lucas, H.; Bercegeay, S.; Le Pendu, J.; Jean, M.; Mirallie, S.; Barriere, P. A fucose-containing epitope potentially involved in gamete interaction on the human zona pellucida. Hum. Reprod. 1994, 9, 1532–1538. [Google Scholar] [CrossRef]
- Noguchi, S.; Hatanaka, Y.; Tobita, T.; Nakano, M. Structural analysis of the N-linked carbohydrate chains of the 55-kDa glycoprotein family (PZP3) from porcine zona pellucida. Eur. J. Biochem. 1992, 204, 1089–1100. [Google Scholar] [CrossRef]
- Noguchi, S.; Nakano, M. Structure of the acidic N-linked carbohydrate chains of the 55-kDa glycoprotein family (PZP3) from porcine zona pellucida. Eur. J. Biochem. 1992, 209, 883–894. [Google Scholar] [CrossRef]
- Mori, E.; Hedrick, J.L.; Wardrip, N.J.; Mori, T.; Takasaki, S. Occurrence of reducing terminal N-acetylglucosamine 3-sulfate and fucosylated outer chains in acidic N-glycans of porcine zona pellucida glycoproteins. Glycoconj. J. 1998, 15, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Töpfer-Petersen, E.; Petrounkina, A.M.; Ekhlasi-Hundrieser, M. Oocyte-sperm interactions. Anim. Reprod. Sci. 2000, 60–61. [Google Scholar] [CrossRef]
- Kudo, K.; Yonezawa, N.; Katsumata, T.; Aoki, H.; Nakano, M. Localization of carbohydrate chains of pig sperm ligand in the glycoprotein ZPB of egg zona pellucida. Eur. J. Biochem. 1998, 252, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Easton, R.L.; Patankar, M.S.; Lattanzio, F.A.; Leaven, T.H.; Morris, H.R.; Clark, G.F.; Dell, A. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J. Biol. Chem. 2000, 275, 7731–7742. [Google Scholar] [CrossRef]
- Noguchi, S.; Nakano, M. Structural characterization of the N-linked carbohydrate chains from mouse zona pellucida glycoproteins ZP2 and ZP3. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1993, 1158, 217–226. [Google Scholar] [CrossRef]
- Dell, A.; Chalabi, S.; Easton, R.L.; Haslam, S.M.; Sutton-Smith, M.; Patankar, M.S.; Lattanzio, F.; Panico, M.; Morris, H.R.; Clark, G.F. Murine and human zona pellucida 3 derived from mouse eggs express identical O-glycans. Proc. Natl. Acad. Sci. USA 2003, 100, 15631–15636. [Google Scholar] [CrossRef]
- Boja, E.S.; Hoodbhoy, T.; Fales, H.M.; Dean, J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 2003, 278, 34189–34202. [Google Scholar] [CrossRef]
- Jimenez-Movilla, M.; Aviles, M.; Gomez-Torres, M.J.; Fernandez-Colom, P.J.; Castells, M.T.; de Juan, J.; Romeu, A.; Ballesta, J. Carbohydrate analysis of the zona pellucida and cortical granules of human oocytes by means of ultrastructural cytochemistry. Hum. Reprod. 2004, 19, 1842–1855. [Google Scholar] [CrossRef]
- Pang, P.C.; Chiu, P.C.; Lee, C.L.; Chang, L.Y.; Panico, M.; Morris, H.R.; Haslam, S.M.; Khoo, K.H.; Clark, G.F.; Yeung, W.S.; et al. Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science 2011, 333, 1761–1764. [Google Scholar] [CrossRef]
- Yonezawa, N.; Mitsui, S.; Kudo, K.; Nakano, M. Identification of an N-glycosylated region of pig zona pellucida glycoprotein ZPB that is involved in sperm binding. Eur. J. Biochem. 1997, 248, 86–92. [Google Scholar] [CrossRef]
- Nakano, M.; Yonezawa, N. Localization of sperm ligand carbohydrate chains in pig zona pellucida glycoproteins. Cells Tissues Organs 2001, 168, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Von Witzendorff, D.; Maass, K.; Pich, A.; Ebeling, S.; Kölle, S.; Kochel, C.; Ekhlasi-Hundrieser, M.; Geyer, H.; Geyer, R.; Töpfer-Petersen, E. Characterization of the acidic N-linked glycans of the zona pellucida of prepuberal pigs by a mass spectrometric approach. Carbohydr. Res. 2009, 344, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Hokke, C.H.; Damm, J.B.; Penninkhof, B.; Aitken, R.J.; Kamerling, J.P.; Vliegenthart, J.F. Structure of the O-linked carbohydrate chains of porcine zona pellucida glycoproteins. Eur. J. Biochem. 1994, 221, 491–512. [Google Scholar] [CrossRef] [PubMed]
- Lay, K.M.; Nakada, T.; Tatemoto, H. Involvement of N-glycosylation of zona glycoproteins during meiotic maturation in sperm-zona pellucida interactions of porcine denuded oocytes. Anim. Sci. J. 2013, 84, 8–14. [Google Scholar] [CrossRef]
- Clark, G.F.; Zimmerman, S.; Lafrenz, D.E.; Yi, Y.J.; Sutovsky, P. Carbohydrate-mediated binding and induction of acrosomal exocytosis in a boar sperm-somatic cell adhesion model. Biol. Reprod. 2010, 83, 623–634. [Google Scholar] [CrossRef]
- Suzuki, K.; Tatebe, N.; Kojima, S.; Hamano, A.; Orita, M.; Yonezawa, N. The Hinge Region of Bovine Zona Pellucida Glycoprotein ZP3 Is Involved in the Formation of the Sperm-Binding Active ZP3/ZP4 Complex. Biomolecules 2015, 5, 3339–3353. [Google Scholar] [CrossRef]
- Ikeda, K.; Yonezawa, N.; Naoi, K.; Katsumata, T.; Hamano, S.; Nakano, M. Localization of N-linked carbohydrate chains in glycoprotein ZPA of the bovine egg zona pellucida. Eur. J. Biochem. 2002, 269, 4257–4266. [Google Scholar] [CrossRef]
- Florman, H.M.; Fissore, R.A. Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: New York, NY, USA, 2015; Volume 1, pp. 149–196. [Google Scholar]
- Georgadaki, K.; Khoury, N.; Spandidos, D.A.; Zoumpourlis, V. The molecular basis of fertilization (Review). Int. J. Mol. Med. 2016, 38, 979–986. [Google Scholar] [CrossRef]
- Okabe, M. Sperm-egg interaction and fertilization: Past, present, and future. Biol. Reprod. 2018, 99, 134–146. [Google Scholar] [CrossRef]
- Zigo, M.; Manaskova-Postlerova, P.; Zuidema, D.; Kerns, K.; Jonakova, V.; Tumova, L.; Bubenickova, F.; Sutovsky, P. Porcine model for the study of sperm capacitation, fertilization and male fertility. Cell Tissue Res. 2020, 380, 237–262. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Carmona, E.; Bou Khalil, M.; Xu, H.; Berger, T.; Gerton, G.L. New insights into sperm-zona pellucida interaction: Involvement of sperm lipid rafts. Front. Biosci. 2007, 12, 1748–1766. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R. Minimum and maximum extracellular Ca2+ requirements during mouse sperm capacitation and fertilization in vitro. J. Reprod. Fertil. 1987, 81, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Gerton, G.L. Differential release of soluble and matrix components: Evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev. Biol. 2003, 264, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M. Mammalian fertilization: The strange case of sperm protein 56. Bioessays 2009, 31, 153–158. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J.; et al. Remodeling of the plasma membrane in preparation for sperm-egg recognition: Roles of acrosomal proteins. Asian J. Androl. 2015, 17, 574–582. [Google Scholar] [CrossRef]
- López-Salguero, J.B.; Fierro, R.; Michalski, J.C.; Jiménez-Morales, I.; Lefebvre, T.; Mondragón-Payne, O.; Baldini, S.F.; Vercoutter-Edouart, A.S.; González-Márquez, H. Identification of lipid raft glycoproteins obtained from boar spermatozoa. Glycoconj. J. 2020, 37, 499–509. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Mammalian sperm-egg interaction: Identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 1980, 20, 873–882. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 1983, 95, 317–324. [Google Scholar] [CrossRef]
- Beebe, S.J.; Leyton, L.; Burks, D.; Ishikawa, M.; Fuerst, T.; Dean, J.; Saling, P. Recombinant mouse ZP3 inhibits sperm binding and induces the acrosome reaction. Dev. Biol. 1992, 151, 48–54. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein’s sperm receptor activity. Proc. Natl. Acad. Sci. USA 1988, 85, 6778–6782. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. Characterization of mouse ZP3-derived glycopeptide, gp55, that exhibits sperm receptor and acrosome reaction-inducing activity in vitro. Biochemistry 1996, 35, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, R.A.; Sakai, Y.; Wassarman, P.M. Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Thall, A.D.; Malý, P.; Lowe, J.B. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 1995, 270, 21437–21440. [Google Scholar] [CrossRef] [PubMed]
- Litscher, E.S.; Juntunen, K.; Seppo, A.; Penttilä, L.; Niemelä, R.; Renkonen, O.; Wassarman, P.M. Oligosaccharide constructs with defined structures that inhibit binding of mouse sperm to unfertilized eggs in vitro. Biochemistry 1995, 34, 4662–4669. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Mori, T.; Takasaki, S. Binding of mouse sperm to beta-galactose residues on egg zona pellucida and asialofetuin-coupled beads. Biochem. Biophys. Res. Commun. 1997, 238, 95–99. [Google Scholar] [CrossRef]
- Johnston, D.S.; Wright, W.W.; Shaper, J.H.; Hokke, C.H.; Van den Eijnden, D.H.; Joziasse, D.H. Murine sperm-zona binding, a fucosyl residue is required for a high affinity sperm-binding ligand. A second site on sperm binds a nonfucosylated, beta-galactosyl-capped oligosaccharide. J. Biol. Chem. 1998, 273, 1888–1895. [Google Scholar] [CrossRef]
- Bleil, J.D.; Greve, J.M.; Wassarman, P.M. Identification of a secondary sperm receptor in the mouse egg zona pellucida: Role in maintenance of binding of acrosome-reacted sperm to eggs. Dev. Biol. 1988, 128, 376–385. [Google Scholar] [CrossRef]
- Avella, M.A.; Baibakov, B.; Dean, J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J. Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef]
- Saling, P.M.; Sowinski, J.; Storey, B.T. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: Sequential relationship to the acrosome reaction. J. Exp. Zool. 1979, 209, 229–238. [Google Scholar] [CrossRef]
- Saling, P.M.; Storey, B.T. Mouse gamete interactions during fertilization in vitro. Chlortetracycline as a fluorescent probe for the mouse sperm acrosome reaction. J. Cell Biol. 1979, 83, 544–555. [Google Scholar] [CrossRef]
- Baibakov, B.; Gauthier, L.; Talbot, P.; Rankin, T.L.; Dean, J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007, 134, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2011, 108, 20008–20011. [Google Scholar] [CrossRef] [PubMed]
- Buffone, M.G.; Hirohashi, N.; Gerton, G.L. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol. Reprod. 2014, 90, 112. [Google Scholar] [CrossRef]
- Foster, J.A.; Gerton, G.L. The Acrosomal Matrix. Adv. Anat. Embryol. Cell Biol. 2016, 220, 15–33. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Clark, G.F.; Dell, A. Molecular models for murine sperm-egg binding. J. Biol. Chem. 2006, 281, 13853–13856. [Google Scholar] [CrossRef]
- Clark, G.F. Molecular models for mouse sperm-oocyte binding. Glycobiology 2011, 21, 3–5. [Google Scholar] [CrossRef]
- Chakravarty, S.; Kadunganattil, S.; Bansal, P.; Sharma, R.K.; Gupta, S.K. Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol. Reprod. Dev. 2008, 75, 75–88. [Google Scholar] [CrossRef]
- Ozgur, K.; Patankar, M.S.; Oehninger, S.; Clark, G.F. Direct evidence for the involvement of carbohydrate sequences in human sperm-zona pellucida binding. Mol. Hum. Reprod. 1998, 4, 318–324. [Google Scholar] [CrossRef]
- Baibakov, B.; Boggs, N.A.; Yauger, B.; Baibakov, G.; Dean, J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J. Cell Biol. 2012, 197, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Yurewicz, E.C.; Sacco, A.G.; Gupta, S.K.; Xu, N.; Gage, D.A. Hetero-oligomerization-dependent binding of pig oocyte zona pellucida glycoproteins ZPB and ZPC to boar sperm membrane vesicles. J. Biol. Chem. 1998, 273, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, N.; Kudo, K.; Terauchi, H.; Kanai, S.; Yoda, N.; Tanokura, M.; Ito, K.; Miura, K.; Katsumata, T.; Nakano, M. Recombinant porcine zona pellucida glycoproteins expressed in Sf9 cells bind to bovine sperm but not to porcine sperm. J. Biol. Chem. 2005, 280, 20189–20196. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, N.; Amari, S.; Takahashi, K.; Ikeda, K.; Imai, F.L.; Kanai, S.; Kikuchi, K.; Nakano, M. Participation of the nonreducing terminal beta-galactosyl residues of the neutral N-linked carbohydrate chains of porcine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2005, 70, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Smith, M.; Wong, N.K.; Khoo, K.H.; Wu, S.W.; Yu, S.Y.; Patankar, M.S.; Easton, R.; Lattanzio, F.A.; Morris, H.R.; Dell, A.; et al. Analysis of protein-linked glycosylation in a sperm-somatic cell adhesion system. Glycobiology 2007, 17, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Turner, K.O.; Meizel, S.; Hedrick, J.L. Zona pellucida-induced acrosome reaction in boar sperm. Biol. Reprod. 1989, 40, 525–530. [Google Scholar] [CrossRef]
- Mattioli, M.; Lucidi, P.; Barboni, B. Expanded cumuli induce acrosome reaction in boar sperm. Mol. Reprod. Dev. 1998, 51, 445–453. [Google Scholar] [CrossRef]
- Kanai, S.; Yonezawa, N.; Ishii, Y.; Tanokura, M.; Nakano, M. Recombinant bovine zona pellucida glycoproteins ZP3 and ZP4 coexpressed in Sf9 cells form a sperm-binding active hetero-complex. FEBS J. 2007, 274, 5390–5405. [Google Scholar] [CrossRef]
- Amari, S.; Yonezawa, N.; Mitsui, S.; Katsumata, T.; Hamano, S.; Kuwayama, M.; Hashimoto, Y.; Suzuki, A.; Takeda, Y.; Nakano, M. Essential role of the nonreducing terminal alpha-mannosyl residues of the N-linked carbohydrate chain of bovine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2001, 59, 221–226. [Google Scholar] [CrossRef]
- Velásquez, J.G.; Canovas, S.; Barajas, P.; Marcos, J.; Jiménez-Movilla, M.; Gallego, R.G.; Ballesta, J.; Avilés, M.; Coy, P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Reprod. Dev. 2007, 74, 617–628. [Google Scholar] [CrossRef]
- Florman, H.M.; First, N.L. The regulation of acrosomal exocytosis. I. Sperm capacitation is required for the induction of acrosome reactions by the bovine zona pellucida in vitro. Dev. Biol. 1988, 128, 453–463. [Google Scholar] [CrossRef]
- Herz, Z.; Northey, D.; Lawyer, M.; First, N.L. Acrosome reaction of bovine spermatozoa in vivo: Sites and effects of stages of the estrous cycle. Biol. Reprod. 1985, 32, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Didion, B.A.; Graves, C.N. In vivo capacitation and acrosome reaction of bovine sperm in estrous and diestrous cows. J. Anim. Sci. 1986, 62, 1029–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shur, B.D.; Hall, N.G. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J. Cell Biol. 1982, 95, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Scully, N.F.; Shaper, J.H.; Shur, B.D. Spatial and temporal expression of cell surface galactosyltransferase during mouse spermatogenesis and epididymal maturation. Dev. Biol. 1987, 124, 111–124. [Google Scholar] [CrossRef]
- Miller, D.J.; Macek, M.B.; Shur, B.D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature 1992, 357, 589–593. [Google Scholar] [CrossRef]
- Shur, B.D. Glycosyltransferases as cell adhesion molecules. Curr. Opin. Cell Biol. 1993, 5, 854–863. [Google Scholar] [CrossRef]
- Gong, X.; Dubois, D.H.; Miller, D.J.; Shur, B.D. Activation of a G protein complex by aggregation of beta-1,4-galactosyltransferase on the surface of sperm. Science 1995, 269, 1718–1721. [Google Scholar] [CrossRef]
- Shi, X.; Amindari, S.; Paruchuru, K.; Skalla, D.; Burkin, H.; Shur, B.D.; Miller, D.J. Cell surface beta-1,4-galactosyltransferase-I activates G protein-dependent exocytotic signaling. Development 2001, 128, 645–654. [Google Scholar]
- Huszar, G.; Sbracia, M.; Vigue, L.; Miller, D.J.; Shur, B.D. Sperm plasma membrane remodeling during spermiogenetic maturation in men: Relationship among plasma membrane beta 1,4-galactosyltransferase, cytoplasmic creatine phosphokinase, and creatine phosphokinase isoform ratios. Biol. Reprod. 1997, 56, 1020–1024. [Google Scholar] [CrossRef]
- Larson, J.L.; Miller, D.J. Sperm from a variety of mammalian species express beta1,4-galactosyltransferase on their surface. Biol. Reprod. 1997, 57, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Rebeiz, M.; Miller, D.J. Porcine sperm surface beta1,4galactosyltransferase binds to the zona pellucida but is not necessary or sufficient to mediate sperm-zona pellucida binding. Mol. Reprod. Dev. 1999, 54, 379–387. [Google Scholar] [CrossRef]
- Tengowski, M.W.; Wassler, M.J.; Shur, B.D.; Schatten, G. Subcellular localization of beta1,4-galactosyltransferase on bull sperm and its function during sperm-egg interactions. Mol. Reprod. Dev. 2001, 58, 236–244. [Google Scholar] [CrossRef]
- Kallajoki, M.; Parvinen, M.; Suominen, J.J. Expression of acrosin during mouse spermatogenesis: A biochemical and immunocytochemical analysis by a monoclonal antibody C 11 H. Biol. Reprod. 1986, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kashiwabara, S.; Baba, T.; Takada, M.; Watanabe, K.; Yano, Y.; Arai, Y. Primary structure of mouse proacrosin deduced from the cDNA sequence and its gene expression during spermatogenesis. J. Biochem. 1990, 108, 785–791. [Google Scholar] [CrossRef]
- Klemm, U.; Maier, W.M.; Tsaousidou, S.; Adham, I.M.; Willison, K.; Engel, W. Mouse preproacrosin: cDNA sequence, primary structure and postmeiotic expression in spermatogenesis. Differentiation 1990, 42, 160–166. [Google Scholar] [CrossRef]
- Kremling, H.; Keime, S.; Wilhelm, K.; Adham, I.M.; Hameister, H.; Engel, W. Mouse proacrosin gene: Nucleotide sequence, diploid expression, and chromosomal localization. Genomics 1991, 11, 828–834. [Google Scholar] [CrossRef]
- Watanabe, K.; Baba, T.; Kashiwabara, S.; Okamoto, A.; Arai, Y. Structure and organization of the mouse acrosin gene. J. Biochem. 1991, 109, 828–833. [Google Scholar] [CrossRef][Green Version]
- Gilboa, E.; Elkana, Y.; Rigbi, M. Purification and properties of human acrosin. Eur. J. Biochem. 1973, 39, 85–92. [Google Scholar] [CrossRef]
- Schleuning, W.D.; Hell, R.; Fritz, H. Multiple forms of human acrosin: Isolation and properties. Hoppe-Seyler’s Z. Physiol. Chem. 1976, 357, 855–865. [Google Scholar] [CrossRef]
- Anderson, R.A., Jr.; Beyler, S.A.; Mack, S.R.; Zaneveld, L.J. Characterization of a high-molecular-weight form of human acrosin. Comparison with human pancreatic trypsin. Biochem. J. 1981, 199, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Drahorad, J.; Peknicova, J. Subcellular immunochemical localization of acrosin in human spermatozoa during the acrosome reaction and zona pellucida penetration. Fertil. Steril. 1988, 50, 133–141. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuda, Y.; Oshio, S.; Kaneko, S.; Nozawa, S.; Mhori, H.; Akihama, S.; Fujimoto, Y. Human acrosin: Purification and some properties. Arch. Androl. 1991, 27, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.D.; Sepúlveda, M.S.; de Ioannes, A.; Barros, C. The polysulphate binding domain of human proacrosin/acrosin is involved in both the enzyme activation and spermatozoa-zona pellucida interaction. Zygote 1998, 6, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Furlong, L.I.; Hellman, U.; Krimer, A.; Tezón, J.G.; Charreau, E.H.; Vazquez-Levin, M.H. Expression of human proacrosin in Escherichia coli and binding to zona pellucida. Biol. Reprod. 2000, 62, 606–615. [Google Scholar] [CrossRef][Green Version]
- Furlong, L.I.; Veaute, C.; Vazquez-Levin, M.H. Binding of recombinant human proacrosin/acrosin to zona pellucida glycoproteins. II. Participation of mannose residues in the interaction. Fertil. Steril. 2005, 83, 1791–1796. [Google Scholar] [CrossRef]
- Schill, W.B. Immunofluorescent localization of acrosin in spermatozoa by boar acrosin antibodies. Naturwissenschaften 1975, 62, 540–541. [Google Scholar] [CrossRef]
- Jones, R.; Brown, C.R. Identification of a zona-binding protein from boar spermatozoa as proacrosin. Exp. Cell Res. 1987, 171, 503–508. [Google Scholar] [CrossRef]
- Jones, R.; Brown, C.R.; Lancaster, R.T. Carbohydrate-binding properties of boar sperm proacrosin and assessment of its role in sperm-egg recognition and adhesion during fertilization. Development 1988, 102, 781–792. [Google Scholar]
- Baba, T.; Kashiwabara, S.; Watanabe, K.; Itoh, H.; Michikawa, Y.; Kimura, K.; Takada, M.; Fukamizu, A.; Arai, Y. Activation and maturation mechanisms of boar acrosin zymogen based on the deduced primary structure. J. Biol. Chem. 1989, 264, 11920–11927. [Google Scholar] [CrossRef]
- Baba, T.; Michikawa, Y.; Kawakura, K.; Arai, Y. Activation of boar proacrosin is effected by processing at both N- and C-terminal portions of the zymogen molecule. FEBS Lett. 1989, 244, 132–136. [Google Scholar] [CrossRef]
- Jones, R. Interaction of zona pellucida glycoproteins, sulphated carbohydrates and synthetic polymers with proacrosin, the putative egg-binding protein from mammalian spermatozoa. Development 1991, 111, 1155–1163. [Google Scholar] [PubMed]
- Puigmulé, M.; Fàbrega, A.; Yeste, M.; Bonet, S.; Pinart, E. Study of the proacrosin-acrosin system in epididymal, ejaculated and in vitro capacitated boar spermatozoa. Reprod. Fertil. Dev. 2011, 23, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zigo, M.; Dorosh, A.; Pohlova, A.; Jonakova, V.; Sulc, M.; Manaskova-Postlerova, P. Panel of monoclonal antibodies to sperm surface proteins as a tool for monitoring localization and identification of sperm-zona pellucida receptors. Cell Tissue Res. 2015, 359, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Easton, M.P.; Munson, M.E.; Doane, M.A. Immunofluorescent localization of bovine acrosin. J. Exp. Zool. 1975, 191, 127–131. [Google Scholar] [CrossRef]
- Mansouri, A.; Phi-van, L.; Geithe, H.P.; Engel, W. Proacrosin/acrosin activity during spermiohistogenesis of the bull. Differentiation 1983, 24, 149–152. [Google Scholar] [CrossRef]
- De los Reyes, M.; Barros, C. Immunolocalization of proacrosin/acrosin in bovine sperm and sperm penetration through the zona pellucida. Anim. Reprod. Sci. 2000, 58, 215–228. [Google Scholar] [CrossRef]
- Gao, Z.; Garbers, D.L. Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J. Biol. Chem. 1998, 273, 3415–3421. [Google Scholar] [CrossRef]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef]
- Gao, Z.; Harumi, T.; Garbers, D.L. Chromosome localization of the mouse zonadhesin gene and the human zonadhesin gene (ZAN). Genomics 1997, 41, 119–122. [Google Scholar] [CrossRef]
- Wilson, M.D.; Riemer, C.; Martindale, D.W.; Schnupf, P.; Boright, A.P.; Cheung, T.L.; Hardy, D.M.; Schwartz, S.; Scherer, S.W.; Tsui, L.C.; et al. Comparative analysis of the gene-dense ACHE/TFR2 region on human chromosome 7q22 with the orthologous region on mouse chromosome 5. Nucleic Acids Res. 2001, 29, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.M.; Garbers, D.L. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J. Biol. Chem. 1995, 270, 26025–26028. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Hickox, J.R.; Winfrey, V.P.; Olson, G.E.; Hardy, D.M. Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem. J. 2003, 375, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Bi, M. Biochemical and Functional Characterization of Zonadhesin: A Sperm Protein Potentially Mediating Species-Specific Sperm-Egg Adhesion during Fertilization. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2002. [Google Scholar]
- Tanphaichitr, N.; Smith, J.; Mongkolsirikieart, S.; Gradil, C.; Lingwood, C.A. Role of a gamete-specific sulfoglycolipid immobilizing protein on mouse sperm-egg binding. Dev. Biol. 1993, 156, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Moase, C.E.; Kamolvarin, N.; Kan, F.W.; Tanphaichitr, N. Localization and role of sulfoglycolipid immobilizing protein 1 on the mouse sperm head. Mol. Reprod. Dev. 1997, 48, 518–528. [Google Scholar] [CrossRef]
- Tantibhedhyangkul, J.; Weerachatyanukul, W.; Carmona, E.; Xu, H.; Anupriwan, A.; Michaud, D.; Tanphaichitr, N. Role of sperm surface arylsulfatase A in mouse sperm-zona pellucida binding. Biol. Reprod. 2002, 67, 212–219. [Google Scholar] [CrossRef]
- Ngernsoungnern, A.; Weerachatyanukul, W.; Saewu, A.; Thitilertdecha, S.; Sobhon, P.; Sretarugsa, P. Rat sperm AS-A: Subcellular localization in testis and epididymis and surface distribution in epididymal sperm. Cell Tissue Res. 2004, 318, 353–363. [Google Scholar] [CrossRef]
- Redgrove, K.A.; Nixon, B.; Baker, M.A.; Hetherington, L.; Baker, G.; Liu, D.Y.; Aitken, R.J. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS ONE 2012, 7, e50851. [Google Scholar] [CrossRef]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef]
- Carmona, E.; Weerachatyanukul, W.; Soboloff, T.; Fluharty, A.L.; White, D.; Promdee, L.; Ekker, M.; Berger, T.; Buhr, M.; Tanphaichitr, N. Arylsulfatase a is present on the pig sperm surface and is involved in sperm-zona pellucida binding. Dev. Biol. 2002, 247, 182–196. [Google Scholar] [CrossRef]
- Kelsey, K.M.; Zigo, M.; Thompson, W.E.; Kerns, K.; Manandhar, G.; Sutovsky, M.; Sutovsky, P. Reciprocal surface expression of arylsulfatase A and ubiquitin in normal and defective mammalian spermatozoa. Cell Tissue Res. 2020, 379, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, R.A.; Armant, D.R.; Millette, C.F. Characterization of fucosyltransferase activity during mouse spermatogenesis: Evidence for a cell surface fucosyltransferase. Biochemistry 1989, 28, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.A.; Cardullo, R.A.; Millette, C.F. Expression and topographical localization of cell surface fucosyltransferase activity during epididymal sperm maturation in the mouse. Gamete Res. 1989, 22, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Chung, M.K.; Koistinen, R.; Koistinen, H.; Seppala, M.; Ho, P.C.; Ng, E.H.; Lee, K.F.; Yeung, W.S. Glycodelin-A interacts with fucosyltransferase on human sperm plasma membrane to inhibit spermatozoa-zona pellucida binding. J. Cell Sci. 2007, 120, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A.; Tulsiani, D.R.; Orgebin-Crist, M.C. Inhibition of the mouse sperm surface alpha-D-mannosidase inhibits sperm-egg binding in vitro. Biol. Reprod. 1991, 44, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Tulsiani, D.R.; Skudlarek, M.D.; Orgebin-Crist, M.C. Human sperm plasma membranes possess alpha-D-mannosidase activity but no galactosyltransferase activity. Biol. Reprod. 1990, 42, 843–858. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C.; Carreras, A. Expression of D-mannose binding sites on human spermatozoa: Comparison of fertile donors and infertile patients. Fertil. Steril. 1991, 56, 113–118. [Google Scholar] [CrossRef]
- Eberspaecher, U.; Roosterman, D.; Krätzschmar, J.; Haendler, B.; Habenicht, U.F.; Becker, A.; Quensel, C.; Petri, T.; Schleuning, W.D.; Donner, P. Mouse androgen-dependent epididymal glycoprotein CRISP-1 (DE/AEG): Isolation, biochemical characterization, and expression in recombinant form. Mol. Reprod. Dev. 1995, 42, 157–172. [Google Scholar] [CrossRef]
- Cohen, D.J.; Ellerman, D.A.; Cuasnicu, P.S. Mammalian sperm-egg fusion: Evidence that epididymal protein DE plays a role in mouse gamete fusion. Biol. Reprod. 2000, 63, 462–468. [Google Scholar] [CrossRef]
- Busso, D.; Cohen, D.J.; Maldera, J.A.; Dematteis, A.; Cuasnicu, P.S. A novel function for CRISP1 in rodent fertilization: Involvement in sperm-zona pellucida interaction. Biol. Reprod. 2007, 77, 848–854. [Google Scholar] [CrossRef]
- Cohen, D.J.; Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Muñoz, M.W.; Battistone, M.A.; Cuasnicú, P.S. Epididymal protein CRISP1 plays different roles during the fertilization process. J. Androl. 2011, 32, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Fujimoto, S.; Takano, H.; Ushiki, T.; Abe, K.; Ishikura, H.; Yoshida, M.C.; Kirchhoff, C.; Ishibashi, T.; Kasahara, M. Characterization of a human glycoprotein with a potential role in sperm-egg fusion: cDNA cloning, immunohistochemical localization, and chromosomal assignment of the gene (AEGL1). Genomics 1996, 32, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Maldera, J.A.; Weigel Muñoz, M.; Chirinos, M.; Busso, D.; Ge Raffo, F.; Battistone, M.A.; Blaquier, J.A.; Larrea, F.; Cuasnicu, P.S. Human fertilization: Epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol. Hum. Reprod. 2014, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Leyton, L.; Saling, P. 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell 1989, 57, 1123–1130. [Google Scholar] [CrossRef]
- Burks, D.J.; Carballada, R.; Moore, H.D.; Saling, P.M. Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science 1995, 269, 83–86. [Google Scholar] [CrossRef]
- Naz, R.K.; Alexander, N.J.; Isahakia, M.; Hamilton, M.S. Monoclonal antibody to a human germ cell membrane glycoprotein that inhibits fertilization. Science 1984, 225, 342–344. [Google Scholar] [CrossRef]
- Naz, R.K.; Phillips, T.M.; Rosenblum, B.B. Characterization of the fertilization antigen 1 for the development of a contraceptive vaccine. Proc. Natl. Acad. Sci. USA 1986, 83, 5713–5717. [Google Scholar] [CrossRef]
- Naz, R.K.; Sacco, A.G.; Yurewicz, E.C. Human spermatozoal FA-1 binds with ZP3 of porcine zona pellucida. J. Reprod. Immunol. 1991, 20, 43–58. [Google Scholar] [CrossRef]
- Naz, R.K.; Brazil, C.; Overstreet, J.W. Effects of antibodies to sperm surface fertilization antigen-1 on human sperm-zona pellucida interaction. Fertil. Steril. 1992, 57, 1304–1310. [Google Scholar] [CrossRef]
- Kadam, A.L.; Fateh, M.; Naz, R.K. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J. Reprod. Immunol. 1995, 29, 19–30. [Google Scholar] [CrossRef]
- Zhu, X.; Naz, R.K. Fertilization antigen-1: cDNA cloning, testis-specific expression, and immunocontraceptive effects. Proc. Natl. Acad. Sci. USA 1997, 94, 4704–4709. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Zhu, X. Molecular cloning and sequencing of cDNA encoding for human FA-1 antigen. Mol. Reprod. Dev. 2002, 63, 256–268. [Google Scholar] [CrossRef]
- Ensslin, M.A.; Shur, B.D. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 2003, 114, 405–417. [Google Scholar] [CrossRef]
- Shur, B.D.; Ensslin, M.A.; Rodeheffer, C. SED1 function during mammalian sperm-egg adhesion. Curr. Opin. Cell Biol. 2004, 16, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Copland, S.D.; Murphy, A.A.; Shur, B.D. The mouse gamete adhesin, SED1, is expressed on the surface of acrosome-intact human sperm. Fertil. Steril. 2009, 92, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, M.; Vogel, T.; Calvete, J.J.; Thole, H.H.; Schmidtke, J.; Matsuda, T.; Topfer-Petersen, E. Molecular cloning and characterization of P47, a novel boar sperm-associated zona pellucida-binding protein homologous to a family of mammalian secretory proteins. Biol. Reprod. 1998, 58, 1057–1064. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Lakamp, A.; Gentzel, M.; Ekhlasi-Hundrieser, M.; Topfer-Petersen, E. Fate of lactadherin P47 during post-testicular maturation and capacitation of boar spermatozoa. Reproduction 2003, 125, 377–387. [Google Scholar] [CrossRef]
- Hagaman, J.R.; Moyer, J.S.; Bachman, E.S.; Sibony, M.; Magyar, P.L.; Welch, J.E.; Smithies, O.; Krege, J.H.; O’Brien, D.A. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 1998, 95, 2552–2557. [Google Scholar] [CrossRef]
- Ramaraj, P.; Kessler, S.P.; Colmenares, C.; Sen, G.C. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J. Clin. Investig. 1998, 102, 371–378. [Google Scholar] [CrossRef]
- Foresta, C.; Indino, M.; Manoni, F.; Scandellari, C. Angiotensin-converting enzyme content of human spermatozoa and its release during capacitation. Fertil. Steril. 1987, 47, 1000–1003. [Google Scholar] [CrossRef]
- Köhn, F.M.; Miska, W.; Schill, W.B. Release of angiotensin-converting enzyme (ACE) from human spermatozoa during capacitation and acrosome reaction. J. Androl. 1995, 16, 259–265. [Google Scholar] [PubMed]
- Köhn, F.M.; Dammshäuser, I.; Neukamm, C.; Renneberg, H.; Siems, W.E.; Schill, W.B.; Aumüller, G. Ultrastructural localization of angiotensin-converting enzyme in ejaculated human spermatozoa. Hum. Reprod. 1998, 13, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Pilch, B.; Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006, 7, R40. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, H.; Sato, S.; Shibuya, M. Localization of angiotensin converting enzyme (dipeptidyl carboxypeptidase) in swine sperm by immunofluorescence. Life Sci. 1984, 35, 1257–1261. [Google Scholar] [CrossRef]
- Gatti, J.L.; Druart, X.; Guerin, Y.; Dacheux, F.; Dacheux, J.L. A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE); evidence that sperm are the source of this ACE. Biol. Reprod. 1999, 60, 937–945. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Mactier, S.; Kohnke, P.L.; Kershaw-Young, C.M.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013, 91, 13–22. [Google Scholar] [CrossRef]
- Zigo, M.; Jonakova, V.; Sulc, M.; Manaskova-Postlerova, P. Characterization of sperm surface protein patterns of ejaculated and capacitated boar sperm, with the detection of ZP binding candidates. Int. J. Biol. Macromol. 2013, 61, 322–328. [Google Scholar] [CrossRef]
- Costa, D.S.; Thundathil, J.C. Characterization and activity of angiotensin-converting enzyme in Holstein semen. Anim. Reprod. Sci. 2012, 133, 35–42. [Google Scholar] [CrossRef]
- Ojaghi, M.; Kastelic, J.; Thundathil, J. Testis-specific isoform of angiotensin-converting enzyme (tACE) is involved in the regulation of bovine sperm capacitation. Mol. Reprod. Dev. 2017, 84, 376–388. [Google Scholar] [CrossRef]
- Ojaghi, M.; Kastelic, J.; Thundathil, J.C. Testis-specific isoform of angiotensin-converting enzyme (tACE) as a candidate marker for bull fertility. Reprod. Fertil. Dev. 2018, 30, 1584–1593. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Identification of a ZP3-binding protein on acrosome-intact mouse sperm by photoaffinity crosslinking. Proc. Natl. Acad. Sci. USA 1990, 87, 5563–5567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Le, T.; Palacios, M.; Bookbinder, L.H.; Wassarman, P.M.; Suzuki, F.; Bleil, J.D. Sperm-egg recognition in the mouse: Characterization of sp56, a sperm protein having specific affinity for ZP3. J. Cell Biol. 1994, 125, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cha, M.C.; Gerton, G.L. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol. Reprod. 2001, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Muro, Y.; Buffone, M.G.; Okabe, M.; Gerton, G.L. Function of the acrosomal matrix: Zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol. Reprod. 2012, 86, 1–6. [Google Scholar] [CrossRef]
- Sullivan, R.; Bleau, G. Interaction between isolated components from mammalian sperm and egg. Gamete Res. 1985, 12, 101–116. [Google Scholar] [CrossRef]
- Sullivan, R.; Robitaille, G. Heterogeneity of epididymal spermatozoa of the hamster. Gamete Res. 1989, 24, 229–236. [Google Scholar] [CrossRef]
- Bérubé, B.; Sullivan, R. Inhibition of in vivo fertilization by active immunization of male hamsters against a 26-kDa sperm glycoprotein. Biol. Reprod. 1994, 51, 1255–1263. [Google Scholar] [CrossRef]
- Bégin, S.; Bérubé, B.; Boué, F.; Sullivan, R. Comparative immunoreactivity of mouse and hamster sperm proteins recognized by an anti-P26h hamster sperm protein. Mol. Reprod. Dev. 1995, 41, 249–256. [Google Scholar] [CrossRef]
- Boué, F.; Bérubé, B.; De Lamirande, E.; Gagnon, C.; Sullivan, R. Human sperm-zona pellucida interaction is inhibited by an antiserum against a hamster sperm protein. Biol. Reprod. 1994, 51, 577–587. [Google Scholar] [CrossRef]
- Boué, F.; Blais, J.; Sullivan, R. Surface localization of P34H an epididymal protein, during maturation, capacitation, and acrosome reaction of human spermatozoa. Biol. Reprod. 1996, 54, 1009–1017. [Google Scholar] [CrossRef][Green Version]
- Van Gestel, R.A.; Brewis, I.A.; Ashton, P.R.; Brouwers, J.F.; Gadella, B.M. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol. Hum. Reprod. 2007, 13, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Parent, S.; Lefievre, L.; Brindle, Y.; Sullivan, R. Bull subfertility is associated with low levels of a sperm membrane antigen. Mol. Reprod. Dev. 1998, 52, 57–65. [Google Scholar] [CrossRef]
- Lessard, C.; Parent, S.; Leclerc, P.; Bailey, J.L.; Sullivan, R. Cryopreservation alters the levels of the bull sperm surface protein P25b. J. Androl. 2000, 21, 700–707. [Google Scholar] [PubMed]
- Frenette, G.; Sullivan, R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 2001, 59, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Calvete, J.J.; Jonakova, V.; Topfer-Petersen, E. Boar spermadhesins AQN-1 and AWN are sperm-associated acrosin inhibitor acceptor proteins. FEBS Lett. 1992, 300, 63–66. [Google Scholar] [CrossRef]
- Sanz, L.; Calvete, J.J.; Schäfer, W.; Mann, K.; Töpfer-Petersen, E. Isolation and biochemical characterization of two isoforms of a boar sperm zona pellucida-binding protein. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1992, 1119, 127–132. [Google Scholar] [CrossRef]
- Veselsky, L.; Jonakova, V.; Sanz, M.L.; Topfer-Petersen, E.; Cechova, D. Binding of a 15 kDa glycoprotein from spermatozoa of boars to surface of zona pellucida and cumulus oophorus cells. J. Reprod. Fertil. 1992, 96, 593–602. [Google Scholar] [CrossRef]
- Dostalova, Z.; Calvete, J.J.; Topfer-Petersen, E. Interaction of non-aggregated boar AWN-1 and AQN-3 with phospholipid matrices. A model for coating of spermadhesins to the sperm surface. Biol. Chem. 1995, 376, 237–242. [Google Scholar] [CrossRef]
- Ensslin, M.; Calvete, J.J.; Thole, H.H.; Sierralta, W.D.; Adermann, K.; Sanz, L.; Topfer-Petersen, E. Identification by affinity chromatography of boar sperm membrane-associated proteins bound to immobilized porcine zona pellucida. Mapping of the phosphorylethanolamine-binding region of spermadhesin AWN. Biol. Chem. 1995, 376, 733–738. [Google Scholar]
- Calvete, J.J.; Carrera, E.; Sanz, L.; Töpfer-Petersen, E. Boar spermadhesins AQN-1 and AQN-3: Oligosaccharide and zona pellucida binding characteristics. Biol. Chem. 1996, 377, 521–527. [Google Scholar] [CrossRef]
- Jonakova, V.; Kraus, M.; Veselsky, L.; Cechova, D.; Bezouska, K.; Ticha, M. Spermadhesins of the AQN and AWN families, DQH sperm surface protein and HNK protein in the heparin-binding fraction of boar seminal plasma. J. Reprod. Fertil. 1998, 114, 25–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veselsky, L.; Peknicova, J.; Cechova, D.; Kraus, M.; Geussova, G.; Jonakova, V. Characterization of boar spermadhesins by monoclonal and polyclonal antibodies and their role in binding to oocytes. Am. J. Reprod. Immunol. 1999, 42, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Harrison, R.A.; Topfer-Petersen, E. Only low levels of spermadhesin AWN are detectable on the surface of live ejaculated boar spermatozoa. Reprod. Fertil. Dev. 2000, 12, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tichá, M.; Kraus, M.; Cechová, D.; Jonáková, V. Saccharide-binding properties of boar AQN spermadhesins and DQH sperm surface protein. Folia Biol. 1998, 44, 15–21. [Google Scholar]
- Jonakova, V.; Manaskova, P.; Kraus, M.; Liberda, J.; Ticha, M. Sperm surface proteins in mammalian fertilization. Mol. Reprod. Dev. 2000, 56, 275–277. [Google Scholar] [CrossRef]
- Manaskova, P.; Peknicova, J.; Elzeinova, F.; Ticha, M.; Jonakova, V. Origin, localization and binding abilities of boar DQH sperm surface protein tested by specific monoclonal antibodies. J. Reprod. Immunol. 2007, 74, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Liberda, J.; Ryslavá, H.; Jelínková, P.; Jonáková, V.; Tichá, M. Affinity chromatography of bull seminal proteins on mannan-Sepharose. J. Chromatogr. B 2002, 780, 231–239. [Google Scholar] [CrossRef]
- Lin, Y.N.; Roy, A.; Yan, W.; Burns, K.H.; Matzuk, M.M. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol. Cell. Biol. 2007, 27, 6794–6805. [Google Scholar] [CrossRef]
- Yu, Y.; Vanhorne, J.; Oko, R. The origin and assembly of a zona pellucida binding protein, IAM38, during spermiogenesis. Microsc. Res. Tech. 2009, 72, 558–565. [Google Scholar] [CrossRef]
- Yatsenko, A.N.; O’Neil, D.S.; Roy, A.; Arias-Mendoza, P.A.; Chen, R.; Murthy, L.J.; Lamb, D.J.; Matzuk, M.M. Association of mutations in the zona pellucida binding protein 1 (ZPBP1) gene with abnormal sperm head morphology in infertile men. Mol. Hum. Reprod. 2012, 18, 14–21. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, J.; Zhang, H.; Wen, Y.; Zhang, H.; Cui, Y.; Tian, J.; Jiang, M.; Liu, X.; Wang, G.; et al. Proteomic Analysis of Dpy19l2-Deficient Human Globozoospermia Reveals Multiple Molecular Defects. Proteom. Clin. Appl. 2019, 13, e1900007. [Google Scholar] [CrossRef]
- Mori, E.; Baba, T.; Iwamatsu, A.; Mori, T. Purification and characterization of a 38-kDa protein, sp38, with zona pellucida-binding property from porcine epididymal sperm. Biochem. Biophys. Res. Commun. 1993, 196, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Kashiwabara, S.; Baba, T.; Inagaki, Y.; Mori, T. Amino acid sequences of porcine Sp38 and proacrosin required for binding to the zona pellucida. Dev. Biol. 1995, 168, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, W.; Yi, Y.J.; Sutovsky, P.; Oko, R. The extracellular protein coat of the inner acrosomal membrane is involved in zona pellucida binding and penetration during fertilization: Characterization of its most prominent polypeptide (IAM38). Dev. Biol. 2006, 290, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Dubova-Mihailova, M.; Mollova, M.; Ivanova, M.; Kehayov, I.; Kyurkchiev, S. Identification and characterization of human acrosomal antigen defined by a monoclonal antibody with blocking effect on in vitro fertilization. J. Reprod. Immunol. 1991, 19, 251–268. [Google Scholar] [CrossRef]
- Foster, J.A.; Klotz, K.L.; Flickinger, C.J.; Thomas, T.S.; Wright, R.M.; Castillo, J.R.; Herr, J.C. Human SP-10: Acrosomal distribution, processing, and fate after the acrosome reaction. Biol. Reprod. 1994, 51, 1222–1231. [Google Scholar] [CrossRef]
- Hamatani, T.; Tanabe, K.; Kamei, K.; Sakai, N.; Yamamoto, Y.; Yoshimura, Y. A monoclonal antibody to human SP-10 inhibits in vitro the binding of human sperm to hamster oolemma but not to human Zona pellucida. Biol. Reprod. 2000, 62, 1201–1208. [Google Scholar] [CrossRef]
- Coonrod, S.A.; Herr, J.C.; Westhusin, M.E. Inhibition of bovine fertilization in vitro by antibodies to SP-10. J. Reprod. Fertil. 1996, 107, 287–297. [Google Scholar] [CrossRef]
- Avilés, M.; Abascal, I.; Martínez-Menárguez, J.A.; Castells, M.T.; Skalaban, S.R.; Ballesta, J.; Alhadeff, J.A. Immunocytochemical localization and biochemical characterization of a novel plasma membrane-associated, neutral pH optimum alpha-L-fucosidase from rat testis and epididymal spermatozoa. Biochem. J. 1996, 318 Pt 3, 821–831. [Google Scholar] [CrossRef]
- Phopin, K.; Nimlamool, W.; Bartlett, M.J.; Bean, B.S. Distribution, crypticity, stability, and localization of α-L-fucosidase of mouse cauda epididymal sperm. Mol. Reprod. Dev. 2012, 79, 208–217. [Google Scholar] [CrossRef]
- Phopin, K.; Nimlamool, W.; Lowe-Krentz, L.J.; Douglass, E.W.; Taroni, J.N.; Bean, B.S. Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol. Reprod. Dev. 2013, 80, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Venditti, J.J.; Donigan, K.A.; Bean, B.S. Crypticity and functional distribution of the membrane associated alpha-L-fucosidase of human sperm. Mol. Reprod. Dev. 2007, 74, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Venditti, J.J.; Bean, B.S. Stabilization of membrane-associated alpha-L-fucosidase by the human sperm equatorial segment. Int. J. Androl. 2009, 32, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, A.; Vanha-Perttula, T. alpha-L-Fucosidase in the reproductive organs and seminal plasma of the bull. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1986, 880, 91–95. [Google Scholar] [CrossRef]
- Peterson, R.N.; Hunt, W.P. Identification, isolation, and properties of a plasma membrane protein involved in the adhesion of boar sperm to the porcine zona pellucida. Gamete Res. 1989, 23, 103–118. [Google Scholar] [CrossRef]
- Zayas-Perez, H.; Casas, E.; Bonilla, E.; Betancourt, M. Inhibition of sperm-zona pellucida binding by a 55 kDa pig sperm protein in vitro. Arch. Androl. 2005, 51, 195–206. [Google Scholar] [CrossRef]
- Redgrove, K.A.; Anderson, A.L.; Dun, M.D.; McLaughlin, E.A.; O’Bryan, M.K.; Aitken, R.J.; Nixon, B. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev. Biol. 2011, 356, 460–474. [Google Scholar] [CrossRef]
- Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J.; Whitelegge, J.; et al. Proteomic Characterization of Pig Sperm Anterior Head Plasma Membrane Reveals Roles of Acrosomal Proteins in ZP3 Binding. J. Cell. Physiol. 2015, 230, 449–463. [Google Scholar] [CrossRef]
- Shur, B.D.; Bennett, D. A specific defect in galactosyltransferase regulation on sperm bearing mutant alleles of the T/t locus. Dev. Biol. 1979, 71, 243–259. [Google Scholar] [CrossRef]
- Lopez, L.C.; Bayna, E.M.; Litoff, D.; Shaper, N.L.; Shaper, J.H.; Shur, B.D. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J. Cell Biol. 1985, 101, 1501–1510. [Google Scholar] [CrossRef]
- Nixon, B.; Lu, Q.; Wassler, M.J.; Foote, C.I.; Ensslin, M.A.; Shur, B.D. Galactosyltransferase function during mammalian fertilization. Cells Tissues Organs 2001, 168, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Fayrer-Hosken, R.A.; Caudle, A.B.; Shur, B.D. Galactosyltransferase activity is restricted to the plasma membranes of equine and bovine sperm. Mol. Reprod. Dev. 1991, 28, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Shur, B.D. Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 1997, 124, 4121–4131. [Google Scholar] [PubMed]
- Lyng, R.; Shur, B.D. Sperm-egg binding requires a multiplicity of receptor-ligand interactions: New insights into the nature of gamete receptors derived from reproductive tract secretions. Soc. Reprod. Fertil. Suppl. 2007, 65, 335–351. [Google Scholar]
- Topfer-Petersen, E.; Friess, A.E.; Nguyen, H.; Schill, W.B. Evidence for a fucose-binding protein in boar spermatozoa. Histochemistry 1985, 83, 139–145. [Google Scholar] [CrossRef]
- Topfer-Petersen, E.; Henschen, A. Acrosin shows zona and fucose binding, novel properties for a serine proteinase. FEBS Lett. 1987, 226, 38–42. [Google Scholar] [CrossRef]
- Baba, T.; Azuma, S.; Kashiwabara, S.; Toyoda, Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 1994, 269, 31845–31849. [Google Scholar] [CrossRef]
- Isotani, A.; Matsumura, T.; Ogawa, M.; Tanaka, T.; Yamagata, K.; Ikawa, M.; Okabe, M. A delayed sperm penetration of cumulus layers by disruption of acrosin gene in rats. Biol. Reprod. 2017, 97, 61–68. [Google Scholar] [CrossRef]
- Adham, I.M.; Nayernia, K.; Engel, W. Spermatozoa lacking acrosin protein show delayed fertilization. Mol. Reprod. Dev. 1997, 46, 370–376. [Google Scholar] [CrossRef]
- Dudkiewicz, A.B. Inhibition of fertilization in the rabbit by anti-acrosin antibodies. Mol. Reprod. Dev. 1983, 8, 183–197. [Google Scholar] [CrossRef]
- Liu, D.Y.; Baker, H.W. Inhibition of acrosin activity with a trypsin inhibitor blocks human sperm penetration of the zona pellucida. Biol. Reprod. 1993, 48, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Honda, A.; Fulka, H.; Tamura-Nakano, M.; Matoba, S.; Tomishima, T.; Mochida, K.; Hasegawa, A.; Nagashima, K.; Inoue, K.; et al. Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proc. Natl. Acad. Sci. USA 2020, 117, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Honda, A.; Kashiwabara, S.I.; Baba, T. Difference of acrosomal serine protease system between mouse and other rodent sperm. Dev. Genet. 1999, 25, 115–122. [Google Scholar] [CrossRef]
- Wassarman, P.M. Zona pellucida glycoproteins. J. Biol. Chem. 2008, 283, 24285–24289. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Naturil-Alfonso, C.; Jiménez-Trigos, E.; Lavara, R.; Vicente, J.S. Influence of zona pellucida thickness on fertilization, embryo implantation and birth. Anim. Reprod. Sci. 2012, 132, 96–100. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Fonseca Balvís, N.; Querejeta-Fernández, A.; Izquierdo-Rico, M.J.; González-Brusi, L.; Lorenzo, P.L.; García-Rebollar, P.; Avilés, M.; Bermejo-Álvarez, P. ZP4 confers structural properties to the zona pellucida essential for embryo development. Elife 2019, 8. [Google Scholar] [CrossRef]
- Abe, H.; Oikawa, T. Ultrastructural evidence for an association between an oviductal glycoprotein and the zona pellucida of the golden hamster egg. J. Exp. Zool. 1990, 256, 210–221. [Google Scholar] [CrossRef]
- Wiesak, T.; Wasielak, M.; Złotkowska, A.; Milewski, R. Effect of vitrification on the zona pellucida hardening and follistatin and cathepsin B genes expression and developmental competence of in vitro matured bovine oocytes. Cryobiology 2017, 76, 18–23. [Google Scholar] [CrossRef]
- Balakier, H.; Sojecki, A.; Motamedi, G.; Bashar, S.; Mandel, R.; Librach, C. Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels? Fertil. Steril. 2012, 98, 77–83. [Google Scholar] [CrossRef]
- Herlyn, H.; Zischler, H. The molecular evolution of sperm zonadhesin. Int. J. Dev. Biol. 2008, 52, 781–790. [Google Scholar] [CrossRef]
- Tardif, S.; Cormier, N. Role of zonadhesin during sperm-egg interaction: A species-specific acrosomal molecule with multiple functions. Mol. Hum. Reprod. 2011, 17, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.M.; Garbers, D.L. Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J. Biol. Chem. 1994, 269, 19000–19004. [Google Scholar] [PubMed]
- Hickox, J.R.; Bi, M.; Hardy, D.M. Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. J. Biol. Chem. 2001, 276, 41502–41509. [Google Scholar] [CrossRef] [PubMed]
- Dudkiewicz, A.B. Purification of boar acrosomal arylsulfatase A and possible role in the penetration of cumulus cells. Biol. Reprod. 1984, 30, 1005–1014. [Google Scholar] [CrossRef]
- White, D.; Weerchatyanukul, W.; Gadella, B.M.; Kamolvarin, N.; Attar, M.; Tanphaichitr, N. Role of sperm sulfogalactosylglycerolipid in mouse sperm-zona pellucida binding. Biol. Reprod. 2000, 63, 147–155. [Google Scholar] [CrossRef]
- Rattanachaiyanont, M.; Weerachatyanukul, W.; Leveille, M.-C.; Taylor, T.; D’Amours, D.; Rivers, D.; Leader, A.; Tanphaichitr, N. Anti-SLIP1-reactive proteins exist on human sperm and are involved in zona-pellucida binding. Mol. Hum. Reprod. 2001, 7, 633–640. [Google Scholar] [CrossRef]
- Weerachatyanukul, W.; Xu, H.; Anupriwan, A.; Carmona, E.; Wade, M.; Hermo, L.; da Silva, S.M.; Rippstein, P.; Sobhon, P.; Sretarugsa, P.; et al. Acquisition of arylsulfatase A onto the mouse sperm surface during epididymal transit. Biol. Reprod. 2003, 69, 1183–1192. [Google Scholar] [CrossRef]
- Schenk, M.; Koppisetty, C.A.; Santos, D.C.; Carmona, E.; Bhatia, S.; Nyholm, P.G.; Tanphaichitr, N. Interaction of arylsulfatase-A (ASA) with its natural sulfoglycolipid substrates: A computational and site-directed mutagenesis study. Glycoconj. J. 2009, 26, 1029–1045. [Google Scholar] [CrossRef]
- Gadella, B.M.; Colenbrander, B.; Golde, L.M.v.; Lopes-Cardozo, M. Boar seminal vesicles secrete arylsulfatases into seminal plasma: Evidence that desulfation of seminolipid occurs only after ejaculation. Biol. Reprod. 1993, 48, 483–489. [Google Scholar] [CrossRef]
- Carmona, E.; Weerachatyanukul, W.; Xu, H.; Fluharty, A.; Anupriwan, A.; Shoushtarian, A.; Chakrabandhu, K.; Tanphaichitr, N. Binding of arylsulfatase A to mouse sperm inhibits gamete interaction and induces the acrosome reaction. Biol. Reprod. 2002, 66, 1820–1827. [Google Scholar] [CrossRef]
- Gonzalez-Cadavid, V.; Martins, J.A.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.; Monteiro-Moreira, A.C.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef]
- Silva, E.; Frost, D.; Li, L.; Bovin, N.; Miller, D.J. Lactadherin is a candidate oviduct Lewis X trisaccharide receptor on porcine spermatozoa. Andrology 2017, 5, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zigo, M.; Manaskova-Postlerova, P.; Jonakova, V.; Kerns, K.; Sutovsky, P. Compartmentalization of the proteasome-interacting proteins during sperm capacitation. Sci. Rep. 2019, 9, 12583. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.L.; O’Gorman, C.; Zhao, J.; Samuel, M.; Walters, E.; Yi, Y.J.; Sutovsky, M.; Prather, R.S.; Wells, K.D.; Sutovsky, P. Transgenic pig carrying green fluorescent proteasomes. Proc. Natl. Acad. Sci. USA 2013, 110, 6334–6339. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.W.; Manandhar, G.; Yi, Y.J.; Gupta, S.K.; Sutovsky, M.; Odhiambo, J.F.; Powell, M.D.; Miller, D.J.; Sutovsky, P. Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS ONE 2011, 6, e17256. [Google Scholar] [CrossRef]
- Zigo, M.; Jonakova, V.; Manaskova-Postlerova, P.; Kerns, K.; Sutovsky, P. Ubiquitin-proteasome system participates in the de-aggregation of spermadhesin and DQH protein during boar sperm capacitation. Reproduction 2019, 157, 283–295. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Jovine, L.; Litscher, E.S. A profile of fertilization in mammals. Nat. Cell Biol. 2001, 3, E59–E64. [Google Scholar] [CrossRef]
- Foster, J.A.; Friday, B.B.; Maulit, M.T.; Blobel, C.; Winfrey, V.P.; Olson, G.E.; Kim, K.S.; Gerton, G.L. AM67, a secretory component of the guinea pig sperm acrosomal matrix, is related to mouse sperm protein sp56 and the complement component 4-binding proteins. J. Biol. Chem. 1997, 272, 12714–12722. [Google Scholar] [CrossRef]
- Kim, K.S.; Foster, J.A.; Gerton, G.L. Differential release of guinea pig sperm acrosomal components during exocytosis. Biol. Reprod. 2001, 64, 148–156. [Google Scholar] [CrossRef]
- Buffone, M.G.; Zhuang, T.; Ord, T.S.; Hui, L.; Moss, S.B.; Gerton, G.L. Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J. Biol. Chem. 2008, 283, 12438–12445. [Google Scholar] [CrossRef]
- Da Ros, V.G.; Maldera, J.A.; Willis, W.D.; Cohen, D.J.; Goulding, E.H.; Gelman, D.M.; Rubinstein, M.; Eddy, E.M.; Cuasnicu, P.S. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 2008, 320, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Ahmad, K. Molecular identities of human sperm proteins that bind human zona pellucida: Nature of sperm-zona interaction, tyrosine kinase activity, and involvement of FA-1. Mol. Reprod. Dev. 1994, 39, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Bhargava, K.K. Antibodies to sperm surface fertilization antigen (FA-1): Their specificities and site of interaction with sperm in male genital tract. Mol. Reprod. Dev. 1990, 26, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.P.; Zhan, Q.T.; Le, F.; Zheng, Y.M.; Jin, F. Angiotensin-converting enzymes play a dominant role in fertility. Int. J. Mol. Sci. 2013, 14, 21071–21086. [Google Scholar] [CrossRef]
- Castilho, C.S.; Fontes, P.K.; Franchi, F.F.; Santos, P.H.; Razza, E.M. Renin-Angiotensin System on Reproductive Biology. In Renin-Angiotensin System Past, Present and Future; Tolekova, A., Ed.; InTech: Rijeka, Croatia, 2017; p. 258. [Google Scholar]
- Reis, A.B.; Araújo, F.C.; Pereira, V.M.; Dos Reis, A.M.; Santos, R.A.; Reis, F.M. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: Implications for male infertility. J. Mol. Histol. 2010, 41, 75–80. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020, 9, 920. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Petit, F.M.; Serres, C.; Bourgeon, F.; Pineau, C.; Auer, J. Identification of sperm head proteins involved in zona pellucida binding. Hum. Reprod. 2013, 28, 852–865. [Google Scholar] [CrossRef]
- Feiden, S.; Wolfrum, U.; Wegener, G.; Kamp, G. Expression and compartmentalisation of the glycolytic enzymes GAPDH and pyruvate kinase in boar spermatogenesis. Reprod. Fertil. Dev. 2008, 20, 713–723. [Google Scholar] [CrossRef]
- Topfer-Petersen, E.; Romero, A.; Varela, P.F.; Ekhlasi-Hundrieser, M.; Dostalova, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia 1998, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jonakova, V.; Ticha, M. Boar seminal plasma proteins and their binding properties. Collect. Czechoslov. Chem. Commun. 2004, 69, 461–475. [Google Scholar] [CrossRef]
- Jonakova, V.; Manaskova, P.; Ticha, M. Separation, characterization and identification of boar seminal plasma proteins. J. Chromatogr. B 2007, 849, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Jonakova, V.; Jonak, J.; Ticha, M. Proteomics of Male Seminal Plasma. In Reproductive Genomics in Domestic Animals; Jiang, Z., Ott, T.L., Eds.; Blackwell Publishing: Oxford, UK, 2010; pp. 339–368. [Google Scholar]
- Ekhlasi-Hundrieser, M.; Gohr, K.; Wagner, A.; Tsolova, M.; Petrunkina, A.; Topfer-Petersen, E. Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol. Reprod. 2005, 73, 536–545. [Google Scholar] [CrossRef]
- Calvete, J.J.; Raida, M.; Gentzel, M.; Urbanke, C.; Sanz, L.; Topfer-Petersen, E. Isolation and characterization of heparin- and phosphorylcholine-binding proteins of boar and stallion seminal plasma. Primary structure of porcine pB1. FEBS Lett. 1997, 407, 201–206. [Google Scholar] [CrossRef]
- Bezouska, K.; Sklenár, J.; Novák, P.; Halada, P.; Havlícek, V.; Kraus, M.; Tichá, M.; Jonáková, V. Determination of the complete covalent structure of the major glycoform of DQH sperm surface protein, a novel trypsin-resistant boar seminal plasma O-glycoprotein related to pB1 protein. Protein Sci. 1999, 8, 1551–1556. [Google Scholar] [CrossRef]
- Fan, J.; Lefebvre, J.; Manjunath, P. Bovine seminal plasma proteins and their relatives: A new expanding superfamily in mammals. Gene 2006, 375, 63–74. [Google Scholar] [CrossRef]
- Plante, G.; Prud’homme, B.; Fan, J.; Lafleur, M.; Manjunath, P. Evolution and function of mammalian binder of sperm proteins. Cell Tissue Res. 2016, 363, 105–127. [Google Scholar] [CrossRef]
- Kim, E.; Park, K.E.; Kim, J.S.; Baek, D.C.; Lee, J.W.; Lee, S.R.; Kim, M.S.; Kim, S.H.; Kim, C.S.; Koo, D.B.; et al. Importance of the porcine ADAM3 disintegrin domain in sperm-egg interaction. J. Reprod. Dev. 2009, 55, 156–162. [Google Scholar] [CrossRef]
- Mori, E.; Fukuda, H.; Imajoh-Ohmi, S.; Mori, T.; Takasaki, S. Purification of N-acetyllactosamine-binding activity from the porcine sperm membrane: Possible involvement of an ADAM complex in the carbohydrate-binding activity of sperm. J. Reprod. Dev. 2012, 58, 117–125. [Google Scholar] [CrossRef]
- Srivastava, N.; Jerome, A.; Srivastava, S.K.; Ghosh, S.K.; Kumar, A. Bovine seminal PDC-109 protein: An overview of biochemical and functional properties. Anim. Reprod. Sci. 2013, 138, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, T.M.; Ignotz, G.G.; Suarez, S.S. PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol. Reprod. 2003, 69, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Somashekar, L.; Selvaraju, S.; Parthipan, S.; Ravindra, J.P. Profiling of sperm proteins and association of sperm PDC-109 with bull fertility. Syst. Biol. Reprod. Med. 2015, 61, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, D.; Singh, I.; Yadav, P.S. Seminal Plasma Proteome: Promising Biomarkers for Bull Fertility. Agric. Res. 2012, 1, 78–86. [Google Scholar] [CrossRef]
- Kelly, V.C.; Kuy, S.; Palmer, D.J.; Xu, Z.; Davis, S.R.; Cooper, G.J. Characterization of bovine seminal plasma by proteomics. Proteomics 2006, 6, 5826–5833. [Google Scholar] [CrossRef]
- Redgrove, K.A.; Aitken, R.J.; Nixon, B. More Than a Simple Lock and Key Mechanism: Unraveling the Intricacies of Sperm-Zona Pellucida Binding. In Binding Protein; Abdelmohsen, K., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 206. [Google Scholar]
- van Gestel, R.A.; Brewis, I.A.; Ashton, P.R.; Helms, J.B.; Brouwers, J.F.; Gadella, B.M. Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol. Hum. Reprod. 2005, 11, 583–590. [Google Scholar] [CrossRef]
- Bou Khalil, M.; Chakrabandhu, K.; Xu, H.; Weerachatyanukul, W.; Buhr, M.; Berger, T.; Carmona, E.; Vuong, N.; Kumarathasan, P.; Wong, P.T.; et al. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev. Biol. 2006, 290, 220–235. [Google Scholar] [CrossRef]
- Gadella, B.M.; Tsai, P.S.; Boerke, A.; Brewis, I.A. Sperm head membrane reorganisation during capacitation. Int. J. Dev. Biol. 2008, 52, 473–480. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Bou Khalil, M.; Weerachatyanukul, W.; Kates, M.; Xu, H.; Carmona, E.; Attar, M.; Carrier, D. Physiological and Biophysical Properties of Male Germ Cell Sulfogalactosylglycerolipid. In Male Fertility and Lipid Metabolsim; De Vriese, S.R., Christophe, A.B., Eds.; AOCS Press: Champaign, IL, USA, 2003; p. 279. [Google Scholar]
- Tanphaichitr, N.; Kongmanas, K.; Faull, K.F.; Whitelegge, J.; Compostella, F.; Goto-Inoue, N.; Linton, J.J.; Doyle, B.; Oko, R.; Xu, H.; et al. Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog. Lipid Res. 2018, 72, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Attar, M.; Kates, M.; Bou Khalil, M.; Carrier, D.; Wong, P.T.; Tanphaichitr, N. A Fourier-transform infrared study of the interaction between germ-cell specific sulfogalactosylglycerolipid and dimyristoylglycerophosphocholine. Chem. Phys. Lipids 2000, 106, 101–114. [Google Scholar] [CrossRef]
- Weerachatyanukul, W.; Probodh, I.; Kongmanas, K.; Tanphaichitr, N.; Johnston, L.J. Visualizing the localization of sulfoglycolipids in lipid raft domains in model membranes and sperm membrane extracts. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2007, 1768, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Weerachatyanukul, W.; Rattanachaiyanont, M.; Carmona, E.; Furimsky, A.; Mai, A.; Shoushtarian, A.; Sirichotiyakul, S.; Ballakier, H.; Leader, A.; Tanphaichitr, N. Sulfogalactosylglycerolipid is involved in human gamete interaction. Mol. Reprod. Dev. 2001, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Asquith, K.L.; Baleato, R.M.; McLaughlin, E.A.; Nixon, B.; Aitken, R.J. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 2004, 117, 3645–3657. [Google Scholar] [CrossRef]
- Kamaruddin, M.; Kroetsch, T.; Basrur, P.K.; Hansen, P.J.; King, W.A. Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia 2004, 36, 327–334. [Google Scholar] [CrossRef]
- Spinaci, M.; Volpe, S.; Bernardini, C.; De Ambrogi, M.; Tamanini, C.; Seren, E.; Galeati, G. Immunolocalization of heat shock protein 70 (Hsp 70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 2005, 72, 534–541. [Google Scholar] [CrossRef]
- Nixon, B.; Aitken, R.J. The biological significance of detergent-resistant membranes in spermatozoa. J. Reprod. Immunol. 2009, 83, 8–13. [Google Scholar] [CrossRef]
- Nixon, B.; Bielanowicz, A.; McLaughlin, E.A.; Tanphaichitr, N.; Ensslin, M.A.; Aitken, R.J. Composition and significance of detergent resistant membranes in mouse spermatozoa. J. Cell. Physiol. 2009, 218, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Naaby-Hansen, S.; Herr, J.C. Heat shock proteins on the human sperm surface. J. Reprod. Immunol. 2010, 84, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Asquith, K.L.; Harman, A.J.; McLaughlin, E.A.; Nixon, B.; Aitken, R.J. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol. Reprod. 2005, 72, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Whelan, D.; Bielanowicz, A.; Skinner, B.; Aitken, R.J.; O’Bryan, M.K.; Nixon, B. Identification of the molecular chaperone, heat shock protein 1 (chaperonin 10), in the reproductive tract and in capacitating spermatozoa in the male mouse. Biol. Reprod. 2008, 78, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Dun, M.D.; Smith, N.D.; Baker, M.A.; Lin, M.; Aitken, R.J.; Nixon, B. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J. Biol. Chem. 2011, 286, 36875–36887. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, N.; Palestini, P.; Botto, L.; Mattioli, M.; Barboni, B. Membrane Dynamics Occuring during Capacitation of Mammalian Spermatozoa. In Spermatozoa. Biology, Motility and Function and Chromosomal Abnormalities; Erickson, B.T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 99–122. [Google Scholar]
- Sutovsky, P. Sperm proteasome and fertilization. Reproduction 2011, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Morales, P.; Sutovsky, P. Regulation of Sperm Capacitation by the 26S Proteasome: An Emerging New Paradigm in Spermatology. Biol. Reprod. 2016, 94, 117. [Google Scholar] [CrossRef]
- Sasanami, T.; Sugiura, K.; Tokumoto, T.; Yoshizaki, N.; Dohra, H.; Nishio, S.; Mizushima, S.; Hiyama, G.; Matsuda, T. Sperm proteasome degrades egg envelope glycoprotein ZP1 during fertilization of Japanese quail (Coturnix japonica). Reproduction 2012, 144, 423–431. [Google Scholar] [CrossRef]
- Sawada, H.; Mino, M.; Akasaka, M. Sperm proteases and extracellular ubiquitin-proteasome system involved in fertilization of ascidians and sea urchins. Adv. Exp. Med. Biol. 2014, 759, 1–11. [Google Scholar] [CrossRef]
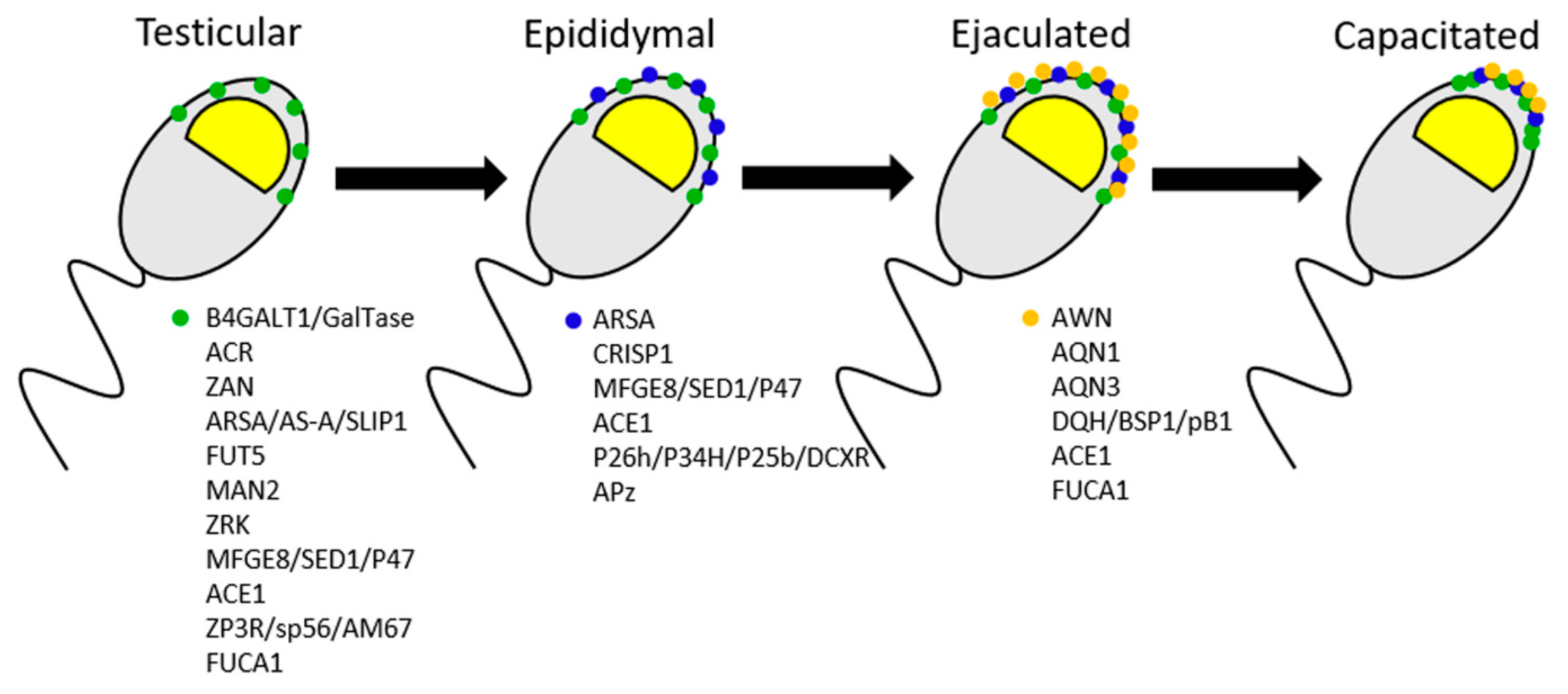

| Mammalian Species | ZP Gene | ZP Protein | Molecular Weight (kDa) | Homology with | References | |||
|---|---|---|---|---|---|---|---|---|
| Mouse | Human | Porcine | Bovine | |||||
| Mouse | ZP1 (ZPB1) | ZP1 | 200 (dimer) | - | 68% | - | - | [21,22,23,24,25] |
| ZP2 (ZPA) | ZP2 | 120 | - | 58% | 55% | 57% | ||
| ZP3 (ZPC) | ZP3 | 83 | - | 68% | 66% | 64% | ||
| ZP4 (ZPB/ZPB2) | not expressed | - | - | - | - | - | ||
| Human | ZP1 (ZPB1) | ZP1 | 65 | 68% | - | - | - | [26,27,28,29] |
| ZP2 (ZPA) | ZP2 | 120 | 58% | - | 64% | 67% | ||
| ZP3 (ZPC) | ZP3 | 58 | 68% | - | 74% | 72% | ||
| ZP4 (ZPB/ZPB2) | ZP4 | 65 | - | - | 68% | 69% | ||
| Porcine | ZP1 (ZPB1) | not expressed | - | - | - | - | - | [30,31,32,33,34,35,36,37,38] |
| ZP2 (ZPA) | ZP2/PZPL | 90 | 55% | 64% | - | 78% | ||
| ZP3 (ZPC) | ZP3/ZP3-β | 55 | 66% | 74% | - | 84% | ||
| ZP4 (ZPB/ZPB2) | ZP4/ZP-α | 55 | - | 68% | - | 76% | ||
| Bovine | ZP1 (ZPB1) | not expressed | - | - | - | - | [39,40,41] | |
| ZP2 (ZPA) | ZP2 | 76 | 57% | 67% | 78% | - | ||
| ZP3 (ZPC) | ZP3 | 47 | 64% | 72% | 84% | - | ||
| ZP4 (ZPB/ZPB2) | ZP4 | 68 | - | 69% | 76% | - | ||
| Protein with ZP-Binding Affinity | Species | Origin | Localization | Binding Activity | References |
|---|---|---|---|---|---|
| β1,4-Galactosyltransferase (B4GALT1/GalTase) | Mouse/rat | Male germ cells | Plasma membrane overlying the acrosome region | Binding to N-acetylglucosamine (GlcNAc) residues of ZP3, an inducer of AE via G-proteins, binds to terminal GlcNAc residues on O-linked oligosaccharides of ZP3 | [119,120,121,122,123,124] |
| Human | Unknown | Binding to ZP is assumed | [125] | ||
| Boar | Anterior part of the sperm head, PM of the acrosome region, periacrosomal region of the sperm head | Binding to N-acetylglucosamine (GlcNAc) residues of ZP3 and/or ZP4; not necessary for sperm to bind ZP | [126,127] | ||
| Bull | Anterior part of the sperm head, periacrosomal region of the sperm head | [126,128] | |||
| Proacrosin/acrosin (ACR) | Mouse/rat | Pachytene spermatocytes | Sperm acrosomal part | Binding non-enzymatically to ZP glycoproteins, mediating the secondary or tight binding of spermatozoa to the zona pellucida following the acrosome reaction | [129,130,131,132,133] |
| Human | Acrosome, sperm surface in acrosomal cap | Binding to the solubilized ZP, interaction with mannose residues in ZP | [134,135,136,137,138,139,140,141] | ||
| Boar | Spermatids | Inner acrosomal membrane and acrosome, sperm surface in acrosomal cap | High-affinity binding activity to sulfated oligosaccharide chains in ZP, secondary binding molecule; mediating or primary binding molecule, ZP-binding activity | [142,143,144,145,146,147,148,149] | |
| Bull | Spermatids | Acrosomal region | [150,151,152] | ||
| Zonadhesin (ZAN) | Mouse | Male germ cells | Outer acrosomal membrane and acrosomal matrix, a portion of ZAN translocates to the apical head region during sperm capacitation | Binding to the extracellular matrix of the oocyte, stimulation of tyrosine kinase activity leading to acrosomal exocytosis | [80,153,154] |
| Human | Male germ cells | Membrane protein, apical head region, acrosome matrix | Binding to ZP3 | [155,156] | |
| Boar | Germ cells—haploid spermatids | Transmembrane protein, apical head region in acrosome matrix | Binding to sulfated carbohydrates in ZP | [153,157,158] | |
| Bull | Male germ cells | Outer acrosomal membrane and acrosomal matrix | Binding to the extracellular matrix of the oocyte (assumed based on the other species) | [159] | |
| Arylsulfatase A (ARSA/AS-A/SLIP1) | Mouse/rat | Male germ cells, epididymal fluid | Acrosomal matrix, sperm surface overlying acrosome | Binding ability to ZP sulfated glycans | [160,161,162,163] |
| Human | Acrosomal matrix, sperm surface overlying acrosome | ZP binding | [164,165] | ||
| Boar | Sperm head surface and acrosome, the head anterior region | Binding to sulfated sugar residues of the acidic ZP glycans present in ZP3α | [166] | ||
| Bull | Convex ridge of the plasma membrane in the acrosomal part | ZP binding, assumed | [167] | ||
| α1–3-Fucosyltransefrase (FUT5) | Mouse | Male germ cells | Sperm head plasma membrane | Binding sites or receptor for ZP, sperm–oocyte recognition | [168,169] |
| Human | Integral membrane protein in the acrosomal region | Interaction with solubilized human zona pellucida | [170] | ||
| α-D-Mannosidase (MAN2) | Mouse | Male germ cells | Plasma membrane overlying the acrosome | Binding molecule or receptor for ZP | [171] |
| Human | Sperm plasma membrane | Role as a ligand for sperm-ZP recognition and binding, sperm surface α-D-mannosidase binds high mannose oligosaccharide units of ZP | [172,173] | ||
| Cysteine-rich secretory protein (CRISP1) | Mouse/rat | Epididymis | Dorsal region of the acrosome | ZP-binding activity | [174,175,176,177] |
| Human | Epididymis | Sperm head plasma membrane? | Binding to ZP-intact human eggs, specific interaction with ZP3 | [178,179] | |
| Zona receptor kinase (ZRK) | Mouse | Sperm head plasma membrane | Binding to the extracellular matrix of the oocyte | [180] | |
| Human | Male germ cells | Sperm surface in the acrosomal region | Receptor for ZP3 | [181] | |
| Fertilization antigen-1 (FA-1) | Mouse | Testis | Sperm surface glycoprotein | [182,183,184,185,186,187,188] | |
| Human | Sperm surface glycoprotein | Recognition and binding to ZP3 | [182,184,185,186,188] | ||
| MFGE8/SED1/P47/lactadherin | Mouse/rat | Male germ cells, Caput epididymis | Sperm plasma membrane overlying the acrosome | Recognition and binding to carbohydrate residues of mZP2 and mZP3 | [189,190] |
| Human | Sperm plasma membrane overlying the acrosome | ZP-binding activity, assumed | [191] | ||
| Boar | Testis | Peripherally associated, the apical ridge of the sperm head or entire acrosome region | ZP-binding activity | [80,149,192,193] | |
| Angiotensin-converting enzyme 1 (ACE1) | Mouse | Spermatids | Sperm plasma membrane overlying the acrosome | ZP-binding activity | [194,195] |
| Human | Spermatids, Seminal plasma | Sperm plasma membrane overlying the acrosome, connecting piece, midpiece | ZP-binding activity, assumed | [196,197,198,199] | |
| Boar | Spermatids, epididymal fluid Seminal plasma | Sperm plasma membrane overlying the acrosome, connecting piece, midpiece | ZP-binding activity | [200,201,202,203] | |
| Bull | Spermatids, epididymal fluid Seminal plasma | Sperm plasma membrane overlying the acrosome, connecting piece, principal piece | ZP-binding activity, assumed | [201,202,203,204,205,206] | |
| ZP3R/sp56/AM67 | Mouse/rat/guinea pig | Male germ cells | Overlying the sperm acrosome, the head of acrosome intact sperm, plasma membrane protein | Binding to terminal galactose residue present on ZP3 O-linked oligosaccharides | [78,207,208,209,210] |
| P26h/P34H/P25b/carbonyl reductase (DCXR) | Mouse/hamster | Epididymis—epididymosomes | Plasma membrane overlying the acrosome | [211,212,213,214] | |
| Human | Epididymis—epididymosomes | Plasma membrane overlying the acrosome | Involved in the primary ZP binding | [215,216] | |
| Boar | Apical plasma membrane | [217] | |||
| Bull | Epididymis—epididymosomes | Plasma membrane overlying the acrosome | [218,219,220] | ||
| Spermadhesins AWN, AQN1, AQN3 | Boar | Seminal plasma | Sperm plasma membrane surface | Binding to Galβ(1–3)-GalNAc and Ga1β(1–4)-GlcNAc carbohydrate structures, ZP-binding activity | [217,221,222,223,224,225,226,227,228,229] |
| Binder of sperm protein DQH/BSP1/pB1 | Boar | Seminal vesicles | Sperm plasma membrane surface, entire sperm head, in the acrosome region | Interaction with sialylated ZP glycoproteins | [230,231,232] |
| Bull | Seminal vesicles | Nonreducing terminal α-mannosyl residues of the N-linked high-mannose-type chains | [108,114,233] | ||
| ZPBP1/sp38/IAM38 | Mouse | Spermatids | Outer and inner acrosomal membrane | Secondary ZP binding | [234,235] |
| Human | Spermatids | Acrosomal matrix | Secondary ZP binding | [236,237] | |
| Boar | Spermatids | Acrosomal matrix, inner acrosomal membrane, sperm surface in capacitated spermatozoa | Secondary ZP binding may be involved in primary ZP binding due to its localization in capacitated spermatozoa | [238,239,240] | |
| Bull | Spermatids | Acrosomal matrix, inner acrosomal membrane, sperm surface in capacitated spermatozoa | Secondary ZP binding may be involved in primary ZP binding due to its localization in capacitated spermatozoa | [2,240] | |
| SPACA2/SP-10/ACV1 | Mouse | Spermatids | Acrosomal matrix | Sperm attachment to ZP and ZP penetration was inhibited by anti-SP-10 antibodies | [241] |
| Human | Spermatids | Acrosomal matrix | SP-10 does not seem to be involved in ZP binding; however, ZP penetration was inhibited by anti-SP-10 antibodies | [241,242,243] | |
| Boar | Spermatids | Acrosomal matrix, sperm surface in capacitated spermatozoa | Surface localization implies the role in primary ZP binding, sperm attachment to ZP and ZP penetration was inhibited by anti-SP-10 antibodies | [80,241] | |
| Bull | Spermatids | Acrosomal matrix | Anti-SP-10 antibodies reduced secondary sperm-ZP binding | [244] | |
| alpha-L-fucosidase (FUCA1) | Mouse/Rat | Spermatids Seminal plasma | Plasma membrane overlying the acrosome, equatorial segment | Anti-FUCA1 antibodies inhibited ZP binging | [245,246,247] |
| Human | Spermatids Seminal plasma | Plasma membrane overlying the acrosome, equatorial segment | ZP binding assumed | [248,249] | |
| Bull | Spermatids Seminal plasma | Unknown | ZP binding assumed | [250] | |
| Adhesion protein z (APz) | Boar | Epididymis | Integral plasma membrane protein | Adhesion of capacitated sperm to the oocyte prior to the acrosomal reaction | [251,252] |
| 26S proteasome | Human | Plasma membrane overlying the acrosome | Component of high-molecular-weight ZP-binding complexes | [253] | |
| Boar | Spermatids | Plasma membrane overlying the acrosome | Component of high-molecular-weight ZP-binding complexes | [254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumova, L.; Zigo, M.; Sutovsky, P.; Sedmikova, M.; Postlerova, P. Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals. Cells 2021, 10, 133. https://doi.org/10.3390/cells10010133
Tumova L, Zigo M, Sutovsky P, Sedmikova M, Postlerova P. Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals. Cells. 2021; 10(1):133. https://doi.org/10.3390/cells10010133
Chicago/Turabian StyleTumova, Lucie, Michal Zigo, Peter Sutovsky, Marketa Sedmikova, and Pavla Postlerova. 2021. "Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals" Cells 10, no. 1: 133. https://doi.org/10.3390/cells10010133
APA StyleTumova, L., Zigo, M., Sutovsky, P., Sedmikova, M., & Postlerova, P. (2021). Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals. Cells, 10(1), 133. https://doi.org/10.3390/cells10010133







